Abstract
Aims
Heavy drinking is associated with hypertension. This study evaluated blood pressure changes occurring during treatment for alcohol dependence.
Participants
Subjects included 1,383 persons participating in the COMBINE Study, a large multicenter treatment study for alcohol dependence.
Measurements
Methods appropriate for repeated measures data were used to assess the relationship of percent drinking days (PDD) to systolic and diastolic blood pressure (BP) over a 16 week treatment period. Modification of these associations by demographic and other variables was assessed.
Findings
Blood pressure reduction was evident only in persons who were above the median BP at baseline. In this group, systolic BP decreased by an average of 12 mm Hg, and diastolic BP decreased by an average of 8 mm Hg. BP reduction occurred during the first month of treatment. This effect was similar regardless of age, sex, body-mass index, reported history of hypertension, and use of anti-hypertensive medications. An observed association between BP and PDD in Caucasians was not evident in African Americans largely due to their lower pre-treatment BP.
Conclusions
Reduction in alcohol consumption has a potent anti-hypertensive effect in alcoholics with higher blood pressure. For hypertensive, alcohol-dependent persons, treatment for alcoholism should be considered a major component of anti-hypertensive therapy.
Keywords: alcohol dependence, blood pressure
INTRODUCTION
Hypertension affects over 30% of the U.S. adult population and is the most common diagnosis in primary health care [1]. For some hypertensive individuals, their initial high blood pressure, or failure to meet blood pressure goals during treatment, is a direct or indirect result of excessive alcohol consumption [2]. Most epidemiologic studies have shown that consumption exceeding about 30 grams of ethanol per day (roughly equivalent to more than 2 standard drinks in the US) is associated with higher blood pressure [3–5], although some evidence suggests that alcohol consumption may be higher than reported [6] in these studies. Blood pressure is reduced quickly with abstinence or a reduction in drinking. Indeed, several short term observational studies have shown that a reduction in blood pressure is observed in days to weeks [7–12]. Most controlled studies evaluating the anti-hypertensive effects of drinking reduction have included hazardous drinkers not meeting criteria for an alcohol use disorder. The most problematic drinkers, particularly among women, are likely underrepresented in population-based surveys and hypertension treatment trials. Therefore, blood pressure changes occurring during alcohol dependence treatment have not been well described. The purpose of this analysis was to document changes in blood pressure occurring during a large national alcohol dependence treatment protocol, to evaluate how demographic variations might affect such changes, and to explore the effect of pharmacotherapies for relapse prevention on blood pressure.
METHODS
Subjects
Subjects included participants in the COMBINE Study, a large multicenter alcohol dependence treatment trial completed in 2004 [13]. Pertinent to this work, COMBINE was a randomized, placebo-controlled trial of pharmacotherapy (naltrexone, acamprosate, or both) and counseling strategies (Combined Behavioral Intervention and/or Medical Management) involving 1,383 alcohol dependent participants recruited from the general population or clinical settings at 11 centers throughout the US. Active treatment continued for 16 weeks. Detailed characteristics of the sample have been published [13]. The mean age was 44, 69% were male, 76% were non-Hispanic white, 12% Hispanic, 8% African American, and 4% other ethnic groups. For this current assessment, blood pressure evaluation at baseline (pre-treatment) and at weeks 4, 8, 12, and 16 of treatment were analyzed, as was the drinking that occurred at baseline and between each blood pressure assessment. This resulted in a total of 6,915 potential blood pressure measurements as defined for this analysis, of which 1,421 (20.5%) were missing.
Assessment of blood pressure
The dependent variables of interest were systolic and diastolic blood pressure. These were measured according to the site and examiners usual routine. Thus we controlled for site effects in all analyses to partially account for non-standardized measurement, and relied on the large sample size to minimize the influence of non-systematic measurement error.
