Abstract
The Arabidopsis FLAGELLIN SENSITIVE2 (FLS2) protein is a leucine-rich repeat receptor-like kinase (LRR-RLK) that plays important roles in pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI). The binding of bacterial flagellin, one of the PAMPs, to the extracellular domain of FLS2 leads to activation of signaling cascades resulting in activation or repression of a specific set of genes involved in plant defense. The mechanisms at the cell membrane that lead to the activation of this signalling pathway are, however, not fully understood. Recently, we have shown that after ligand-treatment the mobility of FLS2 in the cell membrane is reduced and that the activation of FLS2 does not involve its constitutive or ligand-dependent homodimerization. Our data together with recently published reports suggest that FLS2 activation involves its association with other proteins, including BRI1-associated kinase 1 (BAK1), another LRR-RLK, and localization to less mobile areas, probably lipid rafts, in a ligand-dependent manner to initiate PTI.
Key words: PTI, BiFC, flg22, FLS2, FRAP, FRET, membrane protein, RLK
Introduction
The completed genome sequences of several plant species have revealed the existence of a large number of receptor-like kinases (RLKs). For example, model plants Arabidopsis and rice contain over 600 and 1131 RLKs genes, respectively. The kinase domains of plant RLKs are very similar to one another and to the animal Pelle kinases, however, their extracellular domains are different and are accordingly grouped into distinct classes.1 In animals, these kinases play important roles in development and immunity. Similarly, in plants, using genetic, biochemical and molecular analyses, several of them have been shown to be essential for development (e.g., BRI1- brassinosteroid insenstive1, BAK1- BRI1-asociated kinase 1, ERRECTA, CLAVATA) and disease resistance (e.g., FLS2, Xa21 and EFR).1–3
FLAGELLIN SENSITIVE2 (FLS2) is a leucine-rich-repeat (LRR)-RLK, which is required for the perception of bacterial flagellin and activation of defense responses notably through MAPK cascade.2,4,5 Mutations in FLS2 result in enhanced susceptibility to pathogenic bacteria.2,6,7 Using chemical cross-linking FLS2 was shown to bind flg22, an N-terminal conserved peptide of flagellins, directly.8 Site-directed mutagenesis combined with structural modeling and evolutionary conservation suggested that flagellin binds to residues in LRR 9 to 15 of FLS2.9 Functionally, FLS2 is similar to animal Toll-like receptor 5 (TLR5), which also recognizes flagellin through a concave surface of its horse-shoe shaped ectodomain formed by LRR.10 The interaction of flagellin with TLR5 leads to activation of innate and adaptive immune responses ultimately leading to protection against infections.11,12 Similarly, other microbe/pathogen associated molecular patterns ‘PAMP/MAMP’ (for clarity “PAMP” is used here after) such as lipopolysaccharides and ssRNA are recognized by other Toll-like receptors resulting in protection against pathogens.11 Although, the downstream signaling pathways of flg22-FLS2 interaction have been studied at the genetic and biochemical level,5,13 early events in the activation of FLS2 by its ligand on the plasma membrane, are not known. The activation mechanism of several animal and some plant receptors involves ligand-dependent oligomerization as an essential early event and a similar model for plant RLK is also suggested.14–17 In a recent study, we have shown that the mobility of FLS2 in the plasma membrane is reduced in a ligand-dependent manner and that FLS2 does not form homodimers either constitutively or after flg22 treatment.18 Based on our analysis combined with several recent findings,19–22 in this article, we propose a model for the activation of FLS2 (Fig. 1A).
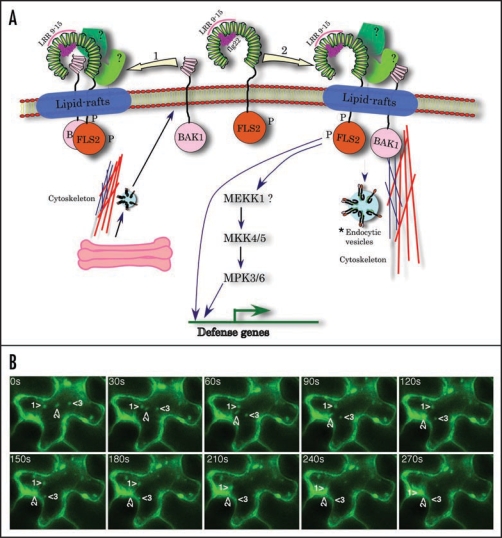
Figure 1.
