Abstract
Flavonoids are a group of secondary metabolites widely distributed in plants that represent a huge portion of the soluble phenolics present in grapevine (Vitis vinifera L.). These compounds play different physiological roles and are often involved in protection against biotic and abiotic stress. Even if the flavonoid biosynthetic pathways have been largely characterized, the mechanisms of their transport and accumulation in cell wall and vacuole are still not completely understood. This review analyses the known mechanisms of flavonoid uptake and accumulation in grapevine, with reference to the transport models and membrane carrier proteins described in other plant species. The effect of different environmental factors on flavonoid biosynthesis and transporters is also discussed.
Key words: ABC proteins, active transport, bilitranslocase, biotic and abiotic stress, flavonoid, secondary metabolites
Introduction
Among the phenolic secondary metabolites naturally-occurring in plant organisms, flavonoids represent a widespread and common group of aromatic compounds. They are phenylpropanoid derivatives, characterized by a common three-ring structure (C6-C3-C6), in which the degree of oxidation and substitution of the third central ring determines the various classes of flavonoids, the most relevant being anthocyanins, flavonols, flavan-3-ols and proanthocyanidins (PAs or condensed tannins).1,2 The large number of possible modifications of flavonoid chemical structure results in a huge multiplicity of different natural products found in overall plant kingdom.3 This complexity explains the occurrence of flavonoids (and their conjugates) in several plant families and the accumulation of their products according to distinct organ and tissue localization, cell types and phenological stage of the plant.1
Some of the major classes of flavonoids represent natural molecules, being anthocyanins the most abundant pigments providing red to purple attractive colouration mainly to floral and fruit organs of the plants. The PAs are colourless complex polymers of flavan-3-ols that confer, upon their oxidation, dark-brown colour to most seed coats.
All these compounds play important physiological functions in the vegetative tissues and organs, such as roots and leaves, where they accumulate in response to several biotic and abiotic stress conditions, or during legume nodulation. They also act as direct UV protectants, general antioxidants against reactive oxygen species and signalling molecules.2,4 In reproductive tissues and organs, like flowers, fruits and seeds, the most predominant role of pigmented flavonoids is to provide colour to these organs for attraction of pollinators and seed-dispersal agents, while colourless or brown-coloured tannins confer resistance to microbial and fungal infections. For explicating this multitude of biological functions, flavonoids have shown to be present and accumulated particularly in the periphery and surface of plant organs, mainly in the tegument or hypodermal cell layers.
Flavonoid Occurence in Grapevine
Grapevine (Vitis vinifera L.) is one of the most important plant fruit crops, characterized by two reproductive organs, like the fruit berry and the seed, which contain a large amount of soluble flavonoids. In fact, in grape, flavonoids are the major portion of soluble phenolics and represent the most concentrated natural antioxidants in the fruit berry.5 The predominant flavonoids, occurring in grape fruit and seed, belong to the classes of tannins, anthocyanins, flavan-3-ols and flavonols.6 These compounds, in addition to phenolic acids (mainly benzoic and hydroxycinnamic acids), contribute in different proportion and manner to organoleptic features of wine and other by-products.7 Since the great economical importance of grape and wine and the nutraceutical potential of grape phenolics, a huge amount of research has been focused on flavonoid occurrence and tissue localization during ripening of the grape berry.5
Flavonoid Accumulation in Grape Berry
From an anatomical point of view, grape flavonoids localize specifically in both the peripheral layers of berry pericarp (skin) and in some layers of the seed coat (testa). The mesocarp of the berry (pulp) contains phenolic hydroxycinnamates, particularly common in white cultivars, and a negligible amount of flavonoids.5,6,8
The grape skin, representing the hydrophobic barrier of the pericarp, is composed by two distinguishable tissues. The outermost, the epidermis, is strongly cutinised, while the inner thick-walled layers of hypodermis (assumed to consist of several layers, depending on the variety), contain most of the skin flavonoids.6,7 In this fraction the major class of flavonoids is represented by anthocyanins, the pigments exclusive of red grapes, tannins and, in a minor extent, simple flavan-3-ols and flavonols.7
Grape skin tannins are polymers of different condensed monomers of flavan-3-ols, mainly made of epicatechin and epigallocatechin, as “extension” subunits, and catechin, as “terminal” units; gallocatechins, like epicatechin gallate, are usually present in traces.6,7,9
The tannins of the skin differ also from those present in the seed fraction for possessing a larger average size, with a mean degree of polymerisation (mDP) of ca. 28, and a lower proportion of galloylated units.10 These features are responsible for the grape skin organoleptic properties, because astringency and bitterness of PAs are known to be inversely correlated to the polymerisation degree.
In red grape varieties, anthocyanins co-localize with tannins in the skin hypodermal layers and their total content ranges from 11.5 to 29.8 mg/g.7 The anthocyanins commonly found in grape include delphinidin, cyanidin, petunidin, peonidin and malvidin 3-glucosides, 3-(6-acetyl)-glucosides and 3-(6-p-coumaroyl)-glucosides, peonidin and malvidin 3-(6-caffeoyl)-glucosides, being malvidin-3-O-glucoside the major anthocyanin present along with its acylated forms.6,7
Free flavan-3-ol monomers, like catechin and epicatechin, are also present in the skin, although in minor concentrations, suggesting a possible role as precursors in the condensation of polymeric tannins.6,9,11 The third component of flavonoids in the skin fraction is constituted by flavonols, the more representative being the corresponding glucosides, galactosides and glucuronides of quercetin, kaempferol and myricetin.5–7,12,13 Flavonols, especially quercetin, are known to behave as UV-protectants and play a role in co-pigmentation with anthocyanins.9,14
Finally, apart from the occurrence of anthocyanins in red cultivars, the flavonoid composition of the skin is similar in both red and white grapes.7,15,16 On the contrary, it varies during the different stages of berry maturation.5,17,18 In fact, PA accumulation occurs from fruit set until 1–2 weeks after véraison (the onset of ripening); then their level decreases between véraison and harvest.19 Differently from tannins, accumulation of anthocyanin pigments in red grapes starts from véraison (8–10 weeks after blooming) and reaches its maximum in the latest phases of fruit maturation. Flavonol synthesis parallels that of tannins, with two distinct periods, the first near flowering and the second beginning after véraison.20 However, their total content has been found to be higher at the initial stages of berry development.9,19
Flavonoid Accumulation in Grape Seed
The seed is the other large source of flavonoids, even though they represent only up to 6% of berry weight.21 In this organ the main group of flavonoids are the flavan-3-ols (comprising monomers and condensed polymers), and traces of flavonols.7 Thus, the seeds contribute mainly to catechins and condensed tannins in the red wine, but their flavanol concentration relies on the maturity of the grape and maceration techniques, followed during vinification process.21
Differently from the pericarp, flavonoids are localized not only in surface tissues of the seed coat, but also in the inner layers.6,7,21 Recently, Cadot et al.21 have identified three histologically distinct integuments (outer, medium and inner) in the seed structure of grape, cv Cabernet Franc. The outer integument, or soft seed coat, is formed by the cuticle, epidermis and parenchymatous tissue, composed of large thin-walled cells. Two lignified thicker cell layers constitute the medium integument, or hard seed coat. The three cellular layers nearby the endosperm form the inner integument.