Assessment of alcohol consumption
Alcohol consumption was treated as the main independent variable of interest for this analysis. Drinking was estimated by self-report using timeline follow-back methodology [14]. This is a detailed, calendar-based recall method employed by trained research personnel that provides an estimate of daily drinking. We chose percent drinking days (PDD) to describe consumption, as the correlation of this measure with blood pressure was slightly higher than other consumption measures at pre-treatment baseline, including total number of drinks consumed, percent heavy drinking days (consuming at least 5 drinks for males, at least 4 for females), and average number of drinks per drinking day. PDD is the percentage of all days on which any amount of alcohol was consumed. Corresponding to blood pressure measurements, we included PDD estimated at baseline and during weeks 0–4, 5–8, 9–12, and 13–16 (i.e. PDD during each 4 week treatment block).
Demographic factors and medication assignment
Demographic variables included in this analysis were age, gender, and ethnicity. Age was included as a continuous variable. Detailed ethnic data was obtained by self-report during baseline assessment. For this analysis we categorized ethnicity as African American, non-Hispanic white, Hispanic, and “other”. Randomization to either naltrexone or acamprosate or their matching placebos was treated dichotomously (i.e., we did not separately evaluate a group randomized to receive both medications or consider counseling assignment).
Other factors affecting blood pressure
A number of factors could confound results of the main effects of alcohol consumption on blood pressure. Some of these important subject characteristics were collected in the COMBINE Study, but typically involved a few missing observations. These factors were self-reported hypertension history (available in n=1,367), reported use of antihypertensive agents at baseline (available in n=1,379), and body mass index (kg/m2) (available in n=1,324). These factors were added to the model as main effects to estimate confounding of the relationship between PDD and blood pressure. We also estimated the influence of baseline alcohol withdrawal on subsequent blood pressure reduction using total Clinical Institute Withdrawal Assessment (CIWA) scores [15] and heart rate.
Analysis
We analyzed up to 5 repeated measures of blood pressure on each subject over a 16-week period, utilizing a random coefficients model (i.e., random intercept and time slope) to account for within subject correlation. We used the observed data to guide our model building strategy, choosing a 2-slope piecewise regression to efficiently model the observed biphasic relationship between blood pressure and time. The fit of maximum likelihood models with fixed effects, random intercept, and random slopes and intercept were compared using likelihood ratio tests. After modeling the change in blood pressure with time, we assessed the importance of alcohol consumption (PDD) by assessing the main effect of PDD on the time slopes for systolic and diastolic pressure. Subsequently, we focused on the interaction of baseline demographic variables and medication assignment with PDD by adding main effect and interaction terms to the model, and conducted stratified analyses in the presence of any statistically significant interaction (p<0.05). We did not include history of hypertension, use of antihypertensive agents, and body mass index in the main analyses due to missing baseline data. However, we did adjust for these secondarily. Furthermore, when demographic interactions with PDD were found, adjustment for these factors was undertaken, in order to estimate whether these factors might mediate the interaction. Finally, because a significant effect of baseline blood pressure on blood pressure change during treatment was found (i.e., there was a significant subject*time interaction), we explored the effects of baseline alcohol withdrawal on the subsequent reduction in blood pressure.
