Introduction
Chronic fatigue syndrome (CFS) is characterized by profound fatigue lasting at least 6 months accompanied by disturbances of sleep, cognition, mood, musculoskeletal pain, and other symptoms (1). Insomnia and insufficient, nonrestorative sleep are among the most common and disabling symptoms (2–6). Clinic-based studies have found that patients with CFS often have poor sleep efficiency (5, 7–12) and, occasionally, intrinsic sleep disorders such as obstructive sleep apnea (2,5,7,8,13). These studies, however, have methodological differences and limitations including the absence of comparison groups (5,8,11,13), failure to include laboratory sleep data (6,14), use of in-home sleep studies (10,14), reporting of clinical sleep disorders without data on sleep architecture (2), and the inclusion of only a single laboratory night (2,5.8,10,12,13). Small, but rigorously conducted, studies have not provided strong evidence for striking abnormalities in sleep architecture among most patients with CFS (15,16). Thus, methodological differences, the lack of control for many genetic and environmental factors, and the inherent limitations of standard electroencephalogram (EEG) likely contribute to the inability to reproducibly detect differences in sleep microarchitecture between CFS and healthy control groups.
Quantitative EEG analysis procedures may be a more sensitive metric for evaluating sleep abnormalities in clinical populations than traditional manual sleep stage scoring (17,18). One study of sleep clinic patients with chronic fatigue demonstrated increased “slow delta” power and a higher cyclic alternating pattern (CAPs) rate in the CFS group (19), Increased alpha activity during sleep also has been inconsistently observed in fibromyalgia (20–25), a disorder closely related to CFS that is characterized by chronic, unexplained, widespread pain (26). The limited studies of quantitative sleep EEG in CFS or other related disorders provided a strong rationale for the present study.
Co-twin control studies offer a powerful alternative to traditional approaches that compare CFS patients to healthy or depressed individuals, while controlling for genetic and numerous environmental factors (27). This research design is particularly valuable in studies of sleep where genetic factors contribute substantially to sleep architecture (28), the number of data points generated is large, and the range of values observed in normal individuals is wide. We therefore compared the power spectral analysis of sleep EEGs between twins discordant for CFS to answer these questions: Does sleep architecture differ between twins with CFS and their non-affected co-twins and is there greater prevalence of alpha-activity phase-locked with delta in the twins with CFS?
Methods
Participants
From 1997 to 1999, 22 sets of CFS discordant twins from the University of Washington CFS Twin Registry were chosen for a 7-day in-person evaluation based on registry information and telephone screening establishing the presence or absence of symptoms consistent with the Centers for Disease Control (CDC) diagnostic criteria of CFS (1,15,29,30). Twins were required to (1) be at least 18 years of age; (2) be reared together; (3) be discordant for CFS (one twin met the CDC CFS criteria, the other did not); (4) be negative for HIV; (5) abstain from alcohol and caffeine and, based on their personal physicians’ advice, discontinue all medications at least 2 weeks prior to the evaluation; and (6) travel to Seattle together.
To determine if a twin met CDC CFS criteria, we used responses to the CFS symptom checklist, diagnoses generated by the Diagnostic Interview Schedule (Version III-A) (32), and information from review of the subject’s medical records. To meet criteria, debilitating fatigue must have been present for at least 6 months with endorsement of at least 4 of 8 CFS symptoms. Exclusionary medical and psychiatric conditions must have been absent. The same inclusion and exclusion criteria (e.g., body mass index, specific psychiatric disorders) and review processes were applied to the fatigued and non-fatigued twins. Medical records covering the last five years were reviewed by a physician knowledgeable about CFS (DB) for exclusionary medical conditions. A psychologist and infectious disease specialist also independently reviewed the twins’ medical charts to verify health status and approve twins for participation. Prior to the scheduled visit, we confirmed that the ill twin still met CFS criteria and that the control twin was devoid of CFS.
Between 2000 and 2003, the twins were contacted about participating in a follow up study. Of the 22 original pairs of twins, 14 agreed to participate in a second week-long evaluation. Written informed consent was obtained from all twins in accordance with regulations of the University of Washington Institutional Review Board. A waiver of consent was obtained from the University of Michigan Institutional Review Board to conduct the statistical analysis of the data at UM.
Depression was assessed using the Diagnostic Interview Schedule, a structured interview based on Diagnostic and Statistical Manual III (32). Monozygosity was initially determined using previously validated self-report methods (33–34), then confirmed with analysis of restriction fragment length polymorphisms. DNA samples were extracted and digested with the restriction endonuclease HaeIII. The restriction fragments were separated by molecular size in agarose gel, Southern blotted onto nylon membrane, and hybridized with a variable number of tandem repeat probes. With 6 probes, the probability of monozygosity can be ascertained with 99.9% certainty (35).
