Abstract
Responses of plant cells to environmental stresses often involve morphological changes, differentiation and redistribution of various organelles and cytoskeletal network. Tobacco BY-2 cells provide excellent model system for in vivo imaging of these intracellular events. Treatment of the cell cycle-synchronized BY-2 cells with a proteinaceous oomycete elicitor, cryptogein, induces highly synchronous programmed cell death (PCD) and provide a model system to characterize vacuolar and cytoskeletal dynamics during the PCD. Sequential observation revealed dynamic reorganization of the vacuole and actin microfilaments during the execution of the PCD. We further characterized the effects cryptogein on mitotic microtubule organization in cell cycle-synchronized cells. Cryptogein treatment at S phase inhibited formation of the preprophase band, a cortical microtubule band that predicts the cell division site. Cortical microtubules kept their random orientation till their disruption that gradually occurred during the execution of the PCD twelve hours after the cryptogein treatment. Possible molecular mechanisms and physiological roles of the dynamic behavior of the organelles and cytoskeletal network in the pathogenic signal-induced PCD are discussed.
Key words: actin microfilament, cell cycle, cryptogein, microtubules, nuclei, programmed cell death, tobacco BY-2 cells, vacuoles
Cryptogein vs. Tobacco BY-2 Cells: A Model System for Visualization of Elicitor-Induced Programmed Cell Death
During adaptation to biotic and abiotic environmental changes, plant cells induce dynamic changes in morphology as well as intracellular positioning and distribution of organelles. Recent development of live cell imaging techniques provides valuable information on these organellar dynamics. However, in planta imaging of the microscopic behavior is still technically difficult. Tobacco BY-2 cells have relatively large size of the cells.1 Various visualizing methods using fluorescent dyes and GFP probes are well established in BY-2 cells.2,3 These advantages make live cell imaging easier in BY-2 cells than the other plant materials.
A proteinaceous elicitor, cryptogein, from an oomycete, Phytophthora cryptogea induce programmed cell death (PCD). Involvement of increase in cytosolic free Ca2+,4,5 production of reactive oxygen species (ROS)4,6 and activation of mitogen-activated protein kinases (MAPKs)7 in cryptogein-induced PCD has extensively studied in BY-2 cells as well as Nicotiana tabacum cv Xanthi8–12 and Nicotiana plumbaginifolia.13 Since exceptionally high level of cell cycle synchronization can be achieved in BY-2 cells,1,3 the cell cycle-dependency of cryptogein-induced PCD was characterized.6 Various cryptogein-induced defense signaling events as well as PCD depended on the cell cycle phases;6,14 cells treated with cryptogein at S or M-G1 phase induced cell cycle arrest at G2 or G1 phase, respectively, prior to the PCD.6 Therefore cell cycle synchronization in BY-2 cells provides a suitable model system not only to observe homogeneous cells but also to induce highly efficient PCD.15 We synchronized the cell cycle at S phase and characterized the cryptogein-induced intracellular events including vacuoles and cytoskeletons.16
Simplification of Vacuolar Structures Before the Cell Death
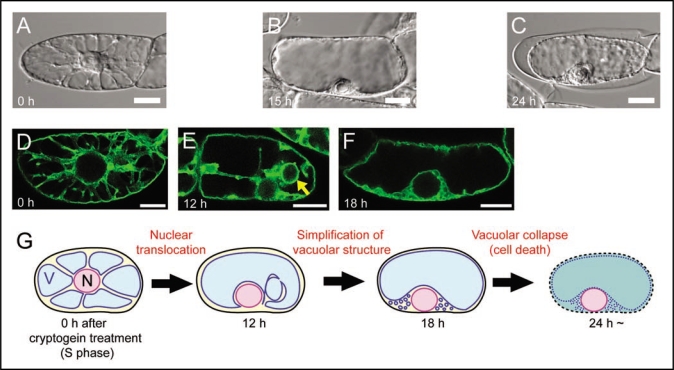
The vacuole contains hydrolytic enzymes for digestive processes, and collapse of vacuoles has been proposed as a crucial event in plant cell death. The vacuolar processing enzyme (VPE), which is localized in the vacuole and possesses the caspase 1 activity, has been shown to be involved in the execution of tobacco mosaic virus (TMV)-induced hypersensitive cell death in Nicotiana.17 However, molecular mechanisms of vacuolar collapse are largely unknown. To find a clue, we characterized vacuolar dynamics in tobacco BY-2 cells treated with cryptogein at S phase using live imaging techniques.16 Time-lapse observation after cryptogein treatment by differential interference contrast (DIC) images revealed that intracellular configuration dynamically altered (Fig. 1A and B) before the cell death (Fig. 1C). Transvauolar strand (TVS), which is a tubular region of cytoplasm connecting nuclei to cell periphery,2 decreased (compare with Fig. 1A and B). Simultaneously, nuclei relocated from cell center to cell periphery (compare with Fig. 1A and B). These intracellular changes implied vacuolar reorganization.
