Abstract
Plants are acutely sensitive to the directional information provided by gravity. They have evolved statocytes, which are specialized cells that sense gravity and, upon integration of the corresponding information with that of other environmental stimuli, control the growth behavior of their organs. The cellular mechanisms that allow statocytes to sense and transduce gravitational information likely involve detecting the sedimentation of, or the tension/pressure exerted by, starch-filled amyloplasts—the presumptive statoliths—within their cytoplasm. Gravity signaling in root statocytes controls the direction of transport of signaling compounds, especially auxin, across the root cap, establishing a lateral gradient that is transmitted to cells in the elongation zone and results in gravitropic curvature. The Arabidopsis J-domain proteins ARG1 and ARL2 function as gravity-signal transducers in root statocytes. In the January issue of The Plant Journal, we reported that ARG1 and ARL2 function non-redundantly in a common gravity signaling pathway required for accumulation of the auxin efflux facilitator PIN3 on the new bottom side of statocytes following gravity stimulation, and lateral redistribution of auxin toward the new lower flank of stimulated roots. Here we present data suggesting that ARG1 physically associates with ARL2, the J-domain co-chaperone HSC70, and actin in vivo. We briefly discuss potential mechanisms by which ARG1 and ARL2 might function in gravity signaling in light of this information.
Key words: gravitropism, statocytes, actin, HSC70/HSP70, auxin
Gravitropic growth in plants requires the ability to sense an organ's orientation within the gravity field, transduce that information into a biochemical signal, and mount a differential cellular-elongation response that corrects growth to follow a defined gravity set point angle. Gravity sensation in roots occurs mainly in statocytes of the root cap columella, either via detection of the position or movement of, or the pressure exerted by, amyloplasts within these cells, or by perceiving the pressure exerted by the statocyte's protoplast within its cell wall.1,2 Several models address the mechanism by which statocytes use amyloplasts as gravity susceptors. One model postulates that gravity signal transduction initiates when sedimenting amyloplasts promote the opening of mechano-sensitive ion channels, either directly or through interaction with the actin cytoskeleton. Alternatively, signal transduction may initiate upon sedimentation, when amyloplast-borne ligands interact with receptors located on sensitive structures within the statocytes.1 Nevertheless, the activated pathway promotes a fast and transient cytoplasmic alkalinization and redistribution of the auxin efflux carrier PIN3 to the lower membrane of the statocytes. These changes contribute to lateral, downward, redistribution of auxin across the cap, resulting in the formation of a lateral auxin gradient that, upon transmission to the elongation zone, promotes tip curvature (reviewed in ref. 1).
Along with experiments demonstrating the importance of amyloplasts in gravity susception, genetic analysis in Arabidopsis has identified ARG1 and ARL2 as gravity signal transducers in root and hypocotyl statocytes.3–6 ARG1 and ARL2 function in a gravity-signaling pathway that links gravistimulation to the accumulation of PIN3 within the PM at the lower side of the statocytes and a redistribution of auxin across the cap.4–6 ARG1 and ARL2 are membrane-associated proteins. While ARG1 localizes throughout the endosomal/secretory pathway, ARL2 associates primarily with the PM, and both proteins are found at the cell plate during cytokinesis.4,6 ARG1 and ARL2 contain J-domains near their N-termini. J-domain proteins typically function as molecular co-chaperones by interacting with HSC70, coupling substrate binding to HSC70 ATPase activity.10 A direct role for HSC70 in gravity signaling has not been reported, though proteomics approaches have identified cytosolic HSC70 isoforms as gravity-regulated proteins within Arabidopsis root tips.7,8