RESULTS
Model selection and predicted blood pressure by time
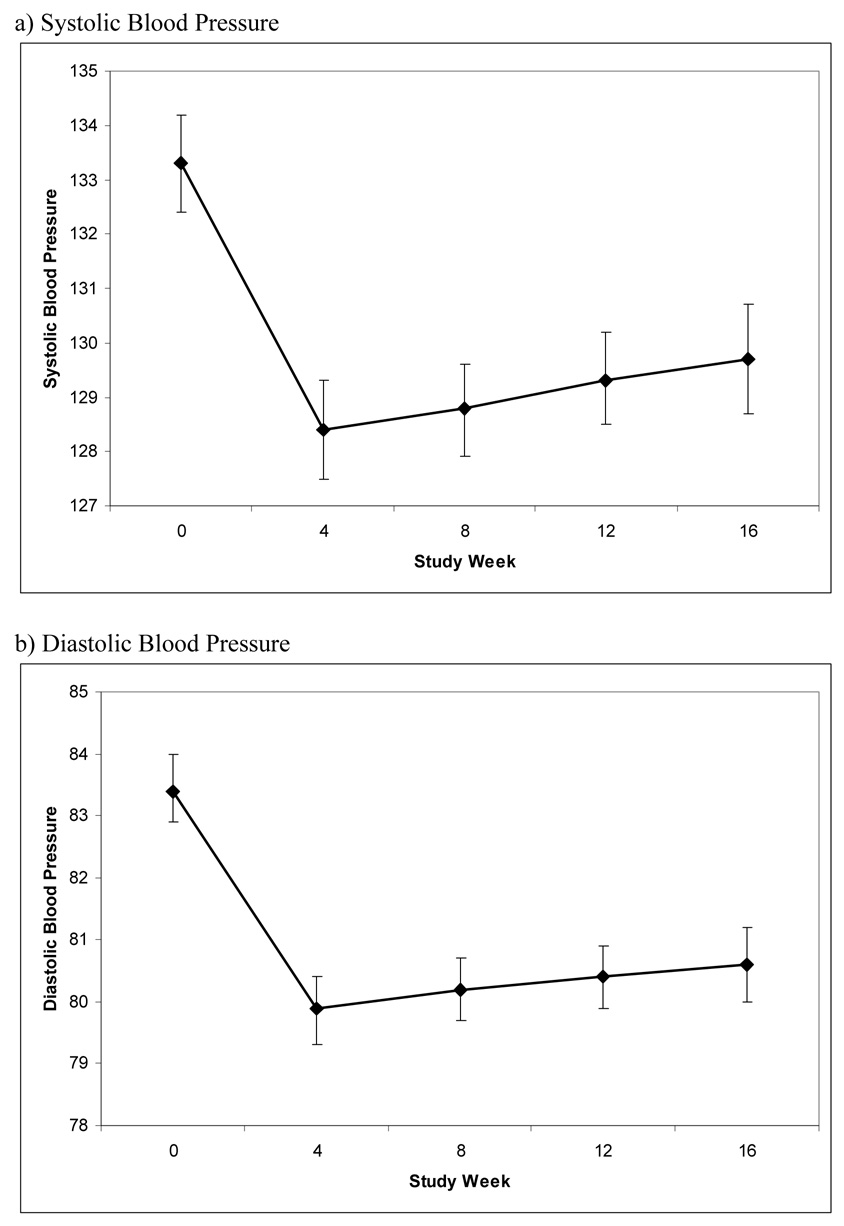
There was an initial drop in systolic and diastolic blood pressure between baseline and week 4, followed by a gently up-sloping curve between weeks 4 and 16. For systolic pressure random intercepts and slopes provided the best fit to the observed data. This demonstrated statistically significant variability in individual changes in blood pressure over time and a relationship of blood pressure change to baseline blood pressure (i.e., on average, people with higher baseline blood pressure had a greater reduction in blood pressure). As an example, the median baseline systolic pressure for the whole sample was 132 mm Hg. For people with a higher-than-average baseline (i.e., > 132 mm Hg), systolic pressure dropped by an average of 12 mm Hg (149 to 137) by week 4. Conversely, those with a baseline systolic pressure less than or equal to 132 had no change by week 4 (120 mm Hg to 121 mm Hg). Diastolic pressure findings were similar; however the slight improvement in fit seen with a random slope for the latter part of the treatment period was not statistically significant. Median baseline diastolic pressure for the whole sample was 84 mm Hg. For people with a higher-than-median baseline (i.e., > 84 mm Hg) diastolic pressure dropped by an average of 8 mm Hg (93 mm Hg to 85 mm Hg) by week 4. Conversely, those with a baseline diastolic pressure less than or equal to 84 had little change (75 mm Hg to 76 mm Hg).
The predicted mean systolic and diastolic blood pressures at each time are illustrated in the figure with 95% confidence intervals.
Figure.
