Abstract
Hyperthermia prolongs the laryngeal chemoreflex (LCR). Under normothermic conditions, adenosine antagonists shorten and adenosine A2A (Ad-A2A) agonists prolong the LCR. Therefore, we tested the hypothesis that SCH-58261, an Ad-A2A receptor antagonist, would prevent thermal prolongation of the LCR when injected unilaterally within the nucleus of the solitary tract (NTS). We studied decerebrate piglets aged 4–13 days. We elicited the LCR by injecting 0.1 ml of water into the larynx and recorded integrated phrenic nerve activity. The laryngeal chemoreflex was prolonged when the body temperature of each piglet was raised ~ 2 degrees C, and SCH-58261 reversed the thermal prolongation of the LCR when injected into the NTS (n = 13), but not when injected in the nucleus ambiguus (n=9). Injections of vehicle alone into the NTS did not alter the thermal prolongation of the LCR (n=9). We conclude that activation of adenosine receptors, perhaps located on GABAergic neurons in the NTS, contributes to thermal prolongation of the LCR.
Keywords: SIDS, laryngeal chemoreflex, hyperthermia, adenosine, nucleus of the solitary tract
1. Introduction
The laryngeal chemoreflex (LCR)—apnea and swallowing in response to water or other foreign liquids in the laryngeal lumen—is much more prominent in newborn animals and human infants than in adults (Haraguchi et al., 1983; Thach, 2001; Xia et al., 2008). The LCR is elicited when fluids with a low chloride content or low pH stimulate receptors in the laryngeal mucosa (Boggs and Bartlett, 1982; Downing and Lee, 1975; Lee et al., 1977). The reflex is a complex behavioral response that may include respiratory inhibition ranging from prolonged apnea to short-lived respiratory disruptions (Downing and Lee, 1975), redistribution of blood flow to vital organs (Grogaard et al., 1982) and activation of airway clearance mechanisms such as coughing and swallowing (Thach, 2001; van der Velde et al., 2003). Moreover, the manifestations of the LCR change as individuals grow older: apnea, respiratory disruption, bradycardia and swallowing are common in neonates, but laryngeal stimulation in adults causes only coughing and swallowing with little respiratory disruption (Thach, 2001).
The LCR is greatly exaggerated in newborn animals that are warmed 1–3 °C above their normal body temperatures (Curran et al., 2005; Xia et al., 2008). For example, the thermal prolongation of the LCR is apparent in lightly anesthetized rat pups from postnatal day 3 (P3) -P25, but the thermal prolongation of the LCR wanes during the first 21 days of life even though the LCR (unmodified by body temperature) persists beyond 21 days of age in rats (Xia et al., 2008). The effect of hyperthermia on the LCR is reversible by body cooling and can be repeated several times in the same animal. The responses of laryngeal water receptors in piglets are not influenced by temperature elevation of 1–2 °C (Xia et al., 2005); whereas focally warming the medulla in the region of the nucleus of the solitary tract (NTS), while keeping body temperature constant, reversibly exaggerated the LCR (Xia et al., 2006). These findings imply that the thermal prolongation of the LCR originates from heating the region of the NTS.
Blocking gamma-aminobutyric acid (GABA) receptors shortened the duration of laryngeal apnea induced by electrical stimulation of the superior laryngeal nerve in normothermic decerebrate neonatal piglets (Abu-Shaweesh et al., 2001). Dialysis of muscimol, a GABAA agonist, in the ventrolateral medulla prolonged the LCR during wakefulness and sleep in normothermic neonatal piglets (van der Velde et al., 2003). Furthermore, unilateral microdialysis of gabazine (a GABAA receptor antagonist) in or near the NTS reversed the thermal prolongation of the LCR in decerebrate piglets (Xia et al., 2007), but did not change the duration of the LCR under normothermic conditions. Thus, the thermal effects on the LCR seem to depend on GABAergic mechanisms in the region of the NTS; whereas the apnea and respiratory disruption associated with superior laryngeal nerve stimulation during normothermia seem to depend on GABAergic mechanisms in the ventral medulla (Czyzyk-Krzeska and Lawson, 1991; Remmers et al., 1986; van der Velde et al., 2003).
Adenosine antagonists shorten the LCR under normothermic conditions (Lee et al., 1977; Martin et al., 2004). This effect has been attributed to blockade of activation of adenosine A2A (Ad-A2A) receptors on GABAergic neurons in the medulla (Martin et al., 2004; Mayer et al., 2006; Wilson et al., 2004). In this setting and others (Hong et al., 2005; Ochi et al., 2000; Phillis, 1998), activation of Ad-A2A receptors seems to enhance GABA release. Since GABAA receptor activation seems to be involved in the apnea and respiratory disruption of the LCR and in the thermal prolongation of the LCR, we tested the hypothesis that an antagonist of Ad-A2A receptors focally injected within the NTS would prevent thermal prolongation of the LCR in decerebrate piglets. We chose as our anatomical control site the nucleus ambiguus, which contains neurons thought to mediate the respiratory inhibition after SLN stimulation (Czyzyk-Krzeska and Lawson, 1991; Jiang and Lipski, 1992; Remmers et al., 1986). It was our expectation that blocking Ad-A2A receptors in the NTS would reverse thermal prolongation of the LCR, and blocking Ad-A2A receptors in the nucleus ambiguus might shorten the LCR under normothermic conditions, but leave the thermal prolongation of the LCR intact.
2. Methods
Experiments were performed on 32 piglets (16 piglets of each gender) ranging in age from 4 to 13 days (8.2 ± 0.5 days; mean ± SEM) with an average weight of 2.8 ± 0.1 kg. The Institutional Animal Care and Use Committee of Dartmouth College approved all surgery and experimental protocols.
2.1 Surgical preparation
Animals were anesthetized with 2% halothane (2-Bromo-2-chloro-1,1,1-trifluoroethane; Halocarbon Laboratories, NJ) in O2. A rectal probe was inserted, and body temperature was maintained between 37 and 38°C using a heating pad. Femoral arterial and venous catheters were inserted to measure blood pressure and administer drugs, respectively. Each animal was tracheostomized and artificially ventilated (Harvard Apparatus Dual Phase Respirator, South Natick, MA) to maintain the end-tidal CO2 concentration at approximately 5%. The carotid sinus regions were exposed bilaterally, and the internal and external carotid arteries were ligated to facilitate decerebration. The vagus nerves were sectioned bilaterally to prevent entrainment of the phrenic rhythm to the mechanical ventilator (Graves et al., 1986; Petrillo et al., 1983). The animal was placed prone, and the head was positioned in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA). The skull was opened, and the animal was decerebrated at the level of the superior colliculi. All brain tissue rostral to the section was removed by suction. Following decerebration, halothane anesthesia was discontinued, and each animal was paralyzed using pancuronium bromide (1 mg/kg, iv; Elkins-Sinn Inc., Cherry Hill, NJ). Supplemental doses of pancuronium were given as required, usually at a rate of 0.5 mg/kg/hr. A phrenic nerve was exposed and sectioned, and the central cut end was placed on a bipolar recording electrode to monitor respiratory output. Phrenic activity was amplified (Gould Universal Amplifier, Cleveland, OH), and the moving time average (“integrated activity”) was calculated electronically (100ms time constant; CWE, Ardmore, PA). Integrated phrenic nerve activity, body temperature, end-tidal CO2, and blood pressure were recorded on a computer (PowerLab, ADI, Australia) for later analysis.