The C-termini of ARG1 and ARL2 contain putative coiled-coil domains with similarity to predicted coiled-coils found in proteins that interact with the cytoskeleton, including Rho-associated kinases, myosin, heavy chain kinesins and tropomyosins.3 Interestingly, a biochemical fractionation experiment detected possible interaction between ARG1 and polymerized actin in plant extracts.4 Furthermore, proteomic analyses of root gravitropism also identified actin as a gravity-regulated protein7,8 whereas other studies strongly suggest a role for the actin cytoskeleton in the modulation of gravity signaling in roots.9
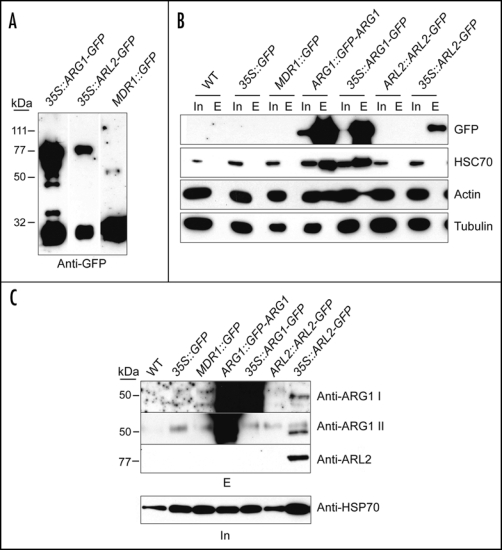
To investigate possible in vivo interactions between ARG1, ARL2, HSC70 and/or actin, we generated transgenic plants expressing GFP fusions to ARG1 or ARL2, in the arg1-2 or arl2-3 mutant backgrounds (Ws alleles), and used them in co-immunoprecipitation experiments. All constructs were tested for functional rescue prior to use. Functional GFP fusions to ARG1 or ARL2 were isolated from plant extracts using immobilized anti-GFP antibodies. Initial experiments indicated that both N and C-terminal GFP fusions to ARG1 and C-terminal fusions to ARL2 could be purified by this method (Fig. 1A). However, we consistently noticed lower relative abundance of ARL2-GFP purified from plants compared to ARG1-GFP, even when both were expressed under the same promoter (Fig. 1A). This is consistent with lower fluorescence intensity and fusion protein abundance of ARL2-GFP compared to ARG1-GFP in the many transgenic lines analyzed (data not shown).
Figure 1.
HSC70, actin and ARL2 co-purify with ARG1 from plant extracts. Total protein extracts from four-week-old liquid grown plants of the indicated genotypes (top) were affinity-purified using anti-GFP antibodies (gift of Richard Vierstra, UW-Madison) cross-linked to Protein A-Sepharose beads and detected with the indicated antibodies. In (A) the affinity-purified eluate was probed with anti-GFP antibody to indicate the presence of both full-length GFP fusions and breakdown products. In (B) western blots of total protein extracts (Input, In) and affinity-purified eluates (E) were probed with anti-HSC70 (diluted 1:10,000; Stressgen, San Diego, CA), monoclonal anti-actin (diluted 1:1000; clone C4 from Chemicon Int., Temecula, CA), or anti-a-tubulin antibodies (Sigma, St Louis, MO). In (C) western blots of affinity-purified eluates (top) were probed with two anti-ARG1 antibodies raised in rabbits against a bacterially expressed His6-ARG1 protein fusion (anti-ARG1 I) and against a peptide corresponding to the 15 C-terminal residues of ARG1 (anti-ARG1 II), both used at 1:1000 dilutions, as well as with a polyclonal anti-ARL2 antibody raised in rabbits against a synthetic ARL2 peptide, used at 1:3000 dilution. Note that anti-ARG1 II did not recognize the ARG1-GFP protein probably because the corresponding C-terminal epitope was masked in this C-terminal fusion. A western blot of total protein extracts used for affinity purification (In, bottom) was probed with anti-HSP70 antibody (gift