Change in Blood Pressure during the COMBINE Study
* Bars represent 95% confidence intervals
There were some missing data at each follow-up time, and we repeated the analysis to estimate blood pressure at each point in time for subjects with complete data (i.e., no missing values). These results did not differ much from the whole sample, showing that ‘missingness’ was not strongly related to blood pressure. For subjects with complete data (n=808 or 4,040 observations), estimated average systolic pressure was 133.8 mm Hg at baseline, 128.2 mm Hg at 4 weeks, 128.7 mm Hg at 8 weeks, 129.2 mm Hg at 12 weeks, and 129.7 mm Hg at 16 weeks. The two piecewise regression slopes were both statistically significant (p<0.001 for the initial drop between baseline and week 4, and p=0.005 for the subsequent modest increase between week 4 and week 16). Corresponding diastolic pressures in those with complete data were 83.6 mm Hg, 79.8 mm Hg, 80.0 mm Hg, 80.1 mm Hg, and 80.2 mm Hg. The initial drop in blood pressure between week 0 and week 4 was statistically significant (p<0.001), but the subsequent increase between week 4 and week 16 was not (p=0.235).
Alcohol effect on blood pressure changes
All analyses were controlled for study site. Adjustment for PDD accounted for most of the initial change in systolic and diastolic pressure. Adding PDD to the systolic blood pressure model resulted in a change in the initial time slope from −4.9 (95% CI −5.8 to −4.1) to −1.5 (95% CI −2.7 to −0.3). Adding PDD to the diastolic blood pressure model resulted in a corresponding change from −3.5 (95% CI −4.0 to −2.9) to −1.1 (95% CI −1.9 to −0.4). PDD adjustment had little impact on the modest increase in blood pressure observed after week 4.
Effect of demographics and study medication on the PDD-blood pressure relationship
There was a significant interaction between age and PDD for systolic pressure (p<0.001) but not diastolic pressure (p=0.108). This same pattern was seen for gender (p=0.044 for systolic pressure; p=0.363 for diastolic pressure). For ethnicity, the effect of PDD on both systolic pressure (p=0.005) and diastolic pressure (p<0.001) differed between African American and non-Hispanic white subjects. Naltrexone treatment did not impact the relationship between PDD and systolic (p=0.884)) or diastolic (p=0.482) pressure. This was also true for acamprosate treatment (p=0.102 for systolic pressure; p=0.207 for diastolic pressure).
The magnitude and directionality of the statistically significant interactions are illustrated in Table 2, which includes stratified estimates of the expected decrease in blood pressure (mm Hg) resulting from a 50% reduction in PDD.
Stratified analyses were run with age dichotomized at the median and ethnicity stratified as African American or non-African American. These analyses were adjusted for the other demographic factors (e.g., analyses by age group were adjusted for gender and ethnicity). Despite statistically significant interactions, the relationship between PDD and systolic blood pressure was clinically similar regardless of gender, and reduction in systolic BP was only slightly greater in older subjects. However, ethnic differences were more clinically relevant, with PDD having little or no association with systolic and diastolic blood pressure among African American participants. African Americans participating in the COMBINE Study had a lower average systolic and diastolic pressure at baseline than other ethnic/racial groups. Because initial analyses showed that a relatively high baseline blood pressure strongly predicted within treatment blood pressure reduction, we further evaluated ethnic differences by performing ethnicity-stratified analyses that adjusted for having baseline pressure greater than the sample average. Although the blood pressure-PDD association remained statistically not significant in the African American group, the magnitude of the ethnic differences became quite small (results included in Table 2). This suggested that ethnic differences in the effect of PDD on blood pressure were largely accounted for by differences in baseline blood pressure.
Interaction of other factors with PDD and effects on PDD-by-demographic interactions
As might be expected, reported use of antihypertensives, reported history of hypertension, and higher BMI were associated with both higher systolic and diastolic blood pressure (all p-values<0.001). Reported use of antihypertensives interacted with PDD for systolic pressure (p=0.015), with less drinking days having a modestly greater effect in lowering blood pressure among people not on antihypertensive medication. For example, a 50% reduction in PDD would lower systolic pressure by 3.1 mm Hg in persons not taking antihypertensives, and by 2.4 mm Hg in persons taking antihypertensives. There was no corresponding interaction for diastolic blood pressure (p=0.769). Reporting a history of hypertension also interacted with PDD for systolic pressure (p<0.001) but not diastolic pressure (p=0.253), with a minimally greater effect of PDD reduction on systolic pressure in persons with a hypertension history. For example, a 50% reduction in PDD for those with a hypertension history would lower systolic pressure by 3.3 mm Hg, and 2.9 mm Hg in those without a hypertension history. BMI did not influence the PDD effect on systolic pressure (p=0.216) or diastolic pressure (p=0.594). We further evaluated the interactions of hypertension history, taking anti-hypertensives, and BMI in the subset of subjects having above average baseline systolic pressure, finding similar results relative to the whole sample. Furthermore, adding these factors to the ethnicity, age, and gender stratified analyses did not significantly alter the association between PDD and blood pressure found previously.
