Abstract
Plant polyphenols including flavonoids and tannins are important constituent of our everyday diet and medical herbals. It is broadly accepted that polyphenols may protect us from toxins, carcinogens and pollutants though the mechanisms of the polyphenols action is still not clear. Here we discuss the ability of polyphenols and especially gallate rich compounds like tannins and catechin gallates to interact with proteins and lipids, establish binding between adjacent bilayer surfaces and initiate membrane aggregation. This phenomena discovered in model experiments could also influence lateral segregation and compartmentalization of cell surface compounds and assist the cell-cell interaction and signal transduction. The involvement of plant polyphenols in communication between cells could be an important factor responsible for anticarcinogenic, vascular and cardioprotective activity of these compounds and speculated to be implicated in the evolution of human brain and intelligence.
Key words: human evolution, polyphenols, flavonoids, tannins, membranes, lipid rafts, bilayer structure, cell communication
Plant polyphenols including flavonoids and tannins are present on our daily diet. The opinion on their influence on the human health is contradictory and ranges widely from positive to skeptic and even to alarming.1–3 Obviously the processes of their functioning in our body should be studied in more details.
The polyphenols are know not only as patent antioxidants but also as cell metabolism regulators.4 The biological functioning of these compounds begins from their interaction with the cell surface and penetration through the plasma membrane into the cytoplasm. They can influence various physical properties of membrane lipids including diffusion, melting temperature, detergent solubility, osmotic stability, permeability to water soluble compounds5–7 and general parameters of lipid packing and intrinsic bilayer curvature related to membrane interaction and fusion. Their penetration through the lipid bilayer inversive correlates with the number of hydroxyl groups and accordingly with the hydrophobicity of molecules.
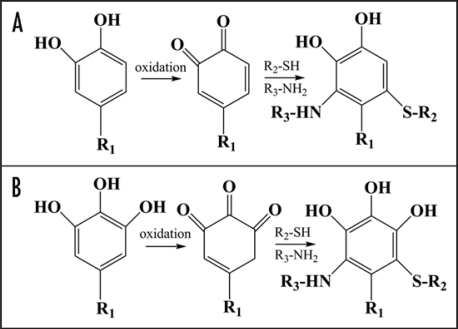
The background of polyphenols functioning is attributed to the ability of these compounds to interact with proteins, lipids and polynucleotides through electrostatic, hydrophobic, and even covalent binding.6–9 The polyphenolic compounds are generally not very active chemically and only electrostatic and hydrophobic forces are responsible for their interaction within cells. However, after oxidation of catechol and gallate10 moieties to quinones (Fig. 1) in presence of peroxidases, polyphenol oxidases, alkaline pH, or transition metals they could be involved in reactions with free nucleophilic functional groups such as sulfhydryl, amine, amide, indole and imidazole substituents;11 and result in covalent binding with different residues including lysine, methionine, cysteine and tryptophane.8,12 We expect that terminal amino groups of phosphatidylethanolamines or sulfolipids of biological membranes can also participate in covalent binding with quinones. The consequence of chemical derivatization of proteins may lead to changes in a-helix and random coiled constituents and be accompanied by modulation of protein physical properties and functioning.8
Figure 1.
A simplified scheme of catechol and gallate moieties oxidation to quinones and subsequent quinones reaction with terminal amino- and sulfa-groups of proteins and lipids (R2 or R3). The catechol and gallate moieties are attached to a larger molecule of polyphenol (R1). For details see reviews.8,12
Among the polyphenolic compounds, tannins express the strongest influence on the lipid bilayer properties. The influence depends on the presence of numerous gallic moieties in the tannin molecule.13 Tannins are able to bind on the membrane surface, establish bridges between apposing bilayers and initiate their interaction and adhesion.14 The process is accompanied by reduction of the membrane dipole potential, lipid interdigitation and the decrease of interbilayer spacing from 15 Å to 5 Å. According to authors13 this happens because tannins (1) are amphipathic and partition into the bilayers interfacial region, (2) are long enough to span the interbilayer space, (3) contain several gallic acids distributed so that they can partition simultaneously into apposing bilayers, and (4) have sufficient gallic acid residues to interact with all lipid headgroups and cover the bilayer surface. The electrostatic interaction between the π electrons in the phenol ring and -N+(CH3)3 groups of phosphatidylcholines is also should be considered.