of Elizabeth Craig, UW-Madison; 1:20,000 dilution) to show relative amounts of protein prior to extraction. Experimental procedures. Plants expressing ARG1::GFP-ARG1 and 35S::ARL2-GFP are described elsewhere (reviewed in refs. 4 and 6, respectively). Plants expressing 35S::ARG1-GFP were generated by transformation with a T-DNA derived from the binary vector pK7FWG225 containing the ARG1 cDNA, whereas plants expressing AtMDR1::GFP were a gift from Edgar Spalding (UW-Madison). For immunoprecipitation, anti-GFP serum was cross-linked to Protein A Sepharose (Amersham, Piscataway, NJ) using the cross-linking reagent disuccinimidyl suberate according to the manufacturer's recommendations (Pierce, Rockford, Ill.). Extracts from liquid-grown plants were generated by grinding in 1:1 (vol:wt) ice cold grinding buffer [20 mM Tris, 1 mM EDTA, 100 mM NaCl, 0.1% Triton X-100 pH7.5, containing 1:100 dilution of protease inhibitor cocktail (Sigma, or Calbiochem, San Diego, CA) and 1 mM phenylmethylsulphonyl fluoride] in a mortar, then adding more buffer to a ratio of 4:1 (vol:wt). Homogenates were cleared by filtering through Miracloth (Calbiochem) and spinning twice at 13,000 g for 15 min each to pellet cellular debris. Protein concentration in the resulting supernatant was then quantified by a modified Lowry assay, using BSA as a standard. Extracts were gently rocked with anti-GFP beads for one hour at room temperature. The beads were washed extensively (five times with ≥5× bead vol.) in extraction buffer, and the bound protein was eluted with low pH (0.1 M glycine pH 2.5) into a small amount of neutralizing 1 M tris buffer (pH 8.0), precipitated with TCA, separated by SDS-PAGE, blotted to PVDF membrane (Millipore, Billerica, MA), and probed with the appropriate antibodies. Positive signals were detected using HRP-conjugated secondary antibodies at 1:20,000 (Sigma).
Proteins that interact with ARG1 or ARL2 are expected to bind to the anti-GFP-coated beads in this assay, along with their GFP-ARG1 or GFP-ARL2 partner, and to co-elute with them upon washing the beads with a low-pH solution. Their identity can be revealed by western blot analysis of bead eluates, using specific antibodies. Figure 1B shows that HSC70 co-purifies with ARG1-GFP and GFP-ARG1 when extracts from plants expressing these fusions are tested in this assay. However, HSC70 was not present in the eluate when plants expressing GFP alone under the control of the CaMV35S or AtMDR1 ectopic promoters were tested (Fig. 1B). Interestingly, we did not detect HSC70 in bead eluates when ARL2-GFP-expressing plants were subjected to this co-immunoprecipitation assay, suggesting that ARG1 and ARL2 differ in their affinity for HSC70. It should however be noted that the low amount of ARL2-GFP precipitated in these experiments does not allow for direct comparison (Fig. 1B). These results indicate an in vivo interaction between ARG1 and HSC70, suggesting that ARG1 is a bona fide co-chaperone.
Figure 1B also shows that actin is present specifically in immunocomplexes containing ARG1. In a parallel experiment, ARL2-GFP precipitates did not contain detectable actin (Fig. 1B). However, here again, the low amount of ARL2 in these precipitates does not allow direct comparison to ARG1. Together these data suggest that ARG1 physically associates with actin in vivo, even though they do not address whether this association involves monomeric or polymerized actin. Interestingly, the biochemical fractionation experiments reported by Boonsirichai et al. (2003) suggested that some ARG1 might associate with polymerized actin in vivo.