A dialysis guide tube (O.D. 310 μm) was placed in the medulla of each animal from the dorsal surface of the medulla, which was exposed as part of the preparation for decerebration. The probe was positioned by using visual landmarks on the dorsal surface of the medulla (the obex primarily), the lateral distance from the midline and an estimate of the depth of the target within the medulla based on previous studies in piglets (Niblock et al., 2005). At least 60 minutes elapsed after placement of the dialysis guide tube before any tests of the LCR were performed. Injections of drugs were made through a broken dialysis probe inserted into the guide tube. The tip of the broken dialysis probe extended 1 mm beyond the tip of the dialysis guide tube. The Ad-A2A receptor antagonist that we used, SCH-58261, is relatively insoluble in water. Therefore, SCH-58261 was dissolved in DMSO at a concentration of 1 μM. Control injections consisted of DMSO alone.
To stimulate the LCR, we placed a pharyngeal catheter (PE-90) through a nostril and positioned the tip just above the larynx. The catheter was filled with water, and 0.1 ml of water was injected into the larynx using a computer controlled syringe pump each time that we elicited the LCR. Water remained in the catheter between tests, and as a consequence, the temperature of the water injected was near body temperature. The larynx was suctioned periodically as needed. At least 5 minutes elapsed between tests of the LCR, and the LCR was not tested unless phrenic respiratory activity was stable.
2.2 Experimental protocols
Studies began with a control period during which the body temperature was held at 37–38 °C, and the LCR was elicited three times. Next, the body temperature was elevated approximately 2.5°C by warming the heating pad. Once the body temperature reached a stable elevated temperature, the LCR was stimulated three more times. At this point in the protocol, 20 nl of SCH-58261 in DMSO or DMSO alone were injected, and testing of the LCR began no sooner than 10 minutes after the injection. The onset of the drug effect seemed delayed in some animals, so we assessed the LCR at 5 min intervals approximately six times while the animal was hyperthermic. After this series of tests, each animal was cooled by swabbing it with isopropyl alcohol until body temperature was reduced to the control value, and the LCR was stimulated a final three times in this follow-up normothermic period after microinjection.
2.3 Neuroanatomy
At the conclusion of each experiment, each piglet was killed with an injection of 500 mg/kg pentobarbital sodium followed by 5–10 ml of saturated potassium chloride administered I.V. Microinjections of 20–50 μl of 1% potassium permanganate were made into the medulla through a broken microdialysis probe passed through the guide tube. Manganese oxide, which is formed by oxidation of the permanganate, stains the tissue brown and indelibly marks the location of the tip of the probe and site of microinjection (Sun et al., 2000). The brainstem was removed from the animal, placed in cryo-embedding medium (Tissue-Tek O.C.T. 458, Sakura Finetek, Torrance, CA) and frozen in isopentane at −70 °C. Brainstems were sectioned (50 μm) in a cryostat at −18 °C, and sections were mounted on gelatinized glass slides, fixed over night in 10% formalin in phosphate buffered saline (pH 7.0) and stained with cresyl violet (Bandroft and Cook, 1994; Luna, 1992). We expressed the location of each probe using three dimensions in millimeters: a mediolateral dimension (midline = 0), a dorsoventral dimension (dorsal surface of the medulla = 0), and a rostrocaudal dimension (obex = 0; rostral, positive; caudal, negative).
2.4 Data analysis and statistics
We defined the duration of respiratory disruption by the LCR as the period of respiratory instability (defined as variability of phrenic amplitude and/or respiratory timing) from the beginning of the breath during which the water stimulus was delivered to the onset of at least five regular breaths. These five breaths did not need to have the same frequency or amplitude as the control breaths; we simply required that they be regular (Curran et al., 2005; van der Velde et al., 2003; Xia et al., 2006). The respiratory disruption measured in this way included both periods of unstable respiratory activity and apneas. We kept the definition of the LCR duration simple and applied it consistently across all animals, but it can be difficult to decide what constitutes five regular breaths to end the LCR. Therefore, we also measured the longest apnea duration of each reflex trial, which is less subject to interpretation. Apnea was defined as a cessation of breathing greater than the duration of the two breaths preceding the breath during which the stimulus was delivered. However, apnea did not occur in all tests of the LCR. Measuring both the LCR duration and apnea duration, when present, provided a more complete analysis of the response since apnea duration alone does not capture the behavioral complexity of the LCR and measuring the LCR duration alone can require subjective judgments. Stimulation of the LCR may induce bradycardia as well as apnea. However, we did not analyze the heart rate responses because the animals were vagotomized.
We analyzed these experiments using a two-way repeated measures analysis of variance (ANOVA, SYSTAT 9.0, SPSS, Inc, Chicago, IL). The average response from each animal in each set of test conditions was used in this analysis. The injection (control versus drug) and temperature (control versus hyperthermia) were repeated within subjects factors. When the ANOVA indicated that significant differences existed among conditions, specific pre-planned comparisons were made using P-values adjusted by the Bonferroni method. Data are presented as the mean ± the standard error of the mean.
To determine whether the control DMSO and test injections of DMSO plus SCH-58261 were made in the same location in the NTS, we performed paired t-tests on each of the three anatomical coordinates describing the location of the injection: rostro-caudal location relative to the obex, lateral location with respect to the midline, and dorso-ventral location with respect to the dorsal surface of the medulla all in millimeters (Systat 9.0). The site of injection was defined by the site of deepest penetration along the track made by dialysis probe; there was usually some tissue disruption at this site, which made it relatively easy to identify in serial sections of the brainstem. Responsiveness to injection of drug plus vehicle or vehicle alone was defined in each animal as the ratio of the average LCR duration during drug and hyperthermia to the LCR duration during hyperthermia alone. Thus, a ratio less than 1.0 indicated that SCH-58261 decreased the hyperthermic prolongation of the LCR.