Effect of baseline blood pressure on subsequent blood pressure change
Subjects below the median had little reduction in blood pressure between baseline and week 4. After adjustment for age, gender, ethnicity, and center, systolic pressure marginally increased by 1.1 mm Hg (p=0.055), and diastolic pressure increased by 0.8 mm Hg (p=0.041). For those above the median, systolic pressure had decreased by 12.0 mm Hg (p<0.001), and diastolic pressure had decreased by 8.5 mm Hg (p<0.001) at week 4. For those with initial hypertension (i.e., systolic pressure ≥ 140 [n=496] or diastolic pressure ≥ 90 [n=437), systolic pressure dropped by 14.1 mm Hg (p<0.001) and diastolic dropped by 9.7 mm Hg (p<0.001). Adjustment for total CIWA score and heart rate at the time of baseline blood pressure, as well as the subset analysis excluding subjects having a total CIWA score > 90th percentile for the sample, had minimal effect on estimated blood pressure changes.
DISCUSSION
This study evaluated changes in blood pressure occurring during alcohol dependence treatment. Similar to findings in largely non-dependent and less heavily drinking populations, observed decreases in systolic and diastolic blood pressure were substantially accounted for by reductions in alcohol consumption, and occurred within the first month of treatment. Blood pressure reduction was most evident in persons having baseline blood pressure above the sample median. Possibly due to their lower average baseline blood pressure, the association between drinking and blood pressure was less evident in African Americans. However, the estimated effect of percent drinking day reduction on blood pressure did not differ substantially between males and females or young and old, and was not related to the type of pharmacotherapy for relapse prevention.
Many cross-sectional epidemiologic studies have demonstrated that the prevalence of hypertension increases with higher average alcohol consumption, with longitudinal studies suggesting that blood pressure changes are positively correlated with drinking changes (i.e., reduced drinking lowers blood pressure). Clinical trials involving counseling or substitution of low alcohol substitutes for hazardous drinkers have confirmed that blood pressure reduction will follow drinking reduction in days to weeks [16, 17]. The present study confirms this effect in dependent, high consumption drinkers, demonstrating an average overall reduction in systolic blood pressure of roughly 5 mm Hg, and in diastolic blood pressure of approximately 3 mm Hg during the first month of treatment. More importantly, this reduction appeared to be limited to persons with higher than average systolic or diastolic pressure at baseline. In these persons, the average reduction in systolic pressure was 12 mm Hg, and the average reduction in diastolic pressure was 8 mm Hg. More generally, due to the dependence of blood pressure reduction on the baseline, those with the highest initial pressure experienced the greatest reduction. This finding is consistent with prior research suggesting that only about half of heavy drinkers experience a pressor effect [4, 7], most of whom will experience blood pressure reduction with reduced drinking. This is clearly a clinically important effect similar in magnitude to changes occurring with intensive hypertension management [18], and was primarily attributable to drinking reduction. While not all subjects having relatively higher baseline blood pressure met criteria for hypertension, blood pressure reduction would still be important given elevated cardiovascular risks in individuals with systolic pressure between 120 and 140 mm Hg, or diastolic pressure between 80 and 89 mm Hg [1]. If maintained in the long-term, such reductions would be expected to result in improved survival [19]. This may magnify gains in life expectancy expected with reduced drinking or abstinence in alcohol dependent populations [20].
