Abstract
The current study estimates the longitudinal effects of severity of prenatal cocaine exposure on language functioning in an urban sample of full-term African-American children (200 cocaine-exposed, 176 noncocaine-exposed) through age 7 years. The Miami Prenatal Cocaine Study sample was enrolled prospectively at birth, with documentation of prenatal drug exposure status through maternal interview and toxicology assays of maternal and infant urine and infant meconium. Language functioning was measured at ages 3 and 5 years using the Clinical Evaluation of Language Fundamentals–Preschool (CELF-P) and at age 7 years using the Core Language Domain of the NEPSY: A Developmental Neuropsychological Assessment. Longitudinal latent growth curve analyses were used to examine two components of language functioning, a more stable aptitude for language performance and a time-varying trajectory of language development, across the three time points and their relationship to varying levels of prenatal cocaine exposure. Severity of prenatal cocaine exposure was characterized using a latent construct combining maternal self-report of cocaine use during pregnancy by trimesters and maternal and infant bioassays, allowing all available information to be taken into account. The association between severity of exposure and language functioning was examined within a model including factors for fetal growth, gestational age, and IQ as intercorrelated response variables and child’s age, gender, and prenatal alcohol, tobacco, and marijuana exposure as covariates. Results indicated that greater severity of prenatal cocaine exposure was associated with greater deficits within the more stable aptitude for language performance (D = −0.071, 95% CI = −0.133, −0.009; p = 0.026). There was no relationship between severity of prenatal cocaine exposure and the time-varying trajectory of language development. The observed cocaine-associated deficit was independent of multiple alternative suspected sources of variation in language performance, including other potential responses to prenatal cocaine exposure, such as child’s intellectual functioning, and other birth and postnatal influences, including language stimulation in the home environment.
Keywords: Prenatal cocaine exposure, Language performance
INTRODUCTION
In the late 1980s and early 1990s, the United States experienced an “epidemic” of cocaine-exposed deliveries due to the burgeoning numbers of women of child-bearing age who were using cocaine and crack cocaine. The phenomenon of “cocaine abuse” by pregnant women received widespread media attention due to observations of serious, life-endangering consequences for the mother and fetus (Burkett et al., 1990; Critchley et al., 1988; Henderson and Torbey, 1988; Mercado et al., 1989; Morild and Stajic, 1990). Known catastrophic cardiovascular and central nervous system effects of cocaine use in adults (Cregler and Mark, 1986; Farrar and Kearns, 1989; Gawin and Ellinwood, 1988; Tarr and Macklin, 1987) clearly raised concerns that maternal use of cocaine, especially crack cocaine, would cause long-term, irreparable damage to the developing infant and young child. However, numerous limitations in study design among the earliest published studies on the effects of in utero cocaine exposure have been noted and results have been inconsistent for most aspects of infant and child developmental outcomes (Bandstra and Burkett, 1991; Carmichael Olson and Toth, 1999; Frank et al., 2001). In an attempt to curtail inappropriate labeling of cocaine-exposed children, perinatal “drug abuse” researchers urged the application of scientifically rigorous prospective study designs with sufficient samples to answer the analytic questions (Mayes et al., 1992; Zuckerman and Frank, 1992a).
Early language development is one of the major domains of investigative interest in perinatal “drug abuse” research since it may impair long-term social adaptation and academic success (Donahue, 1986; Tallal, 1988). Cocaine may affect language development through alterations in the developing monoaminergic neurotransmitter systems (Mayes, 1994, 1999) and through indirect pathways related to cocaine-associated maternal hypertension, decreased uterine blood flow, fetal vasoconstriction and hypoxemia (Mayes, 1994; Moore et al., 1986; Volpe, 1992; Zuckerman and Frank, 1992b) and nutritional deficiencies (Church et al., 1998; Frank et al., 1990; Jacobson et al., 1994; Zuckerman et al., 1989).
Early language development may also be affected by maternal cocaine use by “three different but potentially interactive” pathways, hypothesized by Malakoff and colleagues as follows: (1) subtle disregulation of attentional systems with potential for disrupting an infant’s ability to extract and process available linguistic information, (2) disruptions in parental-child linguistic interactions due to cocaine and other drug use, and (3) impoverished, unstable, and endangering social and caregiving environments (Malakoff et al., 1999).
In a recent systematic review, Frank et al. (2001), reported that “there is no evidence that prenatal cocaine exposure is associated with developmental effects that are different in severity, scope, or kind from the sequelae of multiple other risk factors. Many findings once thought to be specific effects of in utero cocaine exposure are correlated with other factors, including prenatal exposure to tobacco, marijuana, or alcohol, and the quality of the child’s environment.” Their conclusion with respect to language functioning, i.e., that there were no differences in standardized language test results through age 3 years, was based on then available published studies meeting stringent review criteria (Bland-Stewart et al., 1998; Hurt et al., 1997; Kilbride et al., 2000). Subsequently, we reported language findings from the Miami Prenatal Cocaine Study, a longitudinal study of the neurodevelopmental outcome of in utero cocaine exposure conducted in a large sample of urban African-American children, prospectively recruited and followed since birth (Bandstra et al., 2002; Morrow et al., 2003). In these reports, we presented longitudinal evidence demonstrating that prenatally cocaine-exposed children performed more poorly than noncocaine-exposed children on standardized tests of total language performance from infancy through age 3 years (Morrow et al., 2003) and from age 3 through 7 years (Bandstra et al., 2002). Using generalized estimating equation modeling under a general linear model, there was an observed association between prenatal cocaine exposure, expressed dichotomously (yes/no), and deficits in total language functioning after statistically controlling for child sex, visit age, prenatal exposure to other drugs, and other key suspected determinants of language functioning.
The objective of the present investigation was to estimate the influence of severity of prenatal cocaine exposure on the longitudinal development of language functioning in a large sample of full-term African-American children enrolled prospectively at birth and residing in urban neighborhoods. Within the context of the Miami Prenatal Cocaine Study, we sharpen the focus on the issue of cocaine-associated language deficits by shifting the approach used in our prior work (Bandstra et al., 2002; Morrow et al., 2003) to the method of longitudinal latent growth curve (LLG) analysis, thus permitting evaluation of the degree to which aptitude and growth trajectories of language performance might depend upon severity of prenatal cocaine exposure. In the LLG model, each child has a relatively stable aptitude to perform well in verbal and language tasks, expressed each time language performance is tested. Separately, individual children differ in their development or growth of language performance over time, with some demonstrating a steep slope or trajectory and others demonstrating a shallower slope or trajectory of development during the preschool and early childhood years. The LLG allows the variation in language development to be partitioned into these two separate components, while also evaluating whether observed cocaine–language relationships are independent of variables such as other prenatal drug exposures, maternal and infant birth parameters, psychosocial characteristics of the primary caregiver and child at each follow-up visit, and language stimulation in the home environment. Various forms of growth curve analyses have been used previously to model the development of speech articulation (Burchinal and Appelbaum, 1991), verbal reasoning (Smith et al., 2000), and grammatical complexity (Caselli et al., 1999).
METHODS
Study Design
The overall study design for the longitudinal Miami Prenatal Cocaine Study, briefly summarized below, has been extensively detailed in an earlier report (Bandstra et al., 2001b). As part of a larger epidemiological study, 476 mother-infant dyads were enrolled prospectively into a follow-up cohort to evaluate the effects of prenatal cocaine exposure on long-term developmental outcome. Subjects were recruited from the delivery service of the University of Miami School of Medicine Jackson Memorial Medical Center (UM-JMMC). Research staff asked postpartum women for their consent to participate in a study assessing the effect of maternal health habits and lifestyle during pregnancy on infant outcomes. They were informed that they would be asked to answer questions about their use of various medications, tobacco, alcohol, and drugs as well as give permission for drug tests for themselves and their infants. The study was approved by the Institutional Review Board and conducted under a federal Department of Health and Human Services Certificate of Confidentiality.
The current report focuses upon the hypothesized impact of severity of prenatal cocaine exposure on indicators of language functioning at 3, 5, and 7 years. A total of 376 children (200 cocaine-exposed and 176 noncocaine-exposed) from the original follow-up cohort of 476 (79%) completed all three language measures and were included in the present investigation.
Study Participants
The original follow-up sample of 476 infants was recruited between November 1990 and July 1993 during the immediate postnatal period. The study sample was homogeneous with regard to full-term gestational age (≥37 completed weeks), low socioeconomic status, inner-city residence, and African-American race/ethnicity. Exclusion criteria included: maternal HIV/AIDS; prenatal exposure to opiates, methadone, amphetamines, barbiturates, benzodiazepines, or phencyclidine; major congenital malformation; chromosomal aberration; or disseminated congenital infection. Prenatal cocaine exposure was determined by maternal self-report of cocaine use during pregnancy and/or by positive assay on one or more biological markers, including maternal urine, infant urine, and meconium.