3. Results
3.1 Effect of hyperthermia and SCH-58261 on the LCR
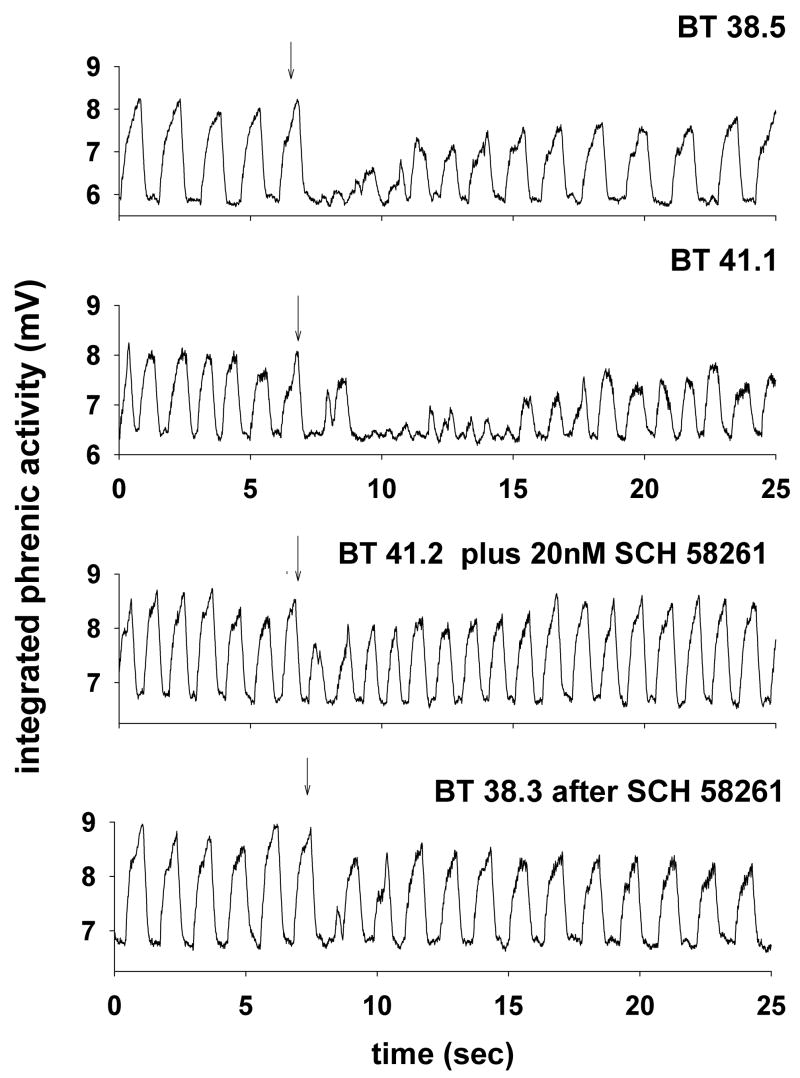
An example of the responses of integrated phrenic nerve activity taken from one piglet during each of the experimental treatments is shown in Fig. 1. The injection site was in the NTS just rostral to the obex this piglet (circled symbol in Fig. 3). In the control condition, body temperature was 38.5 °C, and introducing 0.1 ml of water into the larynx (arrow) caused brief apnea, but regular respiratory activity was quickly restored. After elevating the animal’s body temperature to 41.1 °C, 0.1 ml water injected into the larynx disrupted respiratory activity and regular respiratory activity was not resumed for at least 9 sec. After injection of 20 nl of SCH-58261 into the NTS, the LCR was tested again, and the LCR duration was short, despite the fact that the rectal temperature remained elevated at 41.2 °C. When body temperature was reduced to the control level (38.3 °C), the respiratory disruption following stimulation of the LCR after SCH-58261 injection was brief and of similar duration to the respiratory disruption during hyperthermia after injection of SCH-58261.
Figure 1.
Integrated phrenic activity is shown during four tests of the LCR in a male piglet (age 12 days). The control response to laryngeal injection of 0.1 ml of water (at the downward arrow) at a normal body temperature is shown in the top panel. Elevating the body temperature (second panel) prolonged the duration of the LCR. However, after injecting 20 nM of SCH-58261 into the NTS (the injection site is shown in Fig. 3; symbol with circle around it), the LCR was no longer prolonged even though body temperature remained elevated. In the final condition after SCH-58261 injection (bottom panel), body temperature was restored to the control level, and the duration of the LCR remained brief.
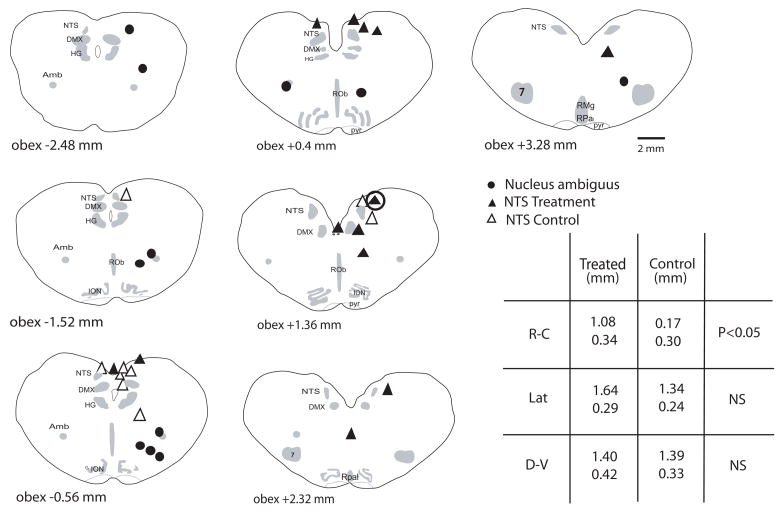
Figure 3.
Schematic cross-sections starting caudal to the obex of the neonatal piglet medulla are shown in which the injection sites within the brainstem are indicated. Injections in the NTS with DMSO alone are indicated by open triangles; injections in the NTS with DMSO and SCH-58261 are indicated by filled triangles; and injections in the nucleus ambiguus with DMSO and SCH-58261 are indicated by filled circles. The symbol with the larger circle around it marks the site of the dialysis probe in the animal from which the data in Fig. 1 were taken. The table in the lower right-hand corner displays the average ± SEM rostro-caudal (RC), lateral (Lat) and dorsoventral (DV) dimensions of the sites of injection in the NS treatment groups. ‘Treated’ indicates the SCH-58261 treatment group; ‘Control’ indicates the animals that received DMSO alone. Only the rostro-caudal dimensions differed statistically between these two groups. Anatomical abbreviations: 7, facial nucleus; RO, raphé obscurus; RP, raphé pallidus; RM, raphé magnus; DMX, dorsal vagal motor nucleus; ION, inferior olivary nucleus; HG, hypoglossal motor nucleus; NTS, nucleus tractus solitarius; NA, nucleus ambiguus.
