Abstract
We have evaluated a small water soluble molecule, biotin ethylenediamine (BED, 286Da), as a permeability tracer across the blood-brain barrier. This molecule was found to have suitable characteristics in that it is stable in plasma, has low plasma protein binding and appears to behave in a similar manner across brain barriers as established permeability markers such as sucrose. BED, together with a 3000Da biotin-dextran (BDA3000), was used to investigate the effectiveness of tight junctions in cortical vessels during development and adulthood of a marsupial opossum (Monodelphis domestica). Marsupial species are born at an early stage of brain development when cortical vessels are just beginning to appear. The tracers were administered systemically to opossums at various ages and localised in brains with light and electron microscopy. In adults the tight junctions restricted the movement of both tracers. In neonates, as soon as vessels grow into the neocortex, their tight junctions are functionally restrictive, a finding supported by presence of claudin-5 in endothelial cells. However, both tracers are also found within brain extracellular space soon after intraperitoneal administration. The main route of entry for the tracers into immature neocortex appears to be via the cerebrospinal fluid over the outer (subarachnoid) and inner (ventricular) surfaces of the brain. These experiments demonstrate that the previously described higher permeability of barriers to small molecules in the developing brain does not seem to be due to leakiness of cerebral endothelial tight junctions, but to a route of entry probably via the choroid plexuses and cerebrospinal fluid.
Indexing words: Brain barrier permeability, cerebrospinal fluid, tight junction, electron microscopy, biotinylated tracers, choroid plexus, claudin-5
INTRODUCTION
The environment within the adult central nervous system (CNS) is different from that of the rest of the body as a result of a series of barrier mechanisms that control the movement of many compounds from blood into the CNS and vice versa. The basic structures of these barriers are tight junctions between endothelial cells of cerebral blood vessels, choroidal epithelial cells, and the cells on the outer surface of the arachnoid membrane (Brightman and Reese, 1969; Nabeshima et al., 1975). Although ultrastructural studies have shown that tight junctions are present at these barrier interfaces early in development, it is still unclear to what degree these are functional and able to limit the entry of water soluble molecules into the brain. In the adult, the tight junctions prevent the paracellular passage of larger molecules such as proteins into the brain and cerebrospinal fluid, CSF (Brightman and Reese, 1969). The tight junctions are also believed to severely restrict, but nevertheless allow the movement of smaller molecules such as inulin (~5000Da) and sucrose (342Da). However, there has been no direct evidence for this, as these molecules cannot be visualised in tissue sections.
Several studies have shown that the penetration of water soluble molecules from blood to brain is greater in young animals and decreases with age (Dziegielewska et al., 1979; Ek et al., 2001; Ferguson and Woodbury, 1969; Habgood et al., 1993). Explanations that have been put forward for this developmentally regulated apparent decrease in blood to brain permeability have been that the “sink effect”, which is due to the CSF constantly being replaced, increases with age (Bass and Lundborg, 1973; Johanson and Woodbury, 1974) or that there are progressive changes in tight junction structure during development (Stewart and Hayakawa, 1987; Kniesel et al., 1996; Wolburg and Lippoldt, 2002). Although there is an increase in the absolute CSF secretion rate during brain maturation, when secretion rate is related to the size of the CSF volume and calculated as a CSF turnover rate, turnover increases only slightly during development and it has been suggested that the change in sink effect is unlikely to account for the large age-related decrease in blood to brain or blood to CSF permeability (Saunders, 1992). The studies that have shown changes in endothelial tight junctional structure in development have perceived this as a gradual maturation of the junctions becoming more restrictive and resulting in a more effective endothelial barrier with age. If this is the case, the changes seen in tight junction structure should therefore parallel the changes in blood-brain barrier permeability; however, where relevant permeability data are available from other studies there is a poor correlation between junctional structure and barrier permeability (cf Habgood et al., 1993 and Kniesel et al., 1996). In the sheep fetus, where both morphological and permeability have been studied over a similar developmental period, no correlation was found between key features of tight junction structure and measured permeability changes (Dziegielewska et al., 1979; Møllgård et al., 1976).
In order to correlate characteristics of brain barrier permeability with underlying morphological characteristics during development, we have made a series of experiments of barrier function in a marsupial species, the grey short-tailed South American opossum (Monodelphis domestica) (Knott et al., 1997; Ek et al., 2001; Ek et al., 2003). The advantage of using a marsupial compared with eutherian mammals is that it is born at an earlier stage of brain development and thus experiments on early brain development can be made in vivo after birth, with less physiological manipulation either of the mother or young. At birth the opossum is comparable to an E13-E14 rat with respect to choroid plexus and cortical development (Dziegielewska et al., 2001; Saunders et al., 1989). The present study follows up previous experiments in the opossum and investigates the functional barrier of the tight junctions of cerebral blood vessels to small water soluble tracers in early development by focusing on the first vessels that grow into the neocortex. The neocortex was chosen since this is the last area of the brain to become vascularized and these vessels are thus the least mature vessels in the brain of the neonate of this species. We have used 3000Da biotin-dextran (BDA3000) and 286Da biotin ethylenediamine (BED) as water soluble tracers. These tracers have been used successfully as axonal tracers and can be visualised both for light and electron microscopy with standard laboratory techniques (Köbbert et al., 2000). The tracer that is generally used in vivo to assess barrier integrity to proteins, horseradish peroxidase (40kDa), is much larger than sucrose or inulin, the traditional markers for quantitative blood-brain barrier permeability studies. Horseradish peroxidase has been reported to have anomalous permeability properties in epithelia and to have toxic effects, which makes it less than ideal as an in vivo tracer (see Discussion). Furthermore, many of the other permeability tracers that are used have never actually been tested to see whether their permeability characteristics are appropriate for their molecular size. BDA3000 has previously been used as a tracer of barrier permeability (Ek et al., 2001; Ek et al., 2003). One of these studies showed that BDA3000 penetrated into CSF from blood to an extent that would be expected if it were transferring by diffusion; this confirmed that this tracer would be suitable for both physiological and morphological studies of brain barrier permeability (Ek et al., 2001). In order to evaluate the suitability of BED as a water soluble tracer across brain barriers, similar experiments investigating the uptake of the BED from blood to CSF were undertaken along with determining its protein binding and stability in plasma. This study shows that BED has good characteristics as a permeability tracer and that even the first vessels that appear in the neocortex possess tight junctions with functional barrier properties, which is supported by the presence of the tight junctional protein claudin–5. However, these tracers do appear to penetrate into the extracellular space within the immature brain. New evidence supports earlier findings (Ek et al., 2003) that these small molecules reach the immature brain via a route across the choroid plexuses into CSF, followed by movement through the inner (ventricular) and outer (pia-arachnoid) surfaces into the brain.
METHODS
All experiments were conducted in accordance with NHMRC and NIH guidelines, and with the approval of the University of Melbourne Animal Ethics Committee.
Stability and plasma protein binding of biotin ethylenediamine (BED)
Plasma from adult rats (n=4) was aliquoted into several replicate 1ml vials and BED (Molecular Probes, USA) added to a concentration of 0.8mg/ml plasma. Vials were incubated at 37°C for 30min and plasma centrifuged in a centrifree partition system (Amicon, USA) with a molecular cut-off of 3000Da. The concentration of BED in filtrate and retentate was determined by HPLC. Samples were diluted (x3) with Acetonitrile/0.1%TFA and left on ice for 10min to denature proteins. Samples were then further diluted (x3) with water, spun at 3000rpm for 5min and the concentration of BED measured in the supernatants using a Hewlett-Packard Series 1100 HPLC fitted with a Zorbax (300SB-C18) C18 reverse phase column (length 25cm, diameter 4.6mm, and particle size 5µm). The column was stabilised using a mobile phase of 3% Acetonitrile and 0.1% TFA. The flow rate was set to 1ml/min and the amount of organic modifier (acetonitrile) gradually increased to 15% over 8min. A UV detector set at 205nm was used to detect BED as it eluted from the column. The amount of BED in samples was determined by comparison with standard curve constructed from chromatograms of standard injections of BED (0.01–10µg) and plotted using HP Chemstation. In order to test the stability of BED in plasma, chromatograms of BED plasma solutions before and after 30min incubation at 37°C were compared.
Sucrose and BED uptake into CSF
Opossums at P16 (n=4) were injected intraperitoneally (ip; 6µl/g body weight) with a mixture of BED (1mg/g body weight) and 14C-sucrose (30kBq/g body weight; Amersham International) in 0.9% w/v isotonic sodium chloride using a glass capillary and animals were killed at 3h by inhalation of halothane (Veterinary Company of Australia). Samples of blood were collected from the heart and CSF from cisterna magna as previously described (Ek et al., 2001). Blood was centrifuged and plasma separated. The concentrations of BED in plasma and CSF samples were measured by HPLC as outlined above and the concentration of sucrose in samples was measured by liquid scintillation counting (Ek et al., 2001). The CSF/plasma concentration ratio of markers was calculated as a measure of plasma to CSF transfer.