The original follow-up sample consisted of 253 cocaine-exposed (with or without concomitant use of alcohol, tobacco, or marijuana) and 223 noncocaine-exposed infants, of whom 147 were drug-free and 76 were exposed to varying combinations of alcohol, tobacco, or marijuana. Criteria for inclusion in the prenatal cocaine exposure group included maternal self-report of cocaine use during pregnancy and/or at least one gas chromatography/mass spectrometry (GC/MS)-confirmed cocaine-positive biological marker, including maternal urine, infant urine, and meconium. Of the 253 identified cocaine-exposed infants in the original follow-up cohort, 40 cases (16%) were identified as cocaine-exposed based solely on positive maternal self-report. Conversely, in 80 cases (32%), the mother denied cocaine use during pregnancy but one or more biological markers were cocaine-positive. Criteria for inclusion in the prenatal noncocaine-exposed group included infants exposed to varying combinations of alcohol, tobacco, or marijuana (ATM) or drug-free infants. Infants characterized as ATM-exposed had mothers with a positive self-report for alcohol, tobacco, and/or marijuana use during pregnancy and/or a positive biological marker for marijuana, as well as a negative self-report and toxicology results for cocaine. Infants characterized as drug-free had mothers with a negative lifetime history for cocaine use, a negative self-report during and 3 months prior to pregnancy, and negative toxicology results on all specimens.
Data Collection Procedures
During the immediate postpartum period, experienced research staff performed a standardized research interview and organized the collection of the biological specimens for analysis. Trained research personnel, blinded to exposure status, performed the Ballard gestational age assessment (Ballard et al., 1979) within 36 hours of delivery, and obtained occipital-frontal head circumference and recumbent crown-heel birth length. Pertinent medical and demographic data were collected from the hospital record at birth. Caregiver and child data relevant to the present study were drawn from caregiver interviews and child neuro-development assessments conducted at 3, 5, and 7 years of age. Examiners blinded to substance exposure status performed all child assessments.
Drug Exposure Measures at Birth
Maternal Interview
A structured standardized postpartum interview to ascertain maternal substance use and additional demographic information was conducted by separate research staff distinct from the child assessment examiners. To enhance timeline recall, targeted recall periods were outlined and anchored to important calendar dates. Drug use questions during pregnancy were asked by trimester and included number of weeks used, most days per week, fewest days per week, usual number of days per week, and usual dose per day.
Creation of Prenatal Substance Exposure Variables
Dosage was measured in number of cigarettes smoked; number of marijuana joints smoked; number of drinks of beer, wine, or hard liquor; and number of cocaine lines/rocks, recorded in increments of usual daily dose, usual number of days per week, and number of weeks used. Standardized definitions (Schneiderman, 1990) were used for determining the one-drink unit for each type of alcohol (beer 12 oz., wine 5 oz., and liquor 1.5 oz.). Pregnancy exposure composites were calculated for each drug by multiplying the number of weeks used by the usual number of days per week and the usual dose per day. The total pregnancy self-report composites are presented descriptively in Table 1 to reflect the median cohort exposure levels based on self-report data. Pregnancy exposure composites for alcohol, tobacco, and marijuana were included in all analysis models.
Table 1.
Maternal self-reported alcohol, tobacco, marijuana, and cocaine use during pregnancy (n = 376).
| Noncocaine-exposed (n = 176) |
Cocaine-exposed (n = 200) |
|||
|---|---|---|---|---|
| Substance | Median (Min, Max)a | % (n) | Median (Min, Max)a | % (n) |
| Alcohol (# drinks) | 61 (2, 1680) | 31 (54) | 102 (1, 3823) | 67 (134) |
| Tobacco (# cigarettes) | 1008 (21, 5880) | 16 (29) | 2058 (1, 8820) | 78 (156) |
| Marijuana (# joints) | 32 (1, 807) | 12 (21) | 28 (1, 1229) | 46 (93) |
| Cocaine/crack (# lines/rocks) | 136 (1, 19320) | 69 (139) | ||
Median values based only on mothers reporting usage, calculated using total exposure composites: (# weeks used) × (usual number of days per week) × (usual dose per day).
Biological Markers (Urine and Meconium)
Screening of urine and meconium for cocaine metabolite (benzoylecgonine) was performed by EMIT® (Syva D.A.U.), at a cut-off of 150 ng/mL urine and 150 ng/gm meconium, respectively, and cocaine-positive specimens were confirmed by GC/MS (Mulé and Casella, 1988). Urine specimens were also assayed by EMIT® for marijuana (cannabinoids), opiates, amphetamines, barbiturates, benzodiazepines, and phencyclidine. Meconium specimens were also assayed by EMIT for marijuana and opiates. Of the total sample, 100% had at least one biological marker, 96% had at least two biological markers, and 68% had all three biological markers.
Child Measures
Language Functioning Measures
The Clinical Evaluation of Language Fundamentals–Preschool (CELF-P) (Wiig et al., 1992), conducted at the age 3- and 5-year research visits, is an individually administered test of expressive and receptive language ability for children ages 3 through 6 years. The test yields standard scores for three receptive subtests (Linguistic Concepts, Sentence Structure, and Basic Concepts) and three expressive subtests (Recalling Sentences in Context, Formulating Labels, and Word Structure), and a composite Total Language score. The CELF-P was standardized on 800 preschoolers, representative of the U.S. population with regard to gender, race/ethnicity, parent education level, and geographical region. Internal consistency estimates for the three composite scores ranged from 0.73 to 0.96 across age groups, and test-retest coefficients for 2- to 4-week intervals were generally high (i.e., 0.87–0.97). The CELF-P has been shown to correlate with the Preschool Language Scale–3 (0.90) and with the Verbal IQ Scale of the Wechsler Preschool and Primary Scale of Intelligence–Revised (0.72). The CELF-P also discriminates with reasonable accuracy (i.e., 71–74%) between children with and without diagnosed language disorders.
The language core domain of the NEPSY: A Developmental Neuropsychological Assessment (Korkman et al., 1997), age-standardized and normed for children aged 3–12, was administered at age 7 years. The NEPSY is an individually administered test of neuropsychological development which measures skills in the core domains of attention/executive functions, language, sensorimotor functioning, visuospatial processing, and memory and learning. The Language Core Domain, the focus of the present study, is comprised of three core subtests (Phonological Processing, Speeded Naming, and Comprehension of Instructions) and a composite standard score for Total Language. The NEPSY was standardized with a stratified sample of 1000 children, ranging from 3 to 12 years old, that was representative of the U.S. population with respect to gender, race/ethnicity, geographic region, and parent education level. The Total Language Core Domain score was shown to correlate well with the Verbal IQ score on the Wechsler Intelligence Scale for Children–Third Edition (0.62) and to discriminate significantly between children with and without diagnosed language disorders. Internal consistency estimates for the Total Language Core Domain score ranged from 0.87 to 0.90 across age groups, and test-retest coefficients for 2- to 10-week intervals ranged from 0.73 to 0.78.
Intellectual Functioning Measures
To control for the potential confounding of cognitive ability on language performance, age-appropriate standardized measures of intellectual functioning were used as covariates in the LLG model. The McCarthy Scales of Children’s Abilities (McCarthy, 1972), an assessment of cognitive and motor development in children ages 2 through 8 years with established reliability and validity, was used as an overall measure of child cognitive functioning at age 3 years. The McCarthy General Cognitive Index (GCI) (standardized mean 100, standard deviation 16) is comprised of the Verbal, Perceptual-Performance, and Quantitative scales. At age 5 years, the Full Scale IQ Score (mean 100, standard deviation 15) of the Wechsler Scale of Preschool and Primary Intelligence–Revised (WPPSI-R) (Wechsler, 1989) was used in the analyses. At the 7-year assessment visit, a short form of the Wechsler Intelligence Scale for Children-III (WISC-III) (Wechsler, 1991) was given. The WISC-III Short Form included the following subtests: Information, Similarities, Vocabulary, Picture Arrangement, and Block Design. An estimated Full Scale IQ Score (mean 100, standard deviation 15) was calculated from these five subtests based on the method developed by Sattler (Sattler, 1992) who reported an internal reliability coefficient of 0.94 and a validity coefficient of 0.90, representing the relationship between the Short Form and the Full Form of the WISC-III.
