Abstract
Previous reports have suggested the primary mode of action of the allelochemical hydroquinone involves disruption of root cell membrane transport. Here we report the effects of hydroquinone on common bean (Phaseolus vulgaris) plants. Growth of leaves, roots and stems were all inhibited by 14 day exposure to 0.01 mM or 0.25 mM hydroquinone. Chlorophyll fluorescence (Fv/Fm) was inhibited by 0.25 mM hydroquinone. The membrane potential of P. vulgaris root cortex cells briefly hyperpolarized and subsequently slowly transiently depolarized upon abrupt exposure to a range of hydroquinone concentrations. Both the hyperpolarization and depolarization were concentration dependent but appeared saturable. Root cells exposed to 0.03 mM hydroquinone hyperpolarized 3.4 mV (+/− 0.6 s.e.) 3 minutes after the start of exposure then depolarized 36.7 mV (+/− 3.9) with no effect evident after 24 hours. Individual recordings showed a response to as little as 0.001 mM hydroquinone. Exposure of P. vulgaris root cells to arbutin, a nontoxic monoglucoside of hydroquinone, produced a similar but much smaller (approximately 25%) electrical response. Exposure of root cells of Antennaria microphylla, a known allelopathic source (donor plant) of hydroquinone, also produced a much smaller hyperpolarization and depolarization response. It is concluded that the electrical response to hydroquinone by P. vulgaris root cells and the changes in membrane transport they represent are not sufficiently large or long lasting enough to disrupt mineral and water uptake leading to plant injury. The possibility, however, that these events are related to initiation of signal transduction events leading to cell death is discussed.
Key words: allelopathy, hydroquinone, membrane potential, depolarization, hyperpolarization, Phaseolus vulgaris, Antennaria microphylla
Introduction
A wide array of plant derived compounds released into the rhizosphere have been found to affect the growth of neighboring plants.1 In general, release of these allelochemicals by “donor plants” results in the inhibition of the development of plants of surrounding “target” species increasing the opportunity for expansion of the donor plants. Small everlasting (Antennaria microphylla Rydb.), an inconspicuous perennial forb native to dry open habitats of western North America, is one example of an allelopathy donor plant.2 Growth inhibition of plants neighboring A. microphylla individuals principally results from release into the soil of hydroquinone,3 in the form of arbutin (a glycoside of hydroquinone), which is rapidly converted to hydroquinone in nonsterile soils.4
A variety of modes of action have be identified for a number of different allelochemicals ranging from inhibition of photosynthetic electron transport, disruption of metabolic enzymes, generation of damaging reactive oxygen species, disruption of plant hormone synthesis and disruption of water and mineral uptake.5,6 Target plants of hydroquinone released by A. microphylla include the invasive plant species leafy spurge (Euphorbia esula L.). Hydroponic treatment of E. esula with 0.25 mM hydroquinone resulted in reduced photosynthesis resulting from sustained stomata closure.7 These results were interpreted to suggest that a disruption of plant water relations by hydroquinone is the primary mode of action leading to reduced photosynthesis and growth.
Disruption of plant water relations by hydroquinone might most easily be effected if the molecule perturbs root cell membrane transport.8 Glass and Bohm9 demonstrated inhibition of 86Rb+ uptake by barley roots by a high concentration of hydroquinone. In the current study we have tested whether hydroquinone affects the electropotential difference across root cell membranes. This membrane potential, as a function of the various fluxes of ions across the membrane, reflects any changes in the activity of membrane transporters.10
While the inspiration for this project was the earlier study by one of us7 of the mechanism of hydroquinone-induced growth inhibition of E. esula, in this study we chose to study the effects of hydroquinone on the roots of the common bean (Phaseolus vulgaris). P. vulgaris seedlings grew vigorously in vermiculite allowing easy isolation of health root tips for experimentation while, in our hands, the slow growing rhizomatous E. esula grew poorly in a loose medium like vermiculite and produced too few root tips for useful study. We report here, however, that both growth and photosynthesis of P. vulgaris are as sensitive to hydroquinone as are those of E. esula. We also describe a complex transient response by the P. vulgaris root cell membrane potential to the onset of hydroquinone treatment.
Results
Treated with hydroquinone in comparable experiments, growth and photosynthesis of P. vulgaris was found to be at least as sensitive to hydroquinone as Barkosky et al.7 found that of E. esula to be. After 14 of hydroponic exposure to hydroquinone growth of P. vulgaris leaves, roots and stems were all inhibited by high concentrations of hydroquinone (Table 1). Leaf area and leaf weight increases were significantly inhibited in plants treated with 0.01 mM and 0.03 mM hydroquinone and significantly more so treated with 0.1 mM and 0.25 mM hydroquinone compared to control plants grown in growth medium without hydroquinone. Stems were significantly shorter after treatment with 0.03 mM hydroquinone and shorter still exposed to 0.1 mM or 0.25 mM. Stems weight, however, was significantly reduced only by exposure to 0.25 mM. By comparison, various growth aspects of E. esula plants were significantly inhibited after 30 day exposures to 0.1 and 0.25 mM hydroquinone.7
Table 1.