Figure 1.
Vacuolar reorganization during cryptogein-induced programmed cell death in S phase-synchronized tobacco BY-2 cells. (A-C) DIC images of BY-2 cells just before (S phase; A), 15 h (B) and 24 h (C) after cryptogein treatment. Cells in (A) and (B) are alive but a cell in (C) is dead, judged from cell shrinkage. (D–F) Vacuolar membranes visualized by GFP-AtVAM3 in BY-2 cells just before (S phase; D), 12 h (E) and 18 h (F) after cryptogein treatment. The arrow in (E) indicates bulb-like vacuolar membrane structures. (G) A schematic model of cryptogein-induced vacuolar reorganization in S phase-synchronized BY-2 cells. After around 12 h, the nucleus relocates from cell center to cell periphery, and transvacuolar strands decrease. Simultaneously, bulb-like vacuolar membrane structures appear. After around 18 h, the bulb-like structures disappear. Consequently, the vacuolar structure becomes simpler before the cell death. The simple shape of the vacuole is suggested as a crucial event for the execution of vacuolar collapse.16 N and V represent the nucleus and the vacuole, respectively. Scale bars: 10 µm.
We therefore visualized the vacuolar membrane (VM) by GFP-AtVAM3 expression18,19 and characterized the cryptogein-induced alteration of the VM structure in detail. At S phase, many TVS were evidently visualized by VM labeling (Fig. 1D). Twelve hours after cryptogein treatment, TVS decreased but spherical intravacuolar VM structures were newly formed (Fig. 1E, arrow). The intravacuolar structures were similar to the ‘bulb’ structures.20 The bulb-like structures would be derived from the excessive VM that comprised the TVS. Just before the cell death, the bulb-like structures disappeared and vacuoles became simple-shaped (Fig. 1F). We assumed that the cryptogein-induced simplification of the vacuolar structure might facilitate the vacuolar rupture in response to water absorption at the last step of the cell death,16 as briefly summarized in Figure 1G.
The vacuolar morphology is regulated by actin microfilaments (MFs) in higher plants.21,22 MFs play a central role in the cryptogein-induced reorganization of the vacuole.16 Artificial disruption of the MF-bundles by application of actin polymerization inhibitor facilitated the vacuoles to disappear the bulb-like VM structures and rapid rupture. These results suggest that the MFs involve in maintenance of bulb-like VM structures and function as a safety lock against the vacuolar rupture to prevent unexpected accidental cell death via keeping the VM reservoir.16 These cryptogein-induced dynamic structural changes of MFs around the vacuolar membranes and the vacuole may therefore be a key step for the PCD as a preparation for the vacuolar collapse.