(A) A hypothetical model for the activation of FLS2. FLS2, after binding flagellin flg22, associates with BAK1 either directly (shown by leftward arrow) or indirectly through other unknown proteins (rightward arrow). In either case the FLS2 complex most likely localizes to lipid-rafts, which include proteins involved in trafficking, signalling and proteolysis. Recent proteomic analysis has shown the presence of BAK1 in plant lipid rafts.34 Engagement of flagellin by FLS2, most likely by its LRR9 to 15,8,9 results in endocytosis of the FLS2 complex either for recycling and/or signalling.20 (A) ‘*’ next to the endocytic vesicle indicates the potential involvement of internalized receptors in activating FLS2 signaling. Evidence for such a mechanism has recently been provided for BRI1 by Geldner et al, (2007).35 The involvement of MEKK1 in FLS2 signaling was provided by Asai et al, (2002).5 However, studies with a mekk1 knockout mutant suggested that MEKK1 is not required for the activation of MPK3 or MPK6 but may play a structural role in FLS2 signaling.38,39 FLS2 may transmit signals independent of endocytosis by activating the MAPK cascade.18,20 Some FLS2-trigged responses may occur independent of MAPK pathway (Prasad et al., unpublished results). The horse-shoe shaped extracellular region of FLS2, which consists of 28 LRR, is derived from the 3D structure of mammalian TLR3. The binding of flagellin to LRR9 to 15 is based on a report by Dunning et al, (2007)9 whereas placing of flg22 on the concave side of LRR is based on mutagenesis and modeling of FLS2 and human TLR5 structure using the known crystal structures of other LRR proteins.9,10 FLS2, FLAGELLIN SENSITIVE2; BAK1, BRI1-ASSOCIATED KINASE1; LRR, leucine rich repeats. (B) FLS2-YFP undergoes exocytosis. FLS2-YFP was expressed in tobacco leaf epidermal cells using the Agrobacterium-mediated transient transformation. Shown are selected images from a time-lapse movie that demonstrate the fusion of several vesicles (marked by numbered arrowheads) with the plasma membrane.
Early Events at the Plasma Membrane Surface in the Activation of FLS2
Most plasma-membrane-localized receptors are activated after binding their cognate ligands to their extracellular domains. Binding of a ligand causes a conformational change in the structure of the receptor, which is transmitted to the cell interior either through trans-membrane vertical changes or through the diffusion and rearrangement of receptors in the horizontal plane of the membrane. This in turn leads to the activation of signaling cascades eventually leading to the expression of specific genes that are required for a specific response. Several plant and animal receptors are activated by forming homodimers or heterodimers in a ligand-dependent manner.17,23–27 For example, BRI1 exists as a homodimer and the activation mechanism of the BRI1 receptor complex includes release of an inhibitory protein (BKI1) followed by heterodimerization with BAK1.14,17 Similarly, several animal toll-like receptors such as TLR2 form heterodimers with TLR1 or TLR6 in response to different ligands,28 suggesting that activation of receptors involves homo and/or heterodimerization.11 Using fluorescence resonance energy transfer (FRET) and bimolecular fluorescence complementation (BiFC), we found that FLS2 does not undergo homodimerization either before or after flg22 treatment.18 In two recently published papers, using co-immunoprecipitation it was shown that FLS2 rapidly associates (within 2 min) with BAK1 in a ligand-dependent manner and this association was shown to be required for FLS2 signaling.21,22 Previously, it was shown that BAK1 also associates with BRI1 and plays a role in brassinosteroid signaling.14,17 These results suggest that BAK1 interacts with multiple LRR-RLKs. The interaction of FLS2 with BAK1 suggests at least two possible scenarios: either FLS2 interacts directly with BAK1 and activates signaling cascade or BAK1 may interact indirectly with FLS2 via some unknown adaptor protein(s) (Fig. 1A). Since BAK1 interaction with BRI1 is direct, the interaction of BAK1 with FLS2 is also likely to be direct. However, this remains to be demonstrated experimentally using either yeast two hybrid or other in vitro assays. In either case, it is important to know that the FLS2 interactome is dynamic in making new associations such as with BAK1, which did not exist before flg22 treatment. Using FRAP analysis we have shown that in the absence of flg22, mobility of FLS2 is slower than would be expected for a protein of similar size in the absence of any interaction with other components. More importantly, mobility of FLS2 was further reduced after flg22 treatment. Together these observations indicate that FLS2 exists in a higher-order molecular complex under normal conditions and that this complex either increases in size and/or is confined to less mobile regions on the cell membrane after engaging flg22 (see below). This means that in addition to recruiting BAK1, the FLS2 complex likely will also include other proteins. This notion is supported by recent findings that another LRR-RLK (SERK1) associates with several other proteins including BRI1 and a hexamer of AAA ATPase CDC48A.29,30 The identification of proteins in the FLS2 complex using proteomic analysis will be an important area of future research in FLS2 signaling.