Plants with mutations in ARG1, ARL2, or both, result in very similar gravity signaling defects, demonstrating that ARG1 and ARL2 function as non-redundant members of the same signaling pathway.5 One interpretation of this data is that ARG1 and ARL2 function in the same signaling complex, the presence of both being required for complex activity in gravity signaling. In support of this model, we have detected an in vivo interaction between ARG1 and ARL2 by immunoprecipitation. Figure 1C shows that endogenous ARG1 (∼45 kDa) co-immunoprecipitates with ARL2-GFP from extracts derived from plants expressing 35S::ARL2-GFP, but not from extracts derived from negative control plants. We were not surprised by the failure to detect ARG1 in precipitates from plants expressing ARL2-GFP under the control of its endogenous promoter (ARL2::ARL2-GFP). Indeed, its expression is restricted to a few cells—therefore, the protein is present at low abundance in whole-plant extracts.6 Similarly, native ARL2 was not detected in ARG1 immunoprecipitates likely due to the low level of endogenous ARL2 expression. Together, these results suggest that ARG1 and ARL2 associate in a complex in vivo, consistent with the function of these proteins in a common signaling pathway.5
The sub-cellular localization of a putative ARG1- and ARL2-containing complex is unknown, but is likely associated with the PM of root statocytes, where ARL2-GFP localizes in cells expressing ARL2::ARL2-GFP.6 Such a complex may serve as a membrane-associated signaling module that recruits cytosolic HSC70 to a membrane site in a gravity-regulated manner. In agreement with this model, we found that HSP70 increases in abundance in a biochemical fraction that includes membrane-associated proteins extracted from Arabidopsis root tips within 10 min of a gravistimulus.8 A similar recruitment model has been proposed for J-protein function in several HSC70-mediated processes, including the regulation of vesicle dynamics and signaling by HSC70.10 In animal systems, HSC70 activity is required for several steps in clatherin-dependent vesicle recycling,11 a process that appears to mediate PIN protein trafficking12. Recruitment of HSC70 activity to clatherin-coated vesicles is mediated mainly by the J-domain protein auxillin, which has paralogs other than ARG1 and ARL2 in plants.10 HSC70 function is also required for Ca2+-induced exocytosis in Drosophila, and depends on the J-domain-containing cysteine-string protein, apparent homologs of which are absent from plants.13 GRV2, a locus required for normal shoot tropisms, encodes a membrane-associated J-domain protein homologous to animal RME-8.14 RME-8 is a membrane-associated J-domain protein that interacts with cytosolic HSC70 and is required for endocytosis and endosomal trafficking in D. melanogaster and C. elegans.15,16 GRV2 appears to regulate dynamics of late vacuolar endosomes,17 which is important for gravity signaling in shoots but not roots.18 GRV2/RME-8, auxillin, and cysteine-string proteins are each specific for endosomal trafficking involving particular molecules or vesicles, thus illustrating the specificity that J-domain proteins can confer to HSC70-mediated processes.
One possibility is that the putative ARG1/ARL2 complex recruits HSC70 to alter actin dynamics in response to gravistimulation. The actin network in root statocytes appears to be intimately associated with the mechanism of gravity sensation, as treatments with low concentrations of the actin-depolymerizing drug Latrunculin B enhances gravity signaling events including amyloplast sedimentation, alkalinization of the statocyte cytoplasm, with subsequent enhancement of both auxin redistribution and organ curvature.9 arg1-2 mutants are deficient in gravity-induced statocyte alkalinization and auxin redistribution, suggesting that ARG1 and microfilaments regulate this gravity signaling response.4
The lateral polarization of the statocytes upon gravistimulation likely involves dramatic changes in vesicle trafficking. Mechanisms that lead to accumulation of PIN3 upon the new bottom side of root statocytes are unknown, but require ARG1 and ARL2 either directly or as upstream signaling components. The prominent localization of ARG1 and ARL2 to the cell plate during cytokinesis suggests that they are present in areas of intense vesicle dynamics. The recent discovery that members of an Arabidopsis retromer complex are required for cell polarization, PIN trafficking, and gravitropic growth suggests they may function in redirection of auxin following gravistimulation.19,20 Animal homologs of these retromer components have been identified in association with clathrin-coated vesicles,21 and disruption of Drosophila Vps35 or human SNX9 affect endocytosis and actin organization.22,23 The mechanisms of clathrin-dependent endocytosis are unresolved, but appear to depend on dynamic cortical microfilaments in animals and yeast suggesting that similar mechanisms may function in plants.24 ARG1 and ARL2 may mediate statocyte polarization via regulating actin and/or vesicle dynamics. Of course, we have not yet excluded the possibility that ARG1 and ARL2 might, in fact, transduce the gravity signal in an HSC70 and actin-independent manner through interactions with yet unidentified molecules. In this case, the interactions identified in this study would be relevant to other, yet uncharacterized, functions potentially associated with ARG1 and ARL2 in plants.4,5 Work aimed at identifying additional ARG1 and ARL2-interacting proteins is underway.