Nearly all flavonoids of the seed are contained in the soft parenchyma of seed coat, the outer integument, between the cuticle and the hard seed coat, whereas tannins localize also in the epidermis and in the last layers of the inner integument.6,21 Prior to véraison, the inner layers of medium integument undergo a process of thickening and strong lignification of their cell walls, whereas the cells of the soft outer integument start to become intensively coloured after véraison. Thus the seed browning, during fruit ripening, is believed to be mainly the result of oxidation of flavan-3-ols and tannins accumulated in the thin-walled cells of the outer integument, providing a physical and chemical barrier to oxygen uptake and pathogen infection.
In comparison to skins, total tannin content is reported to be significantly higher in seeds, although the mDP is generally several-fold lower in the seeds at all stages of berry development.7,19 In addition, the composition of the smaller tannins of seed is usually different from that of the skin.6,7,19
Finally, the composition of flavonoids changes throughout the overall process of seed maturation, together with macroscopic changes in the tissues, such as the colour and hardness. The highest flavanol-3-ols (monomers and condensed tannins) concentration is present at véraison, when the accumulation reaches a maximum, but then declines slowly approaching maturity, with a 90% decrease in monomers and a 60% decrease in PAs.6,19,21,22
Flavonoid Biosynthetic Pathway in Grapevine
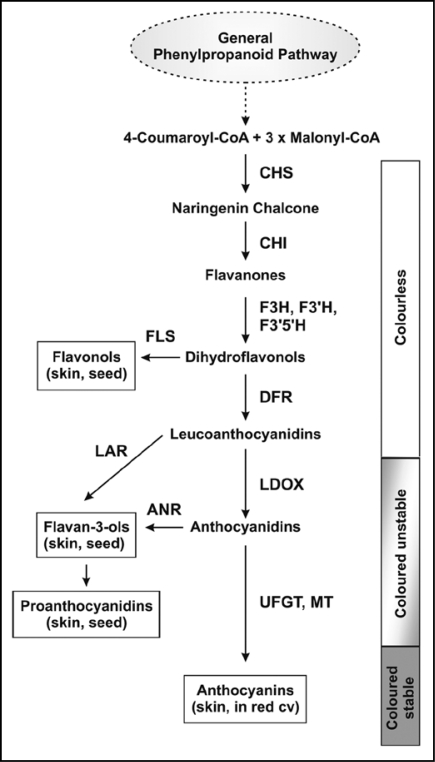
Flavonoids are synthesized along the general phenylpropanoid pathway by the activity of a cytosolic multienzyme complex loosely associated at the cytoplasmic surface of the endoplasmic reticulum (Fig. 1). This pathway has largely been characterized in different plant species,23 but also in V. vinifera where the expression of genes involved in flavonoid synthesis (particularly anthocyanins and PAs) has been well-characterized in berries and seeds of both red and white cultivars.17,18,24–26 The patterns of gene expression show significant differences between organs and cultivars, especially for genes involved in anthocyanin synthesis. In red cultivars, all the genes are expressed in berry skin, although with different temporal patterns. In berry pulp their expression is low and, in particular, phenylalanine ammonia lyase (PAL) and UDP-glucose:flavonoid 3-O-glucosyl transferase (UFGT) genes are not expressed.25 These two genes codify for enzymes involved in the first and in the last step of the anthocyanin pathway, respectively; PAL allows the hydrolysis of ammonia from phenylalanine, whereas UFGT catalyzes the glycosylation of anthocyanidins for the production of the anthocyanins (coloured and stable products). The absence of UFGT has been also shown in seeds.18
Figure 1.
Scheme of flavonoid biosynthetic pathways in grapevine. Anthocyanins are synthesized by a multienzyme complex loosely associated to the endoplasmic reticulum (CHS, chalcone synthase; CHI, chalcone isomerase; F3H, flavanone 3-hydroxylase; F3′H, flavonoid 3′-hydroxylase; F3′5′H, flavonoid 3′5′-hydroxylase; DFR, dihydroflavonol reductase; LDOX, leucoanthocyanidin oxidase; UFGT, UDP-glucose:flavonoid 3-O-glucosyl transferase; MT, methyltransferase). Flavonol and proanthocyanidin syntheses branch off from the anthocyanin pathway (FLS, flavonol synthase; LAR, leucoanthocyanidin reductase; ANR, anthocyanidin reductase).
On the contrary, studies concerning the expression of genes involved in flavonoid synthesis in white cultivars were performed only in berry skin. It has been evidenced that UFGT is not detectable and the expression of other genes is lower, if compared with the skin of red cultivars.18 Recently, Walker et al.27 have found that two very similar genes, belonging to the MYB-family (VvMYBA1 and VvMYBA2), are able to regulate colour in grape berry acting on the UFGT promoter. Interestingly, the white berry gene VvMYBA2 is inactivated by two non-consecutive mutations.