Each pair of twins spent 3 consecutive nights and 1 day in the University of Washington Sleep Research Laboratory in temperature controlled, sound attenuated rooms. All sleep recording equipment was located in a central control room separate from the individual sleeping rooms. Twins were instructed to follow a set sleep schedule for 1 week prior to coming to the laboratory based on an average of their nightly sleep schedule ascertained from a 2-week sleep diary. This schedule was adjusted for twins who traveled to Seattle from Eastern, Central and Mountain time zones.
Throughout the study, the Sleep Research Laboratory investigators and technicians were blind to the illness status of the twins. During the first night, the twins adapted to the laboratory; baseline sleep data from the second night are reported here. The third night was an experimental manipulation night reported elsewhere (37). The twins completed a 10-item post-sleep questionnaire each morning before getting out of bed.
Clinical Characteristics. Body mass index was computed from measured weight and height. Both history of and current major depression were assessed using the National Institute of Mental Health Diagnostic Interview Schedule. Depression was assessed using the Diagnostic Interview Schedule (Version III-A), (35), a structured interview based on Diagnostic and Statistical Manual III. Menopause status was ascertained by asking “To your knowledge, have you reached menopause?” The tenderpoint examination was performed according to the published diagnostic recommendations (36). Participants were considered to have fibromyalgia if they had ≥ 11 tender points and met criteria for widespread pain. Widespread pain was defined by the presence of upper and lower segment, right- and left-sided, and axial pain (36). The age of onset and duration of CFS were computed based on self-reported dates.
Polysomnography. EEG electrodes were positioned at 2 frontal (F7, F8), 2 central (C3, C4), and 2 occipital (O1, O2) locations (International 10–20 system of measurement) and were referenced to the contralateral mastoids. Chin electromyogram electrodes and electrodes for right and left electro-oculogram also were attached. To monitor the twins for sleep-disordered breathing, airflow was measured using a nasal pressure cannula placed in the nose (Pro-Tech Services, Inc. Mukilteo, WA). Chest and abdominal respiratory effort was measured by Piezo Respiratory Effort bands placed around the chest and abdomen (Pro-Tech Services, Inc. Mukilteo, WA). Oxygen saturation was measured from the left or right index finger by a pulse oximeter (EMBLA, Broomfield, CO). Snoring was assessed by a small microphone sensor (Pro-Tech Services, Inc. Mukilteo, WA) placed on the throat, just lateral to the trachea. Electromyogram electrodes were placed on the anterior tibialis of each leg to monitor the occurrence of periodic leg movements during sleep. Two electrodes were placed on the chest to measure the electrocardiogram, according to the modified Lead II configuration.
Electrophysiological signals were recorded and digitized by the EMBLA somnologica data acquisition recording system (A-Ay-101, EMBLA, Broomfield, CO) and displayed and stored on a desktop computer. The sampling rates were set as follows: EEG, electromyogram, periodic leg movements, and electrocardiogram data = 200 Hz; electro-oculogram signal and snoring sensor = 100 Hz; nasal airflow and respiratory effort = 20 Hz, and oximeter = 1 Hz. All digitized data were acquired and stored unfiltered. Prior to each recording session, a standard 50 microvolt, 10 Hz calibration signal was recorded for 5 minutes. Data were displayed in 30 second epochs, on a continuous basis during recording.
Sleep Stage Scoring
All channels of recorded data were displayed on a high-resolution 21-inch color monitor for visual sleep stage scoring. Filter settings for display were set at 0.3 Hz to 40 Hz. Sleep and wake stages were scored in 30 second epochs according to standard criteria (38). Key sleep architectural variables reported here include sleep latency to Stage 1 and sleep latency to Stage 2, time in bed (lights out to final arising), total sleep period (time in minutes from the first epoch of Stage 2 until final awakening), sleep efficiency (total sleep time/time in bed), sleep latency (time from lights out to first epoch of Stage 2 sleep), REM latency (time from sleep latency to first epoch of REM), time spent awake, and the percentage of NREM and REM sleep stages, expressed as a percentage of the sleep period time.
Power Spectral Analysis
On-board power spectral software from the EMBLA Somnologica data acquisition system was used to evaluate power in each of delta (0.5–3.9 Hz), theta (4.0–7.9 Hz), alpha (8.0–11.9 Hz), sigma (12.0–15.9 Hz), and beta (16.0–31.9 Hz) bands. The algorithm used a 512 point fast Fourier Transform with Hamming windows (−53 dB stop band, filter degree 1068, transition bandwidth 0.622 Hz), in 6 second blocks. The resultant power values, expressed in μV2, were then averaged in consecutive 30 second epochs in each frequency band to correspond to visual stage scoring to prepare for averaging data by sleep stage. In addition, relative power measures we also computed for each frequency band, expressed as a proportion of total power per epoch of sleep. Both raw EEG and power spectral data were inspected epoch by epoch for evidence of movement artifact. Epochs with high amplitude artifact were excluded from all EEG analyses. Only data from C3 electrodes are reported here. All-night power spectral data were plotted and inspected visually for evidence of alpha and delta power that were in phase across the night in each subject.