Effect of hydroquinone on growth and on chlorophyll fluorescence of Phaseolus vulgaris after 14 daysa
| Hydroquinone concentration | |||||||
| Plant Variable | Control | 0.001 mM | 0.003 mM | 0.01 mM | 0.03 mM | 0.1 mM | 0.25 mM |
| Leaf area (cm2) | 272 (13)a | 285 (12)a | 269 (9)a | 159 (3)b | 146 (2)b | 100 (4)c | 83 (2)c |
| Leaf weight (mg) | 658 (21)a | 616 (10)a | 651 (11)a | 470 (8)b | 440 (9)b | 378 (14)c | 337 (18)c |
| Stem length (cm) | 26.7 (0.8)ab | 27.9 (1.3)a | 27.2 (0.9)a | 23.1 (0.5)bc | 20.0 (0.2)c | 16.3 (0.7)d | 14.2 (0.4)d |
| Stem weight (mg) | 446 (10)a | 442 (15)a | 439 (14)a | 421 (10)a | 404 (7)a | 411 (21)a | 337 (10)b |
| Root length (cm) | 13.8 (0.4)ab | 11.9 (0.3)a | 12.6 (0.6)a | 12.9 (0.9)a | 12.0 (0.4)a | 15.3 (0.4)bc | 16.6 (0.9)c |
| Root weight (mg) | 158 (10)ab | 157 (14)ab | 174 (14)a | 110 (2)c | 104 (3)c | 118 (12)bc | 128 (13)bc |
| Plant weight (g) | 1.26 (0.02)a | 1.22 (0.02)a | 1.27 (0.02)a | 1.00 (0.01)b | 0.95 (0.02)bc | 0.91 (0.03)c | 0.80 (0.06)d |
| Fv/Fm | 0.72 (0.01)ab | 0.72 (0.00)ab | 0.72 (0.01)ab | 0.74 (0.01)a | 0.74 (0.01)a | 0.71 (0.02)b | 0.62 (0.02)c |
aMean (SE); Control n = 24; 0.001 and 0.003 mM n = 18; 0.01 − 0.25 mM n = 6. a–dIndicate significant differences between means (p < 0.05).
As with E. esula,7 roots of P. Vulgaris treated with higher concentrations of hydroquinone were visibly affected by hydroquinone. There was a marked tendency of those roots to exude mucous. The roots tended to grow longer in length, significantly so treated with the highest concentration (0.25 mM). Root weight, however, was significantly reduced by treatment with intermediate concentrations (0.01 mM and 0.03 mM) suggesting that hydroqinone exposure may induce increased elongation of major roots at the same time as inhibiting lateral root development.
Reduced final total plant weight correlated with exposure to increasing concentrations of hydroquinone. Plants were significantly less massive in a stepwise fashion in plants with roots exposed to 0.01 mM and 0.03 mM smaller than the control plants, plants treated 0.1 mM smaller still, and plants treated with 0.25 mM the smallest.
Tables 1 also shows the effect of root exposure to hydroquinone on the chlorophyll fluorescence parameter Fv/Fm. Fv/Fm is a simple measure of the PSII efficiency and an indication of the level of ongoing photosynthesis.11 Optimal values of around 0.83 are typical of most species.12 Lower Fv/Fm values are indicative of plant stress;13 thus the control plants (0 mM hydroquinone) were not photosynthesizing optimally at the point of harvest. The most likely explanation would seem the lack of aeration of the hydroponic solution (omitted to reduce chemical oxidation of hydroquione in solution) bathing the roots. Further inhibition of P. vulgaris photosynthesis by hydroquinone treatment was limited. Only the highest concentration of hydroquinone (0.25 mM) resulted in a significant decline in PSII light capturing efficiency. The leaves of these plants were also appeared visibly chlorotic. The relation between Fv/Fm and photosynthesis is not always absolute and can change due changes in relative rates of CO2 fixation, and competing processes like photorespiration, nitrogen metabolism, and electron donation to oxygen.13 Nevertheless, this result is consistent with the conclusion by Barkosky et al.7 that hydroquinone induced reduction in photosynthesis is a secondary or downstream consequence of a primary injury to the plant water uptake.
Having demonstrated that P. vulgaris is similar to E. esula in long term injury sensitivity to exposure to hydroquinone, we tested whether growth and photosynthesis inhibition in P. vulgaris is preceded by disruption of root cell membrane transport as evidenced by altered trans-cell membrane electropotential differences (i.e., membrane potential). Root tips immersed in a flowing bathing medium were impaled with KCl filled conventional glass microelectrodes and recordings established from root cortex cells 1–3 cells below the epidermis cells approximately 1–2 cm from the root apex in the elongation or early root hair zone. Successful impalings produced membrane potential recordings that stabilized somewhere within approximately 25 mV of −150 mV (cytoplasmic side relative to extracellular) as is typical of plant cells.14
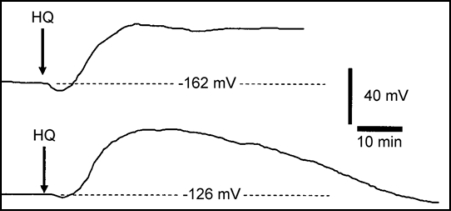
Figure 1 shows two sample recordings illustrating the effect on the membrane potential of abruptly including 0.03 mM hydroquinone in the perfusion stream flowing over the point of microelectrode insertion in the root tip. Though the recording set-up lacked the precision to precisely determine hydroqinone arrival at impaled cells, it is clear that in both recordings the membrane potential begins hyperpolarizing (comes increasingly more negative) essentially immediately following hydroquinone-root contact. The hyperpolarization measures 4 mV in the first (top) recording and about 2 mV in the second when maximal approximately 2–3 minutes following hydro-quinone-root contact or 4–5 minutes after inclusion of hydroquinone in the perfusion stream. In both recordings the hyperpolarization response is immediately followed by depolarization of the membrane potential. In both recordings the magnitude of the depolarization is much larger than the hyperpolarization amounting to a decrease of approximately 44–45 mV relative to the initial prehyperpolarization membrane potential in both cases when maximal 22–24 min after hydroquinone root contact. The two sample recordings differ in that the depolarization of the membrane potential is sustained in one recording (top) and is entirely transient in the other (bottom) recording. The recordings are shown ending abruptly immediately before they failed as marked by a more or less abrupt collapse of the membrane potential to near zero mV.
Figure 1.
Two sample microelectrode recordings of the effect of 0.03 mM hydroquinone on the membrane potential of root cortex cells of the elongation/root hair zone of Phaseolus vulgaris plants. At the time marked HQ following ten minutes of stable recording the perfusion stream directed to the chamber containing the microelectrode impaled root tip was changed to include 0.03 mM hydroquinone which reached the location of the impaled cells approximately 2 min later. Each displayed recording is shown until immediately before it failed.