Effects of Cryptogein on Mitotic Microtubule Structures
Microtubules (MTs) play crucial roles in cell morphogenesis. The cortical MTs regulate the direction of cell elongation and morphogenesis via determination of cellulose microfibril deposition in the innermost layer of the cell wall. MTs also play central roles in cell division by organizing their mitotic structures; preprophase band (PPB) that predicts the cell division site at late G2 phase, mitotic spindle that segregate the duplicated chromosomes at meta-/anaphase, and phragmoplast that form the cell plate at telophase.23
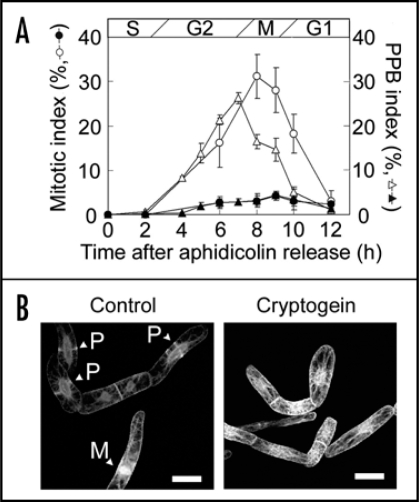
Using synchronously-cultured BY-2 cells stably expressing green fluorescent protein (GFP)-tubulin,23 we further examined effects of cryptogein treatment at S phase on MT-organization (Fig. 2). Cryptogein induced cell cycle arrest at G2 phase (Fig. 2A). In cultured cells of Nicotiana tabacum cv Xanthi, cortical MTs has been reported to be disrupted rapidly by cryptogein.12 In synchronized BY-2 cells, MT-disruption was also observed but its timing was much slower, and the cortical MTs did not disrupt up to twelve hours after cryptogein treatment.16 However, PPB formation was completely inhibited by cryptogein. Seven hours after aphidicolin release (when PPB index peaked in the control cells), cortical MTs kept their random orientation as at S phase (Fig. 2B). Subsequent mitotic MT-structures including mitotic spindle and phragmoplast did not appear. Though the molecular mechanisms for PPB formation have not fully been elucidated yet, its putative regulators such as kinesins24 or MT associated proteins25 may be downregulated by cryptogein treatment. It is still an open question whether inhibition of PPB formation is a cause or a consequence of cryptogein-induced cell cycle arrest at G2 phase. Future multiple labeling of microtubules and their associated proteins as well as the use of transgenic cell lines in which the expression of cell cycle regulators are perturbed would provide new insights into the significance of cytoskeletons in the execution of programmed cell death.
Figure 2.
Cryptogein inhibited preprophase band (PPB) formation. (A) Effect of cryptogein on the PPB and mitotic indexes in tobacco BY-2 cells expressing GFP-tubulin fusion proteins.23 PPB and mitotic indices of non-treated cells (open triangles and open circles, respectively), and cells treated with cryptogein 0.5 h (S phase) after the removal of aphidicolin (solid symbols). Mitotic index was determined by the observation of nuclei/chromosomes stainined with DAPI. The data represent the average of three independent experiments. Error bars indicate the standard error of means (n = 3). (B) Images of BY-2 cells expressing GFP-tubulin fusion proteins. Non-treated cells (Control) and the cells treated with cryptogein were observed 7 h after aphidicolin release. P and M indicates PPB and mitotic apparatus, respectively. Scale bars: 25 µm.
Acknowledgements
We are grateful to Dr. Arata Yoneda of RIKEN and Mr. Takashi Watanabe of Tokyo University of Science for technical assistance. We thank Ms. Tomomi Hayashi of The University of Tokyo for critical reading of the manuscript. This work was supported by the Japan Society for the Promotion of Science (T.H. and Y.K.; Nos. 04392 and 06801), a Grant-in-Aid for Scientific Research on Priority Areas from the Japanese Ministry of Education, Sports, Culture, Science, and Technology on ‘Organelle Differentiation as the Strategy for Environmental Adaptation in Plants’ (S.H. and K.K.; Nos. 17051008 and 17051027) and Institute for Bioinformatics Research and Development (BIRD) from Japan Science and Technology Agency (N.K., T.S. and S.H.).