Light and electron microscopy
Opossum pups at P0 (n=2), P2 (n=2), P5–6 (n=3) and P12 (n=2) were injected ip with BDA3000 or at P2 (n=2) and P5–6 (n=6) with BED using a glass capillary. Animals were killed by an overdose of halothane 20–30min after a BED injection and 25–40min after a BDA3000 injection. The whole brain was dissected out under fixative, sliced in 1mm coronal sections with a razor blade and tissue kept in fixative overnight at 4°C. The whole head was sliced with a razor blade from some P2 and P5 animals and then submerged in fixative in order to obtain sections with intact meninges. Two month old opossums, which have a well formed neocortex and referred to here as “adult”, were anaesthetised with an ip injection of urethane (12.5% w/v solution, 0.02ml/g body weight) and given a bolus injection in the femoral vein with a 0.9% w/v isotonic sodium chloride solution containing BDA3000 (n=2) or BED (n=2) at a dose of 0.2mg/g body weight. Adult animals were decapitated 10 min after the injection and small pieces of the cortex dissected out and immersed fixed overnight. The difference in way of administering tracer solutions to pups and adults (ip versus iv) was due to the size differences of these animals. A pup at P5 weighs around 0.2g which makes it technically very difficult to give an intravenous injection. The concentration of marker was insufficient to affect the osmolality of the injected solutions. Fresh 1.5% glutaraldehyde/1.5% paraformaldehyde solution in 0.07 or 0.1M phosphate buffer adjusted to pH 7.3 at 4°C was used as the primary fixative for all tissue. In young pups the preservation of the tissue was better in the lower buffer concentration. Brain slices/pieces were then sectioned with a vibratome at 50µm. For comparisons, some sections were treated with 0.02% H2O2 for 30min to block endogenous peroxidases. The tissue was then left in a streptavidin/peroxidase complex (DakoCytomation) overnight. Sections were washed and processed with a nickel enhanced DAB reaction for approximately 5min. Some sections were taken away at this stage and mounted in a aqueous mounting medium for light microscopical examination. Most sections were further fixed in a 2% osmium tetroxide solution, dehydrated in increasing concentrations of acetone and flat embedded in Epon812. Semithin sections (0.5–1µm) were cut with a Reichard UltraE microtome, lightly stained with toludine blue and mounted in DPX permanent mounting medium. Ultrathin sections with a light gold interference colour were contrasted with uranyl acetate followed by lead citrate, and examined with a CM10 or CM12 Phillips electron microscope working at 80 or 60kV, respectively. Control brain tissue was obtained from animals not given an injection but otherwise treated in the same way.
Diffusion rate of BED in brains in vitro
In order to test the diffusion rate of tracers through brain tissue whole opossum brains at P5 (n=5) were dissected out and submerged in a solution of BED or BDA3000 (0.34mg/ml tracer in 0.9% saline) for 5, 25, or 60min. Brains were then transferred to the same primary fixative as for electron microscopy (see above) and left overnight. Vibratome sections of brains were cut at 50µm and the sections treated with 0.02% H2O2 for 30min to block endogenous peroxidases. Sections were then left in a streptavidin/peroxidase complex (DakoCytomation) overnight, processed with a nickel enhanced DAB reaction and mounted for light microscopical examination in an aqueous mounting medium (DakoCytomation).
Claudin-5 immunoreactivity
Five micron, paraffin embedded coronal sections from opossum brains at various developmental ages from P0 to P13 and adults were obtained from previous studies (Knott et al., 1997; Ek et al., 2001). Sections were dewaxed in histolene and rehydrated in decreasing concentrations of ethanol, followed by washes in 0.15M phosphate buffered saline with 0.2% Tween20 (PBS/Tween). After consecutive incubations in Peroxidase and Protein Blockers (both from DakoCytomation), sections were incubated overnight at 4°C in anti claudin-5 antibodies (CN35–2500, Zymed). This was followed by a 2h incubations with rabbit anti mouse and mouse PAP, both from DakoCytomation. All antibodies were diluted 1:200 in 2% fish gelatine blocking solution and each incubation was followed by extensive washes in PBS/Tween buffer. The reaction product was developed with DAB+kit (DakoCytomation), washed in distilled water and after dehydration, mounted with permanent mounting medium (DPX). The antibody to claudin-5 was produced using the following synthetic immunogen peptide derived from mouse claudin-5 sequence: PTVPVSQKYEGAALYIGWAASALLMCGGGLVCCGAWVCTGRPEFSFPVKYSAPRRPTANGDYDKKNYV (GenBank Accession No. AAD09758). Western blotting has shown that this antibody only reacts with a 22–23kDa specific claudin protein over a wide range of concentrations of the antibody and protein extracted from rat gastrointestinal tissue, mouse/rat lung (manufacter’s specification sheet) and rat brain microvessels (Hawkins et al., 2004). Western blotting on protein extract from adult opossum brain showed that anti claudin-5 antibody reacts with a protein of an appropriate size (~22kDa). For protein extraction and western blotting the protocol of Hawkins et al. (2004) was followed with the difference that proteins were separated on a 12% polyacrylamide gel (BioRad) and transferred to a nitrocellulose membrane (Amersham). Specificity of claudin-5 antibody was additionally tested in opossum fixed tissue by an absorption method. Lung tissue from mouse was homogenised in phosphate buffered saline with 0.5% Tween20 and protease inhibitors. The homogenate was centrifuged for 5min at 14500rpm, the supernatant removed and mixed with anti claudin-5 antibody solutions to yield final antibody dilutions of 1:200. This was incubated overnight at 4°C and used as the primary antibody on P5 opossum brain sections which were further processed for claudin immunoreactivity as described above. Negative and positive controls were also performed. Negative controls included omission of one of the antibodies and then the sections always appeared blank.. Positive controls included use of tissue with known claudin-5 immunoreactivity (mouse lung).
Image processing
Digitised photographs were processed in Adobe Photoshop 5.5. The brightness and curve functions were used in order to obtain images with background close to white and to enhance contrast.
RESULTS
Stability and plasma binding of biotin ethylenediamine (BED)
The amount of free BED in plasma was determined by centrifugation through a filter with a molecular weight sieve of 3000Da. This showed that 91.1%±0.7% (mean±SEM) remained free and was not bound to protein in plasma. No difference was found in chromatograms of BED plasma solutions before and after 30min of incubation at 37°C indicating that the compound is stable during this time.
CSF/plasma concentration ratios of BED
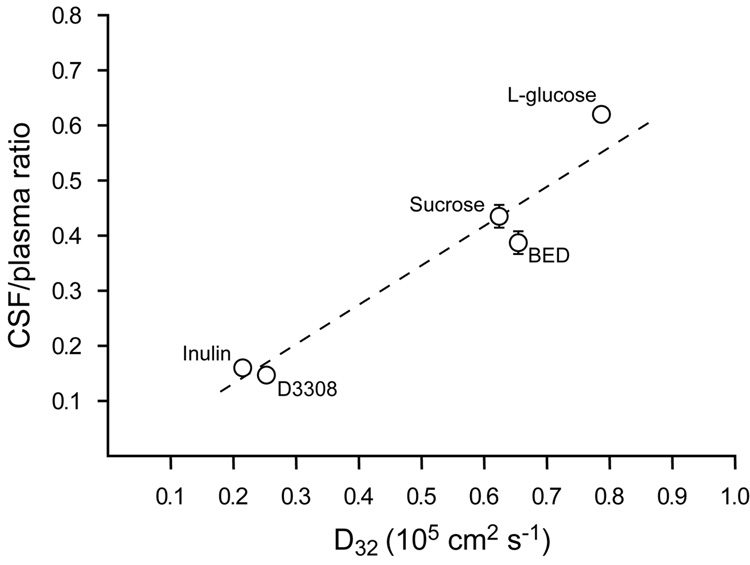
CSF/plasma concentration ratios of BED were measured and compared with ratios obtained with radioactive sucrose in order to check whether the uptake of BED is consistent with its molecular size (286Da). Sucrose (342Da) was chosen since it is of similar size to BED and has previously been used extensively in blood-brain barrier and blood-CSF barrier permeability studies. In P16 animals the CSF/plasma ratio for BED was calculated from its free concentration in plasma, which was determined as described in Methods. The 3h CSF/plasma ratio for sucrose was 43.5%±2.3 (mean±SEM) and 38.8%±2.0 for BED. The uptake of water soluble markers from blood to CSF has been shown to be related to their diffusion coefficient as estimated from the Einstein-Stokes radii (Felgenhauer, 1974). Figure 1 shows the 3h CSF/plasma ratios for BED and sucrose plotted against their diffusion coefficient (D32; body temperature of opossum is 32°C) along with previous data obtained from Ek et al. (2001) for L-glucose (182Da), inulin (5200Da) and fluorescent 3000Da biotin-dextran (D3308). It is apparent from this graph that the uptake of BED from blood to brain is close to what would be expected from its molecular weight.
Figure 1.
Three-hour CSF/plasma concentration ratios for inulin, sucrose, L-glucose, BED (biotin ethylenediamine) and fluorescent 3000Da biotin-dextran (D3308) in P16 opossums plotted against their estimated diffusion coefficients (D32) at 32°C (body temperature of opossum). Estimations of D32 were made from the Einstein-Stokes radii. Values are means±SEM; some error bars are obscured by symbols. Data for L-glucose, inulin and D3308 are from Ek et al. (2001). From this plot it can be seen that the ratios for all molecules are proportional to their D32 suggesting that their blood to CSF transfer route is by passive diffusion, and not subject to any inward transport mechanism.
Tracers in adult brains
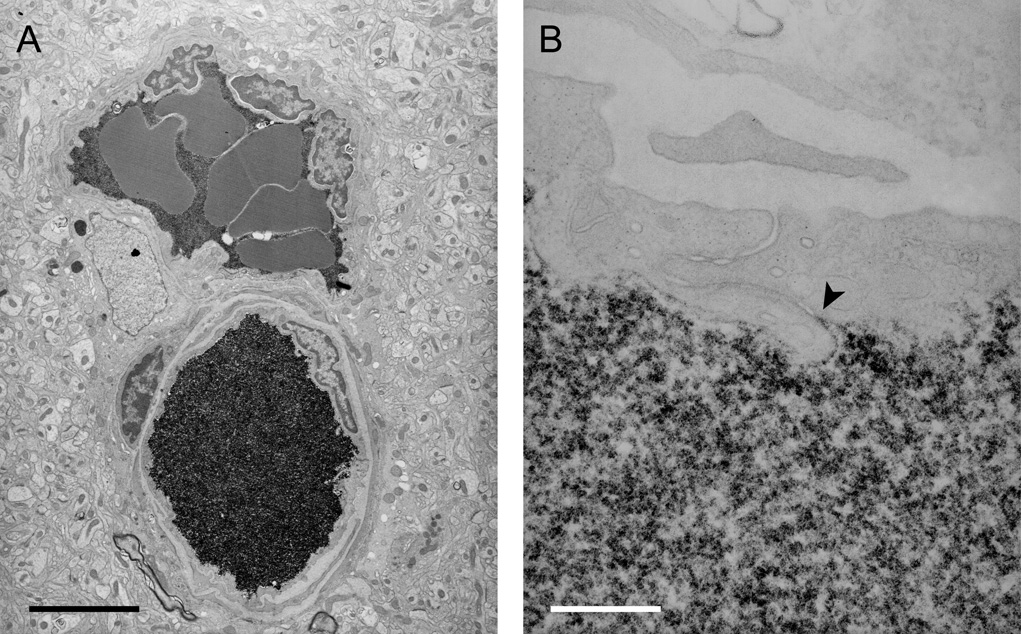
Profiles of blood vessels from the adult cortex were examined under the electron microscope to locate staining for BDA3000 and BED. Vibratome and subsequently ultrathin sections were cut horizontally through brains from the pial surface inwards to obtain as many transverse profiles of vessels as possible, which is advantageous when examining the penetration of the tracer through the endothelial cleft. Blood vessel profiles were examined all the way from the marginal zone down to white matter in the external capsule including all the layers of the neocortex. Only the staining pattern for the smaller tracer BED is illustrated in Figure 2, however, the distribution of the reaction product was similar for both tracers. Under the light microscope the reaction product is only present within blood vessels and the brain parenchyma appears to be devoid of staining. Under the electron microscope it is apparent that although the lumen of all vessels is dense with reaction product, no reaction product can be seen in adjacent brain parenchyma. At higher magnification it is evident that no reaction product is visible in the interendothelial cleft except for the most luminal part, ie the reaction product is not visible beyond the site of the first apposition of adjacent endothelial membranes that form the tight junction (Fig. 2).