Acknowledgments
We thank Greg Sabat, Scott Saracco and Joseph Walker for advice on biochemical technique, Richard Vierstra, Edgar Spalding, Charles Guy, Yan Wang and Elizabeth Craig for reagents, Kanokporn Boonsirichai, Changhui Guan and John Sedbrook for the anti-ARG1 and anti-ARL2 antibodies. This work was supported by grants from the National Science Foundation (MCB-0240084 and IOS0642865) and the UW-Madison College of Agriculture and Life Sciences USDA HATCH program (WIS04784).
Abbreviations
- PM
plasma membrane
- ARG1
altered response to gravity 1
- ARL2
ARG1-like 2
- PIN3
pinformed 3
- HSC70
heat shock cognate 70
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/5749
References
- 1.Perrin RM, Young LS, Murthy UMN, Harrison BR, Wang Y, Will JL, Masson PH. Gravity signal transduction in primary roots. Ann Bot. 2005;96:737–743. doi: 10.1093/aob/mci227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perbal G, Driss-Ecole D. Mechanotransduction in gravisensing cells. Trends Plant Sci. 2003;8:498–504. doi: 10.1016/j.tplants.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Sedbrook JC, Chen R, Masson PH. ARG1 (altered response to gravity) encodes a DnaJ-like protein that potentially interacts with the cytoskeleton. Proc Natl Acad Sci USA. 1999;96:1140–1145. doi: 10.1073/pnas.96.3.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boonsirichai K, Sedbrook JC, Chen R, Gilroy S, Masson PH. ALTERED RESPONSE TO GRAVITY is a peripheral membrane protein that modulates gravity-induced cytoplasmic alkalinization and lateral auxin transport in plant statocytes. Plant Cell. 2003;11:2612–2625. doi: 10.1105/tpc.015560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan C, Rosen E, Boonsirichai K, Poff K, Masson PH. The ARG1-LIKE2 (ARL2) gene of Arabidopsis thaliana functions in a gravity signal transduction pathway that is genetically distinct from the PGM pathway. Plant Physiol. 2003;133:100–112. doi: 10.1104/pp.103.023358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrison BR, Masson PH. ARL2, ARG1 and PIN3 define a gravity signal transduction pathway in root statocytes. Plant J. 2008;53:380–392. doi: 10.1111/j.1365-313X.2007.03351.x. [DOI] [PubMed] [Google Scholar]
- 7.Kamada M, Higashitani A, Isioka N. Proteomic analysis of Arabidopsis root gravitropism. Biol Sci Space. 2005;19:148–154. [Google Scholar]
- 8.Murthy NUM, Young LS, Sabat G, Masson PH. Identification of gravity-regulated root-tip proteins in Arabidopsis. In preparation.