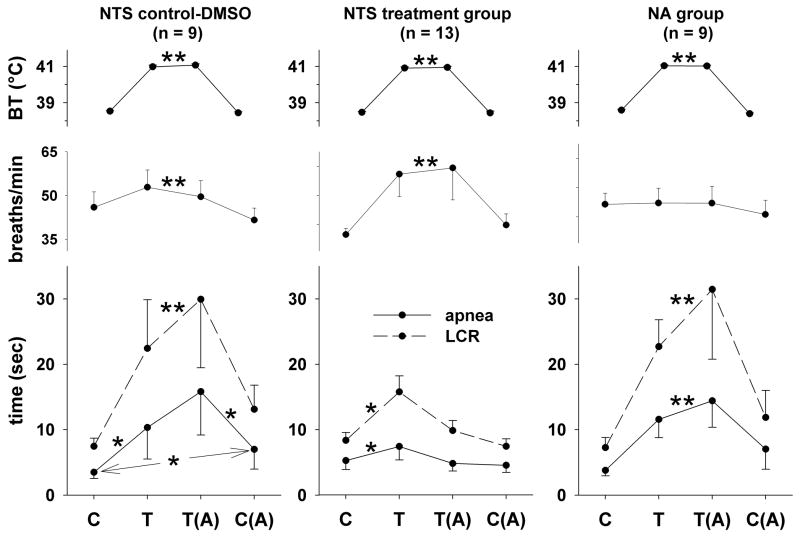
The average values of the LCR duration, the longest apnea duration, respiratory frequency and body temperature during each of the four experimental conditions (control, hyperthermia, hyperthermia after treatment and normothermia after treatment) for each of the three treatment groups are shown in Fig. 2. In the animals treated with SCH-58261 (Fig. 2, middle panels), elevating body temperature by 2.4 °C prolonged the durations of the LCR and of the longest apnea (both P < 0.05). However, the LCR and apnea durations returned to the same range present in the normothermic control condition after unilateral microinjection of SCH-58261 despite the persistent elevation of body temperature. Thus, treatment with SCH-58261 reversed or prevented the thermal prolongation of the LCR. Although the control animals that received NTS injections with DMSO alone demonstrated a similar thermal prolongation of apnea and LCR duration during hyperthermia (Fig. 2, left panels), DMSO injection did not prevent or reverse the thermal prolongation of apnea and the LCR compared to the normothermic condition after DMSO injection. In addition, the normothermic apnea duration was longer after DMSO injection than it had been in the initial control normothermic condition before DMSO injection (P < 0.05). The LCR duration was prolonged by elevated body temperature, and the lengthening of the LCR was statistically indistinguishable before and after DMSO injection in the hyperthermic state. The normothermic LCR duration was increased after DMSO injection, but the difference between the control normothermic response and the normothermic response after DMSO injection was not statistically significant.
Figure 2.
Body temperature (BT), respiratory frequency, the duration of the LCR and the duration of apnea have been plotted as functions of experimental conditions (‘C,’ control normothermia during aCSF dialysis; ‘T,’ hyperthermia; ‘T(A),’ hyperthermia after injection of SCH-58261; and ‘C(A),’ normothermia after injection of SCH-58261) in neonatal piglets. Animals that received DMSO injections into the NTS are shown in the left panels; animals that received injections in the NTS of SCH-58261 dissolved in DMSO are shown in the middle panels; and animals that received injections in the nucleus ambiguus of SCH-58261 dissolved in DMSO are shown in the right panels. Two sets of statistical comparisons were made within each treatment group when the ANOVA indicated that a significant interaction between temperature condition and drug treatment existed: each experimental condition was compared to control (‘C’), and each condition was compared to the preceding condition (the results of this comparison are indicated by the second statistical symbol in any pair of symbols). ‘*’ indicates P < 0.05, and ‘**’ indicates the presence of a significant main effect of temperature (i.e., the ANOVA indicated that there was no significant interaction term).
The locations of the injections in these two groups of animals are shown in Fig. 3. Injections in the SCH-58261 treatment group (filled triangles) were in or near the NTS with the exception of two animals. One injection was deep to the NTS 1.36 mm rostral to the obex, but did pass through the NTS, and the other was in the midline and well below the level of the NTS 2.32 mm rostral to obex. The thermal prolongation of the LCR was less in all of the animals injected with SCH-58261 except in the animal with the deep midline injection. The injections in the DMSO control group were also in or near the NTS (Fig. 3; open triangles), but there were rare ‘misses’ in this group as well (e.g, the injection 0.56 mm caudal to the obex and well below the NTS). Regardless of these ‘misses’, the average LCR duration in the DMSO group was 30 ± 15% longer during hyperthermia after DMSO injection compared to hyperthermia alone. In contrast, the average LCR during hyperthermia after SCH-58261 treatment was 66 ± 9% of the hyperthermic duration of the LCR before injection of the drug, which indicates that the SCH-58261 rather than the DMSO was responsible for reducing the thermal prolongation of the LCR. The injections in the DMSO control group and the SCH-58261 group though in the NTS were not in identical parts of the NTS (the coordinates are shown in the table in Fig. 3). The injections in the DMSO group were significantly more caudal, on average, than the SCH-58261 treatment group (P = 0.02), but there was considerable rostro-caudal overlap, and the lateral and dorso-ventral locations were similar in the two treatment groups.
To determine whether the effects of SCH-58261 were anatomically specific, we injected the drug into the nucleus ambiguus. This was not a chance selection; the normothermic respiratory inhibition associated with the LCR in newborn animals is integrated within the ventral respiratory group of neurons in the region of the nucleus ambiguus (Czyzyk-Krzeska and Lawson, 1991; Remmers et al., 1986). Injections into the nucleus ambiguus gave us the opportunity to determine whether the Ad-A2A receptor antagonist affected only the thermal prolongation of the LCR or other, normothermic characteristics of the LCR as well. The average responses of nine animals injected unilaterally with 20 μM SCH52861 are shown in Fig. 2 (right panels). Hyperthermia significantly prolonged the apnea and LCR durations in these animals, but the treatment with SCH-58261 did not significantly alter the pattern of responses during hyperthermic or normothermic conditions. The injections in the region of the nucleus ambiguus were all in close proximity to the nucleus ambiguus except one animal in which the injection was close to the NTS 2.48 mm caudal to obex (Fig. 3, filled circles). However, the response to SCH-58261 injection was similar in all animals tested; inspection of the individual responses, regardless of the site of injection, gives no reason to believe that there was any location in or adjacent to the nucleus ambiguus where blocking Ad-A2A receptors might reduce the duration or intensity of the LCR during hyperthermia or normothermia.
3.2 Respiratory responses to SCH-58261and hyperthermia
The duration of the LCR is modified by the level of respiratory drive. Interventions that increase the drive to breathe often shorten the duration of the LCR (Litmanovitz et al., 1994; Mitra et al., 1985); for example, increased levels of inspired CO2 significantly shorten the duration of reflex apnea after SLN stimulation (Lawson, 1982). Therefore, we tried to assess whether respiratory activity was altered by hyperthermia and/or SCH-58261 treatment to determine whether the effect of SCH-58261 was really specific to the LCR or a reflection of a more general effect of these treatments on respiratory activity. We recorded peak integrated phrenic nerve activity, but unfortunately this often changed during the experiment owing to movement or drying of the nerve, requiring repositioning or gain changes. Respiratory frequency was recorded accurately, however, and was our most reliable indicator of respiratory drive. The end-tidal CO2 levels were stable across all treatment groups and treatment conditions and equaled 5.1 ± 0.1% on average. In the NTS SCH-58261 treatment group, the respiratory frequency rose significantly during hyperthermia, but was not affected by the drug treatment (a main effect of temperature; P = 0.03). This frequency response is typical of the effect of hyperthermia in decerebrate piglets that we have studied before (Xia et al., 2007). In the NTS DMSO control group, the respiratory frequency demonstrated the same increase during hyperthermia that we saw in the SCH-58261 treatment group, but DMSO injection did not alter the respiration frequency responses to the different treatment conditions. In the nucleus ambiguus treatment group, in contrast to the other two groups, there was no change in respiratory frequency during hyperthermia.