Does FLS2 Localize to Lipid Rafts?
Interestingly, in response to flg22 addition, the mobility of FLS2 was significantly reduced.18 We suggest that this happens because FLS2 associates with other proteins that are then confined to larger and less mobile domains in the membrane (Fig. 1A). It is possible that these domains are similar to lipid rafts, which are plasma membrane microdomains enriched in sphingolipids and sterols.31,32 Lipid rafts, which are detergent-resistant membranes, are thought to function as signaling platforms during the early signaling of several membrane proteins such as toll-like receptors.31,33 Although, we have not demonstrated it biochemically, we suggest that FLS2 after binding to flagellin accumulates in lipid rafts. This conclusion is supported by the observation that the mobility of FLS2 is decreased after ligand binding.18 Interestingly, proteomic analysis of lipid rafts isolated from Medicago and tobacco revealed the enrichment of signaling proteins, especially LRR-RLKs.32,34 Furthermore, BAK1 was found in plant lipid rafts. Hence, it is likely that FLS2, upon binding to flg22, moves to lipid rafts and associates with BAK1.34 Verification of this requires isolation of lipid rafts from Arabidopsis under control and flagellin-treated conditions and identification of their protein complement. Furthermore, research on disrupting lipid rafts by depleting their sterol content is needed to clarify the role of lipid rafts in FLS2 signalling. We speculate that other signaling proteins involved in trafficking such as GTPases, clathrin and cytoskeletal proteins will display a flg22-dependent dynamic localization to FLS2-lipid rafts. The identity of protein in the FLS2 lipid rafts will shed light on FLS2 signalling and will also open the way for studying the signalling mechanism of other RLKs. The question of whether FLS2 localizes to lipid rafts and how this localization is regulated can be addressed by employing fluorescence resonance energy transfer (FRET) or bimolecular fluorescence complementation (BiFC) between a raft-resident protein and FLS2. Accumulation in lipid rafts likely serves as a rendezvous for many different yet to be identified proteins before they can initiate signalling for defense. If and how lipid rafts relay the flagellin signal to downstream intracellular components will be an important future area of research.
Trafficking of FLS2
Using FLS2-GFP driven by its endogenous promoter in transgenic plants, Robatzek et al, (2006)20 have shown that activation of FLS2 by its ligand results in its internalization into vesicles within 20–40 min and eventual loss by 60 min. Based on these data and pulse experiments they have suggested that binding of FLS2 to flg22 results in receptor degradation through endocytosis. Further, they have shown that cytoskeleton and proteosome function are required for internalization. A mutation in a phosphorylation site of FLS2 (T867V) impaired its internalization as well as flg22-induced responses, suggesting that endocytosis is required for FLS2 signaling. However, in our analysis using FLS2-YFP driven by 35S promoter in protoplasts, we observed activation of flg22-induced genes in the absence of apparent internalization of FLS2.18 The absence of internalization could be due to overexpression of FLS2 in our system and/or requirement of cell-wall dependent component(s) for FLS2 internalization. However, it was recently shown that internalization of BRI1 and BAK1 and turnover of BRI are independent of ligand.17,35 Hence, it is possible that some signaling events activated by flg22 through FLS2 are likely dependent upon endocytosis whereas others are not. The finding that a mutation in the PEST-like motif in FLS2 that leads to prevention of flg22-induced endocytosis did not prevent oxidative burst also suggests such a possibility.20,36 We speculate that the flg22-induced increase in ROS could act as an inducer of the reporter genes used in our study. Interestingly, the YXXΦ (X= any amino acid, Φ=hydrophobic residue) motif, which is one of the signals known to mediate endocytosis in animals,37 is present in another LRR-RLK (EF-Tu receptor EFR)3 but not in FLS2. These observations suggest that all RLKs might not follow the same activation mechanism and underscore the need for further research to clarify these issues.