Biosynthesis of PAs shares common steps with anthocyanin pathway, but branches off from this after the reduction of leucocyanidin (or cyanidin) to catechin (or epicatechin) by the enzymatic activity of a leucoanthocyanidin reductase (LAR) or anthocyanidin reductase (ANR), respectively. Expression of genes VvLAR1, VvLAR2 and VvANR in seed and skin from red cultivars show a specific temporal regulation of PA synthesis, starting early during the grape development till the onset of ripening.28 Furthermore, a significant difference has been shown in the relative expression of such genes in the two tissues, with higher values found in grape seed, while VvLAR1 appears to be present only in grape seed. Similarly to what found for anthocyanins, a grapevine transcription factor VvMYBPA1, able to regulate PA formation, has been detected in grape seed and skin.29
Besides the above illustrated pathways, it is interesting to note that recent reports describe the changes of sub-cellular localization of some biosynthetic enzymes during the grape berry development.30,31 Therefore, it would be possible that flavonoid synthesis could be associated to the nucleus, as recently found in Arabidopsis thaliana for chalcone synthase and chalcone isomerase.32
Sub-cellular Compartmentation of Flavonoids in Grapevine
Although the flavonoid biosynthetic pathway takes place in the cytoplasm, the most part of the products are delivered into and stored in distinct compartments, mainly the cell wall and the vacuoles, similarly to many other secondary metabolites.2 Despite the huge amount of works concerning flavonoids in berry and seed tissues, only some reports have examined flavonoid localization or storage at the cellular level in red cultivars, focusing the attention mainly on anthocyanins, flavanols and tannins.
In 1981, Moskowitz and Hrazdina33 determined the qualitative and quantitative composition of anthocyanins isolated from vacuoles of grape berry subepidermal tissue, suggesting that such pigments are in a non-complex form. Moreover, microscopic observations have shown that anthocyanins are present in the vacuoles of the first external layers of the skin hypodermal tissue, except in teinturier varieties that contain anthocyanins in the pulp.34
On the other hand, anthocyanins have been found in cytoplasmic vesicles, named anthocyanoplasts (ACPs), of protoplasts obtained from cell cultures.35 These structures are completely absent in the vacuole, but spherical pigmented inclusions, known as anthocyanic vacuolar inclusions (AVIs), are instead present in this compartment of V. vinifera suspension cell cultures.36 These findings have suggested that ACPs may represent a transport mechanism of anthocyanins in the AVIs inside the vacuole. Recently, AVIs have been found to be membrane-delimited, containing a high amount of mixed acylated (p-coumaroylated) anthocyanins, but also tannins and organic compounds.37,38
Differently from anthocyanins, flavanols and tannins are localized not only in vacuoles, but also in the cell wall of both berry and seed. In studies performed on grape berry skins,39 tannins have been found in different forms, being free tannins inside the vacuole, whereas bound tannins are localized in the internal face of tonoplast (linked to proteins) and in the cell wall (linked to polysaccharides). Lecas and Brilluet40 estimated that the cell wall of grape skin is made of about 15% of insoluble tannins. An analysis of the composition of tannins localized in the cell wall and in the internal cell fraction has been made in skin during berry maturation of cv Cabernet Sauvignon.41 Tannins are mainly present in the internal cell fraction rather than in the cell wall, but the flavanols composition is similar in both the two cell compartments. However, the cell wall fraction exhibits a mDP 2–3 times higher than that of the internal cell fraction. It has also been suggested that the synthesis of tannins, during the first growth period, is followed by a transport to the cell wall.
Only few studies concern the localization of flavonols and tannins in the seed cells. Gény et al.42 showed that there is a significant difference between the composition of tannins from the cell wall and those localized in the internal part of the cell. In particular, tannins from the cell wall show a mDP higher than that of the internal cell fraction, and this difference increases from the véraison to the ripening. The tannin composition is quite similar in the two cell fractions, but the epicatechin gallate proportion is higher in the cell wall. Recently, histochemical investigations in seeds from cv Cabernet Franc showed that the localization of flavan-3-ols is linked to changes in cell walls of the outer integument.21
Although not studied in grape berry, it is important to note that recent works have shown the presence of flavonoids also in the cell nucleus of various plant species.7
Transport Mechanisms of Flavnoids in Grape Cells
The cytosolic localization of flavonoid synthesis implies an efficient mechanism allowing their transport to the final compartments. It has been suggested that flavonoid moieties, depending also on their different substituting groups (acyl, glycosyl and/or methoxyl), are driven to their accumulation sites by a complex vesicle trafficking system involving the Golgi apparatus.43,44 Such a vesicle network requires the participation of specific transporters able to upload flavonoids inside vesicles.
Up to date, a clear and precise understanding of flavonoid transport in plants is far to be elucidated. It is not possible to summarize all the proposed mechanisms in a comprehensive model. Different evidences have been shown, leading to hypothesize that several mechanisms could coexist and overlap (Fig. 2). In particular, it has been demonstrated that flavonoid accumulation could depend not only on species,45 but also on phenological stage,30 tissue and cellular localization,41 environmental factors,26,46 exogenous/endogenous origin47 and substitution of the transported metabolite.48
Figure 2.
Hypothetical scheme of flavonoid transport pathways in grapevine. Flavonoids could be conjugated with glutathione (GSH) through a reaction catalysed by glutathione S-transferases (GSTs). The main transporters localized in grapevine vacuole and plasma membrane are the ATP-binding cassette (ABC) proteins and the bilitranslocase-homologue (BTL-homologue). The multidrug and toxic compound extrusion (MATE) protein, shown to be involved in flavonoid transport in other plant species, has also been added. Transport mediated by vesicle trafficking is indicated by circles (AVIs, anthocyanic vacuolar inclusions; ACPs, anthocyanoplasts). Question marks indicate the lack of information or still hypothetical components and steps in the process.