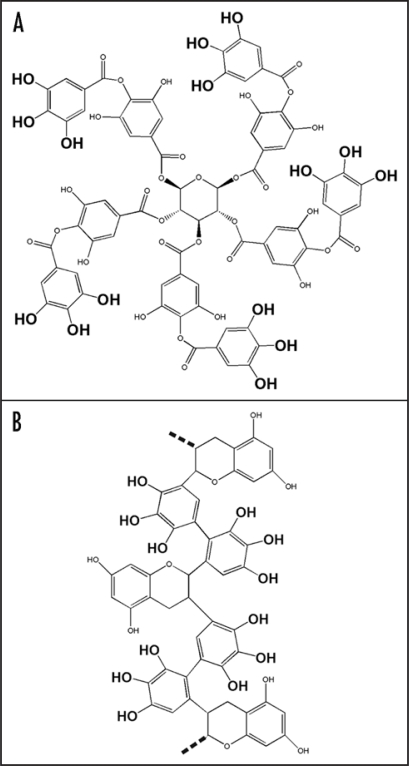
It is necessary to mention that besides tannins the gallate moiety is present also in some antimalarial agents as rufigallol and exifone.15 The tea catechin gallate and a number of related compounds including gallocatechin gallate, epicatechin gallate, epigallocatechin gallate contain the gallic moiety and was found to influence the membrane fluidity.16 Some of the tea gallates reveal anticancer activity correlated with membrane rigidifying effect.17 Not long ago novel black tea pigments rich with gallate moieties and produced by oxidation of tea epagallocatechin-3-o-gallate were discovered (Fig. 2).18 The appearance of this kind of pigments in food is very typical example of enzymatic and thermal browning process accompanying various traditional preparation of beverages (tea, coffee, cacao, beer and so on) and meal (roasted or baked vegetables and meet).11
Figure 2.
Tannin (A) and a fragment of polymeric chain of gallate rich black tea pigment (B). The triplets of neighboring hydroxyl groups of gallic acid are emphasized by a larger font.
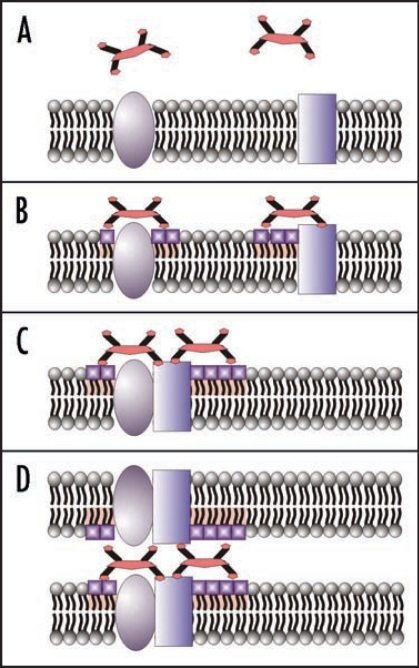
According to our hypotheses (Fig. 3) the polyphenols rich with gallate moieties may attach to the cell surface, change physical properties of lipids, initiate lateral segregation and formation of lipid and protein clusters, and finally, initiate binding of neighbouring cells. The lateral segregation of the bilayer compounds is known as a physical background of membranes compartmentalization and lipid rafts formation.19,20 This process is relevant for cell-cell communication, signalling and metabolism regulation; and, thus, responsible for numerous medically recognizable processes.21 It is known that lipid rafts are enriched with cholesterol and sphingolipids that serve as a platform for gathering of signalling and trafficking proteins.22 Tannins and other gallate rich molecules may specifically interact with -N+(CH3)3 groups of sphingomyelin present in the outer leaflet of plasma membrane. High reactivity of gallates towards nucleophilic moieties of proteins especially in presence of peroxidases may facilitate formation of covalent bridges between adjacent cells.
Figure 3.