- 9.Hou G, Kramer VL, Wang YS, Chen R, Perbal G, Gilroy S, Blancaflor EB. The promotion of gravitropism in Arabidopsis roots upon actin disruption is coupled with the extended alkalinization of the columella cytoplasm and a persistent lateral auxin gradient. Plant J. 2004;39:113–125. doi: 10.1111/j.1365-313X.2004.02114.x. [DOI] [PubMed] [Google Scholar]
- 10.Young JC, Barral JM, Ulrich Hartl F. More than folding: localized functions of cytosolic chaperones. Trends in Biochem Sci. 2003;28:541–547. doi: 10.1016/j.tibs.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Newmyer SL, Schmit SL. Dominant-interfering Hsc70 mutants disrupt multiple stages of the clathrin-coated vesicle cycle in vivo. J Cell Biol. 2001;152:607–620. doi: 10.1083/jcb.152.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhonukshe P, Aniento F, Hwang I, Robinson DG, Mravec J, Stierhof YD, Friml J. Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr Biol. 2007;17:520–527. doi: 10.1016/j.cub.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 13.Bronk P, Wenniger JJ, Dawson Scully K, Guo X, Hong S, Atwood HL, Zinsmaier KE. Drosophila Hsc70-4 is critical for neurotransmitter exocytosis in vivo. Neuron. 2001;30:475–488. doi: 10.1016/s0896-6273(01)00292-6. [DOI] [PubMed] [Google Scholar]
- 14.Silady RA, Kato T, Lukowitz W, Sieber P, Tasaka M, Somerville CR. The gravitropism defective 2 mutants of Arabidopsis are deficient in a protein implicated in endocytosis in Caenorhabditis elegans. Plant Physiol. 2004;136:3095–3103. doi: 10.1104/pp.104.050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang HC, Hull M, Mellman I. The J-domain protein Rme-8 interacts with Hsc70 to control clathrin-dependent endocytosis in Drosophila. J Cell Biol. 2004;164:1055–1064. doi: 10.1083/jcb.200311084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girard M, Poupon V, Blondeau F, McPherson PS. The DnaJ-domain protein RME-8 functions in endosomal trafficking. J Biol Chem. 2005;280:40135–40143. doi: 10.1074/jbc.M505036200. [DOI] [PubMed] [Google Scholar]
- 17.Silady RA, Ehrhardt DW, Jackson K, Faulkner C, Oparka K, Somerville CR. The GRV2/RME-8 protein of Arabidopsis functions in the late endocytic pathway and is required for vacuolar membrane flow. Plant J. 2007;53:29–41. doi: 10.1111/j.1365-313X.2007.03314.x. [DOI] [PubMed] [Google Scholar]
- 18.Morita MT, Tasaka M. Gravity sensing and signaling. Curr Opin Plant Biol. 2004;7:712–718. doi: 10.1016/j.pbi.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Jaillais Y, Fobis Loisy I, Miège C, Rollin C, Gaude T. AtSNX1 defines an endosome for auxin-carrier trafficking in Arabidopsis. Nature. 2006;443:106–109. doi: 10.1038/nature05046. [DOI] [PubMed] [Google Scholar]
- 20.Jaillais Y, Santambrogio M, Rozier F, Fobis Loisy I, Miège C, Gaude T. The retromer protein VPS29 links cell polarity and organ initiation in plants. Cell. 2007;130:1057–1070. doi: 10.1016/j.cell.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 21.Borner GH, Harbour M, Hester S, Lilley KS, Robinson MS. Comparative proteomics of clathrin-coated vesicles. J Cell Biol. 2006;175:571–578. doi: 10.1083/jcb.200607164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korolchuk VI, Schütz MM, Gómez Llorente C, Rocha J, Lansu NR, Collins SM, Wairkar YP, Robinson IM, O'Kane CJ. Drosophila Vps35 function is necessary for normal endocytic trafficking and actin cytoskeleton organisation. J Cell Sci. 2007;120:4367–4376. doi: 10.1242/jcs.012336. [DOI] [PubMed] [Google Scholar]
- 23.Yarar D, Waterman Storer CM, Schmid SL. SNX9 couples actin assembly to phosphoinositide signals and is required for membrane remodeling during endocytosis. Dev Cell. 2007;13:43–56. doi: 10.1016/j.devcel.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Kaksonen M, Toret CP, Drubin DG. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2006;7:404–414. doi: 10.1038/nrm1940. [DOI] [PubMed] [Google Scholar]
- 25.Karimi M, Inze D, Depicker A. GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 2002;7:193–195. doi: 10.1016/s1360-1385(02)02251-3. [DOI] [PubMed] [Google Scholar]