Most of the experimental observations were obtained mainly in A. thaliana. Undoubtly, the well-known characterization of the genome in this plant renders it a paradigmatic model for studying flavonoid translocation and accumulation. On the other hand, A. thaliana is not recognized as a flavonoid-enriched species; in fact, most of the researches have been focused on the seed tegument, the only pigmented tissue in this plant.
Based on these studies, two main transport mechanisms have been identified. The first one regards a primary active transport, driven by ABC proteins, a large family of transporters with extremely differentiated sequences, but sharing a common ATP-binding domain. These enzymes are often involved in the glycosylated flavonoid and aglycone xenobiotic accumulation.47,49,50 In grape cell cultures, a recent report38 has shown the presence of four isoforms of glutathione S-transferases (GSTs), which participate in anthocyanin transport to the vacuole, via a non-covalent (ligandin) activity; here pigments are then stored as AVIs. A strong correlation between pigment accumulation and GST expression profile during grape maturation has been shown.51 The gene expression is induced by sucrose, jasmonic acid and light irradiation, leading to an enhanced anthocyanin accumulation. In addition, GST could promote a rapid removal of anthocyanins from biosynthetic complexes, increasing the turnover of intermediates.38
The second proposed modality utilizes a secondary active transport due to an “antiporter”, as demonstrated in vacuolar uptake of flavonoid-conjugated moieties (bound with sugars or acyl residues).49 This mechanism is also involved in multidrug and toxic extrusion (MATE) activity, as reported in A. thaliana.52
In the framework of secondary metabolite active transport in grape cells, a wide range of molecules (terpenes, benzenoids and phenylpropanoids) are transported, often with a low specificity, by multiple and poorly understood mechanisms, accumulating in exocarp and mesocarp tissues after the initiation of berry ripening. Therefore, it becomes crucial to study in detail this topic, since flavonoids could represent a paradigmatic case, useful to better understand the whole phenomenon.
Recently, in carnation petals it has been described a membrane protein homologue to mammalian bilitranslocase (BTL).53 The latter is a protein able to perform secondary transport of both heme degradation products (bilirubin) and anthocyanins, including aglycones, mono-and di-glycoside derivatives.54,55 Similarly, the carnation BTL-homologue is involved in an electrogenic uptake of bromosulfalein (BSP) and exhibits cross-reactivity with antibodies raised against a BTL-sequence.53
The similarities in molecular mass, immunochemical and kinetic properties between mammalian BTL and its plant homologues, have suggested the hypothesis of a possible involvement of the latter in flavonoid translocation and accumulation in grapevine berries. These organs represent an ideal model to test this assumption, given their high content in flavonoids, the deep knowledge about their biosynthetic pathways and the characterization of the different phenolic compounds accumulated in the fruit.56 Indeed, a BTL-homologue has been found in grape berries in both red57 and white (Braidot, Petrussa, Peresson, and Bertolini, results to be published) cultivars. This protein shares several similarities with the above-mentioned mammalian and carnation protein. In particular, the immunohistochemical identification by an anti-BTL antibody shows a peripheral localization in the skin of the grape berry correlated, as expected, to the high pigment accumulation. In addition, an intriguing evidence has been provided, because a cross-reactivity has also been detected in vascular bundles of berry pulp. These observations confirm that the carrier may be involved in flavonoid accumulation, since it is present in tissues that are directly involved in flavonoid synthesis or translocation. Conversely, parenchymatic cells exhibit no immunohistochemical staining.
The analysis of the BTL-homologue expression profile, in different tissues during berry development, shows a continuous increase in the skin, starting from véraison till harvest stage, similarly to other proteins related to flavonoid biosynthetic pathway.51 On the contrary, the expression profile of the grape BTL-homologue displays a bell-shape pattern in the pulp.57 This apparently conflicting evidence could be rationalized considering that the expression profiles in the skin and in the pulp are very similar to the patterns observed for flavonoid accumulation during maturation, respectively. In the skin pigment accumulation occurs until the latest developmental stages. Differently, accumulation of other secondary metabolites (e.g. anthocyanin precursors and/or colourless flavonoids), reaches its maximum earlier.46 These results suggest that the BTL-like translocator could be responsible not only for the anthocyanin accumulation at epidermal tissue, but also for intermediate metabolite translocation during berry development.
The ability of the carrier to participate to the translocation of non-coloured compounds has been inferred by the immunohistochemical detection of the BTL-homologue in white grape berries (Braidot, Petrussa, Peresson, and Bertolini, results to be published). Its expression is lower in comparison with the red grape, but this finding opens the possibility that the protein would be involved in the transport of a wider range of secondary metabolites, such as intermediates of the flavonoid pathway.
Moreover, the grape BTL-homologue exhibits a secondary active transport (followed as BSP uptake), which is competitively inhibited by the anti-BTL antibody and by quercetin (a colourless flavonoid naturally-occuring in grape berry), both in red57 and white (Braidot, Petrussa, Peresson, and Bertolini, results to be published) cultivars, suggesting that it may transfer also non-coloured flavonoids.
The observation about the localization of the grape BTL-homologue in phloematic tissue, obtained by immunohistochemical analysis, is in agreement with recent reports, which ascribe to sieve tubes an essential role in long distance transport in grape berry after véraison.58 In fact, after the onset of berry véraison, a significant modification occurs in long distance transport, following the inactivation of xylematic flux.59 Due to the decrease of hydrostatic potential gradient in fleshy fruits, like grape berry, sap flow exhibits a shift from xylematic to phloematic transport.58 Furthermore, Zhang et al.60 demonstrated that phloem unloading is modified from symplasmic (i.e. through the plasmodesmata) to apoplasmic pathway (i.e. across the plasma membrane and the apoplasm) during grape berry development, leading to a decreased transport capacity and to a higher resistance to sap flux. The turning point of these phloem unloading pathways happens during or just before the onset of ripening. In this scenario, on the basis of expression profile of the grape BTL-homologue, which appears to start just with véraison and climaxing to full ripening, it is possible to infer that this protein could represent an additional and subsidiary transport mechanism, particularly in physiological conditions associated with a low transport efficiency (e.g. during the late ripening stage).