Prior research has largely been in men, with inconsistent epidemiologic findings on drinking and BP in women [7, 21–27]. Our study suggests that, at least among dependent drinkers, alcohol consumption has similar effects on blood pressure regardless of sex. Similarly, age does not appear to substantially alter the effect of drinking on blood pressure. We did find an unexpected dissociation between percent drinking days and blood pressure in African American participants [26], but this was likely due to baseline ethnic differences in blood pressure. Given the higher prevalence of hypertension among African Americans in the US [28], this was probably a sample-specific finding. African Americans did have a modestly increased report of a high blood pressure diagnosis and were modestly more likely to report antihypertensive medication use, but still had lower estimated blood pressure despite adjustment for these factors (data not shown).
Strengths of this study included the use of a large trial database with repeated and detailed assessment of daily alcohol use over time, repeated blood pressure measurement, and adequate representation of women and several ethnic groups. This allowed an exploration of how these and other variables interact over time. The main limitation of the study is the non-standardized measurement of blood pressure. We were able to correct for some potential bias by adjusting for study site, but measurement error was inevitable since the COMBINE Study was not designed to assess blood pressure as a treatment outcome. Systematic error may have been introduced if blood pressure measurement error was directly related to alcohol consumption. This seems very unlikely, but can not be definitively ruled out. The more probable non-systematic error was partially counteracted by the large sample size and the completeness of the drinking data. About 20% of observations were missing, and these were assumed to be random. Since missing data may very well be related to alcohol consumption, this assumption could have resulted in an overestimation of the average reduction in blood pressure over time. However, missing data would be less likely to influence estimation of the effect of drinking on blood pressure, and evaluating changes in blood pressure in persons with complete data yielded similar results. While the study results suggest important blood pressure reductions occurring with alcohol dependence treatment, future alcoholism treatment trials should include standardized blood pressure assessment. This is particularly important to verify that blood pressure reduction is not related to an initial over-estimation of blood pressure.
This study demonstrates an anti-hypertensive effect of alcohol dependence treatment, and further supports recommendations that hypertensive patients in general medical settings be screened for heavy drinking [29]. Clinicians caring for alcohol dependent individuals with elevated blood pressure can expect some reduction with successful treatment, an effect that has some potential for use as a motivational tool to enhance treatment outcomes. Reduction in blood pressure accompanying abstinence or reduced drinking is largely independent of age, gender, and probably ethnicity, and is limited to persons with relatively higher baseline blood pressure. In such persons, clinically important reductions in blood pressure can be expected during the first month of effective treatment for alcohol use disorders.
Table 1.
Predicted Average Blood Pressure Reduction Associated with a 50% Decrease in Drinking Days by Age Group, Gender, and Ethnicity
| Demographic Variable | Systolic Blood Pressure Reduction (mm Hg) | Diastolic Blood Pressure Reduction (mm Hg) |
|---|---|---|
| Age | ||
| Age < 44 (n=667, obs=2,530) | 2.6 (p<0.001) | --- |
| Age ≥ 44 (n=716, obs=2,962) | 3.2 (p<0.001) | --- |
| Gender | ||
| Female (n=428, obs=1,695) | 3.1 (p<0.001) | --- |
| Male (n=955, obs=3,797) | 3.1 (p<0.001) | --- |
| Ethnicity | ||
| African American (n=108, obs=454) | 1.1 (p=0.515) | 0.49 (p=0.657) |
| Non-African American (n=1,275, obs=5,037) | 3.2 (p<0.001) | 2.3 (p<0.001) |
| Ethnicity with adjustment for baseline BP > sample average | ||
| African American (n=108, obs=454) | 2.0 (p=0.212) | 1.6 (p=0.128) |
| Non-African American (n=1,275, obs=5,037) | 2.4 (p<0.001) | 1.9 (p<0.001) |
These stratified estimates were adjusted for the other demographic variables (i.e., age estimates were adjusted for gender and ethnicity; gender estimates were adjusted for age and ethnicity; ethnic estimates were adjusted for age and gender).
Acknowledgements
This work was partly supported by a Career Development Award from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) (K23AA104188). The COMBINE Study was supported by NIAAA cooperative agreements with each participating center.