Figure 2.
Electron micrographs of the localisation of BED (biotin ethylenediamine) in blood vessels deep inside the cortex of a 2 month old opossum 10min after an intravenous injection. Similar staining is found after an intravenous injection of biotin-dextran (BDA3000).
A) Low power micrograph showing two paired vessels with abundant reaction product within the lumen. No reaction product is visible in the surrounding tissue. Pairs of arteries and veins are characteristic of the vascular pattern in marsupial brains (Wislocki and Campbell, 1937).
B) High power micrograph of an interendothelial cleft showing that the tight junctions in the young adult restrict the passage of BED through the cleft (arrowhead). Scale bars are 4µm in A, 300nm in B.
Tracers in developing brains
Brain sections revealed that at birth there are no blood vessels in the neocortex of the opossum. The first growth of vessels from the pial plexus is into the lateral parts of the neocortex and occurs at around P1–P3; subsequent vascularisation occurs towards the midline (see Fig. 3A). The process is rather slow so that at P5 there are still only a few vessels in the neocortex (Fig. 3B). Most of the tissue that was examined for this study was from opossums at P5 since this gave preparations of the neocortex with a few blood vessels just beginning to appear in the neocortex. The brain vascular system in the North-American opossum has been reported to be different from eutherian mammals (Wislocki and Campbell, 1937; Wislocki, 1958) and it seems that Monodelphis domestica has a brain vascular system that is similar to that of its North-American counterpart. During vascularisation of the neocortex, independent arteries and veins in the pial plexus meet, merge and grow into the brain with a sharp end-loop. This pattern of vascularisation forms individual vascular units resembling trees, that can have multiple branches, and with the arterial and venous vessels always in close proximity of each other as is evident in Figure 2A, Figure 3F and Figure 4B.
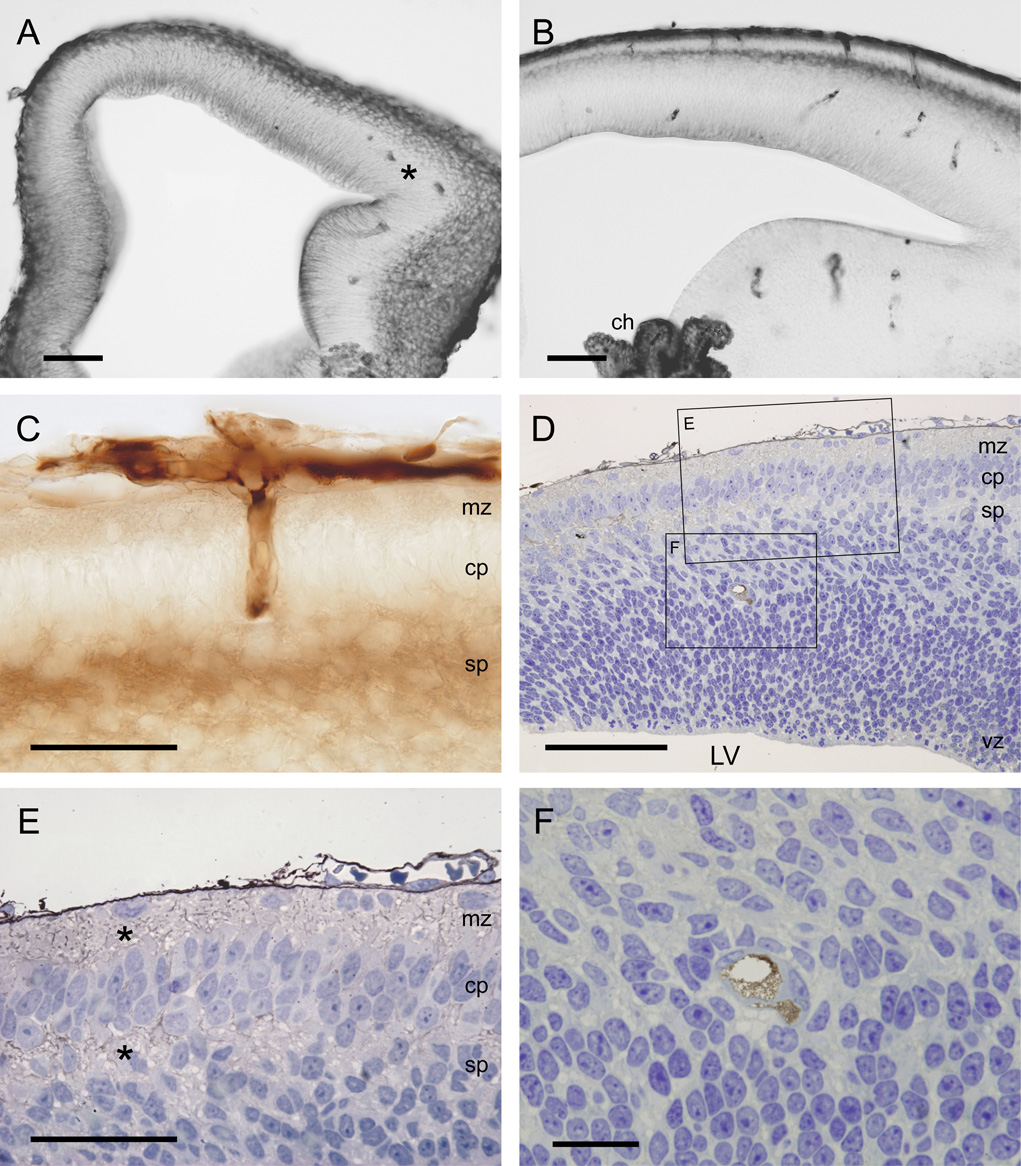
Figure 3.
Light micrographs showing the localisation of BED in neocortex of opossums at P2 (A) and P5 (B–F) 20–25min after an intraperitoneal injection. A–C are vibratome sections (50µm) and D–F semithin sections (0.5µm).
A) Note that at P2 there are no vessels present in the neocortex except for occasional one in the most lateral more developed parts (*).
B) At P5 a few vessels are present within the neocortex. Note also that the staining of BED is most abundant in outer and inner surfaces of the cortex and in the choroid plexus (ch).
C) High power micrograph showing vessels (paired vessels are characteristic of marsupial brains) growing into the neocortex. Note that the staining for BED is most visible within the vessels, in the marginal and subplate zones, similar to the staining for BDA3000 (see 4A).
D) Section of the whole neocortex counterstained with toluidine blue. This section shows that in the cortex the reaction product is most visible within the marginal and the subplate zones (shown in high power in E). Note also how few vessels are visible in the neocortex at this age (one pair in lower box and shown at high power in F).
E) Higher power view of upper box in D. Note reaction product in marginal zone (mz) and subplate zone (sp) indicated by *.
F) Higher power magnification of two paired blood vessels from lower box in D showing that at the light microscopic level it appears that only the lumen of the vessels is stained and no reaction product is visible in the extracellular space surrounding the vessels.
Scale bars are 100µm in A&B, 50µm in C, 100µm in D, 50µm in E and 20µm in F. Abbreviations: LV – lateral ventricle, mz – marginal zone, cp – cortical plate, sp - subplate, vz – ventricular zone.