Statistical Analysis
Data were coded for CFS status, sleep stage (REM, Stage 1, 2 and combined Stage 3 and 4) and frequency band (delta through beta), which were used as repeated measures. MANOVAs evaluated potential statistical differences. Univariate analyses, contrasting twin pairs within each sleep stage, were only computed if a significant overall MANOVA effect was obtained. In addition, within-subject Pearson’s correlation coefficients were computed to evaluate the statistical relationship between all-night alpha and delta power by stage and across the whole night independent of sleep stage. These correlations were coded for twin pair and a within-subject ANOVA evaluated potential differences. Differences in demographic or clinical characteristics were compared between the CFS ill and non-CFS twins with t-tests of Chi-square statistics.
Results
Fourteen twin pairs completed the sleep study. Technical recording problems resulting in missing data occurred on the baseline sleep night in 1 pair of twins, leaving 13 pairs to compare. As shown in Table 1, the demographic and clinical characteristics did not differ between the CFS and non-CFS twins, except for mean number of tender points and the number of participants with fibromyalgia (p<.05). All twins were female, ranging from 29 to 60 years of age. A history of lifetime major depressive disorder was noted in 3 of the CFS twins and 2 of the non-CFS twins, not members of the same family. No CFS or non-CFS twin was currently depressed.
Table I.
Demographic and clinical characteristics of CFS and non-CFS twins
| Characteristic | CFS Twin n = 13 | Non-CFS Twin n = 13 |
|---|---|---|
| Current age, mean years (SD) | 45.4 (10.0) | 45.4 (10.0) |
| Married, number | 10 | 9 |
| High school completion, number | 7 | 9 |
| Body mass index, mean kg/m2 (SD) | 30.1 (7.2) | 29.7 (6.1) |
| Lifetime major depression, number | 3 | 2 |
| Current major depression, number | 0 | 0 |
| Menopause *, number | 6 | 4 |
| Tender points, mean number (SD)1 | 17.5 (1.0) | 10.8 (5.6) |
| Fibromyalgia**, number1 | 9 | 1 |
| Duration of CFS, years (SD) | 9.2 (3.7) | -- |
| Age at onset of CFS, mean years (SD) | 36.8 (3.7) | -- |
One non-CFS twin was missing menopause status;
Fibromyalgia defined as widespread pain and ≥11 tender points; SD = standard deviation;
p<.05
Polysomnography Analysis
As shown in Table 2, the twins did not differ on any of the key polysomnographic measures.
Table II.
Means and standard deviations of selected sleep variables in CFS and non-CFS twins
| Sleep Variable | CFS Twin | Non-CFS Twin |
|---|---|---|
| Total sleep time, minutes | 395.9 (53.4) | 403.9 (35.8) |
| Sleep latency to Stage 1, minutes | 4.5 (4.0) | 3.8 (4.5) |
| Sleep latency to Stage 2, minutes | 6.3 (5.8) | 5.0 (4.7) |
| REM latency, minutes | 75.3 (51.5) | 65.7 (27.0) |
| Stage 1, % | 9.1 (2.2) | 8.3 (3.7) |
| Stage 2, % | 35.2 (8.9) | 38.5 (8.1) |
| Slow wave sleep, % | 18.4 (4.5) | 20.0 (5.0) |
| REM, % | 24.2 (5.5) | 21.4 (5.8) |
| Sleep efficiency, % * | 85.1 (8.4) | 87.4 (8.0) |
| Time awake, minutes | 56.3 (29.8) | 52.4 (36.7) |
| Apnea-hypopnea index, events/hour | 7.0 (6.6) | 4.1 (3.7) |
Total sleep time/time in bed x 100%
Power Spectral Analysis
Table 3 documents only minor differences were observed in the power spectral data in each frequency by stage and by twin pair. MANOVA revealed a significant overall sleep stage main effect (F3,9 = 84.3, p < 0.0001), an EEG frequency band main effect (F4,8 = 872.6, p <. 00001) and sleep stage by frequency band interaction (F1,12 = 140.1, p < 0.0001). However, neither the twin pair main effect nor the interactions were significant, all producing F ratios < 1.0. No further analyses were conducted on spectral analysis by sleep stage.
Table III.