REFERENCES
- 1.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JI, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 2.Miller PM, Anton RF, Egan BM, Basile J, Nguyen SA. Excessive alcohol consumption and hypertension: Clinical implications of current research. Journal of Clinical Hypertension. 2005;7:346–351. doi: 10.1111/j.1524-6175.2004.04463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beilin LJ, Puddey IB. Alcohol and hypertension: an update. Hypertension. 2006;47:1035–1038. doi: 10.1161/01.HYP.0000218586.21932.3c. [DOI] [PubMed] [Google Scholar]
- 4.Estruch R, Coca A, Rodicio JL. High blood pressure, alcohol, and cardiovascular risk. Journal of Hypertension. 2005;23:226–229. doi: 10.1097/00004872-200501000-00039. [DOI] [PubMed] [Google Scholar]
- 5.Klatsky AL. Alcohol and cardiovascular disease-more than one paradox to consider. Alcohol and hypertention: Does it matter? Yes. Journal of Cardiovascular Risk. 2003;10:21–24. doi: 10.1097/01.hjr.0000051960.68260.79. [DOI] [PubMed] [Google Scholar]
- 6.Klatsky AL, Gunderson EP, Kipp H, Udaltsova N, Friedman GD. Higher prevalence of systemic hypertension among moderate alcohol drinkers: an exploration of the role of underreporting. Journal of Studies on Alcohol. 2006;67:421–428. doi: 10.15288/jsa.2006.67.421. [DOI] [PubMed] [Google Scholar]
- 7.Estruch R, Sacanella E, de la Sierra A, Aguilera MT, Antunez E, Nicolas JM, et al. Effects of alcohol withdrawal on 24 hour ambulatory blood pressure among alcohol-dependent patients. Alcoholism: Clinical & Experimental Research. 2003;27:2002–2008. doi: 10.1097/01.ALC.0000100944.02340.46. [DOI] [PubMed] [Google Scholar]
- 8.Minami J, Yoshii M, Todoroki M, Nishikimi T, Ishimitsu T, Fukunaga T, et al. Effects of alcohol restriction on ambulatory blood pressure, heart rate, and heart rate variability in Japanese men. American Journal of Hypertension. 2002;15:125–129. doi: 10.1016/s0895-7061(01)02265-8. [DOI] [PubMed] [Google Scholar]
- 9.Rosito GA, Fuchs FD, Duncan BB. Dose-dependent biphasic effect of ethanol on 24-hour blood pressure in normotensive subjects. American Journal of Hypertension. 1999;12:236–240. doi: 10.1016/s0895-7061(98)00237-4. [DOI] [PubMed] [Google Scholar]
- 10.Aguilera MT, de la Sierra A, Coca A, Estruch R, Fernandez-Sola J, Urbano-Marquez A. Effect of alcohol abstinence on blood pressure: assessment by 24-hour ambulatory blood pressure monitoring. Hypertension. 1999;33:653–657. doi: 10.1161/01.hyp.33.2.653. [DOI] [PubMed] [Google Scholar]
- 11.Rakic V, Puddey IB, Burke V, Dimmitt SB, Beilin LJ. Influence of pattern of alcohol intake on blood pressure in regular drinkers: a controlled trial. Journal of Hypertension. 1998;16:165–174. doi: 10.1097/00004872-199816020-00006. [DOI] [PubMed] [Google Scholar]
- 12.Yamada Y, Tsuritani I, Ishizaki M, Ikai E, Ishida M, Noborisaka Y, et al. Serum gamma-glutamyl transferase levels and blood pressure falls after alcohol moderation. Clinical and Experimental Hypertension. 1997;19:249–268. doi: 10.3109/10641969709080818. [DOI] [PubMed] [Google Scholar]
- 13.Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 14.Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported ethanol consumption. In: Allen J, Litten R, editors. Measuring Alcohol Consumption: Psychosocial and Biological Methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- 15.Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: The revised Clinical Institute Withdrawal Assessment for Alcohol scale (CIWA-Ar) British Journal of Addictions. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 16.Xin X, Jiang H, Frontini MG, Ogden LG, Motsamai OI, Whelton PK. Effects of alcohol reduction on blood pressure: A meta-analysis of randomized controlled trials. Hypertension. 2001;38:1112–1117. doi: 10.1161/hy1101.093424. [DOI] [PubMed] [Google Scholar]
