Abstract
Oxalic acid is thought to be a key factor of the early pathogenic stage in a wide range of necrotrophic fungi. We have recently published that oxalic acid induces Programmed Cell Death (PCD) in Arabidopsis thaliana cells. This cell death results from an early anionic efflux which is a prerequisite for the synthesis of ethylene and the PCD. Complementary experiments have been carried out by using seedlings of A. thaliana. The effects of millimolar concentrations of oxalic acid were analysed on A. thaliana seedlings. A treatment with a 3 mM oxalic acid solution does not alter the development of the plants but induces the transcription of defence related genes which are anion channel dependant. Moreover, our results suggest that a pre-treatment of the seedlings with oxalic acid is able to confer the resistance of A. thaliana against Sclerotium rolfsii. Regarding our results, we suggest that oxalic acid plays two distinct roles, depending on the concentration: a high concentration of oxalic acid induces a large PCD and then contribute to the progression of the fungi. However, at low concentration it is able to induce the establishment of a resistance of the plant against the fungi.
Key words: Arabidopsis thaliana, necrotrophic fungi, oxalic acid, defense-related genes, biocontrol
Introduction
Phytopathogenic fungi such as Sclerotium rolfsii, Botrytis cinerea and Sclerotinia sclerotium are capable of infecting numerous plants. The infection by these fungi leads to considerable losses at harvest time. The early stage of infection involves the production and the accumulation of a large amount of oxalic acid (OA) which appears to be one of the essential determinants of the pathogenecity.1–3 Once produced and accumulated, OA plays a key role, provoking some disease-like symptoms independent of pathogen presence.1,4 Moreover, Sclerotinia sclerotium mutants deficient in oxalate synthesis are no longer pathogenic5 and transgenic plants expressing oxalate decarboxylase show enhanced resistance to phytopathogenic fungus that utilize OA during infection.6,7
New insights regarding the role of OA have been brought in the recent work presented by Errakhi et al.,8 in which the authors investigated the transduction of the signals leading to the death of A. thaliana cells in response to an OA treatment. This study unambiguously shows that OA induces drastic cell death in A. thaliana which fulfils the criteria for PCD: Active gene expression, de novo protein synthesis, cleavage of nuclear DNA and cell shrinkage. Moreover, Errakhi et al.,8 strengthened the evidence that this OA-induced PCD is anion channel dependant. In addition, the authors showed that anion current increase is a necessary upstream event for the synthesis of ethylene which is involved in the induction of PCD. It has also been shown that OA induces increased reactive oxygen species levels in tobacco plants, which correlate to PCD.9
The activation of PCD during pathogen attack is though to be a defence strategy by which the plants try to stop the development of biotrophic pathogens. This strategy can obviously not be used in the case of necrotrophic fungus-plant interactions in which host cell death is beneficial for the pathogen. However, since the hypersensitive response of plant resistant to microbial pathogens involves a complex form of PCD which is associated with the induction of local and systemic defence responses,10 we have then studied the possibility of initiating defence mechanisms in plants by pre-treating them with non-lethal doses of OA before the infection with S. rolfsii. The induction of defence-related genes has been analysed by RT-PCR. Pre-treatment with non-lethal dose of OA are able to induce defence mechanisms and considerably limit the development of the fungus.
