Abstract
We have previously demonstrated that miR399s control phosphate (Pi) homeostasis by regulating the expression of a ubiquitin-conjugating E2 enzyme (UBC24/PHO2) in Arabidopsis. Changes in miR399-dependent PHO2 gene expression modulate Pi uptake, allocation and remobilization. More recently, we provided evidence that miR399s are able to move in the phloem stream and across grafting junctions from the scions overexpressing miR399 to the wild-type rootstocks. Movement of miR399s serves as a long-distance signal to report and balance the Pi status between shoots and roots. Of note, results from grafting experiments indicate that miR399b is less efficient in cleaving the PHO2 mRNA than is miR399f, despite the similar mobility of the two miR399s. We propose that nucleotide 13 of miR399s, which gives rise to the sequence variation among different miR399 species, could be involved in regulating the abundance of PHO2 mRNA through sequence complementarity to the target sequences of PHO2 mRNA and mimicking target sequence of At4/IPS1 noncoding RNAs.
Key words: phosphate, microRNA399, PHO2, UBC24, long-distance movement, At4/IPS1
Regulation of Phosphate Homeostasis by MIR399s and PHO2
MicroRNAs (miRNAs), a novel class of noncoding small RNAs, are crucial in regulating many biological processes, including stress responses.1–5 Previously, we uncovered a mechanism by which miR399s control phosphate (Pi) homeostasis by regulating the expression of UBC24, encoding a ubiquitin-conjugating E2 enzyme in Arabidopsis.6,7 During Pi starvation, the miR399s are upregulated and UBC24 is downregulated. Accumulation of the UBC24 transcript is suppressed in transgenic Arabidopsis overexpressing miR399.6–8 Several features of miR399-overexpressing phenotypes are similar to those described for the pho2 mutant.9,10 The pho2 mutant is in fact caused by a single nucleotide mutation resulting in early termination within the UBC24 gene, which indicates that PHO2 is UBC24.8,11 MiR399-overexpressing, pho2 and PHO2 T-DNA knockout Arabidopsis plants all accumulated a high level of shoot Pi and displayed Pi toxic symptoms as a consequence of enhanced Pi uptake, facilitated translocation of Pi from roots to shoots and retained Pi in old leaves. These observations support the importance of miR399s and PHO2 in the maintenance of Pi homeostasis.
MIR399s as a Long-Distance Signal
Recently, we further revealed a regulatory network of miR399 and PHO2 by systemic signaling.12 The association of Pi translocation and co-expression of miR399s and PHO2 in vascular tissues suggests their involvement in long-distance signaling.7,11 Our grafting results from Arabidopsis and tobacco plants revealed that miR399s are able to move across grafting junctions from the scions overexpressing miR399s to the wild-type rootstocks to suppress the expression of PHO2.12 The expression of PHO2 in the roots is negatively correlated with Pi accumulation in the shoots. We showed that mature miR399s produced in shoots function as a long-distance signal to suppress the expression of PHO2 in the roots. Suppression of PHO2 in the roots rather than shoots is necessary to activate Pi uptake from the rhizosphere and Pi translocation from roots to shoots, because the Pi content remained at normal levels in the pho2 scions grafted onto wild-type rootstocks (Fig. 1A).8 The results of detailed time-course analysis of the accumulation of primary transcripts of miR399s and mature miR399s in shoots versus roots suggest that the movement of miR399s from shoots to roots could be a crucial early defense machinery in response to Pi deficiency.12 Together with findings from Pant et al.,13 we provide the first direct evidence for miRNA functioning as a long-distance signal. In addition to the long-distance movement, local cell-to-cell movement of miR399s to target PHO2 mRNA surrounding the phloem cells is expected after unloading of miR399s from sieve elements of the root because the expression of PHO2 is not restricted in the phloem cells.11
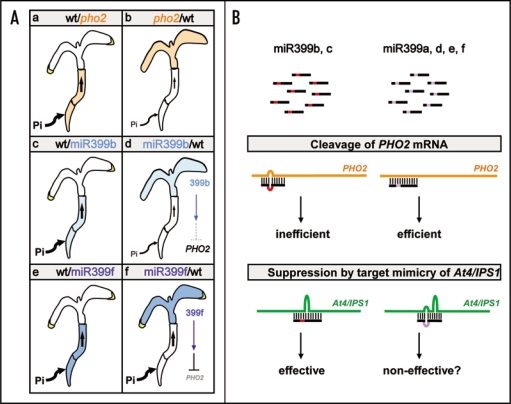
Figure 1.