Average power in each frequency band by sleep stage and twin pair
| CFS Twin | Non-CFS Twin | |
|---|---|---|
| Power, μV2 | Mean (standard deviation) | Mean (standard deviation) |
| Stage 1 | ||
| Beta | 149.4 (29.2) | 140.5 (24.5) |
| Sigma | 122.5 (21.9) | 124.8 (30.1) |
| Alpha | 185.5 (35.5) | 199.7(88.6) |
| Theta | 272.3 (38.6) | 277.9 (72.5) |
| Delta | 530.7 (62.7) | 538.1 (108.8) |
| Stage 2 | ||
| Beta | 130.7 (25.3) | 127.8 (25.1) |
| Sigma | 166.9 (71.1) | 159.2 (51.9) |
| Alpha | 247.9 (87.5) | 247.3 (112.3) |
| Theta | 323.5 (39.2) | 318.2 (66.4) |
| Delta | 774.4 (79.6) | 759.7 (128.6) |
| Slow Wave Sleep | ||
| Beta | 119.8 (20.3) | 118.4 (22.4) |
| Sigma | 149.9 (59.8) | 145.3 (44.3) |
| Alpha | 272.3 (127.4) | 275.6 (125.6) |
| Theta | 375.6 (47.1) | 374.4 (71.0) |
| Delta | 1,276.4 (138.4) | 1,205.1 (331.4) |
| REM | ||
| Beta | 135.5 (31.2) | 128.7 (26.7) |
| Sigma | 95.5 (16.4) | 99.1 (19.4) |
| Alpha | 154.7 (25.7) | 158.1 (61.7) |
| Theta | 240.5 (35.3) | 243.1 (47.2) |
| Delta | 411.0 (55.5) | 397.2 (80.3) |
Relative Power
The statistical analysis of the power measures, expressed relative to total power also failed to produce significant twin pair differences. Less than 2 % difference separated the CFS ill twin from their non-CFS co-twin for relative power in beta, sigma, alpha and theta frequencies (range of probabilities: 0.18–0.93). As a result, data are not presented in tabular form and no further analyses were conducted on the relative power measures.
Alpha-Delta Relationships
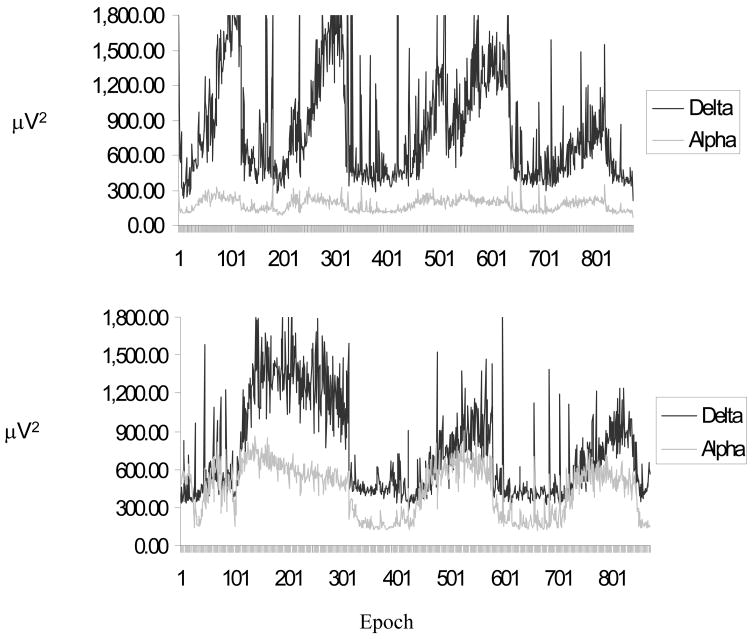
The first evaluation of the relationship between alpha and delta activity was based on visual inspection of the all-night power spectral data, independent of sleep stage. Alpha and delta power were plotted for each twin, examining both the power and phase relationship between these 2 EEG measures. Alpha and delta power appeared to oscillate in phase across the night in 5 of the 13 CFS twins (38.5%). However, the same pattern of in-phase alpha and delta power was seen in the identical twins without CFS in all but 1 twin pair. Thus, if alpha and delta were in-phase in the ill twin, they were also in-phase in their non-CFS twin. In the remaining 8 CFS twins, low levels of alpha power were visually detected with no apparent systematic relationship with delta power. Figure 1 illustrates all-night alpha and delta power in 2 twins with CFS, 1 without elevated alpha activity and 1 with alpha that is periodic both time and phase-locked with delta activity. However, the same pattern of in-phase alpha and delta power was seen in the identical twins without CFS in all but 1 twin pair. Thus, if alpha and delta were in-phase in the CFS ill twin, they were also in-phase in their unaffected co-twins.
Figure 1.
Example of a CFS twin without evidence of phasic alpha, coupled with delta [TOP] and a CFS twin with phasic alpha coupled with delta [BOTTOM], based on all-night power spectral analysis. Shown in successive 30 s epochs.
Pearson’s correlation coefficients confirmed our visual observations. Average correlations between alpha and delta frequencies were 0.79 ± 0.24 in the CFS twins and 0.71 ± 0.22 in the healthy twins. ANOVA did not identify a twin pair difference (F < 1.0). Alpha power was not correlated with the number of arousals in either the CFS or healthy twins, with correlations ranging from − 0.20 to 0.19. Finally, CFS twins with alpha and delta oscillations in phase by visual inspection had stronger correlations than those with low alpha power (r = 0.83 versus 0.58).