Environmental Factors Affecting Flavonoid Biosynthesis, Transport and Accumulation in Grapevine
There are many physiological and environmental factors that could affect the production and the next transport and accumulation of flavonoids in grape. Nevertheless, many of these factors seem to act in a typical bell-shaped manner, where they could improve the final amount of flavonoids just when present at optimal levels. Indeed, a decrease of flavonoid biosynthesis has been observed when either endogenous (e.g. plant hormones), or exogenous factors (e.g. water and temperature stress, light, fertilizer, etc.) are limiting or excessive.
In particular, plant hormones could affect flavonoid biosynthesis in a complex way. Typically, it has been reported that abscisic acid, auxin and ethylene are responsible for an increase of flavonoids, while gibberellic acid and inhibitors of the ethylene receptor decrease their synthesis.61–63
Wounding and pathogenesis have been identified as negative factors for flavonoid biosynthesis. In particular, infections by Botrytis cinerea through degradation induced by laccase lead to a reduced phenolic content in berries.64 Nevertheless, so far the precise role of wounding and pathogenesis in this process is not well understood.
Environmental factors that increase plant vigour (e.g. excessive fertilizer) are reported to negatively influence flavonoid content. Nitrogen and potassium at high levels cause the enhancement of vegetative growth, the delay of ripening and a decrease of colour in grape berry. These effects are due to the induction of metabolic imbalance and competition for sugar translocation between the vegetative part and the berry. Indirectly, excessive fertilizer content could induce the production of especially dense foliage that limits the grape exposure to sunlight.65 The latter, together with temperature, represents one of the main environmental factors responsible for flavonoid biosynthesis. Different light exposures of fruits demonstrate that shading decreases significantly the flavonoid content of the berries.66 The light-dependent biosynthesis would be limited only to flavonols, a result that is consistent with the role that these molecules play in protecting tissues from UV light.66 Nevertheless, shading could also change the proportion between different anthocyanins, as evidenced in Shiraz berries, where malvidin, petunidin and delphinidin glucosides are decreased with respect to peonidin and cyanidin glucosides.66
The explanation of the light effect on flavonoid biosynthesis is complicated by the parallel general decrease of carbon fixation during shading, that could influence the level of secondary metabolites,67 and by the difficulty to discriminate between the effect of the light and that of the temperature. Indeed, it has proposed that “accumulation of anthocyanin is more a function of temperature than of light”.20 Temperature has a great influence on anthocyanin biosynthesis, being this process inhibited at low and high values, with the critical higher temperature identified around 30 °C, depending on different grape varieties.68 Furthermore, anthocyanin content seems to be sensitive to diurnal differences in temperature, being higher in the presence of colder nights with respect to constant (high) temperature.20 Anthocyanin accumulation is also modified by the water status of the plant. It has been reported that excessive water applications could induce a decrease of tannins,22 while a water shortage could influence the berry size, thus indirectly changing the ratio between the berry size and skin surface.69 Since osmotic stress in grape cell cultures has been reported to increase the anthocyanin biosynthesis, this process could be modulated by water supply.20 Recently, it has been suggested that water stress could exert similar effects either when applied before or after véraison.46 In particular, an early water stress is linked to the increase of sugar accumulation and to an acceleration of both anthocyanin synthesis and the onset of ripening.46 This effect seems to be specific for anthocyanin, since PAs and other flavonoids are only slightly affected.20 Furthermore, recent results show that, during water stress, the synthesis of anthocyanins is paralleled by an increase of the expression of flavonoid transporter(s). In particular, it has been shown that, in both skin and pulp, water stress increases the expression of the BTL-related protein(s), detected by immunochemical analysis of microsomes isolated from grapevine.57
Conclusions
From the results above described, it is evident that, even if the synthesis of flavonoids has been well-characterized, several question marks still remain for the transport mechanisms of these metabolites. Up to now, the models of flavonoid transport have been mainly based on genetic approaches, where this process has been correlated to the expression of several specific genes in reproductive organs, during development, or in response to environmental factors. Limited information is available for a direct identification and characterization of proteins involved in the uptake and accumulation of these metabolites. Therefore, it is crucial that the future research would be more focused toward the understanding of the biochemical mechanisms responsible for flavonoid transport and regulation. This would also represent a good reference to identify a general model for the transport of other secondary metabolites in higher plants. Furthermore, since the Vitis genome has been recently published (http://www.genoscope.cns.fr/externe/GenomeBrowser/Vitis/), this plant would represent a better candidate than Arabidopsis for studying the transport and accumulation of flavonoids. Finally, for a comprehensive understanding of this process and for the selection of new grape varieties, the identification of the gene products needs to be integrated with biochemical and physiological studies.
Acknowledgements
The research performed by the authors on this topic was supported by “Tenuta Villanova”, Vineyard and Winery at Farra d'Isonzo (GO), Friuli-Venezia Giulia, Italy, by Regione Autonoma Friuli-Venezia Giulia (L.R. 26/2005 art. 17) and by Ministero dell'Istruzione, Università e Ricerca (PRIN project 2004070118).