As with all plasma membrane localized proteins, FLS2 may also transport through the secretory pathways along the ER→golgi→vesicles→PM route. Time-lapse fluorescence microscopy using transient expression of FLS2-YFP in tobacco epidermal cells has shown that small vesicles that originate in the cell interior merge with plasma membrane (Fig. 1B). Although, the apparent trajectory of FLS2 vesicles is suggestive that FLS2 travels along cytoskeleton tracks (Fig. 1B), the exact mechanism and the nature of the tracks remains to be explored. Using selective inhibitors of F-actin or microtubule (de)ploymerization together with mutants defective in F-actin or microtubule assembly, we intend to address these questions.
Conclusions and Outlook
In the past one year several new findings in the flg22-FLS2 signaling were reported. Some of the results were expected whereas others were not. Our data together with other published reports support a model for FLS2 activation that probably involves heteromerization on the cell membrane, which like several animal plasma membrane proteins, probably happens in microdomains or involves confinement to microdomains such as lipid rafts (Fig. 1A). The requirement of BAK1 for FLS2 signalling was perhaps surprising and raises new questions as to how BAK1 mediates BL and flg22 signalling through interacting with BRI1 or FLS2. How is specificity achieved and what are other regulatory components required for achieving such specificity? Based on what is known, we suggest that BAK1 serves as a common adaptor for both receptor complexes and that other proteins may be required to provide specificity in these two signalling pathways. In the BL signalling it binds to BRI1 and a BL-pathway-specific set of proteins whereas under flagellin signalling it associates with FLS2 and FLS2-pathway-specific proteins. In addition, a crosstalk between these two pathways cannot be ruled out. Flg22-treated Arabidopsis plants show reduced growth and BL signaling has some role in plant defense. Similarly, in animals TOLL-like proteins are involved in both development and immunity. By analogy, one can speculate that in addition to their major role in plant immunity or hormone signalling these two pathways also crosstalk through BAK1 and affect each other. Understanding the mechanism by which PRRs are activated will have implications in developing disease resistant plants as well as in designing novel synthetic receptors with potential applications in developing plant sentinels. The fact that some LRR-RLKs can bind more than one ligand (e.g., BRI can bind BL and systemin) and a single LRR-RLK can interact with multiple receptors (e.g., BAK1 with BRI1, FLS2 and other PRRs) together with the presence of a large number of LRR-RLKS (over 230 in Arabidopsis) suggest that plants have the capability to recognize numerous ligands by combinatorial associations.