Discussion
Sleep Macroarchitecture
None of the polysomnographic measures distinguished the individuals with CFS from their unaffected identical twins. Both groups showed equivalent sleepiness and fell asleep in less than 10 minutes. The ill twins did show a higher apnea-hypopnea index, as reported previously (15,16), but this difference was not statistically significant. REM latency was longer in the twins with CFS, contrary to previous work (11). Once again, the between-group difference did not reach statistical significance in the present study.
Some of the sleep measures were surprising and do require comment. For example, the amount of Stage 2 sleep is considerably lower (38.5%) and the amount of slow-wave sleep considerably higher (20.0%) than what might be expected in a group of well twins with a mean age of 45 years (39). There is no obvious explanation for this outcome. However, the CFS ill twins and their unaffected twins had comparable PSG values. It would seem that sleep may be compromised to some degree in both the groups of twins but the co-twins are more resilient to developing CFS. Familial risk and resilience to disease has become a recent focus in depression and anxiety research (44). Perhaps this model is applicable to sleep and risk for CFS. Furthermore, the critical issue is the comparison of the CFS and non-CFS twin groups, and not necessarily the comparison of the non-CFS twin group to the general population. Importantly, both twins were exposed to the same protocol, PSG equipment, and PSG scoring/analysis. Therefore, the between twin comparison results are valid, despite the apparent discrepancy in some sleep measure when the non-CFS twins is compared to and large highly selected cohorts of healthy individuals (39).
PSA of Sleep EEG
In our co-twin study of CFS discordant monozygotic twins, CFS and non-CFS twins did not differ significantly on power spectral analysis of sleep EEG in REM, Stage 1, Stage 2 or Slow-Wave Sleep. One would expect lower delta power, increased fast frequency beta, or increased alpha power to accompany complaints of sleep disturbance in CFS. Delta power was slightly elevated in the CFS-ill twin, overall, but did not differ from their non-CFS twin. As discussed elsewhere, there was little evidence from objective sleep measures that CFS is associated with significant sleep disturbance (36). Further, relative power measures also failed to distinguish CFS from their unaffected co-twins with less than 2 % difference between them.
A recent paper by Guilleminault et al. (19) pointed toward increases in delta power and across the board reductions in relative power in other frequency bands in CFS subjects compared to non-related controls (19). There are a number of potential explanations for the differences between these findings and our own. First, the between group differences were very small for the relative power differences, 2–5% difference in other bands. Further, when looking at the Guilleminault power spectral analysis data in absolute terms, only delta power differed between controls and patients with CFS, minimizing the apparent differences with our results. Most importantly, we used a co-twin control design, perfectly matching for age and genetic aspects of sleep physiology, as opposed to the non-related “healthy” control group in the Guilleminault paper. This allows us to essentially eliminate genetic influences on sleep physiology. Therefore, discrepancies between studies could be due to differences in the handling of genetic confounds between the two studies.
Alpha-Delta Sleep
It has been suggested that CFS patients will show alpha-delta sleep (18) because of the overlap in symptoms and sleep complaints between fibromyalgia and CFS. We evaluated this relationship between the CFS ill and their unaffected co-twins. Neither total alpha or delta power nor the phase relationship between the two (i.e., alpha-delta sleep) distinguished between those with CFS and their identical non CFS twins. This is consistent with some studies showing alpha activity is not increased among patients with pain disorders (13,21,40).
Our results, however, are contrary to a body of work supporting decreased delta and increased alpha power and alpha-delta sleep in fibromyalgia (18). In particular, the increased alpha sleep is thought to contribute to the frequent sleep complaints reported by fibromyalgia patients, a hypothesis supported by the correlation between alpha activity and perception of shallow sleep (40). A recent study of older women with fibromyalgia identified 3 distinct patterns of alpha activity during sleep: phasic alpha activity that was coupled with an apparent in-phase relationship with delta (the classic alpha-delta pattern); tonic alpha continuous through NREM sleep, and an overall pattern of low alpha activity. Phasic alpha was evident in 50% of the fibromyalgia patients but only 7% of controls. By contrast, low overall alpha characterized over 80% of controls but only 30% of fibromyalgia patients. Despite the prevalence of comorbid CFS and fibroymalgia in the present study, there was no evidence of increased alpha activity or alpha-delta sleep in the CFS group.
In the present study, 5 subjects with CFS and 4/5 of their non-CFS twins showed enhanced alpha that oscillated in phase with delta activity (25). This observation provides little evidence that alpha-delta sleep is more prevalent in, or specific to, CFS. Because monozygotic co-twin control studies almost perfectly control for genetic factors, the enhanced alpha activity and its dynamic relationship with delta likely reflects heritable, disease-independent influences on sleep, as suggested earlier by polysomnography data (15). Further, the CFS and healthy twins were similar in measures of sleep fragmentation and number of arousals associated with enhanced alpha power.