Long-distance movement and differential targeting of miRNA399s. (A) Summarized results of reciprocal grafting between wild-type (wt) plants and pho2 or transgenic plants overexpressing miR399 (miR399b or mR399f). Combinations of grafted plants are designated as scion/rootstock. The wild-type scions accumulate a high level of Pi and display Pi toxicity seen as chlorosis at the leaf tips when grafted onto pho2- or miR399-overexpressing rootstocks (Parts a, c and e). Greater Pi uptake and transport activities are presented as thick black arrows. In pho2/wt grafted plants, the Pi in pho2 scions remains at normal levels because of functional PHo2 in the rootstocks (Part b). MiR399b and miR399f in the scions are able to move across the graft junction to the wild-type rootstocks (Parts d and f); however, the cleavage of PHO2 mRNA by miR399b is not as efficient as that by miR399f, possibly because it is less complementary to the target sequences (Part d). Suppression of PHO2 in the wild-type rootstocks of miR399f/wt grafted plants results in a high level of Pi in the scions (Part f). (B) The sequence variation at nucleotide 13 of different miR399 species (indicated as red or purple) could be critical in determining the cleavage efficiency on PHO2 mRNA and target mimicry effect by At4/IPS1 noncoding RNAs through sequence complementarity.
Detection of miRNAs in the phloem sap of several plant species implies the movement of miRNAs;14,15 however, the expression of artificial miRNAs in the phloem stream under the regulation of SUC2 promoter is not sufficient to move miRNAs into the surrounding cells (Tretter et al., 2008).16 Different biogenesis pathways, whereby small RNAs interact with specific Dicer enzymes or specific RISC complexes, may confine or liberate the mobility of small RNAs.16 The long-distance movement of miR399s we observed suggests that further regulation or an additional mechanism is required for the movement of specific miRNAs, such as miR399s. How cells distinguish cell-autonomous miRNAs from noncell-autonomous miRNAs will be an intriguing topic to pursue.
The Guanosine at Nucleotide 13 of MIR399s
During the process of reciprocal grafting experiments, we were surprised to find the differential targeting of PHO2 mRNA by different miR399 species.12 Although miR399b and miR399f showed a similar mobility from shoots to roots, cleavage of root PHO2 mRNA by miR399b was inefficient as compared with that by miR399f (Fig. 1A). We hypothesized that this differential targeting might reside in the variation at nucleotide 13 of different miR399 species.12 Unlike miR399a, d, e and f, the guanosine (G) at nucleotide 13 of miR399b and c is not base-paired to any of five target sequences on PHO2 mRNA (the sequences of mature miR399b and c are identical) (Fig. 1B). However, this G nucleotide gives miR399b and c better base-pairing to the consensus sequence of At4/IPS1 noncoding RNAs. The function of At4/IPS1 noncoding RNAs was recently identified to inhibit the cleavage of miR399b on PHO2 mRNA by sequestering miR399b through a partial sequence complementary to At4/IPS1.17 This phenomenon was described as “target mimicry,” whereby the activity of miRNA is antagonized by the binding of RNA molecules containing noncleavable mimicking target sequences. The suppression effect of At4/IPS1 was demonstrated by overexpressing At4 or IPS1 in wild-type plants or transgenic plants overexpressing miR399b.17 These transgenic plants displayed reduced Pi content because of elevated PHO2 expression. Because miR399b and c show the highest complementarity to At4/IPS1, inhibition of cleavage activity of miR399b or c may be more effective than that of other miR399 species. Therefore, the effectiveness of suppression by At4/IPS1 on miR399a, d, e and f, which possess an additional mismatch at nucleotide 13, requires further investigation (Fig. 1B).