Behavioral Audiometry
Hearing was assessed at age 3 using play audiometry techniques for children sufficiently mature to respond appropriately to sound stimulation through earphones. For children who were unable to complete the play audiometry task, visual reinforcement audiometry in the sound field was used to obtain reasonable estimates of hearing sensitivity in at least the better ear. All testing and interpretations were performed at the University of Miami Mailman Center for Child Development by a licensed, certified pediatric audiologist. For the purposes of the current longitudinal total language analyses, behavioral audiometry results were coded as normal in at least one ear or bilaterally abnormal. Minimum response levels of 30 dB were considered abnormal.
Blood Lead Levels
At the 3- and 5-year follow-up visits, screening lead levels were performed by capillary sample and processed at the State of Florida Department of Health Laboratory. Abnormal capillary lead levels, i.e., ≥10 μg/dL, were confirmed by repeat specimen obtained by venipuncture. The higher blood level at either the 3- or 5-year visit was categorized dichotomously as <10 and ≥10 μg/dL and used as a single composite covariate in the LLG analyses for this report.
Postnatal Caregiving and Environmental Measures
Psychosocial Interview
A structured psychosocial interview was conducted with the mother/primary caregiver of each child at each assessment visit. The primary caregiver was defined as any family member or custodial guardian responsible for the physical, emotional, and financial well-being of the child. Biological mothers residing with and parenting the child were always prioritized for interview purposes as the primary caregiver. In the present report, selected key postnatal caregiver and child covariates obtained primarily from the 3-year visit were used in the LLG analyses (Table 2).
Table 2.
Caregiver and child demographic sample description at follow-up examsa (n = 376).
| Noncocaine-exposed |
Cocaine-exposed |
|||
|---|---|---|---|---|
| Group n | Mean (SD) | Group n | Mean (SD) | |
| Caregiver education (years) | ||||
| 3-year visit | 176 | 11.3 (1.5) | 198 | 11.2 (1.7) |
| Caregiver unemployment | ||||
| 3-year visit | 175 | 65.1% | 197 | 72.1% |
| Caregiver past year cocaine use | ||||
| 3-year visit | 176 | 1.7% | 194 | 22.2% |
| Caregiver past year alcohol use | ||||
| 3-year visit | 175 | 43.4% | 193 | 40.9% |
| Caregiver past year tobacco use | ||||
| 3-year visit | 175 | 17.7% | 192 | 55.7% |
| Caregiver past year marijuana use | ||||
| 3-year visit | 176 | 12.5% | 194 | 21.1% |
| Biological mother as caregiver | ||||
| 3-year visit | 176 | 97.2% | 199 | 71.9% |
| Child intellectual functioning | Mean (SD) | Mean (SD) | ||
| 3-year visit | 176 | 83.4 (12.7) | 200 | 81.1 (11.7) |
| Child day care | ||||
| 3-year visit | 176 | 31.8% | 199 | 52.8% |
| Child hearing deficit (bilateral) | ||||
| 3-year visit | 172 | 3.5% | 191 | 3.1% |
| Caregiver changes in past year (n) | Mean (SD) | Mean (SD) | ||
| 3-year visit | 174 | 0.11 (0.45) | 193 | 0.28 (0.59) |
| Head start/prekindergarten | ||||
| 5-year visit | 173 | 71.7% | 190 | 60.0% |
| Caldwell HOME language score | Mean (SD) | Mean (SD) | ||
| 4½-year visit | 174 | 5.8 (1.3) | 199 | 5.4 (1.6) |
| Child blood lead ≥10 μg/dL | ||||
| 3- or 5-year visit | 175 | 7.4% | 200 | 14.0% |
Group n’s include only subjects with available language data at the specified visit.
Assessment of Language Stimulation in the Home Environment
The Caldwell Home Observation for Measurement of the Environment (HOME) Inventory–Early Childhood Version (Caldwell and Bradley, 1984), conducted when the children were 4½ years old, is a measure of the quality and quantity of stimulation and support available to a child in the home environment. The Early Childhood Version, used with children ages 3 to 6 years, is administered by a combination of observation and semistructured interview conducted in the home when the child and his/her primary caregiver are present. The HOME Inventory has 55 items and yields a total score and eight subscale scores: Learning Materials, Language Stimulation, Physical Environment, Responsivity, Learning/Academic Stimulation, Modeling, Variety, and Acceptance. The Language Stimulation subscale scores were used in the present analyses. Internal consistency estimates for the HOME total score range from 0.53 to 0.93 and interobserver agreement typically has been reported as 90%. The HOME has been shown to have low to moderate correlations (generally 0.3 to 0.6) with socioeconomic status, IQ and achievement measures, and teacher behavioral ratings, and to have a factor structure that generally supports division of the instrument into the subscales (Sugland et al., 1995).
Statistical Analyses
Longitudinal Latent Growth Curve Analysis (LLG)
The current analyses investigating the association of severity of prenatal cocaine exposure build from the analytic strategy described in our first report on longitudinal language functioning in this sample (Bandstra et al., 2002). In the present report, Muthén and Muthén’s “multiple indicators, multiple causes” structural equations model and latent growth curve analysis adapted for discrete categorical indicator variables was used, as implemented in the Mplus software (Muthén and Muthén, 1998). Figure 1 depicts a conceptual model for the latent growth curve analysis, with both self-report and bioassay methods used to assess levels of prenatal cocaine exposure. Within the framework of the latent growth curve analysis, the intercept of the growth curve (i.e., the relatively time-invariant aptitude for language performance) and the slope of the growth curve (i.e., the time-varying trajectory of language performance) have been expressed as a function of the severity of prenatal cocaine exposure. Other elements within this conceptual model are exogenous covariates that might influence language functioning, as well as three endogenous response variables, i.e., fetal growth, maturation (gestational age), and child IQ. These elements are described in more detail below.
Figure 1.
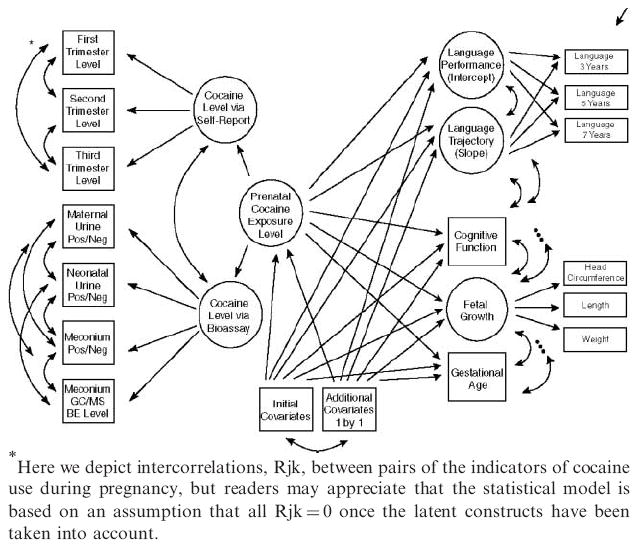
This figure depicts a conceptual model for the longitudinal latent growth curve analysis, the elements of which are presented in the accompany text.
Severity of Prenatal Cocaine Exposure
A latent variable for maternal cocaine/crack usage severity during pregnancy was measured by separate categorical summaries of first, second, and third trimester self-report (0 = no self-reported cocaine/crack use, 1 = 1–24 uses, 2 = 25–180 uses, 3 = 180+uses; note: a priori cut-offs represent approximate thirds of the distribution from the originating birth sample (n = 476), with the condition of a minimum of 30 infants per cell). A second latent variable to quantify cocaine exposure level by bioassay was also constructed, measured by cocaine-positive (yes/no) infant urine, infant meconium, and maternal urine, and log-transformed levels of cocaine metabolite benzoylecgonine determined by GC/MS assays. These self-report and bioassay results were then combined into a single summary latent variable that placed each infant on a single dimension, allowing all available information to be taken into account in estimating level of prenatal cocaine exposure. This approach allowed for bioassay results to characterize cocaine exposure levels for all infants, including the known cocaine-positive infants whose mothers denied cocaine use. Further detailed discussion of this approach has been reported in previous reports with this sample (Bandstra et al., 2001a).
Language Functioning
In order to facilitate interpretation of effect estimates across two different measures, language scores for each assessment visit were standardized by converting the sample mean for each visit to zero, with a standard deviation of 1. As described by Cherlin et al. (1998), the standardization accommodates the need for administration of age-appropriate measures across developmental phases, produces a flat growth curve for the sample as a whole, but permits the study of between-individual differences. Espy et al. (2001) suggest that the standard score metric used to describe children’s academic performance aids in the interpretability of the study results. They analyzed the effects of different environmental measures on individual intellectual growth patterns and found that the results of the growth curve analyses were similar using standardized and raw scores.