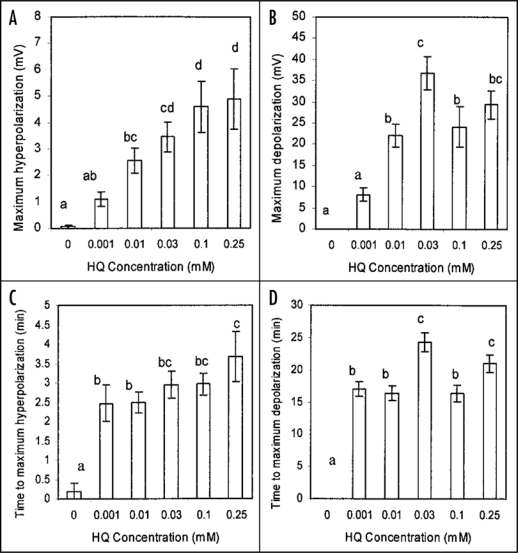
The sample recordings shown in Figure 1 are typical of all others collected over a range of concentrations in that hydroquinone-root contact was always followed first by a small hyperpolarization of short duration followed by a larger depolarization that was or was not sustained except in some (3 of 10) recordings at the lowest hydroquinone concentration tested (0.001 mM) in which there was no initial hyperpolarization. Figure 2 summarizes the results from a number of recordings testing the effect of hydroquinone at a range of hydroquinone concentrations on the P. vulgaris root cortex cell membrane potential. The initial hyperpolarization response (Fig. 2A) proved to be significant at 0.01 mM and above and to be concentration dependent with highest hydroquinone concentrations inducing the greatest responses. The subsequent depolarization response (Fig. 2B) was also significant at 0.01 mM and above. Both the hyperpolarization and depolarization responses show sign of being saturable responses, especially the depolarization response as concentrations above 0.03 mM clearly did induce a still greater depolarization. This suggests hydroquinone might interact, directly or indirectly, with a finite number of membrane transporters. The time to maximal hyperpolarization (Fig. 2C) and to maximal depolarization (Fig. 2D) were not strongly concentration dependent, another indication that both these responses to hydroquinone are saturable phenomena.
Figure 2.
The effect of hydroquinone treatment on the membrane potential of root cortex cells of the elongation/root hair zone of Phaseolus vulgaris plants. Shown in A is the mean maximum hyperpolarization induced following inclusion of hydroquinone in the perfusion stream bathing root tips while B shows the mean maximum magnitude of subsequent depolarization relative to the initial pretreatment membrane potential. C shows the mean time to maximum hyperpolarization from the estimated time of initial hydroquinone-root contact and D shows the mean time the maximum depolarization. Errors bars indicate s.e.; n = 10 or 11 for each concentration except n = 15 for 0 mM. Letters a − d indicate significant differences between means (P < 0.05). Mean initial (immediately pretreatment) membrane potentials (0 mM = −149.5 +/− 5.3 mV; 0.001 mM = −162.8 +/− 6.0 mV; 0.01 mM = −155.1 +/− 4.6 mV; 0.03 mM = −153.6 +/− 6.6 mV; 0.1 mM = −161.5 +/− 7.8 mV; 0.25 mM = −149.7 +/− 11.7 mV;) were not significantly different between treatments.
If the just described root cell membrane potential response to hydroquinone is in some way causally related to hydroquinone-induced injury to P. vulgaris plants we predicted the same response should be absent when root tips of Antennaria microphylla, an allelopathic donor plant and a source of hydroquinone. The membrane potentials of root cortex cells of A. microphylla were, however, found to respond to hydroquinone with a similar initial, comparatively small, hyperpolarization followed by a larger depolarization (Table 2) but these responses were much smaller than the equivalent responses by P. vulgaris root cell membrane potentials. Challenged with 0.03 mM hydroquinone, the mean A. microphylla root cell membrane potential hyperpolarization and depolarization was less than 25% of the equivalent response by P. vulgaris root cell membrane potentials (compare Table 2 with Fig. 2). This result suggests A. microphylla should be more resistant to negative consequences associated with hydroquinone-induced membrane electrical responses.
Table 2.
Effect of 0.03 mM hydroquinoine on the membrane potential of root cortex cells of Antennaria microphylla
| Maximum hyperpolarization | 0.83 +/− 0.17 mV |
| Maximum depolarization | 8.67 +/− 1.96 mV |
| Time to maximum hyperpolarization | 2.5 +/− 0.2 min |
| Time to maximum depolarization | 17.7 +/− 3.2 min |
Shown are the mean maximum hyperpolarization, the mean maximal depolarization, the mean time to maximal hyperpolarization, and the mean time to maximum depolarization of the membrane potential following inclusion of 0.03 mM hydroquinone in the perfusion stream bathing of A. microphylla root cortex cells, each +/− the s.e. of the mean. N = 6. Mean initial membrane potential = -140.2 +/− 7.9.
Hydroquinone accumulates to high levels in the tissue of allelopathic donor plant A. microphylla in the form of arbutin, a nontoxic hydroquinone monoglycoside.3 Secreted into the soil, arbutin is readily deglycoslyated by soil microbes to hydroquinone.4 Detection of low levels of a UDPG-dependent glucosyltransferase in cell free extracts from callus cultures of E. esula suggest that target species themselves have some more limited capacity to detoxify hydroquinone by conversion to arbutin. If the hydroquinone-induced electrical effects on the root cell membrane potentials are causally related to hydroquinone induced injury in susceptible plants, then arbutin could be predicted not to elicit the same hyperpolarization-depolarization response produced by hydroquinone. Table 3 shows that treatment of P. vulgaris root tips with 0.03 mM arbutin did produce a small initial hyperpolarization followed by a depolarization with a similar time course of that induced by hydroquinone. Interpretation of this result is problematic, however, because arbutin is known to be rapidly converted to hydroquinone in nonsterile soils.4 P. Vulgaris may have a small electrical response to arbutin or, in the nonsterile conditions of our experiments, the observed electrical response to the introduction of arbutin may, in fact be a response to hydroquinone of arbutin origin. In either case, the magnitude of both the arbutin-induced hyperpolarization and depolarization was small compared to that induced by hydroquinone (compare Table 3 with Fig. 2) consistent with large electrical responses having a role in injury.