4. Discussion
The main findings in this study are that hyperthermia prolongs the LCR in decerebrate piglets, as we have shown before, and this thermal prolongation of the LCR can be reversed by treatment with an antagonist of Ad-A2A receptors. The reversal of thermal prolongation of the LCR following microinjection of SCH-58261 in the NTS cannot be attributed to any effect of blocking Ad-A2A receptors on respiratory activity since the changes in respiratory activity that we observed were not correlated with changes in LCR duration. These findings are consistent with the hypothesis that hyperthermia increases adenosine release in the NTS, and adenosine binds to Ad-A2A receptors that amplify GABAergic mechanisms that, in turn, prolong the LCR. GABAergic mechanisms were not studied in the current work, but we previously found that blocking GABAA receptors also reversed the thermal prolongation of the LCR (Böhm et al., 2007; Xia et al., 2007).
4.1 Neuronal circuitry of the LCR
The primary afferents of the SLN terminate in the NTS (Hayakawa et al., 2001; Patrickson et al., 1991). The respiratory inhibition associated with SLN stimulation that occurs at normal body temperatures is orchestrated within the ventral respiratory group of neurons. SLN stimulation or water in the larynx inhibits inspiratory and expiratory neurons and stimulates post-inspiratory neurons (Czyzyk-Krzeska and Lawson, 1991; Remmers et al., 1986). The apnea induced is a manifestation of prolonged expiratory time; the post-inspiratory neurons remain persistently depolarized and prevent the normal sequential activation of expiratory neurons that would lead to the next breath (Remmers et al., 1986). The hyperthermic prolongation of the LCR probably enhances the duration of what Remmers et al. called ‘post-inspiratory apneusis.’ But the neuronal elements responsible for thermal enhancement of the LCR seem to reside in or near to the NTS. There are temperature sensitive neurons in and around the NTS (Inoue and Murakami, 1976), and heating just the NTS in decerebrate piglets, and not the rest of the brainstem or body, prolonged the LCR (Xia et al., 2006). GABAergic neurotransmission in and around the NTS is also essential for thermal prolongation of the LCR. Focal administration of gabazine, a GABAA receptor antagonist, reversed the thermal prolongation of the LCR without any apparent shortening of the normothermic LCR (Xia et al., 2007). An Ad-A2A receptor agonist, CGS-21680, administered systemically or intracisternally enhanced the respiratory depression associated with SLN stimulation in anesthetized piglets (Abu-Shaweesh, 2007). The effect of CGS-21680 could be blocked by administration of the GABAA antagonist bicuculline, which implies that CGS-21680 was effective because it enhanced GABA release. A similar mechanism, Ad-A2A receptor agonist enhanced GABA release, has been described in the hypothalamus (Hong et al., 2005), the globus pallidus (Ochi et al., 2000) and the cortex (Phillis, 1998). The response to CGS-21680 provides no information about the anatomical site(s) of action of the Ad-A2A receptors on the LCR during normothermia. In the current study, an Ad-A2A receptor antagonist injected focally in the region of the NTS reversed the thermal prolongation of the LCR. Based on these findings, it is our hypothesis that hyperthermia augments GABAergic neurotransmission within the NTS when the LCR is elicited, and activation of Ad-A2A receptors during hyperthermic conditions amplifies this GABA release within the NTS. It seems likely that this adenosine-dependent process is thermally sensitive (the GABAergic mechanism(s) may or may not be thermally sensitive) since blocking the Ad-A2A receptors completely prevented the thermal prolongation of the LCR. We have not searched exhaustively for other brainstem sites that might mediate the thermal prolongation of the LCR, but our findings are consistent in that focally heating the NTS, focal gabazine administration and focal Ad-A2A receptor antagonist administration, all within or near the NTS, modify the thermal sensitivity of the LCR. No other single anatomical site encompasses all of these findings.
The age-dependence of the manifestations of the LCR has been observed in a variety of studies (Haraguchi et al., 1983; Miller, 1976; Thach, 2001; Xia et al., 2008). Apnea and respiratory disruption diminish after laryngeal stimulation as animals mature, and cough and swallowing become more prominent. The thermal prolongation of the LCR is also age-dependent in neonatal rats and is no longer apparent at approximately P21 (Xia et al., 2008). The Ad-A2A receptor agonist, CGS-21680, causes respiratory depression and apnea when given systemically or intracisternally in neonatal rats at age P14 (Mayer et al., 2006). However, this respiratory depression diminishes as the rat pups mature, and no respiratory disruption is evident when adult rats are given CGS-21680. It seems, therefore, that the inhibitory effects of Ad-A2A receptor activation wane as animals mature, and we may speculate that the loss of thermal responsiveness of the LCR might be attributed to the loss of sensitivity or effectiveness of Ad-A2A receptors during maturation.
The effect of SCH-58261 treatment cannot be attributed to the injection vehicle (DMSO) since DMSO alone did not diminish the thermal prolongation of the LCR. DMSO may have had an effect on the LCR when injected into the NTS. The LCR was longer during normothermic and hyperthermic conditions after DMSO treatment. This is probably a non-specific effect of DMSO, but this finding does not diminish the significance of the SCH-58261 effects since the effect of DMSO was not temperature specific and enhanced rather than diminished the thermal prolongation of the LCR.
It was surprising to us that SCH-58261 administration in the nucleus ambiguus did not alter the normothermic characteristics of the LCR. It may be that the drug effects had diminished by the time we cooled each animal to its normal body temperature and repeated the normothermic LCR trials. The lack of any effect of SCH-58261 leaves open the question where in the brainstem the normothermic effects of adenosine antagonists, which shorten the LCR (Lee et al., 1977), and Ad-A2A agonists, which prolong the LCR, originate. The Ad-A2A receptors are present in the nucleus ambiguus (Zaidi et al., 2006), and there is ample evidence of GABAergic neurotransmission within the nucleus ambiguus that should modify respiratory timing when the SLN or larynx is stimulated (Czyzyk-Krzeska and Lawson, 1991; Remmers et al., 1986).
4.2 Respiratory effects
In this, as in our previous studies, the most consistent thermal effect on respiration in decerebrate piglets was an increase in respiratory frequency (Xia et al., 2007). The most important aspect of the respiratory responses in these animals is that injection of SCH-58261 into the NTS had no discernible effect on respiratory frequency. Therefore, the changes in the LCR in this group following SCH-58261 injection probably arose from the drug treatment rather than from a secondary effect mediated by changes in respiratory activity.