The LLG analysis partitions sources of variation in language functioning into two separate components: the sustained aptitude to perform well, and the time-varying trajectory of growth or change in performance from one age to the next. Analyses are split into these two components in order to determine with more specificity if there is a cocaine-associated deficit in language functioning.
Covariates
Indicators of fetal growth, maturation, and general intelligence were incorporated into the initial model based on a theoretical specification and prior evidence that they might depend upon prenatal cocaine exposure. Fetal growth was measured by a latent construct including birth weight, length, and head circumference, all of which are intercorrelated manifestations of a fetal growth process that has been previously shown to be responsive to prenatal cocaine exposure (Bandstra et al., 2001b). Fetal maturation was measured by gestational age. Intellectual functioning was measured by WISC-III short form given at the 7-year assessment. Additional covariates were included subsequently in the model as specified in the Results section below.
RESULTS
Sample Characteristics
Among the 476 participants in the follow-up study, 376 children completed all three language assessments at 3, 5, and 7 years of age. Of the 100 children excluded because they did not have all three language assessments, 33 children had no language scores, 28 children had only one language score (21 at the 3-year assessment only; 2 at the 5-year assessment only; and 5 at the 7-year assessment only), and 39 children had only 2 language scores (17 at the 3- and 5-year assessments only; 10 at the 3- and 7-year assessments only; and 12 at the 5- and 7-year assessments only). Detailed attrition analyses showed no differences in birth characteristics between the subsample of 376 children with language test results and the subsample of 100 who were missing one or more language test results. There were no differences in attrition by group.
Table 3 includes selected maternal and child characteristics measured at birth for this study subsample, including the key covariates introduced in the longitudinal analyses. In general, cocaine-using mothers were older, utilized less prenatal care, were less likely to be primigravida, and were more likely to be currently unemployed than were noncocaine-using mothers. Cocaine-exposed infants exhibited decrements on birth growth measures and gestational age relative to noncocaine-exposed infants (all p<0.01).
Table 3.
Maternal and infant demographic sample description at birth (n = 376).
| Noncocaine-exposed n = 176 | Cocaine-exposed n = 200 | |
|---|---|---|
| Maternal characteristics | ||
| Maternal age | 24.0 (5.5) | 29.0 (4.8) |
| Prenatal care (≥4 visits) | 86.4% | 68.5% |
| Primigravida | 22.2% | 6.5% |
| Never married | 87.5% | 91.0% |
| Unemployed | 82.4% | 94.5% |
| Education (years) | 11.3 (1.4) | 11.1 (1.4) |
| Infant characteristics | ||
| Birth weight (gms) | 3315 (501) | 2942 (455) |
| Birth length (cm) | 50.8 (2.3) | 48.8 (2.5) |
| Birth head circumference (cm) | 33.8 (1.4) | 33.0 (1.5) |
| Gestational age (weeks) | 39.7 (1.4) | 39.3 (1.4) |
| Apgar score (5 min) | 8.7 (0.7) | 8.7 (0.7) |
| Male | 48.9% | 49.5% |
Note: Numbers represent means (standard deviations) or percentages where indicated.
Table 1 presents data from this study sample on the self-reported rates and median amounts of alcohol, tobacco, marijuana, and cocaine use during pregnancy, including only mothers who admitted use. Median amounts are reported due to the significant skew in self-report data. Patterns of cocaine use ranged from mild/moderate to more severe use. Among cocaine-using mothers compared with noncocaine-using mothers, the percentage who reported alcohol, tobacco, and marijuana use during pregnancy was higher. In addition, the median tobacco use for cocaine-using mothers was higher than for noncocaine-using mothers (all p<0.05).
Table 2 depicts selected social and demographic characteristics measured primarily at the 3-year assessment visit. In general, primary caregivers of cocaine-exposed infants were more likely to report ongoing cocaine, marijuana, and tobacco use. Cocaine-exposed children were less likely to be in the care of their biological mothers and had a higher mean number of caregiving changes in the past year than noncocaine-exposed children. They were also more likely to be in day care, but less likely to be in Head Start or formal prekindergarten programs. The mean Caldwell HOME Language Score was lower for the cocaine-exposed children than for the noncocaine-exposed children (all p<0.05).
Although mothers in the cocaine group were more likely than those in the noncocaine group to report ongoing tobacco, marijuana, and cocaine use at the 3-year visit, there were no group differences in self-reported overall psychological functioning as measured by the Symptom Checklist–90–Revised, a 90-item instrument with a summary score, the Global Severity Index (GSI), that assesses a broad range of psychological symptoms such as depression, anxiety, somatization, obsessive-compulsion, and phobias over the last 7 days (Derogatis, 1993). At the 3-year visit, 42.7% of the mothers or caregivers in the cocaine-exposed group and 48.6% of the mothers or caregivers in the noncocaine-exposed met criteria for a concerning level of psychological symptoms (GSI score or at least two subscale scores ≥63).
Table 4 summarizes mean language scores for the standardized language assessments and examination ages by group at the 3-, 5-, and 7-year visits for the 376 children who had available data for all three exams. At each visit, in addition to the children whose language assessment was missing due to failure to attend, a small number of children attended but did not complete the language assessment due to severe cognitive or behavioral difficulties. However, there were no between-group differences in the noncompletion rates for any of the visits.
Table 4.
Means and standard deviations of language scores from 3 to 7 years.
| Language measure | n | Standard Score mean (SD) | Exam age |
|---|---|---|---|
| CELF-Pa (3-year exam) | |||
| Noncocaine-exposed | 176 | 76.1 (9.8) | 39.7 (1.7) |
| Cocaine-exposed | 200 | 72.9 (9.5) | 39.6 (2.4) |
| CELF-Pa (5-year exam) | |||
| Noncocaine-exposed | 176 | 86.4 (12.1) | 66.2 (2.3) |
| Cocaine-exposed | 200 | 83.4 (12.1) | 65.9 (2.5) |
| NEPSY Language Core Domainb (7-year exam) | |||
| Noncocaine-exposed | 176 | 87.3 (13.3) | 88.6 (4.1) |
| Cocaine-exposed | 200 | 84.1 (12.0) | 88.3 (3.7) |
Clinical Evaluation of Language Fundamentals–Preschool (CELF-P); Standard Score (mean = 100, SD = 15).
NEPSY—A Developmental Neuropsychological Assessment; Standard Score for Core Domains (mean = 100, SD = 15).
Longitudinal Latent Growth Curve Analyses of the Language Measures at 3, 5, and 7 Years
Initial Model
The initial model (see Fig. 1 and Table 5) examined the links between severity of prenatal cocaine exposure and both the relatively stable aptitude for language functioning (i.e., the intercept of the LLG model) and the developmental trajectory of language development (i.e., the slope of the LLG model). Included in the model were the covariates of fetal growth, gestational age, and IQ as described above, as well as terms labeled in Fig. 1 as “initial covariates,” which included age at the time of each assessment session, gender of the child, and prenatal exposure to alcohol, tobacco, and marijuana. Age in months at the time of each assessment was included in the model in order to take into account any possible failure of age standardization to account for age-related differences across levels of prenatal cocaine exposure. A term for child gender was included in order to take into account the frequently observed gender-based differences in longitudinal language development, observed not only in this study sample, but also in many other samples (Bandstra et al., 2002). Terms for the levels of prenatal use of alcohol, tobacco, and marijuana were included to ensure that the estimated size of the cocaine-associated language deficits would be independent of any deficit associated with the use of these other drugs.
Table 5.