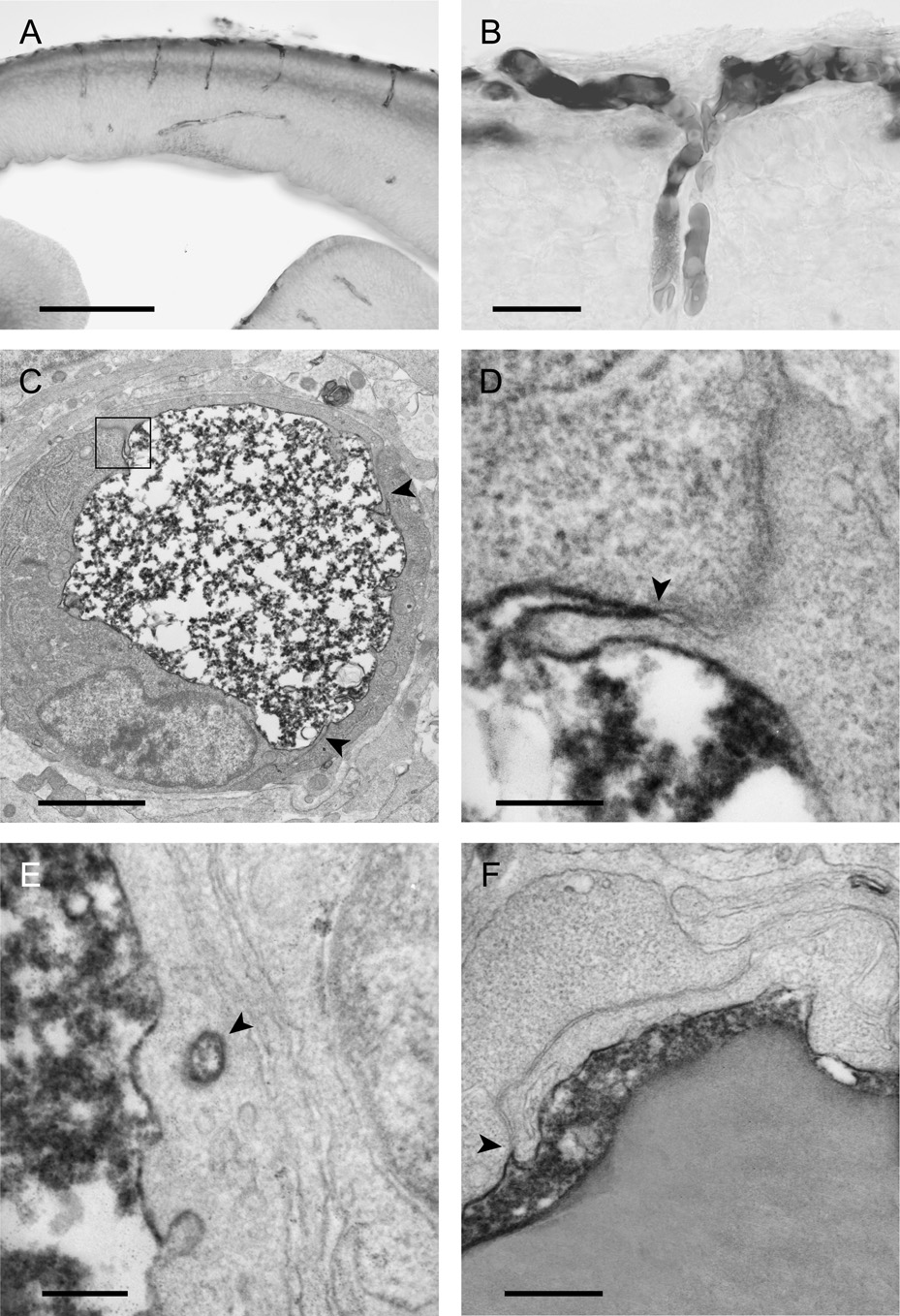
Figure 4.
The localisation of BDA3000 (biotin-dextran) in neocortex of opossums at P5 (A,B), P2 (C–E) and P12 (F) 20–25min (except A which is 40min) after an intraperitoneal injection. A and B are light micrographs of vibratome sections and C–F electron micrographs.
A) Staining is seen within blood vessels and towards the inner and outer surfaces of the cortex.
B) At high magnification it can be seen that two different pial vessels meet and grow into the brain together. Staining can only be seen inside the blood vessels and none in the surrounding tissue.
C) Cross section of a blood vessel which shows that the reaction product is abundant inside the lumen of the vessel, but is not visible in the immediately surrounding tissue. The arrowheads point to the intercellular clefts of the endothelial cell.
D) One of the interendothelial clefts from C (boxed in C) is shown at higher magnification. Arrowhead points to site of the tight junction.
E) Although vesicles that contain the reaction product (arrowhead) are present in the endothelial cells, these are not common.
F) A similar cleft to D at an older age (P12). Note in D and F that the reaction product is only present at the most luminal end of the cleft demonstrating that the tight junctions (arrowheads) between the endothelial cell restrict the movement of the tracer from blood into neural tissue.
Scale bars are 200µm in A, 25µm in B, 2µm in C, 200nm in D&E and 400nm in F.
Under the light microscope the staining pattern within the neocortex appears to be similar for both tracers after an ip injection (compare Fig 3B and Fig 4A). With light microscopy neither tracer is apparent in the tissue immediately around the blood vessels (Fig 3C and Fig 4B). At the electron microscopical level the reaction for BDA3000 is abundant in the lumen of these vessels, but none could be detected in the tissue immediately surrounding the vessels at the ages examined (Fig. 4C–F). Under high magnification it appears that the BDA3000 reaction product is only visible luminal to the site of the tight junctions (Fig. 4D, F), similar to the staining pattern in the adult (Fig. 2B). Although no vessels were found in the neocortex in the opossum at birth we examined many vessels in other parts of the brain at this age including the future piriform cortex, hippocampus, basal ganglia and pons. In no vessels is the reaction product for BDA3000 staining visible in the intercellular cleft, although it is abundant in the lumen of the vessels. These findings were consistent in all ages studied. Occasional vesicles in the endothelial cells are seen that contained the BDA3000 reaction product (Fig. 4E). The number of vesicular profiles appears to decrease somewhat during development. The extracellular spaces of outer layers of the brain, the marginal and the subplate zones, along with the ventricular zone contain large amounts of the reaction product for both BED (Fig. 3) and BDA3000 (Fig. 4A). The extracellular space of other layers of the brain appears devoid of reaction product for BDA3000 at the electron microscopic level (not illustrated). The localisation of the reaction product for BED under the electron microscope is illustrated in Figure 5. There is labelling of the perivascular Virchow-Robin spaces, which extend from the pial surface around the blood vessels penetrating into the neocortex (Fig. 5A). This perivascular staining is also present around some vessels deeper into the neocortex (Fig. 5B). In the marginal zone, some processes contain the BED reaction product (Fig. 5C). The electron microscope reveals that extracellular spaces in other parts of the cortex also contain the BED reaction product (Fig. 5D) although it is much less prominent than in the areas mentioned above. Light microscopic examination of the choroid plexus shows that the reaction product for BED is present on the apical brush border of the epithelial cells and also in some of the epithelial cells from animals at P2 and P5 (Fig. 6). In the meninges the reaction product is visible within blood vessels but also appears present in the subarachnoid space and in the dura (Fig. 6).
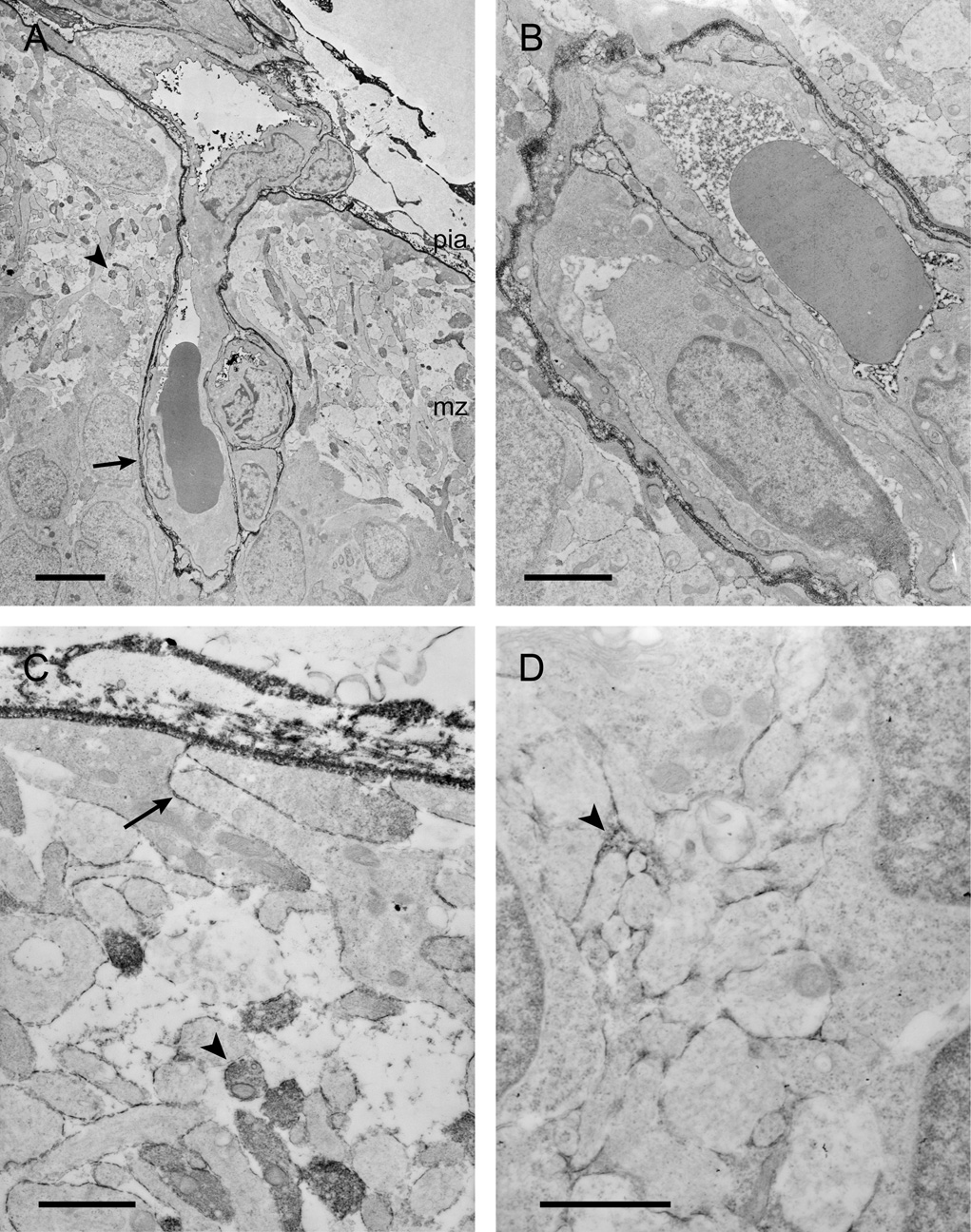
Figure 5.
Electron micrographs showing the distribution of BED in the neocortex of a P5 opossum 25min after an intraperitoneal injection.
A) A blood vessel that is penetrating into the neocortex from the pial plexus. Note that reaction product is visible in the perivascular (Virchow-Robin) space (arrow), inside the lumen of vessels and in the marginal zone. Some processes in the marginal zone are labelled (arrowhead).
B) A pair of blood vessels in the intermediate zone. Note the reaction product within the vessel lumen. Some of the vessels within this area have reaction product in the perivascular space which is presumably an extension of the Virchow-Robin space seen in A.
C) A high power micrograph of the pial surface and underlying marginal zone. Note that extracellular staining is apparent in between the cells (arrow) and some of the processes in the marginal zone (arrowhead).
D) Micrograph of the intermediate zone showing that the reaction product is visible in the extracellular space (arrowhead). This micrograph has been chosen to illustrate the extracellular staining; however, in many regions of the cortex the staining is not as prominent.