Treatment with Non-Lethal Concentration of OA Does Not Affect Seedlings Growth
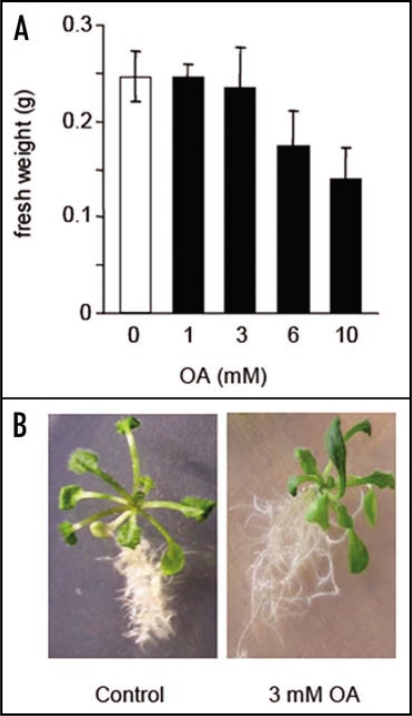
A. thaliana seedlings were grown for 15 days on solid Gamborg medium11 and then transferred on solid Gamborg medium supplemented with increasing concentrations of OA. Because the induction of PCD by OA is independent of the pH-reducing abilities of this organic acid, which is required for sclerotial development,9 the pH of the OA was adjusted to 5.8. The impact of OA on seedling growth were analysed by measuring the fresh weight of the seedling 3 days after the transfer (Fig. 1). A treatment of the seedlings up to 3 mM had no impact on the growth of the plant (Fig. 1A and B). OA had deleterious effects on seedling growth from a concentration of 6 mM (Fig. 1A). Because 3 mM of OA was the highest concentration which did not have any effect on seedlings growth (Fig. 1A and B) we have decided to use it as the pre-treatment concentration.
Figure 1.
Effects of OA on A. thaliana seedling growth. (A) Effects of increasing concentrations of OA on seedling development estimated by the measure of the fresh weight 72 h after the treatment. (B) Typical appearance of seedling 72 h after being treated (right hand) or not (left hand) with a 3 mM OA solution. Results correspond to the means ± sd of 5 replicates.
Treatment with 3 mM OA Upregulates Defence-Related Genes
Ethylene has been shown to be involved OA-induced cell death,8 we therefore examined the effects of OA on the regulation of several defense-related gene transcripts and of hypersensitive response related gene transcripts which are known to be regulated by ethylene, PDF1.2,12 AOX1a/hsr3, coding for the alternative oxidase 1a13 and AAA-ATPase/hsr4, putatively encoding for an AAA type ATPase.14 Other genes classically accumulated during hypersensitive response, PAL1 (encoding for phenyl ammonia-lyase15) and PR1 (pathogenesis-related)12 were analyzed. Because it has also been shown that anion channels activity is one of the early response to OA in A. thaliana cells,8 the regulation of these transcripts has also been analyzed in A. thaliana seedlings treated with 3 mM OA in the presence and absence of anion channel inhibitors.
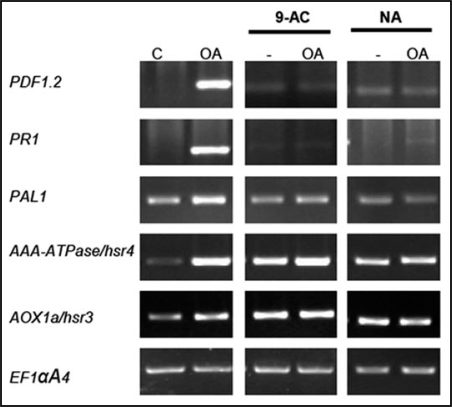
Transcript levels of PAL1, PR1, PDF1.2, Athsr3 and Athsr4 increased after a treatment with 3 mM OA (Fig. 2, left hand). When anion channel inhibitors 9-anthtracen carboxylic acid (9-AC) or niflumic acid (NA) were used as a pre-treatment prior to the addition of 3 mM OA, the OA-induced increases in transcript levels of PAL1, PDF1.2 and PR1 were no longer observed (Fig. 2, middle and right hand). These results suggest that OA is able to induce the activation of ethylene-dependant defense responses and confirm that the anion effluxes are an upstream event in the OA-induced signaling pathway, as previously shown by Errakhi et al.8
Figure 2.
RT-PCR analysis of defence-related gene after an OA treatment. Regulation of PDF1.2, PR1, PAL1, AOX1a/hsr3 and AAA-ATPase/hsr4 after an OA (3 mM) treatment and effect of a pre-treatment with anion channel inhibitors (9-AC, 200 µM and NA, 200 µM) on the transcription of these genes prior to an OA (3 mM) treatment. EF1αA4 is presented as a control. Results presented are representative of 3 experiments.