In the nucleus ambiguus treatment group, the lack of any increase in respiratory frequency is unusual. We have no adequate explanation for this finding. It cannot be attributed to the drug treatment since it was present before the drug was given. It may possibly be related to the placement of the dialysis probe used for injections. However, the probe was placed before any measurements were made, and the baseline respiratory rate is not different from values recorded in other experimental groups. When we placed a relatively large thermode in this location in previous experiments (Xia et al., 2006), respiratory activity was made irregular and slowed, which does suggest that mechanically disrupting the region around the nucleus ambiguus, even when the disruption is unilateral, may alter respiratory activity. The absence of any thermally induced increase in respiratory frequency was unexpected, and it is perhaps more important to emphasize that even when this disruption was present, the thermal prolongation of the LCR remained intact. These findings provide additional support for the hypothesis that the thermal effects on the LCR are generated in the NTS, but not in the region of the ventral respiratory group of neurons.
4.3 Limitations of the methods
As we have noted before (Curran et al., 2005; Xia et al., 2006), the nature of the decerebrate preparation is a major limitation of our studies. Decerebrate animals lack the thermoregulatory mechanisms that exist in structures rostral to the inferior colliculi. Since the piglets in this study were decerebrated and paralyzed, the usual airway clearance mechanisms were impaired. The duration of stimulation of the larynx may have been prolonged since the animals were less able to cough and swallow and clear the larynx. The piglets were mechanically ventilated, which prevented the development of hypoxia and hypercapnia during the LCR, and both of these stimuli may shorten the duration of the LCR in intact animals (Lawson, 1982; van der Velde et al., 2003; Woodson and Brauel, 1992).
A further limitation is that we do not know the exact distribution of the injections that we made. The SCH-58261 diffused from the site of injection, but the volume of diffusion was probably smaller than when we have used dialysis in previous studies since dialysis continually renews the concentration of the drug at the site of dialysis. We can define the site of action of SCH-58261 only broadly: it is in or near the NTS, and it is also clear that SCH-58261 does not have any effect on the LCR when injected in the ventral part of the medulla below the NTS.
Thermal stress has been identified as a risk factor for SIDS, but this does not mean necessarily that infants who died of SIDS had an elevated body temperature. In this respect, our studies of decerebrate piglets may not accurately mimic the thermal risk factors for SIDS. We have elevated the environmental temperature in studies of intact neonatal piglets, and some of the animals began to pant. When panting occurred, it added a thermal respiratory drive that complicated our studies of respiratory inhibition during the LCR (unpublished observations). In this respect, the absence of thermoregulation in decerebrate piglets is actually an advantage since it has permitted us to study the neural circuitry of the LCR without the complication of panting. The occurrence of panting in intact neonatal piglets limits the usefulness of this animal model in studies of interactions among thermal stress, apnea and hypoxia.
4.4 Thermal stresses and the LCR: Implications for SIDS
We have discussed the implications of the thermal prolongation of the LCR in the pathogenesis of SIDS previously (Curran et al., 2005; Leiter and Böhm, 2007; Xia et al., 2007; Xia et al., 2006). Our studies of the LCR and hyperthermia emphasize the importance of interactions among risk factors for SIDS, and the results of these studies are consistent with the triple risk model of SIDS (Filiano and Kinney, 1994), which posits that combinations of rare events may create a lethal ‘Perfect Storm’ from individual factors that are probably not life-threatening when each of them occurs alone. Previous physiological studies have focused on the role of reduced excitatory respiratory reflexes (e.g., ventilatory responses to hypoxia and hypercapnia) in the pathogenesis of SIDS (Hunt, 1981; Hunt, 1992), and the current study emphasizes that enhanced activity of inhibitory neurotransmitters and inhibitory reflexes may also increase the likelihood of SIDS.
One aspect of the association of thermal stress and the risk of SIDS that has not received much emphasis is that the thermally mediated prolongation of the LCR may represent the inappropriate persistence of a fetal pattern of reflex responses into neonatal like. We have argued in the past that inhibitory responses to threats to homeostasis, such as hypoxia, the diving reflex and the LCR, which lead to apnea, bradycardia and redistribution of blood flow, are appropriate fetal responses to life in utero where conservation of limited oxygen resources is the best strategy. However, the same responses are not appropriate in postnatal life where arousal and respiratory stimulation more effectively restore homeostasis (Leiter and Böhm, 2007). Elevated body temperature and even elevated ambient temperature have inhibitory effects on fetal breathing movements. The fetal temperature must be higher than the maternal temperature in order to create a thermal gradient to rid the fetus of excess heat, and the elevated fetal temperature is one of the factors suppressing breathing in utero (Blanco, 1991). Moreover, elevated ambient temperatures can inhibit breathing in newborn animals, especially if they are hypoxic (Blanco, 1991). In the neonatal intensive care nursery, when the temperature in the isolation unit rises, apneas frequently develop even without any increase in core body temperature (Daily et al., 1969; Perlstein et al., 1970). Reflex processes that inhibit respiration, such as the LCR or the inhibitory elements of the Hering-Breuer reflex, are also enhanced by elevated body temperature (Merazzi and Mortola, 1999). In all the foregoing examples, the inhibitory effect of hyperthermia is lost as animals mature; similar inhibitory effects of hyperthermia or elevated ambient temperature are not found in adults. Thus, hyperthermia and elevated ambient temperatures seems to enhance inhibitory mechanisms associated with respiration, and in this way, elevated ambient and/or body temperature may make a significant contribution to the pathogenesis of SIDS. This line of reasoning emphasizes the importance of persistent fetal responses associated with respiratory inhibition as risk factors for SIDS (Leiter and Böhm, 2007) rather than the reduction in excitatory responses that others have emphasized (Hunt, 1992).
The most important aspect of our recent studies is that either adenosine or GABA may enhance the activity of the LCR during hyperthermic conditions. It is interesting to note that adenosine is the neurotransmitter most closely associated with sleep (Rainnie et al., 1994), and sleep enhances the LCR (van der Velde et al., 2003). SIDS occurs most commonly during sleep, and adenosine may provide a mechanistic link between sleep and SIDS as well as between thermal stress, the LCR and SIDS. Finally, adenosine antagonists, caffeine or theophylline, are frequently used as respiratory stimulants in neonates (Schmidt et al., 2006), and these drugs also reduce the duration of the LCR (Lee et al., 1977) and increase CO2 sensitivity (Davi et al., 1978). Hunt et al. (1983) reported on the effects of theophylline therapy in a group of 80 infants who either experienced a near-miss episode of SIDS or were siblings of SIDS victims. Theophylline regularized the respiratory pattern in this group of infants. The infants were treated with theophylline for a variable length of time, and the study had too few subjects to assess the effect of theophylline on the risk of SIDS. However, none of the 80 infants died of SIDS, and this was less than the predicted risk of a SIDS death in this group of infants. Thus, determining the role of excessive inhibition in the pathogenesis of SIDS, whether due to adenosine or GABA, remains an interesting question in SIDS research with potential therapeutic implications.