Table 3.
Effect of 0.03 mM Arbutin on the membrane potential of root cortex cells of Phaseolus vulgaris
| Maximum hyperpolarization | 1.0 +/− 0.36 mV |
| Maximum depolarization | 7.5 +/− 1.96 mV |
| Time to maximum hyperpolarization | 2.7 +/− 0.2 min |
| Time to maximum depolarization | 16.9 +/− 1.5 min |
Shown are the mean maximum hyperpolarization, the mean maximal depolarization, the mean time to maximal hyperpolarization, and the mean time to maximum depolarization of the membrane potential following inclusion of 0.03 mM hydroquinone in the perfusion stream bathing of P.vulgaris root cortex cells, each +/− the s.e. of the mean. N = 10. Mean initial membrane potential = −151.6 +/− 6.4.
Membrane potential recordings of the effect of hydroquinone on P. vulgaris root cells were maintained until they failed usually within an hour of the start of treatment. In most cases the depolarization was followed by at least some repolarization regardless of the hydroquinone concentration. In a few recordings repolarization to at or near the original membrane potential occurred. The bottom recording shown in Figure 1 is an example. To determine whether the hydroquinone-induced depolarization is indeed a transient phenomenon we incubated root tips in bathing medium +/− 0.03 mM hydroquinone for 24 hours then recorded the stable membrane potential. Table 4 shows that when incubated in hydroquinone for a more sustained period membrane potential fully recovers strongly suggesting that, at least at this concentration, any water transport related injury to the plant is not simply the result of a sustained hydroquinone induced depolarization.
Table 4.
Effect of 24 hour pretreatment with hydroquinone on the stable membrane potential of root cortex cells of Phaseolus vulgaris
| Control | hydroquinone |
| −175.0 +/− 8.7 mV | −173.0 +/− 7.9 mV |
N = 11 recordings each.
Discussion
In the work described here, hydroquinone exposure of bean root tips was found to induce a small brief hyperpolarization followed by a larger more sustained depolarization at a range of concentrations (Figs. 1–2). To date other studies of the effects of suspected allelochemicals on root cell membrane potentials are limited to phenolic acids. Glass and Dunlop15 reported that exposure to a number of benzoic and cinnamic acids at 0.5 mM rapidly depolarized the membrane potential of excised barley root tips. The most effective compound tested was salicylic acid which completely but reversibly depolarized the membrane potential within 15 minutes. The salicylic acid-induced depolarization was concentration dependent, however, with the effect disappearing by 0.01 mM. Balke16 found that the 0.5 mM salicylic acid-induced depolarization of oat root cell membrane potentials was preceded by a small hyperpolarization seemingly similar to that reported here induced by hydroquinone. Working with oat coleoptiles Bates and Goldsmith17 reported that 0.001 mM benzoic acid induced a transient 16 mV membrane potential depolarization. Dilute weak acids in general, however, have been shown to produce transient depolarization of oat coleoptile cell membranes.17–18
The cell membrane potential is a complex function of the various ionic fluxes across the cell membrane.10 In plants the cell membrane potential is dominated by a high conductance for K+ 19 and is ultimately maintained by the active transport of H+-ATPase or proton pump.20 The initial hydroquinone-induced hyperpolarization of bean root cell membrane potentials seen here represents an increase of ionic-charge separation across the membrane and could result from either the decrease of a depolarizing current (e.g., closing of channels) or from increased proton pump activity. While nothing in the current experiments distinguishes between these possibilities, membrane hyperpolarizations by both mechanisms are known in plants. For example, hyperosmotic shock has been shown close stretch-activated anion channels restricting the efflux of Cl− 21 and the fungal toxin fusicoccin and plant hormones homobrassinolide and auxins are known to activate the proton pump.22–24
The hydroquinone-induced depolarization found here to follow the initial hyperpolarization of P. vulgaris root cell membrane potentials could result from either an increase in depolarizing current(s) (i.e., opening channels or increasing cotransport) or proton pump inhibition. Membrane potential depolarizations by both of these mechanisms are known. Sustained activation of anion channels is produced by the hormone abscisic acid25–26 in leaf guard cell and auxins treatment triggers an initial transient anion efflux in coleoptiles cells.18 Calcium channel opening and Ca2+ ion influx is implicated in cell membrane depolarizations induced by abscisic acid,27 during the bacteria-induced hypersensitive response,28 and in legume root hair cells responding to the rhizobial Nod factor signal.29
A transient amino acid-induced pH-dependent depolarizationp of oat coleoptile cells has been interpreted as the consequence of multiple amino acid-H+ cotransporters;30–31 and subsequent increased proton pumping.32 In Arabidopsis roots, where there is also evidence of amino acid-H+ cotransport-mediated depolarization,33 six of the protein amino acids have also been shown to be potent activators of a nonspecific Ca2+-conducting channel34–35 activation of which greatly depolarizes the cell.33
Evidence of Proton pump inhibition in roots has also been found to be induced by chemical exposure as two known allelochemicals, juglone36 and sorgoleone37 have been shown to decrease proton pump activity in corn and soybean root plasma membrane microsomes.
What might be the mechanism by which hydroquinone initiates the hyperpolarization and depolarization events in P. vulgaris roots described here? Numerous receptor mediated signal transduction elements exist in plants. Of particular interest is the generation of reactive oxygen species, highly reactive reduced oxygen molecules that are early initiator of the programmed cell death events of the hypersensitive response in plants.38 A transient influx of Ca2+ that is also necessary for plant programmed cell death39,40 has been shown to involve reactive oxygen species activation of plasma membrane hyperpolarization-dependent Ca2+-permeable channels in stomatal guard cells.41 It appears the mode-of-action of at least one allelochemical involves activation of programmed cell death events. The allelopathic donor Centaurea maculosa (spotted knapweed) exudes the potent allelochemical (−)-catechin42 which was found to initiate a rapid rise in reactive oxygen species followed by a surge in cytoplasmic Ca2+ concentration, rapid changes in gene expression, and cell death in the roots of susceptible plants.43 It is possible the depolarization we report here also involves reactive oxygen species activation of a Ca2+ influx.