Estimated cocaine-associated deficit in stable aptitude for language development under multiple covariate models for total language standard scores in children at 3, 5, and 7 years of age.
| Est. difference (D) | 95% C.I. | p value | |
|---|---|---|---|
| Initial model: includes severity of cocaine exposure, fetal growth, gestational age, IQ, child’s age and gender, and self-reported levels of prenatal alcohol, tobacco, and marijuana exposure | −0.071 | −0.133, −0.009 | 0.026 |
| Additional covariates: | |||
| Birth covariates: | |||
| Maternal age | −0.073 | −0.136, −0.009 | 0.025 |
| Maternal education level | −0.064 | −0.125, −0.004 | 0.037 |
| Primigravida (yes/no) | −0.073 | −0.136, −0.009 | 0.025 |
| Marital status (never/ever) | −0.069 | −0.132, −0.007 | 0.028 |
| Maternal employment (never/ever) | −0.068 | −0.130, −0.006 | 0.031 |
| Prenatal care visits (0–3 vs. 4+) | −0.073 | −0.140, −0.005 | 0.034 |
| Apgar score at 5 minutes | −0.067 | −0.129, −0.004 | 0.036 |
| Postnatal covariates: (measured at 3-year visit unless otherwise noted): | |||
| Child resides with biological mother (yes/no) | −0.088 | −0.167, −0.010 | 0.027 |
| Caregiver education level (years) | −0.072 | −0.132, −0.012 | 0.019 |
| Caregiver employment (yes/no) | −0.072 | −0.133, −0.012 | 0.020 |
| Number of caregiver changes (0–36 mos.) | −0.068 | −0.132, −0.003 | 0.041 |
| Past-year reported cocaine use | −0.074 | −0.140, −0.007 | 0.030 |
| Past-year reported marijuana use | −0.078 | −0.143, −0.013 | 0.018 |
| Past-year reported alcohol use | −0.078 | −0.142, −0.013 | 0.019 |
| Past-year reported cigarette use | −0.069 | −0.133, −0.006 | 0.032 |
| Day care placement (yes/no) | −0.075 | −0.138, −0.012 | 0.020 |
| IQ score | −0.041 | −0.079, −0.004 | 0.032 |
| Hearing | −0.070 | −0.131, −0.008 | 0.026 |
| Lead exposure level (ng/dL) | −0.074 | −0.139, −0.009 | 0.025 |
| HOME language stimulation score (4½ years) | −0.060 | −0.120, −0.001 | 0.047 |
| Head Start/Pre-kindergarten placement (5 years) | −0.068 | −0.128, −0.008 | 0.027 |
Results indicated that there was a cocaine-associated deficit (see Fig. 1 and Table 5) across 3, 5, and 7 years in the relatively stable aptitude for language performance with an effect estimate of −0.071, (95% CI = −0.133 to −0.009; p value = 0.026). There was no evidence to support a cocaine-associated deficit on the developmental trajectory of language development across 3, 5, and 7 years. Therefore, further analyses focused on the relatively stable component of language development.
Birth Covariates
In order to probe the robustness of the observed estimate of cocaine-associated deficits in aptitude for language development, 7 covariates measured at birth, including maternal age, educational level, primigravida, marital status, employment status, prenatal care visits, and infant Apgar score at 5 minutes, were introduced one at a time to the model described above and then removed. Sample size constrained the ability to include all the covariates simultaneously. Each estimate conveys the size of the cocaine-associated deficit in the aptitude for language to the extent that this deficit is independent of other covariates controlled in the initial model. Results shown in Table 5 reveal that the cocaine-associated deficit estimate does not change appreciably for any of the covariates included in the model (range of estimates = −0.073 to −0.064; all p<0.05).
Postnatal Covariates
Additional analyses were conducted by adding 14 covariates measured after birth one at a time to the initial model and then removing them. In general, data collected for each covariate at the earliest available study age were used in the LLG model. Covariates measured at 3 years of age included whether the child was residing with biological mother, number of primary caregiver changes since birth, caregiver educational level and employment status, past-year use of cocaine, marijuana, alcohol, and cigarettes, day care placement, IQ, and hearing. The blood lead level covariate was a 3/5-year composite as previously described. Placement in Head Start/Pre-kindergarten was noted at the 5-year visit. The Caldwell HOME Language Stimulation was assessed at age 4½ years.
Results shown in Table 5 indicate that the estimates for the cocaine-associated deficit in aptitude for language did not change appreciably for any of the covariates included in the model (range of estimates = −0.088 to −0.041; all p<0.05). The largest observed reduction in the estimate occurred with the introduction of the covariate of IQ at age 3 years, when the estimate had a value of −0.041 (95% CI = −0.079, −0.004; p = 0.032). However, even when IQ at both 3 years and 7 years has been taken into account, the cocaine-associated deficit in language functioning was still observed.
DISCUSSION
The current report from the Miami Prenatal Cocaine Study extends evidence previously reported (Bandstra et al., 2002) supporting a possible cocaine-associated deficit in language functioning across a developmental span from age 3 to 7 years. Longitudinal latent growth curve analyses were used to examine two components of language performance, a more stable aptitude and a time-varying developmental trajectory, across the three time points and their relationship to varying levels of prenatal cocaine exposure. Severity of prenatal cocaine exposure was characterized using a latent construct combining self-report and bioassays, allowing all available information to be taken into account. The association between severity of exposure and language performance was examined within a model including factors for fetal growth, gestational age, and IQ as intercorrelated response variables and child’s age, gender, and prenatal alcohol, tobacco, and marijuana exposure as additional covariates. Results indicated that greater severity of prenatal cocaine exposure was associated with greater deficits within the more stable aptitude of language performance (D = −0.071, 95% CI = −0.133, −0.009; p = 0.026). There was no relationship between severity of prenatal cocaine exposure and the time-varying trajectory of language development. The observed cocaine-associated deficit was independent of multiple alternative suspected sources of variation in language performance, including other potential responses to prenatal cocaine exposure, such as child’s intellectual functioning, and other birth and postnatal influences, including language stimulation in the home environment. Although the magnitude of the difference between cocaine-exposed and noncocaine-exposed children in language functioning was relatively subtle, Lester et al, (1998) emphasize that even subtle findings may have significant individual, public health, and societal consequences as more prenatally cocaine-exposed children require expensive and resource-consuming special services to prevent or ameliorate language problems. Indeed, Angelilli et al. (1994) observed in a retrospective case-control study in a primary care clinic setting that children ages 24–48 months with language delay were more likely to have been exposed to cocaine in utero than children with normal language development. Furthermore, Delaney-Black et al. (2000) found that 6-year-old children with prenatal cocaine exposure were two-and-a-half times more likely to be classified as having a low language ability than nonexposed children.
Several studies of cocaine-exposed and nonexposed children have observed no association between prenatal cocaine exposure and language development measured by standardized assessments (Bland-Stewart et al., 1998; Espy et al., 1999; Hawley et al., 1995; Hurt et al., 1997; Phelps and Cottone, 1999). Others have noted results consistent with the findings of the present study, but the sample sizes were appreciably smaller and none reported results past the preschool period. These studies have reported cocaine-associated deficits on various standardized tests of total language (Johnson et al., 1997; Singer et al., 2001a), expressive and receptive language (Chapman, 2000; Koren et al., 1998; Nulman et al., 1994, 2001; van Baar and de Graaff, 1994), expressive but not receptive language (Singer et al., 2001a), and receptive but not expressive language (Bender et al., 1995). Additional supportive evidence for cocaine-associated language deficits is provided by studies of small groups of children in whom spontaneous language samples have been coded to assess semantic and syntactical processing elements of language. These studies have documented less complex language skills (Malakoff et al., 1999), differences in discourse pragmatics and syntactic development (Mentis and Lundgren, 1995), phonological delay (Madison et al., 1998), and more restricted and delayed semantic representations (Bland-Stewart et al., 1998) among prenatally cocaine-exposed compared to noncocaine-exposed children.
Establishing a dose-response relationship between prenatal cocaine exposure and specific developmental outcomes is important in defining the teratological influence of cocaine (Lester et al., 1995). To our knowledge, only three studies to date have examined the relationship between severity of prenatal cocaine exposure and language performance. Two of these investigations relied solely on self-report methods to define exposure severity. Hurt and colleagues (Hurt et al., 1997) found no group differences in language outcome between children whose mothers reported frequent cocaine use in pregnancy (defined as more than once per week) and children whose mothers reported less frequent use. Van Baar and de Graaff (1994) constructed a global index of severity of prenatal exposure to all drugs in which higher scores indicated self-report of greater quantities of drugs, higher frequencies of use, and more invasive routes of administration. There was no relationship between this severity measure and language outcome. Singer and colleagues (Singer et al., 2001b) found that more heavily exposed infants (defined by maternal self-report >70th percentile or >75th percentile of meconium benzoylecgonine concentration) had lower auditory comprehension scores than the nonexposed infants, and lower total language scores than the lighter and nonexposed infants. Further work with a consistent approach to define severity with a combination of bioassay and self-report methods is needed to clarify these conflicting findings (Carmichael Olson and Toth, 1999).
The current study utilized a novel approach to examine two components of language functioning, the child’s relatively stable aptitude to perform well in verbal and language tasks, and the time-varying developmental trajectory of performance. This approach has allowed a more precise exploration of the cocaine-associated deficit and reveals that the effect of exposure is expressed in the relatively stable aptitude for language development, but not in the trajectory of language development during the preschool years leading up to age 7.