- 17.Dickinson HO, Mason JM, Nicolson DJ, Campbell F, Beyer FR, Cook JV, et al. Lifestyle interventions to reduce raised blood pressure: a systematic review of randomized controlled trials. Journal of Hypertension. 2006;24:215–233. doi: 10.1097/01.hjh.0000199800.72563.26. [DOI] [PubMed] [Google Scholar]
- 18.The ALLHAT officers and coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs. diuretic. JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 19.Franco OH, Peeters A, Bonneux L, de Laet C. Blood pressure in adulthood and life expectancy with cardiovascular disease in men and women. Life course analysis. Hypertension. 2005;46:280–286. doi: 10.1161/01.HYP.0000173433.67426.9b. [DOI] [PubMed] [Google Scholar]
- 20.Poldrugo F, Chick JD, Moore N, Walburg JA. Mortality studies in the long-term evaluation of treatment of alcoholics. Alcohol and Alcoholism. 1993;2 Suppl:151–155. [PubMed] [Google Scholar]
- 21.Radi S, Lang T, Lauwers-Cances V, Chatellier G, Fauvel JP, Larabi L, et al. One-year hypertension incidence and its predictors in a working population: the IHPAF study. Journal of Human Hypertension. 2004;18:487–494. doi: 10.1038/sj.jhh.1001682. [DOI] [PubMed] [Google Scholar]
- 22.Ohmori S, Kiyohara Y, Kato I, Kubo M, Tanizaki Y, Iwamoto H, et al. Alcohol intake and future incidence of hypertension in a general Japanese population: the Hisayama study. Alcoholism: Clinical and Experimental Research. 2002;26:1010–1016. doi: 10.1097/01.ALC.0000021147.31338.C2. [DOI] [PubMed] [Google Scholar]
- 23.Saremi A, Hanson RL, Tulloch-Reid M, Williams DE, Knowler WC. Alcohol consumption predicts hypertension but not diabetes. Journal of Studies on Alcohol. 2004;65:184–190. doi: 10.15288/jsa.2004.65.184. [DOI] [PubMed] [Google Scholar]
- 24.Thadhani R, Camargo CA, Stampfer MJ, Curhan GC, Willett WC, Rimm EB. Prospective study of moderate alcohol consumption and risk of hypertension in young women. Archives of Internal Medicine. 2002;162:569–574. doi: 10.1001/archinte.162.5.569. [DOI] [PubMed] [Google Scholar]
- 25.Fuchs FD, Chambless LE, Whelton PK, Nieto FJ, Heiss G. Alcohol consumption and the incidence of hypertension: the Atherosclerosis Risk in Communities Study. Hypertension. 2001;37:1242–1250. doi: 10.1161/01.hyp.37.5.1242. [DOI] [PubMed] [Google Scholar]
- 26.Curtis AB, James SA, Strogatz DS, Raghunathan TE, Harlow S. Alcohol consumption and changes in blood pressure among African Americans. The Pitt County Study. American Journal of Epidemiology. 1997;146:727–733. doi: 10.1093/oxfordjournals.aje.a009348. [DOI] [PubMed] [Google Scholar]
- 27.Ascherio A, Hennekens C, Willett WC, Sacks F, Rosner B, Manson J, et al. Prospective study of nutritional factors, blood pressure, and hypertension among US women. Hypertension. 1996;27:1065–1072. doi: 10.1161/01.hyp.27.5.1065. [DOI] [PubMed] [Google Scholar]
- 28.National Center for Health Statistics. Hyattsville, MD: Health, United States, 2006, with Chartbook on Trends in the Health of Americans. 2006 [PubMed]
- 29.Screening for High Blood Pressure. US Dept. of Health and Human Services. Agency for Healthcare Research and Quality; The Guide to Clinical Preventive Services 2005. Recommendations of the US Preventive Services Task Force. Publication No. 05-0570.