Pre-treatment with 3 mM OA Protects Seedlings Against S. rolfsii
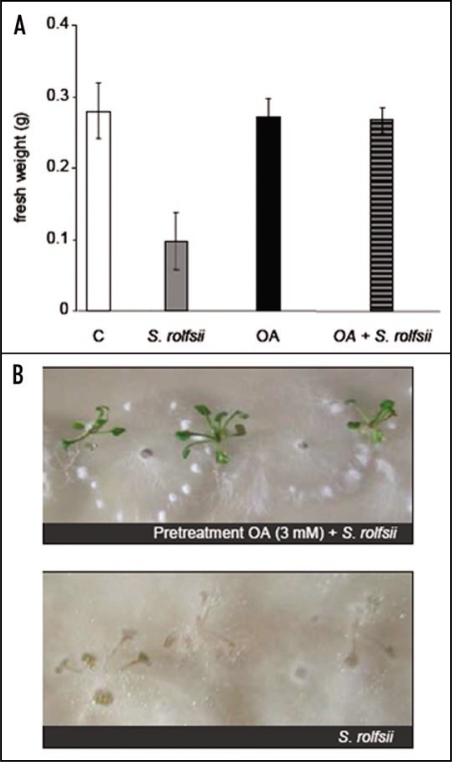
Fifteen days old seedlings were incubated for 24 h on a solid Gamborg medium supplemented with 3 mM OA. They were then infected with S. rolfsii spores. Three days after infection, the effects of the pre-treatment were analyzed by measuring the fresh weight of the seedlings. Three days inoculation with S. rolsfii without any pretreatment resulted in a loss of about 60% of the fresh weight (Fig. 3A). The seedlings were totally submerged by the fungi (Fig. 3B, lower). In contrary, neither seedlings that have been pre-treated for 24 h with 3 mM OA nor seedlings pre-treated for 24 h with 3 mM OA and infected for 72 h with S. rolfsii spores show any loss of their fresh weight (Fig. 3A). Moreover, the development of S. rolsfii was considerably delayed when the seedlings were pre-treated with OA (Fig. 3B, upper).
Figure 3.
Acquisition of protection mechanism of A. thaliana plants against S. rolfsii after an OA (3 mM) pretreatment. (A) Effects of an infection of the seedling with S. rolsfii seedling development estimated by the measure of the fresh weight 72 h after the treatment; C, Control seedlings; S. rolfsii, seedlings infected with S. rolfsii; OA, seedlings treated with a 3 mM solution of OA; OA + S. rolfsii, Seedlings pre treated with 3 mM OA and infected with S. rolfsii. (B) Typical appearance of seedling 72 h after being infected with S. rolfsii (lower). Typical appearance of seedling pretreated with OA (3 mM) 72 h after being infected with S. rolfsii (upper). Results correspond to the means ± sd of 5 replicates.
Conclusion
It has clearly been shown that OA induces PCD on A. thaliana and on tobacco8,9 and that this PCD is anion channel and ethylene dependant.8 The experiments presented in this paper show that a pre-treatment with OA (up to 3 mM) does not affect the development of the plant and induces defense related gene expression and an efficient protection against the proliferation of S. rolsfii. However, OA act differently regarding it concentration: a high concentration of OA (more than 6 mM) induces PCD and facilitates the necrotrophic fungus development while at lower concentration (3 mM) it may act as a protective pre-treatment against the fungus. It has been pointed out that Sclerotinia spp. cause diseases in over 400 species of plant16 and that the all the management strategies established to control the fungi have been largely ineffective.9 Our results bring new perspectives in the control and the limitation of the development of OA-producing necrotrophic fungi, among them, the isolation and the selection of fungus strains that produce non-lethal doses of OA (enough to induce defense mechanisms in plants) and which may enter in competition with the other fungus naturally present in the soil as it has previously been described for Fusarium oxysporum.17
Aknowledgements
The authors would like to thanks E. Paingret and J. Frescura for their help. This work was supported by MESR and by a grant PRAD 04-02.