Important features of the current report include an examination of two components of language development with a growth curve model, the inclusion of a latent construct combining bioassay and self-report data to assess severity in relation to the outcome measure, and the examination of longitudinal data extending through age 7 while accounting for many birth and postnatal covariates. Data for this study emanate from one of the nation’s largest, single-site follow-up studies of prenatal cocaine exposure. The Miami Prenatal Cocaine Study has numerous inherent strengths, including prospective enrollment of study participants at birth and detailed maternal interviews and biological markers to determine exposure to cocaine and other drugs. The cohort is relatively homogeneous with regard to full-term gestation, race-ethnicity, and inner-city residence. Examiner-blinded standardized child assessments of language and cognition, comprehensive primary caregiver interviews, and an observational research measure of language stimulation in the home environment (Caldwell and Bradley, 1984) further strengthen the inferences of the results.
Notwithstanding these strengths, the study has several limitations. The study sample was restricted to full-term, relatively healthy African-American children residing in socially disadvantaged inner-city neighborhoods. This restriction of sample range was intended to promote homogeneity and greater similarities between cocaine-using and nonusing mothers with regard to health and social conditions that might influence child outcome, with a resulting enhanced capacity to infer language effects of prenatal cocaine exposure and somewhat increased statistical power to detect differences between cocaine-exposed and nonexposed children. These sampling techniques, however, result in an associated restriction of inference that precludes generalizing the cocaine–language estimate to other populations or settings. In addition, the cocaine–language association was examined taking into consideration an extensive, albeit not exhaustive, number of pertinent child and caregiver risk factors. Due to sample size restrictions, many of these factors needed to be evaluated individually within the model, precluding the simultaneous examination of multiple influences.
Misclassification due to erroneous maternal self-report or toxicology assays might have occurred, but in studies involving illicit drugs such as cocaine, misclassifications most likely affect the comparison group, and this would tend to draw the study conclusions toward the null. Recruitment of the study cohort in the postpartum period rather than prenatally may have hampered precise measurement of prenatal drug use. Finally, the approach to defining the latent construct of severity will require replication and validation in future work.
In summary, the present analyses focused on the estimated relationship between severity of prenatal cocaine exposure, measured as a latent construct combining bioassay and self-report measures, and language components derived from the CELF-P at 3 and 5 years and the NEPSY at 7 years. The evidence tends to support an inference of a modest cocaine-associated deficit for the stable aptitude for language performance during early childhood through age 7 years, with a gradient in which greater severity of cocaine exposure is associated with increased deficits in language functioning. There was no evidence to support a cocaine-associated deficit with the time-varying trajectory of language functioning. The cocaine-associated language deficit was not appreciably altered by inclusion of key demographic and psychosocial risk factors measured at birth and postnatally.
These findings suggest that professionals working with substance-using women with young children should monitor the language functioning of the children since early detection of subtle impairments can provide opportunities for intervention. Kilbride et al. (2000) found that among prenatally cocaine-exposed children who remained with their biological mothers, those who had received case management services had higher verbal scores at 36 months than those who had received routine management. Language difficulties during the toddler and preschool years can lead to learning, social, and/or academic problems during later years, thus highlighting the emphasis on careful early surveillance and standardized assessments when warranted. Although the findings from the current study are suggestive of subtle impairment in child language functioning, further evidence from other scientifically rigorous study designs with sufficient sample sizes are needed to make more definitive recommendations for professionals working with substance-using pregnant and postpartum women with young children. It remains critical to understand the mechanisms by which prenatal cocaine exposure may impact child language functioning, whether through teratological and/or environmental factors, and more broadly the ways in which substance use by mothers affects the outcome of their children. With this information, effective interventions targeting factors critical to child development in language and related neuropsychological domains can be designed and implemented.
Future research directions include the examination of receptive vs. expressive language functioning using the analytic strategy applied in this study of total language and replication with larger samples to allow for simultaneous inclusion of multiple covariates thought to impact the relationship between prenatal exposure and language functioning. Finally, the performance of similar studies examining the effects of prenatal cocaine exposure on the development of language in children of other linguistic cultures would permit probing of the generalizability of these findings.
Acknowledgments
We are indebted to the participating families and staff of the University of Miami Perinatal Chemical Addiction Research and Education (CARE) Program and the physicians and nurses of the UM/Jackson Memorial Medical Center Neonatal Services for their contributions to this research. We specifically thank Dr. Arnise L. Johnson and Dr. Veronica Accornero for supervising the research staff; Manya Glavach, M.S.Ed., Cherie O. Pagan, M.A., and Luz Ajuria-Londono for data quality assurance; M. Joy McKenzie, M.Ed., and Audrey Josey, M.A., for conducting the Caldwell HOME Inventory assessments; Dr. Ana T. Dausa and Dr. Robert C. Fifer for conducting and interpreting the audiometry assessments; and Dr. Bernard W. Steele and Dr. Niou-Ching Wu for performing the toxicology assays. The Spanish and French translations were graciously provided by Dr. Teresa del Moral and Dr. Monique Ernst, respectively.
This research was supported by the National Institutes of Health National Institute on Drug Abuse (RO1 DA 06556), the NIH Center for Research Resources, University of Miami General Clinical Research Center (MO1-RR 05280), and NIDA Research Training Award (T32 DA 07292 P.I.: J.C. Anthony; Trainee: A. L. Vogel). Services for participating families were partially supported by awards from the State of Florida Healthy Start Program, the Health Foundation of South Florida, and the Kenneth A. Lattman Foundation.
ABBREVIATIONS
- CELF-P
Clinical Evaluation of Language–Preschool
- GCI
General Cognitive Index
- LLG
Longitudinal latent growth curve
- HOME
Home Observation for Measurement of the Environment
- IQ
Intelligence Quotient
- WPPSI-R
Wechsler Scale of Preschool and Primary Intelligence–Revised
- WISC-III
Wechsler Intelligence Scale for Children–III
Biographies

Emmalee S. Bandstra, M.D., is Professor of Pediatrics and Obstetrics and Gynecology at the University of Miami School of Medicine and an Adjunct Professor of Mental Hygiene in the School of Public Health at Johns Hopkins University. Clinically licensed and board-certified in pediatrics and neonatal-perinatal medicine, Dr. Bandstra serves as an attending physician in the UM/Jackson Memorial Hospital neonatal intensive care unit. During the past 15 years, she has been the Director of the UM Perinatal Chemical Addiction Research and Education (CARE) Program and has been the principal investigator of the National Institute on Drug Abuse-funded Miami Prenatal Cocaine Study as well as several other federal, state, and private foundation prevention-intervention projects in the field of perinatal substance abuse.

April L. Vogel, Ph.D., is an Assistant Professor of Clinical Pediatrics at the University of Miami School of Medicine Department of Pediatrics in the Perinatal Chemical Addiction Research and Education Program. She received her Ph.D. in Clinical Psychology from the University of Miami and completed a NIDA-sponsored post-doctoral fellowship in the epidemiology of drug dependence at Johns Hopkins University Bloomberg School of Public Health with on-site assignment at the University of Miami. She is a licensed clinical psychologist and has research interests in the areas of the long-term effects of prenatal cocaine exposure, substance use and parenting, and developmental trajectories for adolescent risk behaviors.

Connie E. Morrow, Ph.D., Research Associate Professor of Pediatrics and Psychology at the University of Miami School of Medicine, has been the Psychology Research Director on the NIDA-funded Miami Prenatal Cocaine Study since 1992. Dr. Morrow is a licensed Clinical Psychologist specializing in Pediatric and Child Clinical Psychology. In addition, Dr. Morrow is Principal Investigator of the Miami site of the “Starting Early, Starting Smart Initiative” funded by SAMHSA and the Casey Family Program. This randomized study is focused on enhancing early childhood development and school readiness for infants and young children at risk due to caregiver mental health or substance abuse issues, by providing parenting and behavioral health services through integrated access within the pediatric health care setting.

Lihua Xue, M.S., M.A., is a Database Analyst at the University of Miami Perinatal Chemical Addiction Research and Education Program where she oversees data management and data analysis for the Miami Prenatal Cocaine Study. She received her Master’s degree from the University of Miami Department of Management Science and worked previously at the Statistical Bureau in China.

James C. Anthony, Ph.D., a psychiatric epidemiologist, is Professor and Chairman of Epidemiology at Michigan State University. He was formerly affiliated with the Johns Hopkins University Bloomberg School of Public Health and School of Medicine, where he was a professor in the departments of mental health, epidemiology, psychiatry, and behavioral sciences. Much of his work involves epidemiological research on the suspected hazards of psychoactive drug use, including the clinical sydromes of drug dependence. He has been working as an active collaborator in the Miami Prenatal Cocaine Study for the past four years.