Importantly, the normal complex biochemistry of plant cells appears to homeostatically buffer changes in reactive oxygen species. Thus normal small fluctuations in the level of reactive oxygen species does not lead to activation of programmed cell death. For example, mutant or transgenic plants with disruptions to phenolic acid metabolism,44 porphyrin biosynthesis,45 or fatty acid synthesis46 all exhibit spontaneous premature cell death apparently mediated by the events of programmed cell death.
We report here that a two week exposure to hydroquinone significantly inhibited growth of P. vulgaris plants only at concentrations of 0.01 mM and higher while growth inhibition of E. esula was earlier documented after 30 day exposures to 0.1 and 0.25 mM hydroquinone.7 In both of these studies the plants were undoubtedly exposed to changing concentrations of hydroquinone because hydroponic solutions containing freshly dissolved hydroquinone were only replaced twice weekly and microbial metabolism can be expected to diminish the concentration by more than 50% over the course of 3–4 days.4 Nevertheless, it seems likely species like E. esula encounter still lower hydroquinone concentrations in soil water in the natural environment than found here to produce injury. Indeed, growth inhibition of plants by putative allelochemicals in the lab almost always requires application of concentrations clearly higher than are encountered by plants in nature.47 Estimates of soil allelochemical levels are problematic.48 For example solvent based extraction methods tend to yield results that do not reflect actual soil water concentrations. While soil water concentration is a critical factor determining phytotoxicity of allelochemicals,49 most estimates of soil content are results are reported in µg/g soil. Natural soil water concentrations of allelochemicals, however, are probably quite low. Bais et al.42 give estimates for soil (−)-catechin content that equate to as high as 1.3 nM while soil monomeric phenolics of alpine Picea forests were found to range up to 1.4 µM.50 Our report may be the first describing a root electrical response to an allelochemical at concentrations likely to be encountered by plant roots in nature.
Einhellig47 has argued that individual allelochemicals at levels found noninhibitory in the laboratory in a natural setting may act additively or synergistically to produce injury in the presence of other allelochemicals or that sensitivity to allelopathy may increase in the face of plant stress (e.g., pathogen attack, herbivory, nutrient deficiency, drought). Indeed plants subjected to multiple allelochemicals, water stress, or nutrient deficiency showed increased susceptibility to individual allelochemicals.51–53 As evident from the chlorophyll fluorescence results (Table 1), the P. vulgaris plants treated hydroponically with hydroquinone for 14 days in the current study were additionally stressed (presumably by root anoxia) and therefore could be expected to have had increased hydroquinone sensitivity.
Results from the earlier study of the allelopathic effects of hydroquinone on the target plant E. esula7 suggested a working model where hydroquinone acts at the level of the root. Disruption of cell membrane transport would disrupt water uptake to the shoot leading to reduced growth and photosynthesis and ultimately to plant death. In this study the hyperpolarization and depolarization induced in our short term hydroquinone exposure experiments are of small magnitude and are transient (Fig. 1, Table 4); these perturbations of the membrane potential by themselves are clearly not sufficiently large or long lasting enough to disrupt mineral and water uptake leading to long term reduced plant growth and inhibition of photosynthesis. Thus our results do not immediately support our working model.
The membrane potential of root cells of P. vulgaris does appear quite sensitive, however, to the low concentrations of hydroquinone target plants are likely to encounter in nature as most recordings at the lowest concentration tested (1.0 µM) clearly showed both a hyperpolarization and depolarization response. Perhaps the transient membrane potential changes seen in our short term hydroquinone exposure experiments are reflective of the initiation of the events programmed cell death. In the root tips of our otherwise unstressed healthy lab-grown plants, however, normal homeostatic biochemistry may arrest and reverse the process initiated by a sudden exposure to hydroquinone. In plants of allelopathic target species subjected to chronic hydroquinone exposure in nature, however, and in the presence of additional stress the events of programmed cell death might proceed unchecked and root cell death ensues. The much smaller electrical effects of hydroquinone on A. microphylla root cell membrane potentials suggest that this plant could reverse programmed cell death more easily.
Materials and Methods
Plant material.
Phaseolus vulgaris L cv. Contender (Southern States Cooperative Inc.; Richmond, Virginia) seeds were first selected for intactness and uniform weight (0.425 to 0.525 g), imbibed for 24 h on moist paper towels, and then planted individually in vermiculite in 500 ml pots. Seedlings were grown under greenhouse conditions (ambient light with temperature maintained about 20°C and below 35°C). Plants were watered every 1 to 2 days as needed, and treated once weekly with 0.25 strength Miracle-Gro (Scotts Miracle-Gro Products Inc., Port Washington, NY). Antennaria microphylla rhizomatous cuttings were obtained from Fourth Corner Nurseries, Bellingham, WA. These were planted in vermiculite and grown as above.
Growth and chlorophyll fluorescence assays.
After 13 days P. vulgaris plants of similar size and development (i.e., partially expanded monofoliates and emerging first trifoliate) were uprooted, rinsed free of vermiculite, and transferred to opaque approximately 600 ml plastic containers containing 0.5 strength Hoagland's nutrient solution.54 The stem of each plant was wrapped in cotton wadding and secured through in a notch in the plastic container lid which, once snapped in place kept the roots in darkness. After 48 hr the plants were exposed to a range of hydroquinone concentrations by replacement of the nutrient solution with fresh 0.5 strength Hoagland's augmented with hydroquinone. Plants were exposed to hydroquinone for 14 days. Treatment solutions were replaced twice weekly with hydroquinone dissolved immediately before use. To limit chemical oxidation of hydroquinone55 in the hydroponic solutions, as in the earlier test of hydroquinone sensitivity of E. esula7 no aeration and was provided before or during hydroquinone treatment.