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/6431
References
- 1.Nagata T, Nemoto Y, Hasezawa S. Tobacco BY-2 cell line as the HeLa cell in the cell biology of higher plants. Int Rev Cytol. 1992;132:1–30. [Google Scholar]
- 2.Yoneda A, Kutsuna N, Higaki T, Oda Y, Sano T, Hasezawa S. Recent progress in living cell imaging of plant cytoskeleton and vacuole using fluorescent protein-transgenic lines and 3-D imaging. Protoplasma. 2007;230:129–139. doi: 10.1007/s00709-006-0237-4. [DOI] [PubMed] [Google Scholar]
- 3.Kumagai-Sano F, Hayashi T, Sano T, Hasezawa S. Cell cycle synchronization of tobacco BY-2 cells. Nat Protoc. 2006;1:2617–2621. doi: 10.1038/nprot.2006.381. [DOI] [PubMed] [Google Scholar]
- 4.Kadota Y, Goh T, Tomatsu H, Tamauchi R, Higashi K, Muto S, Kuchitsu K. Cryptogein-induced initial events in tobacco BY-2 cells: pharmacological characterization of molecular relationship among cytosolic Ca2+ transients, anion efflux and production of reactive oxygen species. Plant Cell Physiol. 2004;45:160–170. doi: 10.1093/pcp/pch020. [DOI] [PubMed] [Google Scholar]
- 5.Kadota Y, Furuichi T, Ogasawara Y, Goh T, Higashi K, Muto S, Kuchitsu K. Identification of putative voltage-dependent Ca2+-permeable channels involved in cryptogein-induced Ca2+ transients and defense responses in tobacco BY-2 cells. Biochem Biophys Res Commun. 2004;317:823–830. doi: 10.1016/j.bbrc.2004.03.114. [DOI] [PubMed] [Google Scholar]
- 6.Kadota Y, Watanabe T, Fujii S, Higashi K, Sano T, Nagata T, Hasezawa S, Kuchitsu K. Crosstalk between elicitor-induced cell death and cell cycle regulation in tobacco BY-2 cells. Plant J. 2004;40:131–142. doi: 10.1111/j.1365-313X.2004.02197.x. [DOI] [PubMed] [Google Scholar]
- 7.Kadota Y, Fujii S, Ogasawara Y, Maeda Y, Higashi K, Kuchitsu K. Continuous recognition of the elicitor signal for several hours is prerequisite for induction of cell death and prolonged activation of signaling events in tobacco BY-2 cells. Plant Cell Physiol. 2006;47:1337–1342. doi: 10.1093/pcp/pcj098. [DOI] [PubMed] [Google Scholar]
- 8.Tavernier E, Wendehenne D, Blein JP, Pugin A. Involvement of free calcium in action of cryptogein, a proteinaceous elicitor of hypersensitive reaction in tobacco cells. Plant Physiol. 1995;109:1025–1031. doi: 10.1104/pp.109.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pugin A, Frachisse JM, Tavernier E, Bligny R, Gout E, Douce R, Guern J. Early events induced by the elicitor cryptogein in tobacco cells: involvement of a plasma membrane NADPH oxidase and activation of glycolysis and the pentose phosphate pathway. Plant Cell. 1997;9:2077–2091. doi: 10.1105/tpc.9.11.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourque S, Ponchet M, Binet MN, Ricci P, Pugin A, Lebrun-Garcia A. Comparison of binding properties and early biological effects of elicitins in tobacco cells. Plant Physiol. 1998;118:1317–1326. doi: 10.1104/pp.118.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lebrun-Garcia A, Ouaked F, Chiltz A, Pugin A. Activation of MAPK homologues by elicitors in tobacco cells. Plant J. 1998;15:773–781. doi: 10.1046/j.1365-313x.1998.00269.x. [DOI] [PubMed] [Google Scholar]