References
- Angelilli ML, Fischer H, Delaney-Black V, Rubinstein M, Ager JW, Sokol RJ. History of in utero cocaine exposure in language-delayed children. Clin Pediat. 1994;33:514–516. doi: 10.1177/000992289403300901. [DOI] [PubMed] [Google Scholar]
- Ballard JL, Novak KK, Driver M. A simplified score for assessment of fetal maturation of newly born infants. J Pediatr. 1979;95(5 Pt 1):769–774. doi: 10.1016/s0022-3476(79)80734-9. [DOI] [PubMed] [Google Scholar]
- Bandstra ES, Burkett G. Maternal-fetal and neonatal effects of in utero cocaine exposure. Semin Perinatol. 1991;15(4):288–301. [PubMed] [Google Scholar]
- Bandstra ES, Morrow CE, Anthony JC, Accornero VH, Fried PA. Longitudinal investigation of task persistence and sustained attention in children with prenatal cocaine exposure. Neurotoxicol Teratol. 2001a;23:545–559. doi: 10.1016/s0892-0362(01)00181-7. [DOI] [PubMed] [Google Scholar]
- Bandstra ES, Morrow CE, Anthony JC, Churchill SS, Chitwood DD, Steele BM, Ofir AY, Xue L. Intrauterine growth of full-term infants: Impact of prenatal cocaine exposure. Pediatrics. 2001b;108:1309–1319. doi: 10.1542/peds.108.6.1309. [DOI] [PubMed] [Google Scholar]
- Bandstra ES, Morrow CE, Vogel AL, Fifer RC, Ofir AY, Dausa AT, Xue L, Anthony JC. Longitudinal influence of prenatal cocaine exposure on child language functioning. Neurotoxicol Teratol. 2002;24(3):297–308. doi: 10.1016/s0892-0362(02)00192-7. [DOI] [PubMed] [Google Scholar]
- Bender SL, Word CO, DiClemente RJ, Crittenden MR, Persaud NA, Ponton LE. The developmental implications of prenatal and/or postnatal crack cocaine exposure in preschool children: A preliminary report. J Dev Behav Pediatr. 1995;16(6):418–424. doi: 10.1097/00004703-199512000-00005. [DOI] [PubMed] [Google Scholar]
- Bland-Stewart LM, Seymour HN, Beeghly M, Frank DA. Semantic development of African-American children prenatally exposed to cocaine. Semin Speech Lang. 1998;19(2):167–186. doi: 10.1055/s-2008-1064043. [DOI] [PubMed] [Google Scholar]
- Burchinal M, Appelbaum MI. Estimating individual development functions: methods and their assumptions. Child Dev. 1991;62(1):23–43. [Google Scholar]
- Burkett G, Bandstra ES, Cohen J, Steele BM, Palow D. Cocaine-related maternal death. Am J Obstet Gynecol. 1990;163(1):40–41. doi: 10.1016/s0002-9378(11)90662-0. [DOI] [PubMed] [Google Scholar]
- Caldwell BM, Bradley RH. In: Manual for the Home Observation for Measurement of the Environment (H.O.M.E.) Revised. Caldwell BM, editor. Little Rock: University of Arkansas; 1984. [Google Scholar]
- Carmichael Olson H, Toth SK. Samples in research on prenatal cocaine exposure: vexing problems and practical solutions. J Drug Issues. 1999;29(2):237–252. [Google Scholar]
- Caselli C, Casadio P, Bates E. A comparison of the transition from first words to grammar in English and Italian. J Child Lang. 1999;26(1):69–111. doi: 10.1017/s0305000998003687. [DOI] [PubMed] [Google Scholar]
- Chapman KT. Developmental outcomes in two groups of infants and toddlers: Prenatally exposed and noncocaine exposed: Part 2. Infant Toddler Interv. 2000;10(2):81–96. [Google Scholar]
- Cherlin A, Chase-Lansdale P, McRae C. Effects of parental divorce on mental health throughout the life course. Am Sociol Rev. 1998;63(2):239–249. [Google Scholar]
- Church MW, Crossland WJ, Holmes PA, Overbeck GW, Tilak JP. Effects of prenatal cocaine on hearing, vision, growth, and behavior. Ann N Y Acad Sci. 1998;846:12–28. doi: 10.1111/j.1749-6632.1998.tb09723.x. [DOI] [PubMed] [Google Scholar]
- Cregler LL, Mark H. Medical complications of cocaine abuse. N Engl J Med. 1986;315(23):1495–1500. doi: 10.1056/NEJM198612043152327. [DOI] [PubMed] [Google Scholar]
- Critchley HO, Woods SM, Barson AJ, Richardson T, Lieberman BA. Fetal death in utero and cocaine abuse: case report. Br J Obstet Gynaecol. 1988;95(2):195–196. doi: 10.1111/j.1471-0528.1988.tb06851.x. [DOI] [PubMed] [Google Scholar]
- Delaney-Black V, Covington C, Templin T, Kershaw T, Nordstrom-Klee B, Ager J, Clark N, Surendran A, Martier S, Sokol RJ. Expressive language development of children exposed to cocaine prenatally: literature review and report of a prospective cohort study. J Commun Disord. 2000;33(6):463–480. doi: 10.1016/s0021-9924(00)00033-2. [DOI] [PubMed] [Google Scholar]
- Derogatis L. Symptom Checklist-90-R. Minnesota: National Computer Systems; 1993. [Google Scholar]
- Donahue M. Linguistic and Communicative Development in Learning-disabled Children. In: Ceci SJ, editor. Handbook of Cognitive, Social, and Neuropsychological Aspects of Learning Disabilities. Hillsdale, NJ: Lawrence Erlbaum Associates; 1986. pp. 263–289. [Google Scholar]
- Espy KA, Kaufmann P, Glisky M. Neuropsychologic function in toddlers exposed to cocaine in utero: a preliminary study. Dev Neuropsychol. 1999;15(3):447–460. [Google Scholar]
- Espy KA, Molfese VJ, DiLalla LF. Effects of environmental measures on intelligence in young children: growth curve modeling of longitudinal data. Merrill Palmer Q. 2001;47(1):42–73. [Google Scholar]
- Farrar HC, Kearns GL. Cocaine: clinical pharmacology and toxicology. J Pediatr. 1989;115(5 Pt 1):665–675. doi: 10.1016/s0022-3476(89)80640-7. [DOI] [PubMed] [Google Scholar]
- Frank DA, Augustyn M, Knight WG, Pell T, Zuckerman B. Growth, development, and behavior in early childhood following prenatal cocaine exposure: a systematic review. JAMA. 2001;285(12):1613–1625. doi: 10.1001/jama.285.12.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DA, Bauchner H, Parker S, Huber AM, Kyei-Aboagye K, Cabral H, Zuckerman BS. Neonatal body proportionality and body composition after in utero exposure to cocaine and marijuana. J Pediatr. 1990;117(4):622–626. doi: 10.1016/s0022-3476(05)80702-4. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Ellinwood EH., Jr Cocaine and other stimulants. Actions, abuse, and treatment. N Engl J Med. 1988;318(18):1173–1182. doi: 10.1056/NEJM198805053181806. [DOI] [PubMed] [Google Scholar]
- Hawley TL, Halle TG, Drasin RE, Thomas NG. Children of addicted mothers: effects of the “crack epidemic” on the caregiving environment and the development of preschoolers. Am J Orthopsychiatry. 1995;65(3):364–379. doi: 10.1037/h0079693. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Torbey M. Rupture of intracranial aneurysm associated with cocaine use during pregnancy. Am J Perinatol. 1988;5(2):142–143. doi: 10.1055/s-2007-999673. [DOI] [PubMed] [Google Scholar]
- Hurt H, Malmud E, Betancourt LM, Brodsky NL, Giannetta JM. A prospective evaluation of early language development in children with in utero cocaine exposure and in control subjects. J Pediatr. 1997;130(2):310–312. doi: 10.1016/s0022-3476(97)70361-5. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Sokol RJ. Effects of prenatal exposure to alcohol, smoking, and illicit drugs on postpartum somatic growth. Alcohol Clin Exp Res. 1994;18(2):317–323. doi: 10.1111/j.1530-0277.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Seikel JA, Madison CL, Foose SM, Rinard KD. Standardized test performance of children with a history of prenatal exposure to multiple drugs/cocaine. J Commun Disord. 1997;30(1):45–72. doi: 10.1016/s0021-9924(96)00055-x. [DOI] [PubMed] [Google Scholar]
- Kilbride H, Castor C, Hoffman E, Fuger KL. Thirty-six-month outcome of prenatal cocaine exposure for term or near-term infants: impact of early case management. J Dev Behav Pediatr. 2000;21(1):19–26. doi: 10.1097/00004703-200002000-00004. [DOI] [PubMed] [Google Scholar]