Study Limitations
One study (19) has revealed increased quantitative sleep EEG abnormalities in CFS, in the form of cyclic alternating pattern (CAP), a physiological measure of NREM sleep thought to be associated with sleep disruption (41). Cyclic alternating patterns were increased in chronically fatigued patients from a sleep clinic. However, the comparison group was healthy control subjects who were nearly a decade younger (19). It is not clear to what degree age may have played a factor in the between-group differences. Nevertheless, the approach by the Stanford group provides a finer gradient analysis of the EEG and as such, may be more sensitive to subtle sleep EEG disturbances. The present study was limited by a 6 second sampling window for the PSA. This is substantially longer than standard practice (43) and could have contributed to the lack of difference between CFS ill and non-CFS twins. Unfortunately, this limitation was imposed by the EMBLA software. We are currently conducting a more detailed PSA analysis of the original sleep study in these twins to determine if between group differences are greater with a narrower sampling window.
There is an additional limitation to the present study. We attempted to adjust for circadian factors by both stabilizing the participant’s sleep schedule prior to traveling to Seattle and adjusting the study sleep schedule to the time zone of their residence. Nevertheless, the circadian effects of being exposed to a different light-dark cycle, as well as jet-lag itself, may have affected our results.
Despite these limitations, we view the inclusion of the identical but unaffected twin as a control group to be a significant strength over previous studies. Our results do suggest that heritability of quantitative sleep EEG characteristics may be stronger than CFS-related factors under baseline conditions. As demonstrated in a recent publication, sleep paradigms that challenge the adaptive response may be needed to bring out differences between individuals with CFS and comparison groups, including their non-CFS twins (37). Further, it may be that some aspects of sleep are abnormal in both ill and non-CFS co-twins, reflecting a familial vulnerability but that the non-CFS twin developed greater resilience. Understanding the role that sleep may play in vulnerability and resilience to disease is an important next step.
Conclusion
In conclusion, we did not find evidence of sleep macro- or micro-architectural changes in our study of twins discordant for CFS. This finding suggests that power spectral characteristics of sleep EEG do not differentiate twins with CFS from their unaffected co-twins. Thus, the sleep measures cannot explain their chronic disabling fatigue. Studies that challenge sleep regulation in patients with CFS have demonstrated abnormalities in the dissipation of delta activity during NREM sleep and thus may be more likely to elicit between-group differences than a standard baseline sleep assessment (36). The cyclic alternating pattern also may be affected by fatiguing illnesses (19), an intriguing finding that should be followed up in well controlled studies of persons with CFS. Taken together, these findings underscore the need to continue to seek novel ways to understanding the mechanisms underlying the sleep disturbances in CFS.
Acknowledgments
This research was supported by NIH grant U19AI38429 (Drs. Buchwald and Goldberg) and NR04011 (Center on Women’s Health Gender Research; Drs. Landis and Lentz). We are also grateful for the time and effort given by the twins who participated in this study and to Joan Stephens for secretarial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fukuda K, Staus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. International chronic fatigue syndrome case definition study group. Ann Int Med. 1994;121:953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 2.Buchwald D, Pascualy R, Bombardier C, Kith P. Sleep disorders in patients with chronic fatigue. Clin Infect Dis. 1994;18(suppl 1):S68–S72. doi: 10.1093/clinids/18.supplement_1.s68. [DOI] [PubMed] [Google Scholar]
- 3.Komaroff AL, Buchwald D. Symptoms and signs of chronic fatigue syndrome. Rev Infect Dis. 1991;13:S8–S11. doi: 10.1093/clinids/13.supplement_1.s8. [DOI] [PubMed] [Google Scholar]
- 4.Komaroff AL, Buchwald DS. Chronic fatigue syndrome: An update. In: Coggins C, Hancock E, Levitt L, editors. Annual Review of Medicine. Palo Alto, CA: 1998. pp. 1–13. [DOI] [PubMed] [Google Scholar]