- 12.Binet MN, Humbert C, Lecourieux D, Vantard M, Pugin A. Disruption of microtubular cytoskeleton induced by cryptogein, an elicitor of hypersensitive response in tobacco cells. Plant Physiol. 2001;125:564–572. doi: 10.1104/pp.125.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lecourieux D, Mazars C, Pauly N, Ranjeva R, Pugin A. Analysis and effects of cytosolic free calcium increases in response to elicitors in Nicotiana plumbaginifolia cells. Plant Cell. 2002;14:2627–2641. doi: 10.1105/tpc.005579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadota Y, Watanabe T, Fujii S, Maeda Y, Ohno R, Higashi K, Sano T, Muto S, Hasezawa S, Kuchitsu K. Cell cycle dependence of elicitor-induced signal transduction in tobacco BY-2 cells. Plant Cell Physiol. 2005;46:156–165. doi: 10.1093/pcp/pci008. [DOI] [PubMed] [Google Scholar]
- 15.Kadota K, Kuchitsu K. Regulation of elicitor-induced defense responses by Ca2+ channels and cell cycle in tobacco BY-2 cells. In: Nagata T, Matsuoka K, Inze D, editors. Biotechnology in agriculture and forestry 58 Tobacco BY-2 cells: from cellular dynamics to omics. Vol. 58. Berlin: Springer; 2006. pp. 207–221. [Google Scholar]
- 16.Higaki T, Goh T, Hayshi T, Kutsuna N, Kadota Y, Hasezawa S, Sano T, Kuchitsu K. Elicitor-induced cytoskeletal rearrangement relates to vacuolar dynamics and execution of cell death: in vivo imaging of hypersensitive cell death in tobacco BY-2 cells. Plant Cell Physiol. 2007;48:1414–1425. doi: 10.1093/pcp/pcm109. [DOI] [PubMed] [Google Scholar]
- 17.Hatsugai N, Kuroyanagi M, Yamada K, Meshi T, Tsuda S, Kondo M, Nishimura M, Hara-Nishimura I. A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death. Science. 2004;305:855–858. doi: 10.1126/science.1099859. [DOI] [PubMed] [Google Scholar]
- 18.Uemura T, Yoshimura SH, Takeyasu K, Sato MH. Vacuolar membrane dynamics revealed by GFP-AtVam3 fusion protein. Genes Cells. 2002;7:743–753. doi: 10.1046/j.1365-2443.2002.00550.x. [DOI] [PubMed] [Google Scholar]
- 19.Kutsuna N, Hasezawa S. Dynamic organization of vacuolar and microtubule structures during cell cycle progression in synchronized tobacco BY-2 cells. Plant Cell Physiol. 2002;43:965–973. doi: 10.1093/pcp/pcf138. [DOI] [PubMed] [Google Scholar]
- 20.Saito C, Ueda T, Abe H, Wada Y, Kuroiwa T, Hisada A, Furuya M, Nakano A. A complex and mobile structure forms a distinct subregion within the continuous vacuolar membrane in young cotyledons of Arabidopsis. Plant J. 2002;29:245–255. doi: 10.1046/j.0960-7412.2001.01189.x. [DOI] [PubMed] [Google Scholar]
- 21.Higaki T, Kutsuna N, Okubo E, Sano T, Hasezawa S. Actin microfilments regulate vacuolar structures and dynamics: dual observation of actin microfilaments and vacuolar membrane in living tobacco BY-2 cells. Plant Cell Physiol. 2006;47:839–852. doi: 10.1093/pcp/pcj056. [DOI] [PubMed] [Google Scholar]
- 22.Sheahan MB, Rose RJ, McCurdy DW. Actin-filament-dependent remodeling of the vacuole in cultured mesophyll protoplasts. Protoplasma. 2007;230:141–152. doi: 10.1007/s00709-006-0236-5. [DOI] [PubMed] [Google Scholar]
- 23.Kumagai F, Yoneda A, Tomida T, Sano T, Nagata T, Hasezawa S. Fate of nascent microtubules organized at the M/G1 interface, as visualized by synchronized tobacco BY-2 cells stably expressing GFP-tubulin: time-sequence observations of the reorganization of cortical microtubules in living plant cells. Plant Cell Physiol. 2001;42:723–732. doi: 10.1093/pcp/pce091. [DOI] [PubMed] [Google Scholar]
- 24.Vanstraelen M, Inze D, Geelen D. Mitosis-specific kinesins in Arabidopsis. Trends Plant Sci. 2006;11:167–175. doi: 10.1016/j.tplants.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Sedbrook JC. MAPs in plant cells: delineating microtubule growth dynamics and organization. Curr Opin Plant Biol. 2004;7:632–640. doi: 10.1016/j.pbi.2004.09.017. [DOI] [PubMed] [Google Scholar]