Abbreviations
- ABC
ATP-binding cassette
- ACP
anthocyanoplast
- ANR
anthocyanidin reductase
- ATP
adenosine triphosphate
- AVI
anthocyanic vacuolar inclusion
- BSP
bromosulfalein
- BTL
bilitranslocase
- GST
glutathione S-transferase
- LAR
leucoanthocyanidin reductase
- MATE
multidrug and toxic extrusion
- mDP
mean degree of polymerisation
- MYB
myeloblastosis
- PA
proanthocyanidin
- PAL
phenylalanine ammonia lyase
- UDP
uridine diphosphate
- UFGT
UDP-glucose:flavonoid 3-O-glucosyl transferase
- UV
ultraviolet
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/6686
References
- 1.Schwinn KE, Davies KM. Flavonoids. In: Davies KM, editor. Plant pigments and their manipulation. Annual Plant Reviews. Vol. 14. Boca Raton FL: CRC Press, Blackwell Publishing; 2004. pp. 92–149. [Google Scholar]
- 2.Kitamura S. Transport of flavonoids: from cytosolic synthesis to vacuolar accumulation. In: Grotewold E, editor. Science of flavonoids. Berlin D: Springer; 2006. pp. 123–146. [Google Scholar]
- 3.Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 4.Gould KS, Lister C. Flavonoid functions in plants. In: Andersen ØM, Markham KR, editors. Flavonoids: chemistry, biochemistry and applications. Boca Raton FL: CRC Press, Taylor and Friends Group; 2006. pp. 397–442. [Google Scholar]
- 5.Conde C, Silva P, Fontes N, Dias ACP, Tavares RM, Sousa MJ, Agasse A, Delrot S, Gerós H. Biochemical changes throughout grape berry development and fruit and wine quality. Food. 2007;1:1–22. [Google Scholar]
- 6.Adams DO. Phenolics and ripening in grape berries. Am J Enol Vitic. 2006;57:249–256. [Google Scholar]
- 7.Pinelo M, Arnous A, Meyer AS. Upgrading of grape skins: significance of plant cell-wall structural components and extraction techniques for phenol release. Trends Food Sci Tech. 2006;17:579–590. [Google Scholar]
- 8.Castellarin SD, Di Gaspero G, Marconi R, Nonis A, Peterlunger E, Paillard S, Adam-Blondon A-F, Testolin R. Colour variation in red grapevines (Vitis vinifera L.): genomic organisation, expression of flavonoid 3′-hydroxylase, flavonoid 3′,5′-hydroxylase genes and related metabolite profiling of red cyanidin-/blue delphinidin-based anthocyanins in berry skin. BMC Genomics. 2006;7:17–29. doi: 10.1186/1471-2164-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doshi P, Adsule P, Banerjee K. Phenolic composition and antioxidant activity in grapevine parts and berries (Vitis vinifera L.) cv Kishmish Chornyi (Sharad Seedless) during maturation. Int J Food Sci Technol. 2006;41:1–9. [Google Scholar]
- 10.Souquet J-M, Cheynier V, Brossaud F, Moutounet M. Polymeric proanthocyanidins from grape skins. Phytochemistry. 1996;43:509–512. [Google Scholar]
- 11.Downey MO, Harvey JS, Robinson SP. Synthesis of flavonols and expression of flavonol synthase genes in the developing grape berries of Shiraz and Chardonnay (Vitis vinifera L.) Austr J Grape Wine Res. 2003;9:110–121. [Google Scholar]
- 12.Hollman PCH, Arts ICW. Flavonols, flavones and flavanols—Nature, occurrence and dietary burden. J Sci Food Agric. 2000;80:1081–1093. [Google Scholar]
- 13.Makris DP, Kallithrakab S, Kefalas P. Flavonols in grapes, grape products and wines: burden, profile and influential parameters. J Sci Food Comp Anal. 2006;19:396–404. [Google Scholar]
- 14.Winkel-Shirley B. Biosynthesis of flavonoids and effects of stress. Curr Opin Plant Biol. 2002;5:218–223. doi: 10.1016/s1369-5266(02)00256-x. [DOI] [PubMed] [Google Scholar]
- 15.Borbalán AMA, Zorro L, Guillén DA, Barroso CG. Study of the polyphenol content of red and white grape varieties by liquid chromatography-mass spectrometry and its relationship to antioxidant power. J Chromatogr A. 2003;1012:31–38. doi: 10.1016/s0021-9673(03)01187-7. [DOI] [PubMed] [Google Scholar]
- 16.Masa A, Vilanova M. Flavonoid and aromatic characterisation of cv. Albarín blanco (Vitis vinifera L.) Food Chem. 2008;107:273–281. [Google Scholar]
- 17.Bogs J, Ebadi A, McDavid D, Robinson SP. Identification of the flavonoid hydroxylases from grapevine and their regulation during fruit development. Plant Physiol. 2006;140:279–291. doi: 10.1104/pp.105.073262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boss PK, Davies C, Robinson SP. Expression of anthocyanin biosynthesis pathway genes in red and white grapes. Plant Mol Biol. 1996;32:565–569. doi: 10.1007/BF00019111. [DOI] [PubMed] [Google Scholar]
- 19.Downey MO, Harvey JS, Robinson SP. Analysis of tannins in seeds and skins of Shiraz grapes throughout berry development. Australian J Grape Wine Res. 2003;9:15–27. [Google Scholar]
- 20.Downey MO, Dokoozlian NK, Krstic MP. Cultural practice and environmental impacts on the flavonoid composition of grapes and wine: a review of recent research. Am J Enol Vitic. 2006;57:257–268. [Google Scholar]
- 21.Cadot Y, Miñana-Castelló MT, Chevalier M. Anatomical, histological, and histochemical changes in grape seeds from Vitis vinifera L. cv Cabernet franc during fruit development. J Agric Food Chem. 2006;54:9206–9215. doi: 10.1021/jf061326f. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy JA, Matthews MA, Waterhouse AL. Changes in grape seed polyphenols during fruit ripening. Phytochemistry. 2000;55:77–85. doi: 10.1016/s0031-9422(00)00196-5. [DOI] [PubMed] [Google Scholar]
- 23.Winkel-Shirley B. The biosynthesis of flavonoids. In: Grotewold E, editor. Science of flavonoids. Berlin D: Springer; 2006. pp. 71–95. [Google Scholar]