Scale bars are 5µm in A, 2µm in B, and 1µm in C&D.
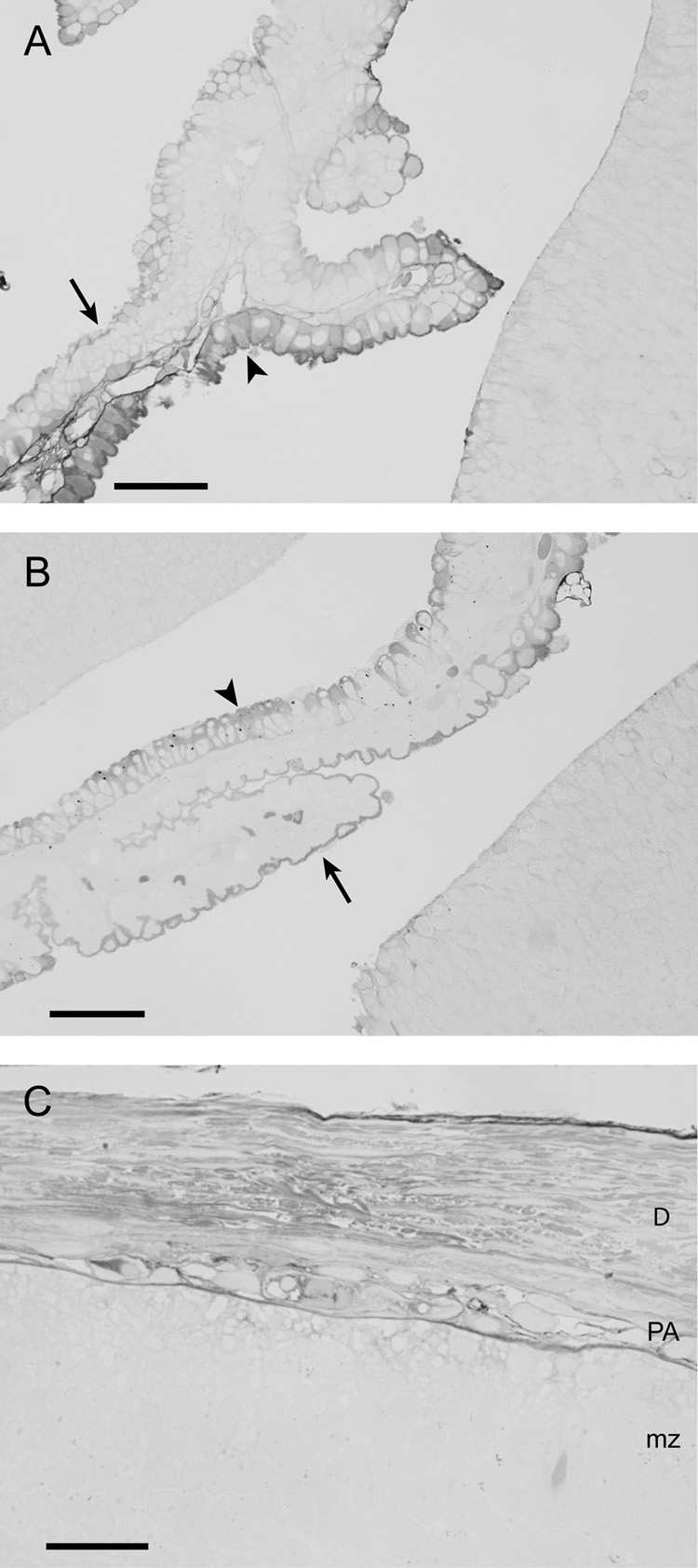
Figure 6.
Localisation of BED in choroid plexus at P2 (A), P5 (B) and in the meninges at P5 (C) 15min after an ip injection (1µm sections).
A&B) Many epithelial cells of the choroid plexus contain the tracer (arrowheads). Note also the staining of the brush border of the epithelial cells (arrows) indicating the presence of the tracer within CSF.
C) Apart from the tracer visible inside pial/arachnoid vessels, staining is also visible in the dura, subarachnoid space and extracellular space of the marginal zone. Note that there are no blood vessels present within the dura at this stage of development. Scale bars are 50µm in A&B and 25µm in C. Abbreviations: D – Dura mater, PA – pia-arachnoid, mz – marginal zone.
Diffusion of tracers in brains in vitro
In order to be sure that the penetration of marker into the surfaces of the brain was not due to an effect of brain fixation after ip injection of marker, brains at P5 were dissected out and immersed in isotonic saline containing individual tracers (see Methods). The results from these experiments are illustrated in Figure 7. After 5min BED has diffused into the subplate area from the outer surface of the brain and into the ventricular zone from the lateral ventricle. Little staining is visible in the cortical plate which is probably due to the small extracellular space of this layer compared to the marginal and subplate zones. After 25min of submersion in BED the whole cortex appears to have been penetrated by the tracer (Fig. 7B). Submersion in BDA3000 shows that the diffusion of this tracer is slower since after 25min it penetrated into the cortex to a similar extent as BED after 5min (compare Figs 7A and 7C).
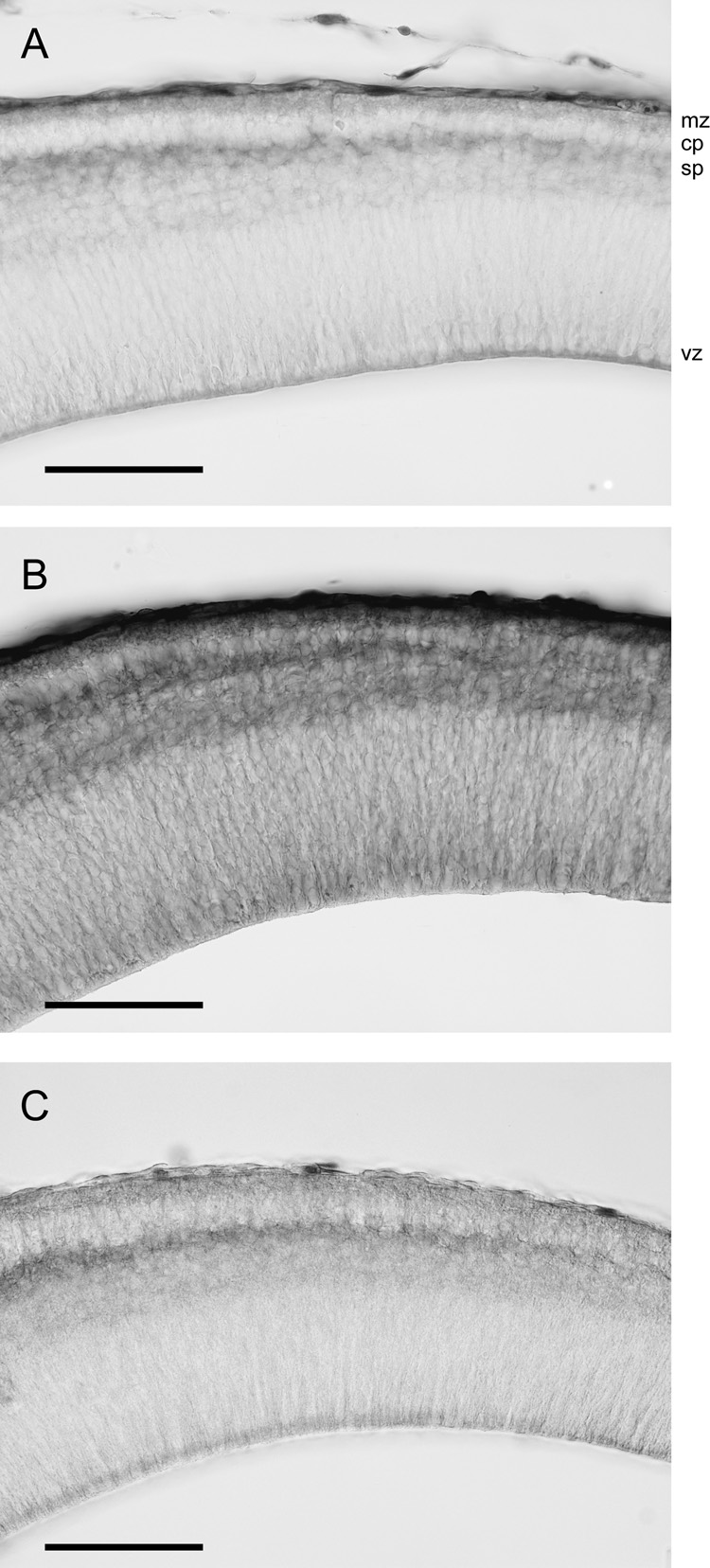
Figure 7.
Penetration of tracers through the neocortex at P5 after submersion of brains in solutions containing BED or BDA3000.
A) After 5min submersion of the brain BED has penetrated into the ventricular zone presumably from the lateral ventricle and into the subplate zone from the outer surface of the brain.
B) After 25min submersion in BED solution the tracer penetrates the whole neocortex.
C) After 25min submersion BDA3000 has a similar distribution in the neocortex as BED after 5min (see A).
Scale bar is 100µm for all micrographs. Abbreviations: mz – marginal zone, cp – cortical plate, sb – subplate, vz – ventricular zone.
Claudin-5 immunoreactivity
The localisation of claudin-5 was examined in opossum brains at various ages between P0 and adulthood. The results show that in the brain at birth, blood vessels in the midbrain and on the pial surface are positive for claudin-5 (Fig. 8A, C). Similar staining for claudin-5 of the first vessels that penetrate into the neocortex at P5 is shown in Figure 8B and at higher magnification in Figure 8D. No staining is visible in the parenchyma of the brain. This staining pattern is similar at all ages studied, but weaker in adults. Pre-absorption of claudin antibodies using lung homogenate abolished claudin-5 immunoreactivity in brain sections (Fig. 8E).