- Koren G, Nulman I, Rovet J, Greenbaum R, Loebstein M, Einarson TR. Long-term neurodevelopmental risks in children exposed in utero to cocaine: The Toronto Adoption Study. Ann N Y Acad Sci. 1998;846:306–313. [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp S. NEPSY A Developmental Neuropsychological Assessment Manual. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- Lester BM, Freier K, LaGasse LL. Prenatal cocaine exposure and child outcome: what do we really know? In: Lewis M, Bendersky M, editors. Mothers, Babies, and Cocaine: The Role of Toxins in Development. Hillsdale: LEA Publishers; 1995. pp. 19–39. [Google Scholar]
- Lester BM, LaGasse LL, Seifer R. Cocaine exposure and children: the meaning of subtle effects. Science. 1998;282(5389):633–634. doi: 10.1126/science.282.5389.633. [DOI] [PubMed] [Google Scholar]
- Madison CL, Johnson JM, Seikel JA, Arnold M, Schultheis L. Comparative study of the phonology of preschool children prenatally exposed to cocaine and multiple drugs and nonexposed children. J Commun Disord. 1998;31(3):231–243. doi: 10.1016/s0021-9924(97)00091-9. [DOI] [PubMed] [Google Scholar]
- Malakoff ME, Mayes LC, Schottenfeld R, Howell S. Language production in 24-month-old inner-city children of cocaine-and-other-drug-using mothers. J App Dev Psychol. 1999;20(1):159–180. [Google Scholar]
- Mayes LC. Developing brain and in utero cocaine exposure: effects on neural ontogeny. Dev Psychopathol. 1999;11(4):685–714. doi: 10.1017/s0954579499002278. [DOI] [PubMed] [Google Scholar]
- Mayes LC. Neurobiology of prenatal cocaine exposure effect on developing monoamine systems. Inf Men Health J. 1994;15(2):121–133. [Google Scholar]
- Mayes LC, Granger RH, Bornstein MH, Zuckerman BS. The problem of prenatal cocaine exposure. A rush to judgment. JAMA. 1992;267(3):406–408. [PubMed] [Google Scholar]
- McCarthy D. Manual for the McCarthy Scales of Children’s Abilities. Cleveland, OH: The Psychological Corporation; 1972. [Google Scholar]
- Mentis M, Lundgren K. Effects of prenatal exposure to cocaine and associated risk factors on language development. J Speech Hear Res. 1995;38(6):1303–1318. doi: 10.1044/jshr.3806.1303. [DOI] [PubMed] [Google Scholar]
- Mercado A, Johnson G, Jr, Calver D, Sokol RJ. Cocaine, pregnancy, and postpartum intracerebral hemorrhage. Obstet Gynecol. 1989;73(3 Pt 2):467–468. [PubMed] [Google Scholar]
- Moore TR, Sorg J, Miller L, Key T, Resnik R. Hemodynamic effects of intravenous cocaine on the pregnant ewe and fetus. Am J Obstet Gynecol. 1986;155:883–888. doi: 10.1016/s0002-9378(86)80044-8. [DOI] [PubMed] [Google Scholar]
- Morild I, Stajic M. Cocaine and fetal death. Forensic Sci Int. 1990;47(2):181–189. doi: 10.1016/0379-0738(90)90212-h. [DOI] [PubMed] [Google Scholar]
- Morrow CE, Bandstra ES, Anthony JC, Ofir AY, Xue L, Reyes M. The influence of prenatal cocaine exposure on early language development: longitudinal findings from 4 months through three years of age. J Dev Behav Pediatr. 2003;24(1):39–50. doi: 10.1097/00004703-200302000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulé S, Casella GA. Confirmation and quantitation of cocaine, benzoylecgonine, ecgonine methyl ester in human urine by GC/MS. J Anal Toxicol. 1988;12(3):153–155. doi: 10.1093/jat/12.3.153. [DOI] [PubMed] [Google Scholar]
- Muthén BO, Muthén L. Mplus Software. The Comprehensive Modeling Program for Applied Researchers User’s Guide. Los Angeles: Muthén & Muthén; 1998. [Google Scholar]
- Nulman I, Rovet J, Altmann D, Bradley C, Einarson TR, Koren G. Neurodevelopment of adopted children exposed in utero to cocaine. CMAJ. 1994;151(11):1591–1597. [PMC free article] [PubMed] [Google Scholar]
- Nulman I, Rovet J, Greenbaum R, Loebstein M, Wolpin J, Pace-Asciak P, Koren G. Neurodevelopment of adopted children exposed in utero to cocaine: The Toronto Adoption Study. Clin Invest Med. 2001;24(3):129–137. [PubMed] [Google Scholar]
- Phelps L, Cottone JW. Long-term developmental outcomes of prenatal cocaine exposure. J Psychoeduc Assess. 1999;17(4):343–353. [Google Scholar]
- Sattler JM. In: Assessment of Children: Revised and Updated Third Edition. Sattler JM, editor. San Diego, CA: 1992. [Google Scholar]
- Schneiderman JF. Nonmedical drug and chemical use in pregnancy. In: Koren G, editor. Maternal-Fetal Toxicology: A Clinician’s Guide. New York, NY: Marcel Dekker, Inc.; 1990. pp. 301–320. [Google Scholar]
- Singer LT, Hawkins S, Huang J, Davillier M, Baley J. Developmental outcomes and environmental correlates of very low birthweight, cocaine-exposed infants. Early Hum Dev. 2001a;64(2):91–103. doi: 10.1016/s0378-3782(01)00182-7. [DOI] [PubMed] [Google Scholar]
- Singer LT, Arendt R, Minnes S, Salvator A, Siegel AC, Lewis BA. Developing language skills of cocaine-exposed infants. Pediatrics. 2001b;107(5):1057–1064. doi: 10.1542/peds.107.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KE, Landry SH, Swank PR. Does the content of mothers’ verbal stimulation explain differences in children’s development of verbal and nonverbal cognitive skills? J Sch Psychol. 2000;38(1):27–49. [Google Scholar]
- Sugland BW, Zaslow M, Smith JR, Brooks-Gunn J, Coates D, Blumenthal C, Moore KA, Griffin T, Bradley R. The Early Childhood HOME Inventory and HOME-Short Form in differing racial/ethnic groups: are there differences in underlying structure, internal consistency of subscales, and patterns of prediction? J Fam Issues. 1995;16(5):632–663. [Google Scholar]
- Tallal P. Developmental language disorders. In: Kavanagh JF, Truss TJ, editors. Learning Disabilities: Proceedings of the National Conference. Parkton, MD: York Press; 1988. pp. 181–272. [Google Scholar]
- Tarr JE, Macklin M. Cocaine. Pediatr Clin North Am. 1987;34(2):319–331. doi: 10.1016/s0031-3955(16)36217-4. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Effect of cocaine use on the fetus. N Engl J Med. 1992;327(6):399–407. doi: 10.1056/NEJM199208063270607. [DOI] [PubMed] [Google Scholar]
- van Baar A, de Graaff BM. Cognitive development at preschool-age of infants of drug-dependent mothers. Dev Med Child Neurol. 1994;36(12):1063–1075. doi: 10.1111/j.1469-8749.1994.tb11809.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children–Third Edition (WISC-III) San Antonio, TX: Psychological Corporation; 1991. [Google Scholar]
- Wechsler D. Wechsler Preschool and Primary Scale of Intelligence-Revised (WPPSI-R) San Antonio, TX: Psychological Corporation; 1989. [Google Scholar]
- Wiig EH, Secord W, Semel E. Clinical Evaluation of Language Fundamentals-Preschool: Examiner’s Manual. New York, NY: Psychological Corporation; 1992. [Google Scholar]
- Zuckerman BS, Frank DA. Crack kids: not broken. Pediatrics. 1992a;89(2):337–339. [PubMed] [Google Scholar]
- Zuckerman BS, Frank DA. Prenatal cocaine and marijuana exposure: research and clinical implications. In: Zagon S, Slotkin TA, editors. Maternal Substance Abuse and the Developing Nervous System. Boston, MA: Academic Press; 1992b. pp. 125–154. [Google Scholar]
- Zuckerman BS, Frank DA, Hingson R, Amaro H, Levenson SM, Kayne H, Parker S, Vinci R, Aboagye K, Fried LE. Effects of maternal marijuana and cocaine use on fetal growth. N Engl J Med. 1989;320(12):762–768. doi: 10.1056/NEJM198903233201203. [DOI] [PubMed] [Google Scholar]