In addition to Arabidopsis, other plant species show the sequence variation at nucleotide 13 of miR399s (miRBase, Release 11.0, http://microrna.sanger.ac.uk/sequences/index.shtml). As was found in Arabidopsis, the G at nucleotide 13 of miR399s in rice, Medicago and Populus results in mismatches to all the five potential target sequences but better base-pairing to the homologues of At4/IPS1 in these species. This observation suggests that the sequence at nucleotide 13 could be critical in regulating the targeting efficiency of miR399s by adjusting base-pairing to the real target sequences of PHO2 or mimicking target sequences of At4/IPS1.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/6488
References
- 1.Jones-Rhoades MW, Bartel DP, Bartel B. MicroRNAs and their regulatory roles in plants. Annu Rev of Plant Biol. 2006;57:19–53. doi: 10.1146/annurev.arplant.57.032905.105218. [DOI] [PubMed] [Google Scholar]
- 2.Mallory AC, Vaucheret H. Functions of microRNAs and related small RNAs in plants. Nat Genet. 2006;38:31–36. doi: 10.1038/ng1791. [DOI] [PubMed] [Google Scholar]
- 3.Zhang B, Pan X, Cobb GP, Anderson TA. Plant microRNA: A small regulatory molecule with big impact. Dev Biol. 2006;289:3–16. doi: 10.1016/j.ydbio.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 4.Chiou TJ. The role of microRNAs in sensing nutrient stress. Plant Cell Environ. 2007;30:323–332. doi: 10.1111/j.1365-3040.2007.01643.x. [DOI] [PubMed] [Google Scholar]
- 5.Sunkar R, Chinnusamy V, Zhu J, Zhu JK. Small RNAs as big players in plant abiotic stress responses and nutrient deprivation. Trends Plant Sci. 2007;12:301–309. doi: 10.1016/j.tplants.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Fujii H, Chiou TJ, Lin SI, Aung K, Zhu JK. A miRNA involved in phosphate-starvation response in Arabidopsis. Curr Biol. 2005;15:2038–2043. doi: 10.1016/j.cub.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Chiou TJ, Aung K, Lin SI, Wu CC, Chiang SF, Su CL. Regulation of phosphate homeostasis by microRNA in Arabidopsis. Plant Cell. 2006;18:412–421. doi: 10.1105/tpc.105.038943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bari R, Datt Pant B, Stitt M, Scheible WR. PHO2, microRNA399 and PHR1 define a phosphate-signaling pathway in plants. Plant Physiol. 2006;141:988–999. doi: 10.1104/pp.106.079707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delhaize E, Randall PJ. Characterization of a phosphate-accumulator mutant of Arabidopsis thaliana. Plant Physiol. 1995;107:207–213. doi: 10.1104/pp.107.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong B, Rengel Z, Delhaize E. Uptake and translocation of phosphate by pho2 mutant and wild-type seedlings of Arabidopsis thaliana. Planta. 1998;205:251–256. doi: 10.1007/s004250050318. [DOI] [PubMed] [Google Scholar]
- 11.Aung K, Lin SI, Wu CC, Huang YT, Su CL, Chiou TJ. pho2, a phosphate overaccumulator, is caused by a nonsense mutation in a microRNA399 target gene. Plant Physiol. 2006;141:1000–1011. doi: 10.1104/pp.106.078063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin SI, Chiang SF, Lin WY, Chen JW, Tseng CY, Wu PC, Chiou TJ. Regulatory network of microRNA399 and PHO2 by systemic signaling. Plant Physiol. 2008;147:732–746. doi: 10.1104/pp.108.116269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pant BD, Buhtz A, Kehr J, Scheible WR. MicroRNA399 is a long-distance signal for the regulation of plant phosphate homeostasis. Plant J. 2008;53:731–738. doi: 10.1111/j.1365-313X.2007.03363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoo BC, Kragler F, Varkonyi-Gasic E, Haywood V, Archer-Evans S, Lee YM, Lough TJ, Lucas WJ. A systemic small RNA signaling system in plants. Plant Cell. 2004;16:1979–2000. doi: 10.1105/tpc.104.023614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buhtz A, Springer F, Chappell L, Baulcombe DC, Kehr J. Identification and characterization of small RNAs from the phloem of Brassica napus. Plant J. 2008;53:739–749. doi: 10.1111/j.1365-313X.2007.03368.x. [DOI] [PubMed] [Google Scholar]
- 16.Tretter EM, Alvarez JP, Eshed Y, Bowman JL. Activity range of Arabidopsis small RNAs derived from different biogenesis pathways. Plant Physiol. 2008;147:58–62. doi: 10.1104/pp.108.117119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franco-Zorrilla JM, Valli A, Todesco M, Mateos I, Puga MI, Rubio-Somoza I, Leyva A, Weigel D, Garcia JA, Paz-Ares J. Target mimicry provides a new mechanism for regulation of microRNA activity. Nat Genet. 2007;39:1033–1037. doi: 10.1038/ng2079. [DOI] [PubMed] [Google Scholar]