Polyphenols rich of gallate moieties can interact with the cell surface (A). Then gallates may initiate the phospholipid rigidifying process (physically modified lipids are emphasized by shape and color) (B) and lead to aggregation of some proteins and lipids in clusters (D). Polyphenol molecules could serve as bridges between surfaces of two neighbor cells and initiate cells binding and formation of similar clusters in the membrane of the opposite cell.
Plant polyphenols as catechins of tea23,24 are involved in cell-cell interactions responsible for their anticarcinogenic activity by upregulating the receptor and signaling cascades of communication between cells. Well known cardiovascular protective effect of plant polyphenols can also be explained by influence of these compounds on interaction between endothelial cells.25
The cell-cell communication and signal transduction is very important also for functioning of neural networks. In human evolution the plant polyphenol consumption would be considerable improved after invention of the fire cooking. It was supposed that the thermal treatment of meat and vegetables, mostly wild tubers of antic savanna, was an important factor of the brain evolution.26 The increase of protein, lipid and carbohydrates availability contributes the most noticeable gain in human intellectual development.27 The role of polyphenols in neuronal communication and brain evolution is still has to be uncovered. Flavonoids are known to protect the brain from oxidation stress and prevent the aging and numerous pathological processes including neuronal damage during Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis.28,29 It seems the mechanism of polyphenols action on the brain functioning could not be restricted by prevention against oxidation stress because these compounds could also participate in the intracellular signal transduction cascades and modulation of brain functioning.30 This may be explained by interactions of polyphenols with specific proteins and plasma membrane compartments responsible for signal transduction as well as for memory formation and storage.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/6359
References
- 1.Kroon P, Williamson G. Polyphenols: dietary components with established benefits to health? J Sci Food Agricult. 2005;85:1239–1240. [Google Scholar]
- 2.Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 3.Zhou SF, Xue CC, Yu XQ, Wang G. Metabolic activation of herbal and dietary constituents and its clinical and toxicological implications: an update. Curr Drug Metab. 2007;8:526–553. doi: 10.2174/138920007781368863. [DOI] [PubMed] [Google Scholar]
- 4.Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med. 2004;36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Arora A, Byrem TM, Nair MG, Strasburg GM. Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Arch Biochem Biophys. 2000;373:102–109. doi: 10.1006/abbi.1999.1525. [DOI] [PubMed] [Google Scholar]
- 6.Hendrich AB. Flavonoid-membrane interactions: possible consequences for biological effects of some polyphenolic compounds. Acta Pharm Sinica. 2006;27:27–40. doi: 10.1111/j.1745-7254.2006.00238.x. [DOI] [PubMed] [Google Scholar]
- 7.Oteiza PI, Erlejman AG, Verstraeten SV, Keen CL, Fraga CG. Flavonoid-membrane interactions: a protective role of flavonoids at the membrane surface? Clin Dev Immunol. 2005;12:19–25. doi: 10.1080/10446670410001722168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kroll J, Rawel HM, Rohn S. Reactions of plant phenolics with food proteins and enzymes under special consideration of covalent bounds. Food Sci Technol Res. 2003;9:205–218. [Google Scholar]
- 9.Hadi SM, Bhat SH, Azmi AS, Hanif S, Shamim U, Ullah MF. Oxidative breakage of cellular DNA by plant polyphenols: a putative mechanism for anticancer properties. Semin Cancer Biol. 2007;17:370–376. doi: 10.1016/j.semcancer.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Rawel HM, Ranters H, Rohn S, Kroll J. Assessment of the reactivity of selected isoflavones against proteins in comparison to quercetin. J Agric Food Chem. 2004;52:5263–5271. doi: 10.1021/jf0354850. [DOI] [PubMed] [Google Scholar]