On day 14 chlorophyll fluorescence was determined using an OS-30 Chlorophyll Fluorometer (Opti-Sciences, Tyngsboro, MA, USA). Plants were dark-adapted 10 min and chlorophyll fluorescence measured at one mid-leaf location on one expanded trifoliate of each plant.
Following chlorophyll fluorescence determination plants were harvested. For each plant stems, leaves, and roots were separated and weighed. Stem length and longest root length was measured and total leaf area for each plant was determined using a CI-202 Model Portable leaf area meter (CID,Inc., Vancouver, WA, USA).
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Electrophysiology.
Vermiculite grown P. vulgaris seedlings similar to those selected for growth and chlorophyll fluorescence assays were uprooted and rinsed free of vermiculite. Root tips, approximately 2 cm in length, were excised and immediately transferred to a bathing medium consisting of 1.0 mM CaCl2, 0.1 mM 2-(4-morpholino)-ethane sulfonic acid (MES)/1, 3-bis[tris{hydro xymethyl}methylamino]propane (Btp) pH 6.0, and 0.1 mM KCl. Following at least one hour of recovery in bathing medium individual root tips were secured to a plexiglass stage with thin strands of Qubitac (Qubit Systems, Kingston, Ontario, Canada) then mounted horizontally in a perfusion chamber similar to that described by Mertz and Higinbotham.56
The perfusion chamber itself was mounted on the stage of a horizontally positioned microscope so that each root tip could be viewed in longitudinal profile. The mounted root tip was perfused by bathing medium flowing though the length of the approximately 5 ml chamber at approximately 5.0 ml/min. Bathing medium was either gravity fed or delivered via a peristaltic pump though Tygon tubing. Solution changes were made by turning a valve in the line changing the source of the perfusion stream between two reservoirs so that approximately 2 min later the solution changed at the mid-chamber point of microelectrode impalement of the root tip (determined by including stain in the perfusion stream).
Root cells were impaled and the electropotential difference across the membrane (i.e., membrane potential) recorded while root tips were illuminated by the microscope light (approximately 80 µmol · m−2 · s −1). The membrane potential, defined as the difference between the microelectrode inserted into a cell and a reference electrode in the bathing medium in the perfusion stream, was measured by impaling root cortex cells of the elongation zone and early root hair zone approximately 1–2 cm from the root tip with a conventional microelectrode filled with 300 mM or 1.0 M KCl. The microelectodes were pulled using a vertical pipette puller (Model PP-830; Narishige International USA, Long Island, NY) from capillary glass (TW150F-4; World Precision Instruments [WPI], Sarasota, FL). The reference electrodes used were DRIREF-5SH (WPI). Vertical microelectrode impalements were made using a three dimensional micromanipulators (either No. 6507; Narishige or KITE-R; WPI) mounted above the perfusion chamber. The microscope-perfusion chamber set-ups were located inside grounded Faraday cages atop custom-built vibration isolation tables (concrete slabs resting on 10 cm thick foam rubber). The measured membrane potentials were amplified using high impedance electrometers (either model Duo 773 or model Electro 705; WPI) and recorded on chart recorders (model L-200 E; Linseis Inc, Princeton-Jct., NJ). Tip resistances were generally 10–30 MΩ. Successful impalings (evident as a steadily decreasing potential initially at least −80 mV) were of the first to third sub-epidermal (cortex) cells. These were allowed to stabilize at least ten min until the recorded potential was changing no more than 0.5 mV · min−1 at which point the perfusion stream was changed to include hydroquinone at a range of concentrations. Recordings were generally continued until they failed, invariably within 100 min of the start of hydroquinone treatment. Any recordings that failed within 25 minutes of the start of hydroquinone treatment and completion of the characteristic hydroquinone-induced depolarization were discarded.
Statistical analysis.
Data were analyzed using one-way analysis of variance with means separated using Duncan's Multiple range test. Analyses were conducted using SPSS 12.0 (SPSS Inc., Chicago, IL).
Aknowledgements
Support for this project by National Institutes of Health (NIH, USA) Grant Number P20 RR016741 from the IDeA Network of Biomedical Research Excellence / Biomedical Research Infrastructure Network (INBRE/BRIN) Program of the National Center for Research Resources is gratefully acknowledged. The publication contents are, however, solely the responsibility of the authors and conclusions drawn are not to be seen as official views of the NIH.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/5965
References
- 1.Einhellig FA. Allelopathy: Current status and future goals. In: Inderjit, Dakshini KMM, Einhellig FA, editors. Allelopathy: Organisms, Processes, and Applications. Washington, DC: American Chemical Society; 1995. pp. 1–24. [Google Scholar]
- 2.Selleck GW. The antibiotic effects of plants in laboratory and field. Weed Sci. 1972;20:189–194. [Google Scholar]
- 3.Manners GD, Galitz GD. Allelopathy of small everlasting (Antennaria microphylla): Identification of constituents phytotoxic to leafy spurge (Euphorbia esula) Weed Sci. 1985;34:8–12. [Google Scholar]
- 4.Weidenhamer JD, Romeo JT. Allelochemicals of Polygonella myriopylla: Chemistry and soil degradation. J Chem Ecol. 2004;30:1067–1082. doi: 10.1023/b:joec.0000028468.97851.7a. [DOI] [PubMed] [Google Scholar]