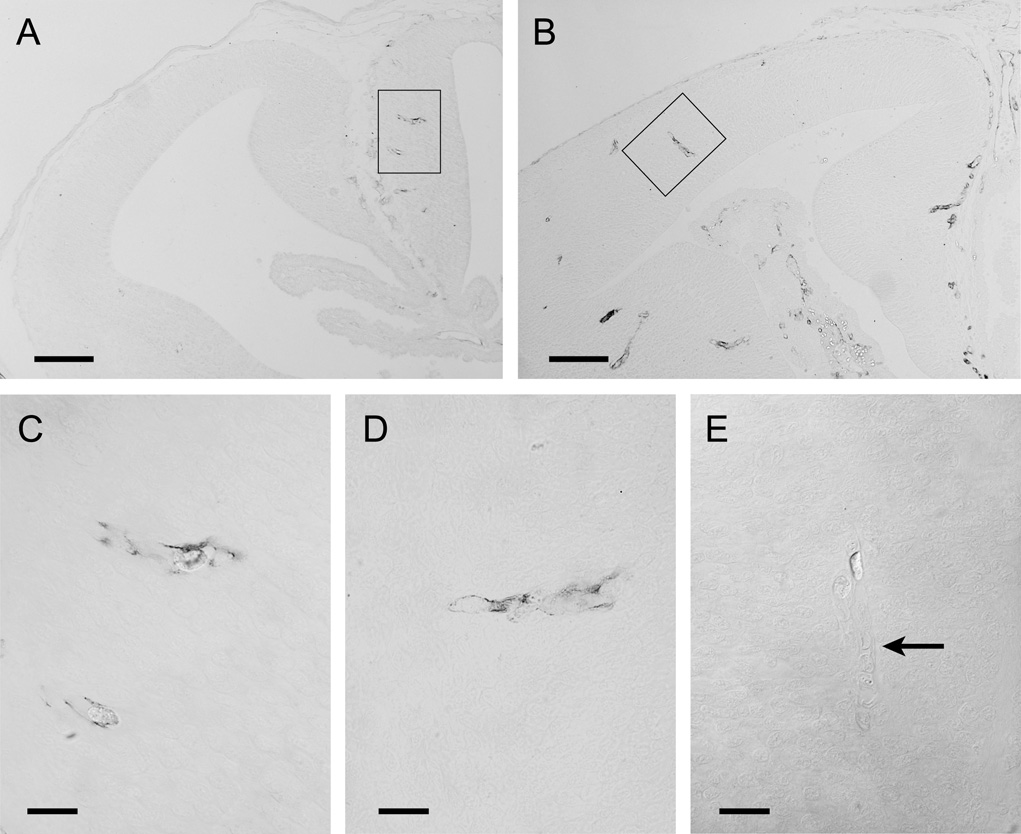
Figure 8.
Localisation of claudin-5 at P0 (A&C) and P5 (B&D), and negative control of claudin-5 staining at P5 (E) in opossum brains.
A) Although no vessels are present in the neocortex at P0, vessels in older parts of the brain are already immunoreactive for claudin-5 (box).
B) At P5, vessels in the neocortex are also immunoreactive for claudin-5. Note that staining in the choroid plexus is only within the stroma and not between the epithelial cells where the tight junctions of the blood-CSF barrier are located.
C) Two claudin-5 positive blood vessels shown at high power (box in A).
D) One cortical vessel positive for claudin-5 shown at high power (box in B).
E) Micrograph viewed with Nomarski optics showing that after absorption of anti claudin-5 antibody in adult lung homogenate, which is known to contain claudin-5, no staining is visible in brain blood vessels (arrow).
Scale bars are 100µm in A&B and 20µm in C–E.
DISCUSSION
Progress in understanding the nature of the brain barriers in the developing brain has been hampered by the lack of suitable small water soluble tracers that can be both measured quantitatively in blood, brain and CSF and visualised at the EM level. The present study shows that this problem can be overcome by using tracers that have been utilised for axonal tracing and investigating gap junction coupling of cells (Köbbert et al., 2000; Penn et al., 1994). We have previously tested the use of biotin-dextrans (Ek et al., 2001) as permeability tracers across brain barriers. The present study evaluates the use of a much smaller molecule, biotin ethylenediamine (286Da), for similar experiments. Felgenhauer (1974) and Dziegielewska et al. (1979) showed that CSF/plasma concentration ratios for a range of water soluble molecules were related to their estimated diffusion coefficients (D, see Fig. 1). A molecule’s CSF/plasma ratio can thus be predicted by its diffusion coefficient. In order to evaluate BED as a permeability tracer we measured its transfer from plasma to CSF and compared it to other small water soluble markers. CSF/plasma ratio for BED was found to be close to what would have been expected from its molecular weight (Fig. 1). This shows that the transfer mechanism of BED from blood into the CNS would be expected to involve similar processes to these other permeability markers and other water soluble molecules. This together with other tests performed showed that BED has favourable characteristics as a low molecular weight permeability tracer in that it is easy to visualise at the EM level with standard laboratory techniques, can be measured quantitively with HPLC, has a low binding to plasma proteins, is stable in plasma and seems to behave similarly to other small water soluble permeability markers across the brain barriers.
The present study shows that in the adult, neocortical blood vessels possess tight junctions that restrict the movement of molecules as small as BED (286Da) from blood into the brain (Fig. 2). This is an important observation because it is widely believed that there is a cut-off of 400–500Da in molecular size for penetration of lipid insoluble molecules from blood into brain in the adult (eg Pardridge, 1998). It is also thought that small molecules such as sucrose cross epithelial interfaces via tight junctions (see Madara, 1998) and that under some pathological conditions cerebral endothelial tight junctions may become more permeable to sucrose (Huber et al., 2001). However, since sucrose was not visualised in experiments that were interpreted as demonstrating tight junction permeability, this has not been definitively established. The present results showing that a molecule as small as BED does not appear to cross cerebral endothelial tight junctions suggest that earlier studies of epithelial and endothelial permeability to small molecules need to be repeated using such a visualizable marker. In development, the first blood vessels that grow into the neocortex also possess tight junctions that restrict blood to brain penetration of small molecules. The larger of the two molecules used in these studies, BDA3000, seems to be stopped by the tight junctions of the first blood vessels that grow into the neocortex and also of blood vessels in other parts of the brain. The smaller molecule BED was found in the extracellular spaces of the developing brain shortly after an ip injection. Because of this rapid penetration (probably via a CSF route, see below) we were not able to obtain the clear picture of tight junction exclusion of BED in the developing brain (see Fig. 3) that we saw in the mature brain (Fig. 2) and for BDA3000 in the immature brain (see Fig. 4). The extracellular staining for BED was strongest in the outer layers of the brain and in the ventricular zone. Many of the perivascular spaces were stained and reaction product was also present within the interendothelial cleft. The staining pattern of the large amount of reaction product found in the outer layers of the brain (Fig 3 and Fig 5) and in the ventricular zone indicates that much of the tracer penetrated into the brain from the CSF over the outer and inner (ventricular) surfaces of the brain. That this route of penetration occurs in the developing brain is supported by the experiments in which the brain was dissected out and simply submerged in a solution of BED (Fig. 7). In these experiments the tracer could only have entered the brain from the outer and inner surfaces and not through blood vessels. Five minutes of submersion in a BED solution yielded similar staining pattern of the neocortex as 20min after an ip injection of the tracer in vivo (compare Fig. 3B with Fig. 7A). These immersion studies also show that BED can penetrate throughout the whole neocortex within 25min and that BDA3000, as expected, has a much slower diffusion rate into the brain. The BED staining of perivascular spaces in the intermediate zone of the cortex (Fig. 5B) may be due to diffusion of tracer along perivascular (Virchow-Robin) spaces (Fig. 5A) from the CSF filled subarachnoid space. However, a more direct “leak” of tracer across the endothelial wall or cleft cannot be excluded. It is possible that there are two routes of entry into the brain for such small molecules in early brain development, one via the CSF and another across the blood vessels. Given how few blood vessels are present within the neocortex at this early age and the large amount of staining in the outer and inner layers of the neocortex, it seems likely that the main route of entry is via the CSF.
The importance of the paracellular pathway (generally considered to be via tight junctions) on permeability characteristics of epithelia has been extensively studied over the past 30 years (Diamond, 1974; Claude, 1978; Mitic and Anderson, 1998; Madara, 1998; Tsukita et al., 2001; Tsukita and Furuse, 2000, 2002). Much less work has been done on the permeability properties of tight junctions in cerebral blood vessels, although it seems to have been generally assumed that results obtained from epithelia would also apply to cerebral blood vessels (eg Wolburg and Lippoldt, 2002). The relative contribution of the transcellular versus paracellular transfer of various water soluble compounds and electrolytes across epithelia is still debated. Based on the transepithelial measurements of electrical resistance by Frömter and Diamond (1972) and freeze fracture studies of Claude and Goodenough (1973) it has been proposed that there is a correlation between epithelial permeability and tight junctional complexity (Claude and Goodenough, 1973). However, there are some exceptions to this correlation (see Stevenson et al. 1988 for a striking example and summary of literature). Also the size of the microelectrodes (1.5µm tip diameter) used for the transepithelial resistance measurements might not be able to distinguish between a paracellular pathway via tight junctions (estimated to be only 6–8Å, Milhorat et al., 1973) or a route through the cell membrane adjacent to the site of the intercellular tight junction. Actual measurements of transepithelial permeability have generally involved classical physiological markers such as radiolabelled sucrose or inulin. As pointed out in the Introduction, these have not been visualised in tissue sections, nevertheless it has been assumed that a molecule as small as sucrose crosses a tight epithelium such as the rabbit gallbladder via the tight junctions (van Os et al., 1974). The basis for this assumption seems to have been that since sucrose is used as an extracellular marker, it could only pass across an epithelial interface via the junctions (eg Flamion et al., 1995). Use of tracers that can be visualised at the EM level has usually involved horseradish peroxidase (HRP). This is a foreign protein of molecular size (40kDa) substantially greater than sucrose (342Da) and inulin (5000Da), thus it is inappropriate to extrapolate findings from HRP studies to tracer studies with low molecular weight. One of the few ultrastructural studies of epithelial permeability to employ a wide range of molecular size markers showed that HRP behaved anomalously in that it crossed the epithelium (parotid gland stimulated to secrete by isoproterenol) whereas the smaller microperoxidase and cytochrome c remained in the luminal spaces of acini and ducts (Mazariegos et al., 1984). These authors suggested that HRP may have membrane damaging effects; this is something which has been reported by other authors (eg Ross et al., 1977). Thus one has to be cautious in interpreting studies using only HRP to test blood-brain barrier integrity because it may have deleterious effects on cell membranes, effects that might be greater in the more delicate developing brain. Although it has generally been assumed that findings from epithelia can be extrapolated to endothelial cells, Ohno et al. (1978) in an extensive study of nonelectrolyte permeability in adult rats considered that their data were not consistent with permeability of cerebrovascular tight junctions to the molecules studied (glycerol, mannitol, sucrose and inulin).