- 24.Sparvoli F, Martin C, Scienza A, Gavazzi G, Tonelli C. Cloning and molecular analysis of structural genes involved in flavonoid and stilbene biosynthesis in grape (Vitis vinifera L.) Plant Mol Biol. 1994;24:743–755. doi: 10.1007/BF00029856. [DOI] [PubMed] [Google Scholar]
- 25.Boss PK, Davies C, Robinson SP. Analysis of the expression of anthocyanin pathway genes in developing Vitis vinifera L. cv Shiraz grape berries and the implications for pathway regulation. Plant Physiol. 1996;111:1059–1066. doi: 10.1104/pp.111.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castellarin SD, Pfeiffer A, Sivilotti P, Degan M, Peterlunger E, Di Gaspero G. Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant Cell Environ. 2007;30:1381–1399. doi: 10.1111/j.1365-3040.2007.01716.x. [DOI] [PubMed] [Google Scholar]
- 27.Walker AR, Lee E, Bogs J, McDavid DAJ, Thomas MR, Robinson SP. White grapes arose through the mutation of two similar and adjacent regulatory genes. Plant J. 2007;49:772–785. doi: 10.1111/j.1365-313X.2006.02997.x. [DOI] [PubMed] [Google Scholar]
- 28.Bogs J, Downey M, Harvey JS, Ashton AR, Tanner GJ, Robinson SP. Proanthocyanidin synthesis and expression of genes encoding leucoanthocyanidin reductase and anthocyanidin reductase in developing grape berries and grapevine leaves. Plant Physiol. 2005;139:652–663. doi: 10.1104/pp.105.064238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bogs J, Jaffe FW, Takos AM, Walker AR, Robinson SP. The grapevine transcription factor VvMYBPA1 regulates proanthocyanidin synthesis during fruit development. Plant Physiol. 2007;143:1347–1361. doi: 10.1104/pp.106.093203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen JY, Wen PF, Kong WF, Pan QH, Wan SB, Huang WD. Changes and subcellular localizations of the enzymes involved in phenylpropanoid metabolism during grape berry development. J Plant Physiol. 2006;163:115–127. doi: 10.1016/j.jplph.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Tian L, Wan S-B, Pan Q-H, Zheng Y-J, Huang W-D. A novel plastid localization of chalcone synthase in developing grape berry. Plant Sci. 2008 doi: 10.1016/j.plantsci.2008.03.012. [DOI] [Google Scholar]
- 32.Saslowsky DE, Warek U, Winkel BS. Nuclear localization of flavonoid enzymes in Arabidopsis. J Biol Chem. 2005;280:23735–23740. doi: 10.1074/jbc.M413506200. [DOI] [PubMed] [Google Scholar]
- 33.Moskowitz AH, Hrazdina G. Vacuolar contents of fruit subepidermal cells from Vitis species. Plant Physiol. 1981;68:686–692. doi: 10.1104/pp.68.3.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheynier V. Flavonoids in wine. In: Andersen ØM, Markham KR, editors. Flavonoids: chemistry, biochemistry and applications. Boca Raton FL: CRC Press, Taylor and Friends Group; 2006. pp. 263–318. [Google Scholar]
- 35.Calderón AA, Pedrenó MA, Muñoz R, Ros Barceló A. Evidence for the non-vacuolar localization of anthocyanoplasts (anthocyanin-containing vesicles) in suspension cultured grapevine cells. Phyton. 1993;54:91–98. [Google Scholar]
- 36.Conn S, Zhang W, Franco C. Anthocyanic vacuolar inclusions (AVIs) selectively bind acylated anthocyanins in Vitis vinifera L. (grapevine) suspension culture. Biotechnol Lett. 2003;25:835–839. doi: 10.1023/a:1024028603089. [DOI] [PubMed] [Google Scholar]
- 37.Zhang H, Wang L, Deroles S, Bennett R, Davies K. New insight into the structures and formation of anthocyanic vacuolar inclusions in flower petals. BMC Plant Biol. 2006;6:29–43. doi: 10.1186/1471-2229-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang W, Conn S, Franco C. Characterisation of anthocyanin transport and storage in Vitis vinifera L. cv Gamay Fréaux cell suspension cultures. J Biotechnol. 2007;131S:S196–S210. doi: 10.1016/j.jbiotec.2007.07.373. [DOI] [Google Scholar]
- 39.Amrani-Joutei K, Glories Y, Mercier M. Localisation des tanins dans la pellicule de baie de raisin. Vitis. 1994;33:133–138. (Fre). [Google Scholar]
- 40.Lecas M, Brillouet JM. Cell wall composition of grape berry skins. Phytochemistry. 1994;35:1241–1243. [Google Scholar]
- 41.Gagné S, Saucier C, Gény L. Composition and cellular localization of tannins in Cabernet Sauvignon skins during growth. J Agric Food Chem. 2006;54:9465–9471. doi: 10.1021/jf061946g. [DOI] [PubMed] [Google Scholar]
- 42.Gény L, Saucier C, Bracco S, Daviaud F, Glories Y. Composition and cellular localization of tannins in grape seeds during maturation. J Agric Food Chem. 2003;51:8051–8054. doi: 10.1021/jf030418r. [DOI] [PubMed] [Google Scholar]
- 43.Grotewold E. The challenges of moving chemicals within and out of cells: insights into the transport of plant natural products. Planta. 2004;219:906–909. doi: 10.1007/s00425-004-1336-0. [DOI] [PubMed] [Google Scholar]
- 44.Grotewold E. The genetics and biochemistry of floral pigments. Annu Rev Plant Biol. 2006;57:761–780. doi: 10.1146/annurev.arplant.57.032905.105248. [DOI] [PubMed] [Google Scholar]
- 45.Cortell JM, Halbleib M, Gallagher AV, Righetti TL, Kennedy JA. Influence of vine vigor on grape (Vitis vinifera L. cv Pinot Noir) anthocyanins. 1. Anthocyanin concentration and composition in fruit. J Agric Food Chem. 2007;55:6575–6584. doi: 10.1021/jf070195v. [DOI] [PubMed] [Google Scholar]
- 46.Castellarin SD, Matthews MA, Di Gaspero G, Gambetta GA. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta. 2007;227:101–112. doi: 10.1007/s00425-007-0598-8. [DOI] [PubMed] [Google Scholar]