- 11.Bittner S. When quinones meet amino acids: chemical, physical and biological consequences. Amino Acids. 2006;30:205–224. doi: 10.1007/s00726-005-0298-2. [DOI] [PubMed] [Google Scholar]
- 12.Rohn S, Rawel HM, Kroll J. Antioxidant activity of protein-bound quercetin. J Agric Food Chem. 2004;52:4725–4729. doi: 10.1021/jf0496797. [DOI] [PubMed] [Google Scholar]
- 13.Huh NW, Porter NA, McIntosh TJ, Simon SA. The interaction of polyphenols with bilayers: conditions for increasing bilayer adhesion. Biophys J. 1996;71:3261–3277. doi: 10.1016/S0006-3495(96)79519-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon SA, Disalvo EA, Gawrisch K, Borovyagin V, Toone E, Schiffman SS, Needham D, McIntosh TJ. Increased adhesion between neutral lipid bilayers: interbilayer bridges formed by tannic acid. Biophys J. 1994;66:1943–1958. doi: 10.1016/S0006-3495(94)80988-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winter RW, Cornell KA, Johnson LL, Ignatushchenko M, Hinrichs DJ, Riscoe MK. Potentiation of the antimalarial agent rufigallol. Antimicrob Agents Chemother. 1996;40:1408–1411. doi: 10.1128/aac.40.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuchiya H. Effects of green tea catechins on membrane fluidity. Pharmacology. 1999;59:34–44. doi: 10.1159/000028303. [DOI] [PubMed] [Google Scholar]
- 17.Tsuchiya H, Nagayama M, Tanaka T, Furusawa M, Kashimata M, Takeuchi H. Membrane-rigidifying effects of anti-cancer dietary factors. Biofactors. 2002;16:45–56. doi: 10.1002/biof.5520160301. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka T, Matsuo Y, Kouno I. A novel black tea pigment and two new oxidation products of epigallocatechin-3-O-gallate. J Agric Food Chem. 2005;53:7571–7578. doi: 10.1021/jf0512656. [DOI] [PubMed] [Google Scholar]
- 19.Hancock JF. Lipid rafts: contentious only from simplistic standpoints. Nat Rev Mol Cell Biol. 2006;7:456–462. doi: 10.1038/nrm1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simons K, Vaz WL. Model systems, lipid rafts and cell membranes. Annu Rev Biophys Biomol Struct. 2004;33:269–295. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- 21.Simons K, Ehehalt R. Cholesterol, lipid rafts and disease. J Clin Invest. 2002;110:597–603. doi: 10.1172/JCI16390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kusumi A, Suzuki K. Toward understanding the dynamics of membrane-raft-based molecular interactions. Biochim Biophys Acta. 2005;1746:234–251. doi: 10.1016/j.bbamcr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Trosko JE. Dietary modulation of the multistage, multimechanisms of human carcinogenesis: effects on initiated stem cells and cell-cell communication. Nutr Cancer. 2006;54:102–110. doi: 10.1207/s15327914nc5401_12. [DOI] [PubMed] [Google Scholar]
- 24.Trosko JE. Gap junctional intercellular communication as a biological “Rosetta stone” in understanding, in a systems biological manner, stem cell behavior, mechanisms of epigenetic toxicology, chemoprevention and chemotherapy. J Membr Biol. 2007;218:93–100. doi: 10.1007/s00232-007-9072-6. [DOI] [PubMed] [Google Scholar]
- 25.Siegel-Axel DI, Gawaz M. Platelets and endothelial cells. Semin Thromb Hemost. 2007;33:128–135. doi: 10.1055/s-2007-969025. [DOI] [PubMed] [Google Scholar]
- 26.Wrangham RW, Jones JH, Laden G, Pilbeam D, Conklin-Brittain NL. The raw and the stolen: cooking and the ecology of human origins. Curr Anthropol. 1999;40:567–594. [PubMed] [Google Scholar]
- 27.Leonard WR. Food for thought. Dietary change was a driving force in human evolution. Sci Am. 2002;287:106–115. [PubMed] [Google Scholar]
- 28.Schaffer S, Eckert GP, Schmitt-Schillig S, Muller WE. Plant foods and brain aging: a critical appraisal. Forum Nutr. 2006;59:86–115. doi: 10.1159/000095209. [DOI] [PubMed] [Google Scholar]
- 29.Lau FC, Shukitt-Hale B, Joseph JA. The beneficial effects of fruit polyphenols on brain aging. Neurobiol Aging. 2005;26:128–132. doi: 10.1016/j.neurobiolaging.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Belcher SM, Zsarnovszky A. Estrogenic actions in the brain: estrogen, phytoestrogens, and rapid intracellular signaling mechanisms. J Pharmacol Exp Ther. 2001;299:408–414. [PubMed] [Google Scholar]