Abstract
The ability of some animals to sense magnetic fields has long captured the human imagination. In our recent paper, we explored how radical pair effects in the protein cryptochrome may underlie the magnetic orientation sense of migratory birds. Here we explain our model and discuss its relationship to experimental results on plant cryptochromes, as well as discuss the next steps in refining our model, and explore alternate but related possibilities for modeling and understanding cryptochrome as a magnetic sensor.
Key words: cryptochrome, radical pair machanism, avian orientation, magnetic field effect, Arabidopsis thaliana, avian magnetoreception, magnetic sensor
The ability of some animals to sense magnetic fields is a long-standing open problem in biology. Over the past 50 years, scientific studies have shown that a wide variety of living organisms have the ability to perceive magnetic fields and can use information from the earth's magnetic field in orientation behavior. The best-studied example of animal magnetoreception is the case of migratory birds, who use the earth's magnetic field, as well as a variety of other environmental cues, to find their way during migration.
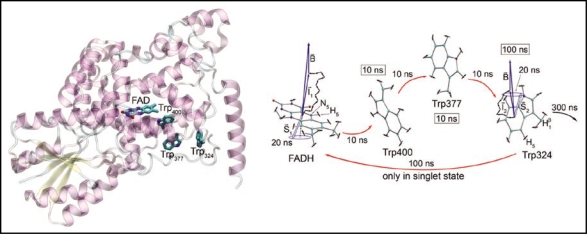
The two prevailing hypotheses for the mechanism of avian magnetoreception are an iron-mineral-based explanation, wherein birds use small deposits of magnetic iron minerals1,2,12 in the base of their beaks for magnetic orientation, and a radical-pair-based explanation, in which a magnetically sensitive chemical reaction in the eye of the bird enables perception of the magnetic field via its effects on reaction products. The latter hypothesis is based on the idea that a radical pair reaction may take place in the protein cryptochrome in the retina of the bird.3,4 Cryptochrome contains a blue-light-absorbing chromophore, flavin adenine dinucleotide (FAD); this FAD cofactor is reduced via a series of light-induced electron transfers from a chain of three tryptophans that bridge the space between FAD and the protein surface (see Fig. 1). The hypothesis explored in our paper4 is that a radical pair reaction takes place between FAD and the tryptophans in the photoreduction pathway which modulates the signaling activity of cryptochrome. The specifics of this idea are outlined in Figure 1.
Figure 1.
Right: Cryptochrome internally binds the FAD cofactor and contains a three-tryptophan photoreduction pathway conserved from photolyase, consisting of Trp400, Trp377, and Trp324, with Trp400 nearest the FAD and Trp324 closest to the protein surface. After the FAD cofactor absorbs a photon, bringing it into an excited state, it is protonated from a nearby acidic residue, and then electron transfer proceeds from Trp400. At this stage, the semireduced FADH and Trp400+ comprise a radical pair—that is, each partner has an unpaired electron, and the spins of those electrons are in a correlated state. Cryptochrome is thought to be in its active, signaling state when the FAD cofactor is in this semireduced FADH form. An electron is then transferred from Trp377 to Trp400 and from Trp324 to Trp377, forming radical pairs FADH + Trp377+ and FADH + Trp324+ in the process. The Trp324 radical is then deprotonated. Before this final deprotonation, it is possible for the electron to back transfer from the tryptophan to FADH. If this occurs, FADH reverts to the oxidized FAD form, and cryptochrome is no longer in its active state. Left: This schematic of the electron transfer pathway in cryptochrome shows the estimated lifetimes of each of the radical pair states. The system spends most of its time in the FADH + Trp324 radical pair state. Also shown are the electron and nuclear spins on the FADH and Trp324 radicals. Each nuclear spin adds a small contribution to the local magnetic field. The unpaired electron spins are shown here in the singlet (antiparallel) state. They precess around the local magnetic field, which consists of contributions from the external field and from each of the nuclear spins, causing interconversion to the triplet (parallel) state and back again. This singlet-triplet interconversion is the basis of the radical pair effect in the following sense. Electron back-transfer from Trp324 to FADH proceeds only when the unpaired electrons on each radical are in the singlet state. Cryptochrome remains in its active state so long as this back-transfer is impeded. Therefore, singlet-triplet interconversion influences the time cryptochrome can spend in its active state, and so this magnetic-field-driven effect can alter the protein's signaling behavior.
That magnetic field effects do occur in cryptochrome is supported indirectly by experiments done by Margaret Ahmad and co-workers, as reported in their recent paper5 on the effects of magnetic fields on cryptochrome-dependent responses in Arabidopsis thaliana seedlings. In our paper, Magnetic Field Effects in Arabidopsis thaliana Cryptochrome-1 (4), we sought to evaluate this possibility computationally, to see whether a magnetic field effect in the FADH - tryptophan radical pair is reasonable. We found that it is possible to see a change in cryptochrome activation yield (the amount of time cryptochrome stays in its active state) of about 10%.
Unfortunately, the magnetic field dependence of cryptochrome activation seen in our calculations cannot be taken as exact because of several limitations. Chief among these are that the models of the radical pair did not include all nuclei, and the hyperfine coupling constants were taken from DNA photolyase, which is a protein highly similar to cryptochrome in structure, but which does not necessarily have precisely the same hyperfine coupling for the FAD cofactor and the tryptophans in the photoreduction pathway as does cryptochrome. However, the suggested theory is general and with the knowledge of correct hyperfine coupling constants for the radical pair partners it can be used to calculate the activation yield precisely. Although it would be ideal to obtain hyperfine parameters from experiment, it is also possible to calculate the hyperfine coupling constants with advanced ab initio techniques using the Gaussian package.6 Our preliminary calculations of the hyperfine couplings in tryptophan radicals compare well with the values used in our paper.4 This sort of calculation creates the opportunity not only to refine our current picture of the radical pair mechanism in cryptochrome, but also to explore other possible radical pairs in the system.