- 47.Frangne N, Eggmann T, Koblischke C, Weissenböck G, Martinoia E, Klein M. Flavone glucoside uptake into barley mesophyll and Arabidopsis cell culture vacuoles. Energization occurs by H+-antiport and ATP-binding cassette-type mechanisms. Plant Physiol. 2002;128:726–733. doi: 10.1104/pp.010590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matern U, Reichenbach C, Heller W. Efficient uptake of flavonoids into parsley (Petroselinum hortense) vacuoles requires acylated glycosides. Planta. 1986;167:183–189. doi: 10.1007/BF00391413. [DOI] [PubMed] [Google Scholar]
- 49.Klein M, Weissenböck G, Dufaud A, Gaillard C, Kreuz K, Martinoia E. Different energization mechanisms drive the vacuolar uptake of a flavonoid glucoside and a herbicide glucoside. J Biol Chem. 1996;271:29666–29671. doi: 10.1074/jbc.271.47.29666. [DOI] [PubMed] [Google Scholar]
- 50.Goodman CD, Casati P, Walbot V. A multidrug resistance-associated protein involved in anthocyanin transport in Zea mays. Plant Cell. 2004;16:1812–1826. doi: 10.1105/tpc.022574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ageorges A, Fernandez L, Vialet S, Merdinoglu D, Terrier N, Romieu C. Four specific isogenes of the anthocyanin metabolic pathway are systematically co-expressed with the red colour of grape berries. Plant Sci. 2006;170:372–383. [Google Scholar]
- 52.Debeaujon I, Peeters AJ, Léon-Kloosterziel KM, Koornneef M. The TRANSPARENT TESTA12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium. Plant Cell. 2001;13:853–871. doi: 10.1105/tpc.13.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Passamonti S, Cocolo A, Braidot E, Petrussa E, Peresson C, Medic N, Macrì F, Vianello A. Characterization of electrogenic bromosulfophthalein transport in carnation petal microsomes and its inhibition by antibodies against bilitranslocase. FEBS J. 2005;272:3282–3296. doi: 10.1111/j.1742-4658.2005.04751.x. [DOI] [PubMed] [Google Scholar]
- 54.Passamonti S, Vrhovsek U, Mattivi F. The interaction of anthocyanins with bilitranslocase. Biochem Biophys Res Commun. 2002;296:631–636. doi: 10.1016/s0006-291x(02)00927-0. [DOI] [PubMed] [Google Scholar]
- 55.Passamonti S, Vrhovsek U, Vanzo A, Mattivi F. The stomach as a site for anthocyanins absorption from food. FEBS Lett. 2003;544:210–213. doi: 10.1016/s0014-5793(03)00504-0. [DOI] [PubMed] [Google Scholar]
- 56.Coombe BG. Research on development and ripening of the grape berry. Am J Enol Vitic. 1992;43:101–110. [Google Scholar]
- 57.Braidot E, Petrussa E, Bertolini A, Peresson C, Ermacora P, Loi N, Passamonti S, Terdoslavich M, Macrì F, Vianello A. Evidence for a putative flavonoid translocator similar to mammalian bilitranslocase in grape berries (Vitis vinifera, L.) during ripening. Planta. 2008;228:203–213. doi: 10.1007/s00425-008-0730-4. [DOI] [PubMed] [Google Scholar]
- 58.Bondada BR, Matthews MA, Shackel KA. Functional xylem in the post-veraison grape berry. J Exp Bot. 2005;56:2949–2957. doi: 10.1093/jxb/eri291. [DOI] [PubMed] [Google Scholar]
- 59.Coombe BG, McCarthy M. Dynamics of grape berry growth and physiology of ripening. Austr J Grape Wine Res. 2000;6:131–135. [Google Scholar]
- 60.Zhang XY, Wang XL, Wang XF, Xia GH, Pan QH, Fan RC, Wu FQ, Yu XC, Zhang DP. A shift of phloem unloading from symplasmic to apoplasmic pathway is involved in developmental onset of ripening in grape berry. Plant Physiol. 2006;142:220–232. doi: 10.1104/pp.106.081430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jeong ST, Goto-Yamamoto N, Kobayashi S, Esaka A. Effects of plant hormones and shading on the accumulation of anthocyanins and the expression of anthocyanin biosynthetic genes in grape berry skins. Plant Sci. 2004;167:247–252. [Google Scholar]
- 62.Deikman J, Hammer PE. Induction of anthocyanin accumulation by cytokinins in Arabidopsis thaliana. Plant Physiol. 1995;108:47–57. doi: 10.1104/pp.108.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dan DH, Lee CH. The effects of GA3, CPPU and ABA applications on the quality of Kyoho (Vitis vinifera L. × lambrusca L.) grape. Acta Horticolt. 2004;653:193–197. [Google Scholar]
- 64.Slomczynski D, Nakas JP, Tanenbaum SW. Production and characterization of laccase from Botrytis cinerea 61–34. Appl Environ Microbiol. 1995;61:907–912. doi: 10.1128/aem.61.3.907-912.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Delgado R, Martin P, del Alamo M, Gonzalez MR. Changes in the phenolic composition of grape berries during ripening in relation to vineyard nitrogen and potassium fertilisation rates. J Sci Food Agric. 2004;84:623–630. [Google Scholar]
- 66.Downey MO, Harvey JS, Robinson SP. The effect of bunch shading on berry development and flavonoid accumulation in Shiraz grapes. Austr J Grape Wine Res. 2004;10:55–73. [Google Scholar]
- 67.Smart R, Smith SM, Winchester RV. Light quality and quantity effects on fruit ripening for Cabernet Sauvignon. Am J Enol Viticolt. 1988;39:250–258. [Google Scholar]
- 68.Dokoozlian NK, Kliewer WM. Influence of light on grape berry growth and composition varies during fruit development. J Am Soc Hortic Sci. 1996;121:869–874. [Google Scholar]
- 69.Roby G, Harbertson JF, Adams DA, Matthews MA. Berry size and vine water deficits as factors in winegrape composition: anthocyanins and tannins. Austr J Grape Wine Res. 2004;10:100–107. [Google Scholar]