Abbreviations
- 9-AC
9-anthtracen carboxylic acid
- NA
niflumic acid
- OA
oxalic acid
- PCD
programmed cell death
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/6634
References
- 1.Noyes RD, Hancock JG. Role of oxalic acid in the sclerotinia wilt of sunflower. Physiol Plant Pathol. 1981;18:123–132. [Google Scholar]
- 2.Dutton MV, Evans CS. Oxalate production by fungi: Its role in pathogenicity and ecology in the soil environment. Can J Microbiol. 1996;42:881–895. [Google Scholar]
- 3.Guimaraes RL, Stotz HU. Oxalate production by Sclerotinia sclerotiorum deregulates guard cells during infection. Plant Physiol. 2004;136:3703–3711. doi: 10.1104/pp.104.049650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bateman DF, Beer SV. Simultaneous production and synergistic action of oxalic acid and polygalacturonase during pathogenesis by Sclerotiorum rolfsii. Phytopathol. 1965;55:204–211. [PubMed] [Google Scholar]
- 5.Godoy JA, Pardo JM, Pintor-Toro JA. A tomato cDNA inducible by salt stress and abscisic acid: nucleotide sequence and expression pattern. Plant Mol Biol. 1990;15:695–705. doi: 10.1007/BF00016120. [DOI] [PubMed] [Google Scholar]
- 6.Kesarwani M, Azam M, Natarajan K, Mehta A, Datta A. Oxalate decarboxylase from Collybia velutipes. Molecular cloning and its overexpression to confer resistance to fungal infection in transgenic tobacco and tomato. J Biol Chem. 2000;275:7230–7238. doi: 10.1074/jbc.275.10.7230. [DOI] [PubMed] [Google Scholar]
- 7.Livingstone DM, Hampton JL, Phipps PM, Grabau EA. Enhancing resistance to Sclerotinia minor in peanut by expressing a barley oxalate oxidase gene. Plant Physiol. 2005;137:1354–1362. doi: 10.1104/pp.104.057232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Errakhi R, Meimoun P, Lehner A, Vidal G, Briand J, Corbineau F, et al. Anion channel activity is necessary to induce ethylene synthesis and programmed cell death in response to oxalic acid. J Exp Bot. 2008;59:3121–3129. doi: 10.1093/jxb/ern166. [DOI] [PubMed] [Google Scholar]
- 9.Kim KS, Min JY, Dickman MB. Oxalic acid is an elicitor of plant programmed cell death during Sclerotinia sclerotiorum disease development. Mol Plant Microbe Interact. 2008;21:605–612. doi: 10.1094/MPMI-21-5-0605. [DOI] [PubMed] [Google Scholar]
- 10.Heath MC. Hypersensitive response-related death. Plant Mol Biol. 2000;44:321–334. doi: 10.1023/a:1026592509060. [DOI] [PubMed] [Google Scholar]
- 11.Bouizgarne B, El-Maarouf-Bouteau H, Madiona K, Biligui B, Monestiez M, Pennarun AM, et al. A putative role for fusaric acid in biocontrol of the parasitic angiosperm Orobanche ramosa. Mol Plant Microbe Interact. 2006;19:550–556. doi: 10.1094/MPMI-19-0550. [DOI] [PubMed] [Google Scholar]
- 12.Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol. 2005;43:205–227. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- 13.Ederli L, Morettini R, Borgogni A, Wasternack C, Miersch O, Reale L, et al. Interaction between nitric oxide and ethylene in the induction of alternative oxidase in ozone-treated tobacco plants. Plant Physiol. 2006;142:595–608. doi: 10.1104/pp.106.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugimoto M, Yamaguchi Y, Nakamura K, Tatsumi Y, Sano H. A hypersensitive response-induced ATPase associated with various cellular activities (AAA) protein from tobacco plants. Plant Mol Biol. 2004;56:973–985. doi: 10.1007/s11103-004-6459-y. [DOI] [PubMed] [Google Scholar]
- 15.Dixon RA. Natural products and plant disease resistance. Nature. 2001;411:843–847. doi: 10.1038/35081178. [DOI] [PubMed] [Google Scholar]
- 16.Boland GJ, Hall R. Index of plant hosts of Sclerotinia sclerotiorum. Can J Plant Pathol. 1994;16:93–100. [Google Scholar]
- 17.Fravel D, Olivain C, Alabouvette C. Fusarium oxysporum and its biocontrol. New Phytol. 2003;157:493–502. doi: 10.1046/j.1469-8137.2003.00700.x. [DOI] [PubMed] [Google Scholar]