In light of work being done by Margaret Ahmad and co-workers (not yet published), it has been suggested recently that the radical pair reaction in cryptochrome may not occur between the FAD cofactor and tryptophan, but in some other radical pair within the protein. It is possible that rather than occurring in the FAD photoreduction process, the radical pair reaction actually takes place in the reoxidation reaction wherein the semireduced FADH is brought back to the oxidized FAD form. One possible radical pair in the back reaction is between FAD and an oxygen molecule which is thought to be involved in the reoxidization process. This radical pair is of particular interest because an oxygen radical would be devoid of hyperfine interactions. Such a radical pair, where one radical has no hyperfine coupling, would be consistent with studies on the effects of weak radio-frequency oscillating magnetic fields on migratory bird orientation. Thorsten Ritz and co-workers found that appropriate orientation behavior depended not only on the strength and angle of the oscillating field, but also that the minimum field strength necessary to disrupt orientation depended on the frequency of the oscillating field in a resonance-like behavior that would be predicted by just such a radical pair7–9 (personal communication with T. Ritz).
The scientific community is still a long way from a complete understanding of avian magnetoreception. The best that may be said of our understanding of it is that birds do demonstrably perceive and use magnetic field information, and that their responses to magnetic fields under different conditions—light intensity and color, magnetic field strength and presence and frequency of oscillating fields—belies a complex phenomenon which is probably the result of multiple receptors which interact in unknown ways.10,11 However, disorientation responses to low-intensity oscillating magnetic fields are strongly suggestive of the involvement of a radical-pair mechanism, making the exploration of radical pair effects in cryptochrome a useful endeavor. Much remains to be done. Even if cryptochrome is confirmed as magnetoreceptor, it remains for biologists to determine how its signaling modulation enters into a bird's sensory perception and ultimately its orientation behavior. Nevertheless, radical pair effects in cryptochrome seem promising as a possible source of magnetoreception in birds, and continued investigation may yet shed light on this complex behavior.
Acknowledgements
This work is supported by grants from the NIH P41-RR05969 and NSF MCB-0744057. The authors gladly acknowledge supercomputer time provided by Pittsburgh Supercomputing Center and the National Center for Supercomputing Applications via Large Resources Allocation Committee grant MCA93S028 and the Turing Xserve Cluster.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/5809
References
- 1.Fleissner G, Holtkamp-Rötzler E, Hanzlik M, Winklhofer M, Fleissner G, Petersen N, Wiltschko W. Ultrastructural analysis of a putative magnetoreceptor in the beak of homing pigeons. J Comp Neurol. 2003;458:350–360. doi: 10.1002/cne.10579. [DOI] [PubMed] [Google Scholar]
- 2.Fleissner G, Stahl B, Thalau P, Falkenberg G, Fleissner G. A novel concept of Fe-mineral based magnetoreception: histological and physicochemical data from the upper beak of homing pigeons. Naturwissenschaften. 2007;94:631–642. doi: 10.1007/s00114-007-0236-0. [DOI] [PubMed] [Google Scholar]
- 3.Ritz T, Adem S, Schulten K. A model for photoreceptor-based magnetoreception in birds. Biophys J. 2000;78:707–718. doi: 10.1016/S0006-3495(00)76629-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solov'yov IA, Chandler DE, Schulten K. Magnetic field effects in Arabidopsis thaliana Cryptochrome-1. Biophys J. 2007;92:2711–2726. doi: 10.1529/biophysj.106.097139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad M, Galland P, Ritz T, Wiltschko R, Wiltschko W. Magnetic intensity affects cryptochrome-dependent responses in Arabidopsis thaliana. Planta. 2006;225:615–624. doi: 10.1007/s00425-006-0383-0. [DOI] [PubMed] [Google Scholar]
- 6.Frisch MJ, et al. Gaussian 03, Revision C.02. Wallingford CT: Gaussian, Inc.; 2004. [Google Scholar]
- 7.Ritz T, Thalau P, Phillips JB, Wiltschko R, Wiltschko W. Resonance effects indicate a radical-pair mechanism for avian magnetic compass. Nature. 2004;429:177–180. doi: 10.1038/nature02534. [DOI] [PubMed] [Google Scholar]
- 8.Thalau P, Ritz T, Stapput K, Wiltschko R, Wiltschko W. Magnetic compass orientation of migratory birds in the presence of a 1.315 MHz oscillating field. Naturwissenschaften. 2005;92:86–90. doi: 10.1007/s00114-004-0595-8. [DOI] [PubMed] [Google Scholar]
- 9.Wang K, Ritz T. Zeeman resonances for radical-pair reactions in weak static magnetic fields. Mol Phys. 2006;104:1649–1658. [Google Scholar]
- 10.Wiltschko R, Ritz T, Stapput K, Thalau P, Wiltschko W. Two different types of light-dependent responses to magnetic fields in birds. Curr Biol. 2005;15:1518–1523. doi: 10.1016/j.cub.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 11.Wiltschko R, Stapput K, Ritz T, Thalau P, Wiltschko W. Magnetoreception in birds: different physical processes for two types of directional responses. HFSP J. 2007;1:41–48. doi: 10.2976/1.2714294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solov'yov I, Greiner W. Theoretical analysis of an iron mineral-based magnetoreceptor model in birds. Biophys J. 2007;93:1493–1509. doi: 10.1529/biophysj.107.105098. [DOI] [PMC free article] [PubMed] [Google Scholar]