Acknowledgments
We acknowledge the technical assistance of Soo-Yeon Kang. This work was supported by grants 36379 and 42707 from the NICHD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Abu-Shaweesh JM. Activation of central adenosine A2A receptors enhances superior laryngeal nerve stimulation-induced apnea in piglets via a GABAergic pathway. J Appl Physiol. 2007;103:1205–1211. doi: 10.1152/japplphysiol.01420.2006. [DOI] [PubMed] [Google Scholar]
- Abu-Shaweesh JM, Dreshaj IA, Haxhiu MA, Martin RJ. Central GABAergic mechanisms are involved in apnea induced by SLN stimulation in piglets. J Appl Physiol. 2001;90:1570–1576. doi: 10.1152/jappl.2001.90.4.1570. [DOI] [PubMed] [Google Scholar]
- Bandroft JD, Cook HC. The central and peripheral nervous system. In: Bandroft JD, editor. Manual of Histological Techniques and Their Diagnostic Application. New York: Churchill Livingstone; 1994. pp. 350–351. [Google Scholar]
- Blanco C. Role of the brain stem in the changes at birth: initiation of continuous breathing and its maintenance. In: Hanson MA, editor. The Fetal and Neonatal Brain Stem. Cambridge: Cambridge University Press; 1991. pp. 106–126. [Google Scholar]
- Boggs DF, Bartlett D., Jr Chemical specificity of a laryngeal apneic reflex in puppies. J Appl Physiol. 1982;53:455–462. doi: 10.1152/jappl.1982.53.2.455. [DOI] [PubMed] [Google Scholar]
- Böhm I, Xia L, Leiter JC, Bartlett D., Jr GABAergic processes mediate thermal prolongation of laryngeal reflex apnea in decerebrate piglets. Respir Physiol Neurobiol. 2007;156:229–233. doi: 10.1016/j.resp.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Curran AK, Xia L, Leiter JC, Bartlett D., Jr Elevated body temperature enhances the laryngeal chemoreflex in decerebrate piglets. J Appl Physiol. 2005;98:780–786. doi: 10.1152/japplphysiol.00906.2004. [DOI] [PubMed] [Google Scholar]
- Czyzyk-Krzeska MF, Lawson EE. Synaptic events in ventral respiratory neurones during apnoea induced by laryngeal nerve stimulation in neonatal piglet. J Physiol. 1991;436:131–147. doi: 10.1113/jphysiol.1991.sp018543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daily WJ, Klaus MH, Meyer HB. Apnea in premature infants: monitoring, incidence, heart rate changes, and an effect of environmental temperature. J Pediatr. 1969;43:510–518. [PubMed] [Google Scholar]
- Davi MJ, Sankaran K, Simons KJ, Simons ER, Seshia MM, Rigatto H. Physiologic changes induced by theophylline in the treatment of apnea in preterm infants. J Pediatr. 1978;92:91–95. doi: 10.1016/s0022-3476(78)80084-5. [DOI] [PubMed] [Google Scholar]
- Downing SE, Lee JC. Laryngeal chemosensitivity: A possible mechanism of sudden infant death. Pediatrics. 1975;55:640–649. [PubMed] [Google Scholar]
- Filiano JJ, Kinney HC. A perspective on neuropathological findings in victims of the Sudden Infant Death Syndrome: The triple-risk model. Biol Neonate. 1994;65:194–197. doi: 10.1159/000244052. [DOI] [PubMed] [Google Scholar]
- Graves C, Glass L, Laporta D, Meloche R, Grassino A. Respiratory phase locking during mechanical ventilation in anesthetized subjects. Am J Physiol. 1986;250:R902–R909. doi: 10.1152/ajpregu.1986.250.5.R902. [DOI] [PubMed] [Google Scholar]
- Grogaard J, Lindstrom DP, Stahlman MT, Marchal F, Sundell H. The cardiovascular response to laryngeal water administration in young lambs. J Dev Physiol. 1982;4:353–370. [PubMed] [Google Scholar]
- Haraguchi S, Fung RQ, Sasaki R. Effect of hyperthermia on the laryngeal closure reflex. Implications in the sudden infant death syndrome. Ann Otol Rhinol Laryngol. 1983;92:24–28. doi: 10.1177/000348948309200106. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Takanaga A, Maeda S, Seki M, Yajima Y. Subnuclear distribution of afferents from the oral, pharyngeal and laryngeal regions in the nucleus tractus solitarii of the rat: a study using transganglionic transport of cholera toxin. Neurosci Res. 2001;39:221–232. doi: 10.1016/s0168-0102(00)00218-2. [DOI] [PubMed] [Google Scholar]
- Hong ZY, Huang ZL, Qu WM, Eguchi N, Urade Y, Hayaishi O. An adenosine A2A receptor agonist induces sleep by increasing GABA release in the tuberomammillary nucleus to inhibit histaminergic systems in rats. J Neurochem. 2005;92:1542–1549. doi: 10.1111/j.1471-4159.2004.02991.x. [DOI] [PubMed] [Google Scholar]
- Hunt CE. Abnormal hypercarbic and hypoxic sleep arousal responses in near-miss SIDS infants. Pediatr Res. 1981;15:1462–1464. doi: 10.1203/00006450-198111000-00015. [DOI] [PubMed] [Google Scholar]
- Hunt CE. The cardiorespiratory control hypothesis for Sudden Infant Death Syndrome. Clinics Perinatol. 1992;19:757–771. [PubMed] [Google Scholar]
- Hunt CE, Brouillette RT, Hanson D. Theophylline improves pneumogram abnormalities in infants at risk for sudden infant death syndrome. J Pediatr. 1983;103:969–974. doi: 10.1016/s0022-3476(83)80734-3. [DOI] [PubMed] [Google Scholar]
- Inoue S, Murakami N. Unit responses in the medulla oblongata of rabbit to changes in local and cutaneous temperature. J Physiol. 1976;259:339–356. doi: 10.1113/jphysiol.1976.sp011469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Lipski J. Synaptic inputs to medullary respiratory neurons from superior laryngeal afferents in the cat. Brain Res. 1992;584:197–206. doi: 10.1016/0006-8993(92)90895-g. [DOI] [PubMed] [Google Scholar]
- Lawson EE. Recovery from central apnea: effect of stimulus duration and end-tidal CO2 partial pressure. J Appl Physiol. 1982;53:105–109. doi: 10.1152/jappl.1982.53.1.105. [DOI] [PubMed] [Google Scholar]
- Lee JC, Stoll BJ, Downing SE. Properties of the laryngeal chemoreflex in neonatal piglets. Am J Physiol. 1977;233:R30–R36. doi: 10.1152/ajpregu.1977.233.1.R30. [DOI] [PubMed] [Google Scholar]
- Leiter JC, Böhm I. Mechanisms of pathogenesis in the Sudden Infant Death Syndrome (SIDS) Respir Physiol Neurobiol. 2007;159:127–138. doi: 10.1016/j.resp.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Litmanovitz I, Dreshaj I, Miller MJ, Haxhiu MA, Martin RJ. Central chemosensitivity affects respiratory muscle responses to laryngeal stimulation in the piglet. J Appl Physiol. 1994;76:403–408. doi: 10.1152/jappl.1994.76.1.403. [DOI] [PubMed] [Google Scholar]