A considerable amount of work has been carried out by the group of Tsukita on the molecular structure of tight junctions, particularly in epithelia but also more recently in cerebral endothelia (see reviews, Tsukita and Furuse, 2000; Tsukita et al., 1999; Tsukita et al., 2001). A recent developmental study from that group (Nitta et al., 2003) shows for the first time that it may be possible to dissociate blood-brain barrier permeability to large (protein) molecules from that to small polar molecules by selective knockout of a junctional constituent, namely claudin-5. Thus in the claudin-5 knockout mice, at E18.5, several low molecular weight markers (sulfo-NHS-biotin, 443Da; Hoechst H33258, 562Da; gadolinium-dethylene-triamine-pentaacetic acid, 742Da) penetrated into the brain, whereas microperoxidase (1862Da) and a dextran (10kDa) did not. However, this study did not include ultrastructural evidence for the route of penetration of small polar molecules from blood into brain. There is a similar lack of ultrastructural evidence in papers claiming to correlate changes in molecular constituents (eg claudins, occludin) of tight junctions in developing brain blood vessels and their permeability (eg Kniesel and Wolburg, 2000; Lee et al., 2003). The experiments of Nitta et al. (2003) were of short duration (5min or less) and thus the penetration of small molecules into immature brain via CSF, as suggested in Ek et al. (2001) and in this paper, might not have had time to occur. No information on the choroid plexus in the knockout mice was provided.
Given that the tight junctional protein claudin-5 has been implicated in brain endothelial cell tight junction function in adults (Nitta el al., 2003), we examined the presence of this protein in the developing opossum brain using immunocytochemistry. This showed that claudin-5 is present in cerebral endothelial cells at the time of birth in the opossum and also in the endothelial cells of the first vessels that grow into the neocortex (Fig. 8). That claudin-5 is present early in development is consistent with previous studies, which have shown that claudin-5 is present early in development of both mouse and human brain vessels (Morita et al., 1999; Virgitino et al., 2004) However, in the human it was noted that the immunolocalisation of claudin-5 did change from being mainly in the cytoplasm of endothelial cells in 12-week human fetuses to more linear bands in 14-week fetuses (Virgitino et al., 2004). The early presence of claudin-5 in developing brain blood vessels supports our findings based on permeability and ultrastructural evidence that cerebral blood vessel tight junctions are functional at an early stage of development.
The current study indicates that in development the main route of entry from blood to brain is via the CSF. In order to reveal possible blood to CSF pathways, the localisation of BED in the choroid plexus and within the meninges was also examined in some animals. Staining of some choroidal epithelial cells suggests that there is an intracellular route through the epithelial cells thereby by-passing the epithelial tight junctions (Fig. 6). Previous experiments with BDA3000 have shown similar intracellular staining (Ek et al. 2001, 2003) although the proportion of epithelial cells that are positive for BED appears to be higher than for BDA3000. In the meninges the tracer is localised not only within the blood vessels but also seen in the subarachnoid space and in the dura. The dura does not seem to contain blood vessels at these stages of development so the tracer that is found in the subarachnoid space could possibly come from either leaky pial/arachnoid vessels or have entered the CSF at some other site, such as the choroid plexus, and then been carried to the subarachnoid space by CSF flow. Ultrastructural studies of these vessels in the rat have shown that pial vessels are fenestrated in early development (a characteristic associated with leaky vessels) and later lose these fenestrations as they penetrate into the brain parenchyma (Stewart and Hayakawa, 1994). However, fenestrations have not been proven to account for the greater permeability of pial vessels. Bauer et al. (1993, 1995) found in mouse embryos that fenestrations disappeared as soon as blood vessels first penetrated into the immature neocortex and that these vessels were impermeable to Trypan blue (a low molecular weight dye that binds to albumin). Further studies of the barrier properties of both the choroid plexus and the pial vessels are needed in order to understand the overall significance of these different routes of entry from blood to CSF and the brain.
The present study has investigated barrier properties of blood vessels in early stages of brain development. This was done in a marsupial species because of the advantage that the pups are born at an early stage of brain development. That the blood to brain permeability of water soluble molecules is higher in younger animals has led to several hypothesis about its explanation. One of the most common suggestions is that the tight junctions in cerebral vessels, although present at an early stage of development, are less functionally restrictive to the passage of molecules (Risau et al., 1986; Kniesel et al., 1996; Wolburg and Lippoldt, 2002). The few developmental studies of neural endothelial tight junctions have mostly been morphological studies using freeze fracture or transmission electron microscopy; few have involved visualising permeability routes for probes across the endothelia. Morphological studies have produced contradictory results. Some indicated changes in structure of the tight junctions (Kniesel et al., 1996; Schulze and Firth, 1992; Stewart and Hayakawa, 1987; Stewart and Hayakawa, 1994). Others found no changes during development (Møllgård and Saunders, 1975; Xu and Ling, 1994). The studies that have shown differences in junctional structure between developing and adult animals have proposed this as a possible explanation for the higher blood to brain permeability in young animals although the correlations of blood-brain barrier permeability and tight junctional indexes during development are quite poor (see Introduction). Stewart and Hayakawa (1987) injected HRP into embryonic rats as young as E15 and Xu and Ling (1994) injected both lanthanum and ferritin into P1 rats but neither study found evidence of the probes passing through cerebral tight junctions. The former authors nevertheless suggested that changes in the structure are responsible for the permeability changes in the blood-brain barrier in development. The latter authors suggested that the main route across the endothelium is through pinocytotic vesicles in developing animals. Delorme et al. (1970) and later Wakai and Hirokawa (1978) reported that HRP can pass through tight junctions in chick embryos up to E10 and E15, respectively. There could be a difference between the developing mammalian and avian blood vessels, however, the studies of Wakai and Hirokawa (1978) have been criticised over the large amount of HRP that was administered to the embryos, which could be expected to have harmful effects (Saunders, 1992). The present study has used much smaller sized tracers in low concentrations and shows that the tight junctions restrict the movement of small molecules in early brain development and that the higher blood to brain permeability of water soluble molecules is likely to be due to a route from blood to brain via the choroid plexus and the CSF.
Supplementary Material
Acknowledgement
We would like to thank the National Institutes of Health (Grant Number 5RO1NS043949) and University of Melbourne (Melbourne University Early Career Grant) for financial support.
Supporting grants: National Institutes of Health Grant number 5RO1 NS043949
Melbourne University Early Career Grant
Literature cited
- Bass NH, Lundborg P. Postnatal development of bulk flow in the cerebrospinal fluid system of the albino rat: clearance of carboxyl-(14C)inulin after intrathecal infusion. Brain Res. 1973;52:323–332. doi: 10.1016/0006-8993(73)90668-9. [DOI] [PubMed] [Google Scholar]
- Bauer HC, Bauer H, Lametschwandtner A, Amberger A, Ruiz P, Steiner M. Neovascularization and the appearance of morphological characteristics of the blood-brain barrier in the embryonic mouse central nervous system. Brain Res Dev Brain Res. 1993;75:269–278. doi: 10.1016/0165-3806(93)90031-5. [DOI] [PubMed] [Google Scholar]
- Bauer H, Sonnleitner U, Lametschwandtner A, Steiner M, Adam H, Bauer HC. Ontogenic expression of the erythroid-type glucose transporter (Glut 1) in the telencephalon of the mouse: correlation to the tightening of the blood-brain barrier. Brain Res Dev Brain Res. 86:317–325. doi: 10.1016/0165-3806(95)00044-e. [DOI] [PubMed] [Google Scholar]
- Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969;40:648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claude P. Morphological factors influencing transepithelial permeability: a model for the resistance of the zonula occludens. J Membr Biol. 1978;3:219–232. doi: 10.1007/BF01870332. [DOI] [PubMed] [Google Scholar]
- Claude P, Goodenough DA. Fracture faces of zonulae occludentes from "tight" and "leaky" epithelia. J Cell Biol. 1973;58:390–400. doi: 10.1083/jcb.58.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme P, Gayet J, Grignon G. Ultrastructural study on transcapillary exchanges in the developing telencephalon of the chicken. Brain Res. 1970;22:269–283. doi: 10.1016/0006-8993(70)90471-3. [DOI] [PubMed] [Google Scholar]
- Diamond JM. Tight and leaky junctions of epithelia: a perspective on kisses in the dark. Fed Proc. 1974;33:2220–2224. [PubMed] [Google Scholar]