- 5.Krupp LB, Jandorf L, Coyle PK, Mendelson WB. Sleep disturbance in chronic fatigue syndrome. J Psychosom Res. 1993;37:325–331. doi: 10.1016/0022-3999(93)90134-2. [DOI] [PubMed] [Google Scholar]
- 6.Morriss RK, Wearden AJ, Battersby L. The relation of sleep difficulties to fatigue, mood and disability in chronic fatigue syndrome. J Psychosom Res. 1997;42:597–605. doi: 10.1016/s0022-3999(97)89895-9. [DOI] [PubMed] [Google Scholar]
- 7.Whelton CL, Salit I, Moldofsky H. Sleep, Epstein-Barr virus infection, musculoskeletal pain, and depressive symptoms in chronic fatigue syndrome. J Rheumatol. 1992;19:939–943. [PubMed] [Google Scholar]
- 8.Krupp LB, Mendelson WB. Sleep disorders in chronic fatigue syndrome. In: Horne J, editor. Sleep ‘90. Bochum: Pontenagel Press; pp. 261–263. [Google Scholar]
- 9.Zubieta JK, Demitrack MA, Shipley JE, Engleberg ND, Eiser A, Douglas A. Sleep EEG in chronic fatigue syndrome: comparison with major depression. Bio Psychiatry. 1993;33:73A. [Google Scholar]
- 10.Morriss R, Sharpe M, Sharpley AL, Cowen P, Hawton K, Morris J. Abnormalities of sleep in patients with the chronic fatigue syndrome. Br J Med. 1993;306:1161–1164. doi: 10.1136/bmj.306.6886.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morehouse RL, Flanigan M, MacDonald DD, Braha D, Shapiro C. Depression and short REM latency in subjects with chronic fatigue syndrome. Psychosom Med. 1998;60:347–351. doi: 10.1097/00006842-199805000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Sharpley A, Clements A, Hawton K, Sharpe M. Do patients with “pure” chronic fatigue syndrome (neurasthenia) have abnormal sleep? Psychosom Med. 1997;59:592–596. doi: 10.1097/00006842-199711000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Manu P, Lane TJ, Matthews DA, Castriotta RJ, Watson RK, Abeles M. Alpha-delta sleep in patients with a chief complaint of chronic fatigue. South Med J. 1994;87(4):465–470. doi: 10.1097/00007611-199404000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Stores G, Fry A, Crawford C. Sleep abnormalities demonstrated by home polysomnography in teenagers with chronic fatigue syndrome. J Psychosom Res. 1998;45(1):85–91. doi: 10.1016/s0022-3999(98)00024-5. [DOI] [PubMed] [Google Scholar]
- 15.Ball N, Buchwald DS, Schmidt D, Goldberg J, Ashton S, Armitage R. Monozygotic twins discordant for chronic fatigue syndrome: objective measures of sleep. J Psychosom Res. 2004;56:207–212. doi: 10.1016/S0022-3999(03)00598-1. [DOI] [PubMed] [Google Scholar]
- 16.Reeves WC, Hein C, Maloney EM, Youngblood LS, Unger ER, Decker MJ. Sleep characteristics of persons with chronic fatigue syndrome and non-fatigued controls: results from a population-based study. BMC Neurol. 2006;6:41. doi: 10.1186/1471-2377-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armitage R. Microarchitectural findings in sleep EEG in depression: diagnostic implications. Biol Psychiatry. 1995;37:72–84. doi: 10.1016/0006-3223(94)00082-E. [DOI] [PubMed] [Google Scholar]
- 18.Moldofsky H. Sleep and pain. Sleep Med Rev. 2001;5:387–398. doi: 10.1053/smrv.2001.0179. [DOI] [PubMed] [Google Scholar]
- 19.Guilleminault C, Poyares D, Rosa A, Kirisolglu C, Almeida T, Lopes MC. Chronic fatigue, unrefreshing sleep and nocturnal polysomnography. Sleep Med. 2006;7:513–520. doi: 10.1016/j.sleep.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Landis CA, Lentz MJ, Rothermel J, Buchwald D, Shaver JL. Decreased sleep spindles and spindle activity in midlife women with fibromyalgia and pain. Sleep. 2004;27(4):741–50. doi: 10.1093/sleep/27.4.741. [DOI] [PubMed] [Google Scholar]
- 21.Lentz MJ, Landis CA, Rothermel J, Shaver JL. Effects of selective slow wave sleep disruption on musculoskeletal pain and fatigue in middle aged women. J Rheumatol. 1999;26:1586–1592. [PubMed] [Google Scholar]
- 22.Branco J, Atalaia A, Paiva T. Sleep cycles and alpha-delta sleep in fibromyalgia syndrome. J Rheumatol. 1994;21:1113–1117. [PubMed] [Google Scholar]
- 23.Horne JA, Shackell BS. Alpha-like EEG activity in non-REM sleep and the fibromyalgia (fibrositis) syndrome. Electroencephalogr Clin Neurophysiol. 1991;79:271–276. doi: 10.1016/0013-4694(91)90122-k. [DOI] [PubMed] [Google Scholar]