Acknowledgements
This work was supported by a grant from Defense Advanced Research Project Agency. We thank Dr. Irene Day and Julie Thomas for their comments on this review.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/5472
References
- 1.Shiu SH, Karlowski WM, Pan R, Tzeng YH, Mayer KF, Li WH. Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell. 2004;16:1220–1234. doi: 10.1105/tpc.020834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomez Gomez L, Boller T. FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell. 2000;5:1003–1011. doi: 10.1016/s1097-2765(00)80265-8. [DOI] [PubMed] [Google Scholar]
- 3.Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JD, Boller T, Felix G. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell. 2006;125:749–760. doi: 10.1016/j.cell.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 4.Chisholm ST, Coaker G, Day B, Staskawicz BJ. Host-microbe interactions: shaping the evolution of the plant immune response. Cell. 2006;124:803–814. doi: 10.1016/j.cell.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- 6.Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JD, Felix G, Boller T. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature. 2004;428:764–767. doi: 10.1038/nature02485. [DOI] [PubMed] [Google Scholar]
- 7.Felix G, Duran JD, Volko S, Boller T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 1999;18:265–276. doi: 10.1046/j.1365-313x.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- 8.Chinchilla D, Bauer Z, Regenass M, Boller T, Felix G. The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception. Plant Cell. 2006;18:465–476. doi: 10.1105/tpc.105.036574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunning FM, Sun W, Jansen KL, Helft L, Bent AF. Identification and mutational analysis of Arabidopsis FLS2 leucine-rich repeat domain residues that contribute to flagellin perception. Plant Cell. 2007 doi: 10.1105/tpc.106.048801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersen Nissen E, Smith KD, Bonneau R, Strong RK, Aderem A. A conserved surface on Toll-like receptor 5 recognizes bacterial flagellin. J Exp Med. 2007;204:393–403. doi: 10.1084/jem.20061400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akira S, Takeda K. Toll-like receptor signalling. Nature Review of Immunology. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 13.Gomez Gomez L, Boller T. Flagellin perception: a paradigm for innate immunity. Trends Plant Sci. 2002;7:251–256. doi: 10.1016/s1360-1385(02)02261-6. [DOI] [PubMed] [Google Scholar]
- 14.Belkhadir Y, Chory J. Brassinosteroid signaling: a paradigm for steroid hormone signaling from the cell surface. Science. 2006;314:1410–1411. doi: 10.1126/science.1134040. [DOI] [PubMed] [Google Scholar]
- 15.Gendron JM, Wang ZY. Multiple mechanisms modulate brassinosteroid signaling. Curr Opin Plant Biol. 2007;10:436–441. doi: 10.1016/j.pbi.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson KL, Ingram GC. Sending the right signals: regulating receptor kinase activity. Curr Opin Plant Biol. 2005;8:648–656. doi: 10.1016/j.pbi.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Russinova E, Borst JW, Kwaaitaal M, Cano Delgado A, Yin Y, Chory J, de Vries SC. Heterodimerization and endocytosis of Arabidopsis brassinosteroid receptors BRI1 and AtSERK3 (BAK1) Plant Cell. 2004;16:3216–3229. doi: 10.1105/tpc.104.025387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ali GS, Prasad KV, Day I, Reddy AS. Ligand-Dependent Reduction in the Membrane Mobility of FLAGELLIN SENSITIVE2, an Arabidopsis Receptor-Like Kinase. Plant Cell Physiol. 2007;48:1601–1611. doi: 10.1093/pcp/pcm132. [DOI] [PubMed] [Google Scholar]
- 19.Kemmerling B, Schwedt A, Rodriguez P, Mazzotta S, Frank M, Qamar SA, Mengiste T, Betsuyaku S, Parker JE, Mussig C, Thomma BP, Albrecht C, de Vries SC, Hirt H, Nurnberger T. The BRI1-associated kinase 1, BAK1, has a brassinolide-independent role in plant cell-death control. Curr Biol. 2007;17:1116–1122. doi: 10.1016/j.cub.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 20.Robatzek S, Chinchilla D, Boller T. Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes Dev. 2006;20:537–542. doi: 10.1101/gad.366506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heese A, Hann DR, Gimenez Ibanez S, Jones AM, He K, Li J, Schroeder JI, Peck SC, Rathjen JP. The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci USA. 2007;104:12217–12222. doi: 10.1073/pnas.0705306104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nurnberger T, Jones JD, Felix G, Boller T. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature. 2007;448:497–500. doi: 10.1038/nature05999. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Li X, Meisenhelder J, Hunter T, Yoshida S, Asami T, Chory J. Autoregulation and homodimerization are involved in the activation of the plant steroid receptor BRI1. Dev Cell. 2005;8:855–865. doi: 10.1016/j.devcel.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Wen J, Lease KA, Doke JT, Tax FE, Walker JC. BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell. 2002;110:213–222. doi: 10.1016/s0092-8674(02)00812-7. [DOI] [PubMed] [Google Scholar]
- 25.Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 26.Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell. 2002;110:669–672. doi: 10.1016/s0092-8674(02)00966-2. [DOI] [PubMed] [Google Scholar]
- 27.Yu X, Sharma KD, Takahashi T, Iwamoto R, Mekada E. Ligand-independent dimer formation of epidermal growth factor receptor (EGFR) is a step separable from ligand-induced EGFR signaling. Mol Biol Cell. 2002;13:2547–2557. doi: 10.1091/mbc.01-08-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farhat K, Riekenberg S, Heine H, Debarry J, Lang R, Mages J, Buwitt-Beckmann U, Roschmann K, Jung G, Wiesmuller KH, Ulmer AJ. Heterodimerization of TLR2 with TLR1 or TLR6 expands the ligand spectrum but does not lead to differential signaling. J Leukoc Biol. 2007 doi: 10.1189/jlb.0807586. In Press. [DOI] [PubMed] [Google Scholar]
- 29.Karlova R, Boeren S, Russinova E, Aker J, Vervoort J, de Vries S. The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE1 protein complex includes BRASSINOSTEROID-INSENSITIVE1. Plant Cell. 2006;18:626–638. doi: 10.1105/tpc.105.039412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aker J, Hesselink R, Engel R, Karlova R, Borst JW, Visser AJWG, de Vries SC. In vivo hexamerization and characterization of the Arabidopsis AAA ATPase CDC48A complex using forster resonance energy transfer-fluorescence lifetime imaging microscopy and fluorescence correlation spectroscopy. Plant Physiology. 2007;145:339–350. doi: 10.1104/pp.107.103986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grennan AK. Lipid rafts in plants. Plant Physiol. 2007;143:1083–1085. doi: 10.1104/pp.104.900218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lefebvre B, Furt F, Hartmann MA, Michaelson LV, Carde JP, Sargueil-Boiron F, Rossignol M, Napier JA, Cullimore J, Bessoule JJ, Mongrand S. Characterization of lipid rafts from Medicago truncatula root plasma membranes: a proteomic study reveals the presence of a raft-associated redox system. Plant Physiol. 2007;144:402–418. doi: 10.1104/pp.106.094102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Triantafilou M, Morath S, Mackie A, Hartung T, Triantafilou K. Lateral diffusion of Toll-like receptors reveals that they are transiently confined within lipid rafts on the plasma membrane. J Cell Sci. 2004;117:4007–4014. doi: 10.1242/jcs.01270. [DOI] [PubMed] [Google Scholar]
- 34.Morel J, Claverol S, Mongrand S, Furt F, Fromentin J, Bessoule JJ, Blein JP, Simon-Plas F. Proteomics of plant detergent-resistant membranes. Mol Cel Prot. 2006;5:1396–1411. doi: 10.1074/mcp.M600044-MCP200. [DOI] [PubMed] [Google Scholar]
- 35.Geldner N, Hyman DL, Wang X, Schumacher K, Chory J. Endosomal signaling of plant steroid receptor kinase BRI1. Genes Dev. 2007;21:1598–1602. doi: 10.1101/gad.1561307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salomon S, Robatzek S. Induced endocytosis of the receptor kinase FLS2. Plant Signal Behav. 2006;1:293–295. doi: 10.4161/psb.1.6.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurten RC. Sorting motifs in receptor trafficking. Adv Drug Deliv Rev. 2003;55:1405–1419. doi: 10.1016/j.addr.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Suarez-Rodriguez MC, Adams-Phillips L, Liu Y, Wang H, Su SH, Jester PJ, Zhang S, Bent AF, Krysan PJ. MEKK1 is required for flg22-induced MPK4 activation in Arabidopsis plants. Plant Physiol. 2007;143:661–669. doi: 10.1104/pp.106.091389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ichimura K, Casais C, Peck SC, Shinozaki K, Shirasu K. MEKK1 is required for MPK4 activation and regulates tissue-specific and temperature-dependent cell death in Arabidopsis. J Biol Chem. 2006;281:36969–36976. doi: 10.1074/jbc.M605319200. [DOI] [PubMed] [Google Scholar]