- Luna LG. Histopathologic Methods and Color Atlas of Special Stains and Tissue Artifacts. Gaithersburg: American Histolabs, Inc; 1992. [Google Scholar]
- Martin RJ, Wilson CG, Abu-Shaweesh JM, Haxhiu MA. Role of inhibitory neurotransmitter interactions in the pathogenesis of neonatal apnea: Implications for management. Semin Perinatol. 2004;28:273–278. doi: 10.1053/j.semperi.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Mayer CA, Haxhiu MA, Martin RJ, Wilson CG. Adenosine A2A receptors mediate GABAergic inhibition of respiration in rats. J Appl Physiol. 2006;100:91–97. doi: 10.1152/japplphysiol.00459.2005. [DOI] [PubMed] [Google Scholar]
- Merazzi D, Mortola JP. Effects of changes in ambient temperature on the Hering-Breuer reflex of the conscious newborn rat. Ped Res. 1999;45:370–376. doi: 10.1203/00006450-199903000-00014. [DOI] [PubMed] [Google Scholar]
- Miller AJ. Characterization of the postnatal development of superior laryngeal nerve fibers in the postnatal kitten. J Neurobiol. 1976;7:483–494. doi: 10.1002/neu.480070603. [DOI] [PubMed] [Google Scholar]
- Mitra J, Prabhakar NR, Haxhiu MA, Cherniack NS. The effects of hypercapnia and cooling the ventral medullary surface on capsaicin induced respiratory reflexes. Respir Physiol. 1985;60:377–385. doi: 10.1016/0034-5687(85)90065-9. [DOI] [PubMed] [Google Scholar]
- Niblock MM, Luce CJ, Belliveau RA, Paterson DS, Kelley ML, Sleeper LA, Filiano J, Kinney HC. Comparative anatomical assessment of the piglet as a model for the developing human medullary serotonergic system. Brain Res Rev. 2005;50:169–183. doi: 10.1016/j.brainresrev.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Ochi M, Koga K, Kurokawa M, Kase H, Nakamura JI, Kuwana Y. Systemic administration of adenosine A2A receptor antagonist reverses increased GABA release in the globus pallidus of unilateral 6-hydroxydopamine-lesioned rats: A microdialysis study. Neurosci. 2000;100:53–62. doi: 10.1016/s0306-4522(00)00250-5. [DOI] [PubMed] [Google Scholar]
- Patrickson JW, Smith TE, Zhou SS. Afferent projections of the superior and recurrent laryngeal nerves. Brain Res. 1991;539:169–174. doi: 10.1016/0006-8993(91)90702-w. [DOI] [PubMed] [Google Scholar]
- Perlstein PH, Edwards NK, Sutherland JM. Apnea in premature infants and incubator-air-temperature changes. N Engl J Med. 1970;282:461–466. doi: 10.1056/NEJM197002262820901. [DOI] [PubMed] [Google Scholar]
- Petrillo GA, Glass L, Trippenbach T. Phase locking of the respiratory rhythm in cats to a mechanical ventilator. Can J Physiol Pharmacol. 1983;61:599–607. doi: 10.1139/y83-092. [DOI] [PubMed] [Google Scholar]
- Phillis JW. Inhibitory action of CGS 21680 on cerebral cortical neurons is antagonized by bicuculline and picrotoxin - is GABA involved? Brain Res. 1998;807:193–198. doi: 10.1016/s0006-8993(98)00756-2. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Grunze HC, McCarley RW, Greene RW. Adenosine inhibition of mesopontine cholinergic neurons: implications for EEG arousal. Science. 1994;263:689–692. doi: 10.1126/science.8303279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmers JE, Richter DW, Ballantyne D, Bainton CR, Klein JP. Reflex prolongation of stage I of expiration. Pflugers Arch. 1986;407:190–198. doi: 10.1007/BF00580675. [DOI] [PubMed] [Google Scholar]
- Schmidt BJ, Roberts RS, Davis PB, Doyle LW, Barrington KJ, Ohisson A, Solimano A, Tin W. Caffeine therapy for apnea of prematurity. N Eng J Med. 2006;354:2112–2121. doi: 10.1056/NEJMoa054065. [DOI] [PubMed] [Google Scholar]
- Sun MH, Hildebrandt L, Curran AK, Darnall RA, Chen G, Filiano JJ. Potassium permanganate can mark the site of microdialysis in brain sections. J Histotech. 2000;23:151–154. [Google Scholar]
- Thach BT. Maturation and transformation of reflexes that protect the laryngeal airway from liquid aspiration from fetal to adult life. Am J Med. 2001;111:69S–77S. doi: 10.1016/s0002-9343(01)00860-9. [DOI] [PubMed] [Google Scholar]
- van der Velde L, Curran A, Filiano JJ, Darnall RA, Bartlett D, Jr, Leiter JC. Prolongation of the laryngeal chemoreflex after inhibition of the rostroventral medulla in piglets: A role in SIDS? J Appl Physiol. 2003;94:1883–1895. doi: 10.1152/japplphysiol.01103.2002. [DOI] [PubMed] [Google Scholar]
- Wilson CG, Martin RJ, Jaber M, Abu-Shaweesh JM, Jafri A, Haxhiu MA, Zaidi S. Adenosine A2A receptors interact with GABAergic pathways to modulate respiration in neonatal piglets. Respir Physiol Neurobiol. 2004;141:201–211. doi: 10.1016/j.resp.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Woodson GE, Brauel G. Arterial chemoreceptor influences on the laryngeal chemoreflex. Otolaryngol Head Neck Surg. 1992;107:775–782. doi: 10.1177/019459988910700612.1. [DOI] [PubMed] [Google Scholar]
- Xia L, Damon T, Niblock MM, Bartlett D, Jr, Leiter JC. Unilateral microdialysis of gabazine in the dorsal medulla reverses thermal prolongation of the laryngeal chemoreflex in decerebrate piglets. J Appl Physiol. 2007;103:1864–1872. doi: 10.1152/japplphysiol.00524.2007. [DOI] [PubMed] [Google Scholar]
- Xia L, Damon TA, Leiter JC, Bartlett D., Jr Focal warming of the nucleus of the solitary tract prolongs the laryngeal chemoreflex in decerebrate piglets. J Appl Physiol. 2006;102:54–62. doi: 10.1152/japplphysiol.00720.2006. [DOI] [PubMed] [Google Scholar]
- Xia L, Leiter JC, Bartlett D., Jr Laryngeal water receptors are insensitive to body temperature in neonatal piglets. Respir Physiol Neurobiol. 2005;150:82–86. doi: 10.1016/j.resp.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Xia L, Leiter JC, Bartlett D., Jr Laryngeal apnea in rat pups: effects of age and body temperature. J Appl Physiol. 2008;104:269–274. doi: 10.1152/japplphysiol.00721.2007. [DOI] [PubMed] [Google Scholar]
- Zaidi SIA, Jafri A, Martin RJ, Haxhui MA. Adenosine A2A receptors are expressed by GABAergic neurons of medulla oblongata in developing rat. Brain Res. 2006;1071:42–53. doi: 10.1016/j.brainres.2005.11.077. [DOI] [PubMed] [Google Scholar]