- Dziegielewska KM, Ek J, Habgood MD, Saunders NR. Development of the choroid plexus. Microsc Res Tech. 2001;52:5–20. doi: 10.1002/1097-0029(20010101)52:1<5::AID-JEMT3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Dziegielewska KM, Evans CA, Malinowska DH, Møllgård K, Reynolds JM, Reynolds ML, Saunders NR. Studies of the development of brain barrier systems to lipid insoluble molecules in fetal sheep. J Physiol. 1979;292:207–231. doi: 10.1113/jphysiol.1979.sp012847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek CJ, Habgood MD, Dziegielewska KM, Potter A, Saunders NR. Permeability and route of entry for lipid-insoluble molecules across brain barriers in developing Monodelphis domestica. J Physiol. 2001;536:841–853. doi: 10.1111/j.1469-7793.2001.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek CJ, Habgood MD, Dziegielewska KM, Saunders NR. Structural characteristics and barrier properties of the choroid plexuses in developing brain of the opossum (Monodelphis domestica) J Comp Neurol. 2003;460:451–464. doi: 10.1002/cne.10661. [DOI] [PubMed] [Google Scholar]
- Felgenhauer K. Protein size and cerebrospinal fluid composition. Klin Wochenschr. 1974;52:1158–1164. doi: 10.1007/BF01466734. [DOI] [PubMed] [Google Scholar]
- Ferguson RK, Woodbury DM. Penetration of 14C-inulin and 14C-sucrose into brain, cerebrospinal fluid, and skeletal muscle of developing rats. Exp Brain Res. 1969;7:181–194. doi: 10.1007/BF00239028. [DOI] [PubMed] [Google Scholar]
- Flamion B, Spring KR, Abramow M. Adaptation of inner medullary collecting duct to dehydration involves a paracellular pathway. Am J Physiol. 1995;268:F53–F63. doi: 10.1152/ajprenal.1995.268.1.F53. [DOI] [PubMed] [Google Scholar]
- Frömter E, Diamond J. Route of passive ion permeation in epithelia. Nat New Biol. 1972;235:9–13. doi: 10.1038/newbio235009a0. [DOI] [PubMed] [Google Scholar]
- Habgood MD, Knott GW, Dziegielewska KM, Saunders NR. The nature of the decrease in blood-cerebrospinal fluid barrier exchange during postnatal brain. J Physiol. 1993;468:73–83. doi: 10.1113/jphysiol.1993.sp019760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BT, Abbruscato TJ, Egleton RD, Brown RC, Huber JD, Campos CR, Davis TP. Nicotine increases in vivo blood-brain barrier permeability and alters cerebral microvascular tight junction protein distribution. Brain Res. 2004;1027:48–58. doi: 10.1016/j.brainres.2004.08.043. [DOI] [PubMed] [Google Scholar]
- Huber JD, Witt KA, Hom S, Egleton RD, Mark KS, T. P. Davis TP. Inflammatory pain alters blood-brain barrier permeability and tight junctional protein expression. Am J Physiol Heart Circ Physiol. 2001;280:H1241–H1248. doi: 10.1152/ajpheart.2001.280.3.H1241. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Woodbury DM. Changes in CSF flow and extracellular space in the developing rat. In: Vernadikis A, Weiner N, editors. Drugs and the developing brain. New York: Plenum Press; 1974. pp. 281–286. [Google Scholar]
- Kniesel U, Risau W, Wolburg H. Development of blood-brain barrier tight junctions in the rat cortex. Brain Res Dev Brain Res. 1996;96:229–240. doi: 10.1016/0165-3806(96)00117-4. [DOI] [PubMed] [Google Scholar]
- Kniesel U, Wolburg H. Tight junctions of the blood-brain barrier. Cell Mol Neurobiol. 2000;20:57–76. doi: 10.1023/A:1006995910836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott GW, Dziegielewska KM, Habgood MD, Li ZS, Saunders NR. Albumin transfer across the choroid plexus of South American opossum (Monodelphis domestica) J Physiol. 1997;499:179–194. doi: 10.1113/jphysiol.1997.sp021919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köbbert C, Apps R, Bechmann I, Lanciego JL, Mey J, Thanos S. Current concepts in neuroanatomical tracing. Prog Neurobiol. 2000;62:327–351. doi: 10.1016/s0301-0082(00)00019-8. [DOI] [PubMed] [Google Scholar]
- Lee S-W, Kim WJ, Choi YK, Song HS, Son MJ, Gelman IH, Kim Y-J, Kim K-W. SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat Med. 2003;9:900–906. doi: 10.1038/nm889. [DOI] [PubMed] [Google Scholar]
- Madara JL. Regulation of the movement of solutes across tight junctions. Ann Rev Physiol. 1998;60:143–159. doi: 10.1146/annurev.physiol.60.1.143. [DOI] [PubMed] [Google Scholar]
- Mazariegos MR, Tice LW, Hand AR. Alteration of tight junctional permeability in the rat parotid gland after isoproterenol stimulation. J Cell Biol. 1984;98:1865–1877. doi: 10.1083/jcb.98.5.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milhorat TH, Davis DA, Lloyd BJJ. Two morphologically distinct blood-brain barriers preventing entry of cytochrome c into cerebrospinal fluid. Science. 1973;180:76–78. doi: 10.1126/science.180.4081.76. [DOI] [PubMed] [Google Scholar]
- Mitic LL, Anderson JM. Molecular architecture of tight junctions. Ann Rev Physiol. 1998;60:121–142. doi: 10.1146/annurev.physiol.60.1.121. [DOI] [PubMed] [Google Scholar]
- Morita K, Sasaki H, Furuse M, Tsukita S. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol. 1999;147:185–194. doi: 10.1083/jcb.147.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møllgård K, Malinowska DH, Saunders NR. Lack of correlation between tight junction morphology and permeability properties in developing choroid plexus. Nature. 1976;264:293–294. doi: 10.1038/264293a0. [DOI] [PubMed] [Google Scholar]
- Møllgård K, Saunders NR. Complex tight junctions of epithelial and of endothelial cells in early foetal brain. J Neurocytol. 1975;4:453–468. doi: 10.1007/BF01261375. [DOI] [PubMed] [Google Scholar]
- Nabeshima S, Reese TS, Landis DM, Brightman MW. Junctions in the meninges and marginal glia. J Comp Neurol. 1975;164:127–169. doi: 10.1002/cne.901640202. [DOI] [PubMed] [Google Scholar]
- Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno K, Pettigrew KD, Rapoport SI. Lower limits of cerebrovascular permeability to nonelectrolytes in the conscious rat. Am J Physiol. 1978;235:H299–H307. doi: 10.1152/ajpheart.1978.235.3.H299. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. CNS drug design based on principles of blood-brain barrier transport. J Neurochem. 1998;70:1781–1792. doi: 10.1046/j.1471-4159.1998.70051781.x. [DOI] [PubMed] [Google Scholar]
- Penn AA, Wong RO, Shatz CJ. Neuronal coupling in the developing mammalian retina. J Neurosc. 1994;14:3805–3815. doi: 10.1523/JNEUROSCI.14-06-03805.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahner C, Mitic LL, Anderson JM. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology. 2001;120:411–422. doi: 10.1053/gast.2001.21736. [DOI] [PubMed] [Google Scholar]
- Ross MD, Nuttall AL, Wright CHG. Horseradish peroxidase acute ototoxicity and the uptake and movement of the peroxidase in the auditory system of the guinea pig. Acta Otolaryngol. 1977;84:187–201. doi: 10.3109/00016487709123957. [DOI] [PubMed] [Google Scholar]
- Risau W, Hallmann R, Albrecht U. Differentiation-dependent expression of proteins in brain endothelium during development of the blood-brain barrier. Dev Biol. 1986;117:537–545. doi: 10.1016/0012-1606(86)90321-0. [DOI] [PubMed] [Google Scholar]
- Saunders NR. Ontogenic development of brain barrier mechanism. In: Bradbury MWB, editor. Handbook of Experimental Pharmacology: Physiology and Pharmacology of the Blood-Brain Barrier. Berlin: Springer-Verlag; 1992. pp. 327–369. [Google Scholar]
- Saunders NR, Adam E, Reader M, Møllgård K. Monodelphis domestica (grey short-tailed opossum): an accessible model for studies of early neocortical development. Anat Embryol. 1989;180:227–236. doi: 10.1007/BF00315881. [DOI] [PubMed] [Google Scholar]
- Schulze C, Firth JA. Interendothelial junctions during blood-brain barrier development in the rat: morphological changes at the level of individual tight junctional contacts. Brain Res Dev Brain Res. 1992;69:85–95. doi: 10.1016/0165-3806(92)90125-g. [DOI] [PubMed] [Google Scholar]
- Stevenson BR, Anderson JM, Goodenough DA, Mooseker MS. Tight junction structure and ZO-1 content are identical in two strains of Madin-Darby canine kidney cells which differ in transepithelial resistance. J Cell Biol. 1988;107:2401–2408. doi: 10.1083/jcb.107.6.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PA, Hayakawa EM. Interendothelial junctional changes underlie the developmental 'tightening' of the blood-brain barrier. Brain Res. 1987;429:271–281. doi: 10.1016/0165-3806(87)90107-6. [DOI] [PubMed] [Google Scholar]
- Stewart PA, Hayakawa K. Early ultrastructural changes in blood-brain barrier vessels of the rat embryo. Brain Res Dev Brain Res. 1994;78:25–34. doi: 10.1016/0165-3806(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M. Pores in the wall: claudins constitute tight junction strands containing aqueous pores. J Cell Biol. 2000;149:13–16. doi: 10.1083/jcb.149.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Furuse M. Claudin-based barrier in simple and stratified cellular sheets. Curr Opin Cell Biol. 2002;14:531–536. doi: 10.1016/s0955-0674(02)00362-9. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M, Itoh M. Structural and signalling molecules come together at tight junctions. Curr Opin Cell Biol. 1999;11:628–633. doi: 10.1016/s0955-0674(99)00016-2. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- van Os CH, de Jong MD, Slegers JF. Dimensions of polar pathways through rabbit gallbladder epithelium. The effect of phloretin on nonelectrolyte permeability. J Membr Biol. 1974;15:363–382. doi: 10.1007/BF01870095. [DOI] [PubMed] [Google Scholar]
- Virgintino D, Errede M, Robertson D, Capobianco C, Girolamo F, Vimercati A, Bertossi M, Roncali L. Immunolocalization of tight junction proteins in the adult and developing human brain. Histochem Cell Biol. 2004;122:51–59. doi: 10.1007/s00418-004-0665-1. [DOI] [PubMed] [Google Scholar]
- Wakai S, Hirokawa N. Development of the blood-brain barrier to horseradish peroxidase in the chick embryo. Cell Tissue Res. 1978;195:195–203. doi: 10.1007/BF00236719. [DOI] [PubMed] [Google Scholar]
- Wislocki GB. The fine structure of the mammalian choroid plexus. In: Wolsteholme GEW, O'Connor CM, editors. Ciba foundation symposium on the cerebrospinal fluid. London: J&A Churchill; 1958. pp. 55–79. [Google Scholar]
- Wislocki GB, Campbell CP. The unusual manner of vascularization of the brain of the opossum (Didelphis virginiana) Anat Rec. 1937;67:177–189. [Google Scholar]
- Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- Xu J, Ling EA. Studies of the ultrastructure and permeability of the blood-brain barrier in the developing corpus callosum in postnatal rat brain using electron dense tracers. J Anat. 1994;184:227–237. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.