- 5.Einhellig FA. Mechanism of action of allelochemicals. In: Inderjit, Dakshini KMM, Einhellig FA, editors. Allelopathy: Organisms, Processes, and Applications. Washington, DC: American Chemical Society; 1995. pp. 96–116. [Google Scholar]
- 6.Weir TL, Park SW, Vivanco JM. Biochemical and physiological mechanisms mediated by allelochemicals. Curr Opin Plant Biol. 2004;7:472–479. doi: 10.1016/j.pbi.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Barkosky RR, Butler JL, Einhellig FA. Mechanisms of hydroquinone-induced growth reduction in leafy spurge. J Chem Ecol. 1999;25:1611–1621. [Google Scholar]
- 8.Steudle E, Peterson CA. How does water get through roots? J Expt Bot. 1998;49:775–788. [Google Scholar]
- 9.Glass ADM, Bohm BA. The uptake of simple phenols by barley roots. Planta. 1971;100:93–105. doi: 10.1007/BF00385211. [DOI] [PubMed] [Google Scholar]
- 10.Hille B. Ion channels of excitable membranes. 3rd. Sunderland, MA: Sinauer; 2001. [Google Scholar]
- 11.Krause GH, Weis E. Chlorophyll fluorescence and photosynthesis: The basics. Ann Rev Plant Physiol Plant Mol Biol. 1991;42:313–349. [Google Scholar]
- 12.Björkman O, Demmig B. Photon yield of O2 evolution and chlorophyll fluorescence at 77k among vascular plants of diverse origins. Planta. 1987;170:489–504. doi: 10.1007/BF00402983. [DOI] [PubMed] [Google Scholar]
- 13.Maxwell K, Johnson GN. Chlorophyll fluorescence - A practical guide. J Expt Bot. 2000;51:659–668. doi: 10.1093/jxb/51.345.659. [DOI] [PubMed] [Google Scholar]
- 14.Higinbotham N. Electropotentials of plant cells. Ann Rev Plant Physiol. 1973;24:25–46. [Google Scholar]
- 15.Glass ADM, Dunlop J. Influence of phenolic acids on ion uptake. IV. Depolarization of the membrane potentials. Plant Physiol. 1974;54:855–858. doi: 10.1104/pp.54.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balke NE. Effects of allelochemicals on mineral uptake and associated physiological processes. In: Alonzo AC, editor. The Chemistry of Allelopathy: Biochemical Interactions Among Plants. Washington, DC: American Chemical Society; 1985. pp. 161–178. [Google Scholar]
- 17.Bates GW, Goldsmith MHM. Rapid response of the plasma membrane potential in oat, Avena sativa, coleoptiles to auxin and other weak acids. Planta. 1983;159:231–237. doi: 10.1007/BF00397530. [DOI] [PubMed] [Google Scholar]
- 18.Keller CP, Van Volkenburgh E. The electrical response of Avena coleoptile cortex to auxin. Planta. 1996;198:404–412. [Google Scholar]
- 19.Pitman MG, Mertz SM, Jr, Graves JS, Pierce WS, Higinbotham N. Electrical potential differences in cells of barley roots and their relation to ion uptake. Plant Physiol. 1971;47:76–81. doi: 10.1104/pp.47.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sze H, Li X, Palmgren MG. Energization of plant cell membranes by H+-pumping ATPases: Regulation and biosynthesis. Plant Cell. 1999;11:677–689. doi: 10.1105/tpc.11.4.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beffagna N, Buffoli B, Busi C. Modulation of reactive oxygen species production during osmotic stress in Arabidopsis thaliana cultured cells: Involvement of the plasma membrane Ca2+-ATPase and H+-ATPase. Plant Cell Physiol. 2005;46:1326–1339. doi: 10.1093/pcp/pci142. [DOI] [PubMed] [Google Scholar]
- 22.Bunney TD, Van Den Wijngaard PWJ, De Boer AH. 14-3-3 protein regulation of proton pumps and ion channels. Plant Mol Biol. 2002;50:1041–1051. doi: 10.1023/a:1021231805697. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Ramirez J, Reboutier D, Brault M, Trouverie J, Pennarun AM, Amiar Z, Biligui B, Galagovsky L, Rona JP. Brassinosteroids regulate plasma membrane anion channels in addition to proton pumps during expansion of Arabidopsis thaliana cells. Plant Cell Physiol. 2005;46:1494–1504. doi: 10.1093/pcp/pci162. [DOI] [PubMed] [Google Scholar]
- 24.Senn AP, Goldsmith MHM. Regulation of electrogenic proton pumping by auxin and fusicoccin as related to the growth of Avena coleoptiles. Plant Physiol. 1988;88:131–138. doi: 10.1104/pp.88.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D. Guard cell signal transduction. Ann Rev Plant Physiol Plant Mol Biol. 2001;52:627–658. doi: 10.1146/annurev.arplant.52.1.627. [DOI] [PubMed] [Google Scholar]
- 26.Raschke K, Shabahang M, Wolf R. The slow and quick anion conductance in whole guard cells: Their voltage-dependent alternation, and the modulation of their activities by abscisic acid and CO2. Planta. 2003;217:639–650. doi: 10.1007/s00425-003-1033-4. [DOI] [PubMed] [Google Scholar]
- 27.Schroeder JI, Hagiwara S. Repetitive increases in cytosolic Ca2+ of guard cells by abscisic acid activation of nonselective Ca2+ permeable channels. Proc Natl Acad Sci USA. 1990;87:9305–9309. doi: 10.1073/pnas.87.23.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pike SM, Zhang XC, Gassmann W. Electrophysiological characterization of the Arabidopsis avRpt2-specific hypersensitive response in the absence of other bacterial signals. Plant Physiol. 2005;138:1009–1017. doi: 10.1104/pp.104.047142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehrhardt DW, Wais R, Long SR. Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell. 1996;85:673–681. doi: 10.1016/s0092-8674(00)81234-9. [DOI] [PubMed] [Google Scholar]