- 24.Moldofsky H, Scarisbrick P, England R, Smythe H. Musculoskeletal symptoms and non-REM sleep disturbance in patients with “fibrositis syndrome” and healthy subjects. Psychosom Med. 1975;37:341–351. doi: 10.1097/00006842-197507000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Roizenblatt S, Moldofsky H, Benedito-Silva AA, Tufik S. Alpha sleep characteristics in fibromyalgia. Arthritis and Rheumatism. 2001;44:222–230. doi: 10.1002/1529-0131(200101)44:1<222::AID-ANR29>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 26.Wallace DJ. The fibromyalgia syndrome. Ann Med. 1997;29(1):9–21. doi: 10.3109/07853899708998739. [DOI] [PubMed] [Google Scholar]
- 27.Hubrec Z, Robinette CD. The study of human twins in medical research. NEJM. 1984;310:435–441. doi: 10.1056/NEJM198402163100706. [DOI] [PubMed] [Google Scholar]
- 28.Linkowski P. EEG sleep patterns in twins. J Sleep Res. 1999;8(suppl 1):11–13. doi: 10.1046/j.1365-2869.1999.00002.x. [DOI] [PubMed] [Google Scholar]
- 29.Watson NF, Jacobsen C, Goldberg J, Kapur V, Buchwald D. Subjective and objective sleepiness in monozygotic twins discordant for chronic fatigue syndrome. Sleep. 2004;27(5):973–7. doi: 10.1093/sleep/27.5.973. [DOI] [PubMed] [Google Scholar]
- 30.Watson NF, Kapur V, Arguelles LM, Goldberg J, Schmidt DF, Armitage R, Buchwald D. Comparison of subjective and objective measures of insomnia in monozygotic twins discordant for chronic fatigue syndrome. Sleep. 2003;26(3):324–328. doi: 10.1093/sleep/26.3.324. [DOI] [PubMed] [Google Scholar]
- 31.Buchwald D, Herrell R, Ashton S, Belcourt M, Schmaling K, Goldberg J. The Chronic Fatigue Twin Registry: method of construction, composition, and zygosity assignment. Twin Res. 1999;2:203–211. doi: 10.1375/136905299320565870. [DOI] [PubMed] [Google Scholar]
- 32.Eisen S, Neuman R, Goldberg J, Rice J, True W. Determining zygosity in the Vietnam era twin registry: an approach using questionnaires. Clin Genet. 1989;35:423–432. doi: 10.1111/j.1399-0004.1989.tb02967.x. [DOI] [PubMed] [Google Scholar]
- 33.Torgersen S. The determination of twin zygosity by means of a mailed questionnaire. Acta Genet Med Gemellology (Roma) 1979;28:225–236. doi: 10.1017/s0001566000009077. [DOI] [PubMed] [Google Scholar]
- 34.Keith L, Machin G. Zygosity testing: current status and evolving issues. J Reprod Med. 1997;42:699–707. [PubMed] [Google Scholar]
- 35.Robins LN, Helzer JE, Ratcliff KS, Seyfreid W. Diagnostic Interview Schedule (DIS): Version III-A. St. Louis, Department of Psychiatry; Washington University School of Medicine: 1985. [Google Scholar]
- 36.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33(2):160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 37.Armitage R, Landis C, Hoffmann R, Lentz M, Watson NF, Goldberg J, Buchwald D. The impact of a 4-hour sleep delay on slow wave activity in twins discordant for chronic fatigue syndrome. Sleep. 2007;30(5):657–62. doi: 10.1093/sleep/30.5.657. [DOI] [PubMed] [Google Scholar]
- 38.Rechtschaffen A, Kales A. In: A Manual of Standardized Terminology, Techniques and Scoring System For Sleep Stages of Human Subjects. Berger RJ, Dement WC, Jacobson A, Johnson LC, Jouvet M, Monroe LJ, Oswald I, Roffwarg HP, Roth B, Walter RD, editors. Public Health Service, U.S. Government Printing Office; Washington DC: 1968. [Google Scholar]
- 39.Walsleben JA, Kapur VK, Newman AB, Shahar E, Bootzin RR, Rosenberg CE, Connor G, Nieto FJ. Sleep and reported daytime sleepiness in normal subjects: the Sleep Heart Health Study. Sleep. 2004 Mar 15;27(2):293–8. doi: 10.1093/sleep/27.2.293. [DOI] [PubMed] [Google Scholar]
- 40.Drewes AM, Nielsen BM, Taagholt SJ, Bjerregard K, Sevendsen L, Gade J. Sleep intensity in fibromyalgia: focus on the microstructure of the sleep process. Br J Rheumatol. 1995;34(7):629–35. doi: 10.1093/rheumatology/34.7.629. [DOI] [PubMed] [Google Scholar]
- 41.Ferre A, Guilleminault C, Lopes MC. Cyclic alternating pattern as a sign of brain instability during sleep. Neurologia. 2006;21(6):304–11. [PubMed] [Google Scholar]
- 42.Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: The behavioral modeal and a neurocognitive perspective. J Sleep Res. 1997;6:179–188. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- 43.Armitage R, Hoffmann R, Fitch T, Trivedi M, Rush AJ. Temporal characteristics of delta activity during NREM sleep in depressed outpatients and healthy adults: group and sex effects. Sleep. 2000;23(5):607–617. [PubMed] [Google Scholar]
- 44.Conner KM, Zhang W. Recent advances in the understanding and treatment of anxiety disorders. Resilience: determinants, measurement, and treatment responsiveness. CNS Spectr. 2006;10(Suppl 12):5–12. doi: 10.1017/s1092852900025797. [DOI] [PubMed] [Google Scholar]