- 30.Etherton B, Rubinstein B. Evidence for amino acid-H+ cotransport in oat coleoptiles. Plant Physiol. 1978;61:933–937. doi: 10.1104/pp.61.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinraide TB, Ethertom B. Electrical evidence for different mechanisms of uptake of basic, neutral, and acidic amino acids in oat coleoptiles. Plant Physiol. 1980;65:1085–1089. doi: 10.1104/pp.65.6.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinraide TB, Ethertom B. Energy coupling in H+-amino acid cotransport: ATP dependence of the spontaneous electrical repolarization of the cell membranes in oat coleoptiles. Plant Physiol. 1982;69:648–652. doi: 10.1104/pp.69.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qi Z, Stephens NR, Spalding EP. Calcium entry mediated by GLR3.3, an Arabidopsis glutamate receptor with a broad agonist profile. Plant Physiol. 2006;142:963–971. doi: 10.1104/pp.106.088989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dennison KL, Spaulding EP. Glutamate-gated calcium fluxes in Arabidopsis. Plant Physiol. 2000;124:1511–1514. doi: 10.1104/pp.124.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demidchik V, Essah P, Tester M. Glutamate activates cation currents in the plasma membrane of Arabidopsis root cells. Planta. 2004;219:167–175. doi: 10.1007/s00425-004-1207-8. [DOI] [PubMed] [Google Scholar]
- 36.Hejl AM, Koster KL. Juglone disrupts root plasma membrane H+-ATPase activity and impairs water uptake, root respiration, and growtrh in soybean (Glycine max) and corn (Zea mays) J Chem Ecol. 2004;30:453–471. doi: 10.1023/b:joec.0000017988.20530.d5. [DOI] [PubMed] [Google Scholar]
- 37.Hejl AM, Koster KL. The allelochemical sorgoleone inhibits root H+-ATPase and water uptake. J Chem Ecol. 2004;30:2181–2191. doi: 10.1023/b:joec.0000048782.87862.7f. [DOI] [PubMed] [Google Scholar]
- 38.Torres MA, Jones DG, Dangl JL. Reactive oxygen species signaling in response to pathogens. Plant Physiol. 2006;141:373–378. doi: 10.1104/pp.106.079467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dangl JL, Dietrich RA, Richberg MH. Death don't have no mercy: Cell death programs in plant-microbe interactions. Plant Cell. 1996;8:1793–1807. doi: 10.1105/tpc.8.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ali R, Ma W, Lemtiri-Chlieh F, Tsaltas D, Leng Q, Von Bodman S, Berkowitz GA. Death don't have no mercy and neither does calcium: Arabidopsis CYCLIC NUCLEOTIDE GATED CHANNEL2 and innate immunity. Plant Cell. 2007;19:1081–1095. doi: 10.1105/tpc.106.045096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Schroeder JI. Calcium channels activated by hydrogen peroxide mediate abscisic acid signaling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- 42.Bais HP, Walker TS, Stermitz FR, Hufbauer RA, Vivanco JM. Enantiomeric-dependent phytotoxic and antimicrobial activity of (±)-Catechin: A rhizosecreted racemic mixture from spotted knapweed. Plant Physiol. 2002;128:1173–1179. doi: 10.1104/pp.011019. [DOI] [PubMed] [Google Scholar]
- 43.Bais HP, Vepachedu R, Gilroy S, Callaway RM, Vivanco JM. Allelopathy and exotic plant invasion: From molecules and genes to species interactions. Science. 2003;301:1377–1380. doi: 10.1126/science.1083245. [DOI] [PubMed] [Google Scholar]
- 44.Tamagnone L, Merida A, Stacey N, Plaskitt K, Parr A, Chang CF, Lynn D, Dow JM, Roberts K, Martin C. Inhibition of phenolic acid metabolism results in precocious cell death and altered morphology in leaves of transgenic tobacco plants. Plant Cell. 1998;10:1801–1816. doi: 10.1105/tpc.10.11.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu G, Yalpani N, Briggs SP, Johal GS. Aporphyrin pathway impairment is responsible for the phenotype of a dominant disease lesion mimic mutant of maize. Plant Cell. 1998;10:1095–1105. doi: 10.1105/tpc.10.7.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mou Z, He Y, Dai Y, Liu X, Li J. Deficiency in fatty acid synthase leads to premature cell death and dramatic alterations in plant morphology. Plant Cell. 2000;12:405–417. doi: 10.1105/tpc.12.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Einhellig FA. An integrated view of allelochemicals amid multiple stresses. In: Inderjit, Dakshini KMM, Foy CL, editors. Principles and Practices in Plant Ecology: Allelochemical Interactions. Boca Raton, FL: CRC Press; 1999. pp. 479–494. [Google Scholar]
- 48.Qasem JR, Hill TA. On the difficulties with allelopathy methodology. Weed Res. 1989;29:345–347. [Google Scholar]
- 49.Kobayashi K. Factors affecting phytotoxic activity of allelochemicals in soil. Weed Biol Manag. 2004;4:1–7. [Google Scholar]
- 50.Gallet C, Pellissier F. Phenolic compounds in natural solutions of a coniferous forest. J Chem Ecol. 1997;23:2401–2412. [Google Scholar]
- 51.Einhellig FA, Rasmussen JA. Synergistic inhibitory effects of vanillic and p-hydroxybenzoic acid on radish and grain sorghum. J Chem Ecol. 1978;4:425–436. [Google Scholar]
- 52.Einhellig FA. Interactive effects of allelochemicals and environmental stress. In: Chou CH, Walter GR, editors. Phytochemical Ecology: Allelochemicals, Mycotoxins and Insect Pheromones and Allomones. Taipei, Taiwan: Institute of Botany, Academia Sinica; 1989. pp. 101–118. Monograph Series No. 9. [Google Scholar]
- 53.Williamson GB, Obee EM, Weidenhamer JD. Inhibition of Schizachyrium scoparium (Poaceae) by the allelochemical hydrocinnamic acid. J Chem Ecol. 1992;18:2095–2210. doi: 10.1007/BF00981930. [DOI] [PubMed] [Google Scholar]
- 54.Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. Calif Agric Ext Serv Circ No. 1950:347. [Google Scholar]
- 55.Flaig W, Salfeld JC, Haider K. Intermediate stages in the formation of natural humic acids and of comparable synthetic compounds. Landwirtsch Forsh. 1963;16:85–96. [Google Scholar]
- 56.Mertz SM, Jr, Higinbotham N. Transmembrane electropotential in barley roots as related to cell type, cell location, and cutting and aging effects. Plant Physiol. 1976;57:123–128. doi: 10.1104/pp.57.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]