Abstract
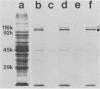
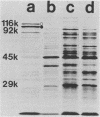
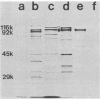
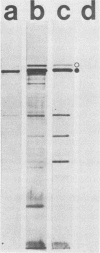
Cell-free culture supernatant (CFCS) prepared from Haemophilus influenzae type b (Hib) was examined for the presence of soluble Hib proteins. Two proteins with apparent molecular weights of 100,000 (100K) and 116K were predominant in the CFCS, and antibodies directed against these proteins could be detected by radioimmunoprecipitation or Western blot analyses of serum from adult rats immunized with Hib. Radioimmunoprecipitation analyses and sodium dodecyl sulfate-polyacrylamide gel electrophoresis of these two proteins demonstrated that the 100K CFCS protein was also present on the cell surface of Hib, whereas the 116K CFCS protein was only detectable in culture supernatants. Both the 100K and 116K CFCS proteins were immunogenic in human infants with Hib meningitis and in infant rats systemically infected with Hib. In addition, the first detectable antibodies produced in these Hib-infected rats against Hib proteins were specific for the 100K protein in both its CFCS and cell-associated forms. These two CFCS proteins were also immunogenic in rats immunized with CFCS in the absence of Hib infection. Monoclonal antibody directed against the 100K protein reacted with 34 of 55 Hib strains examined by using a colony blot radioimmunoassay. The immunogenicity of the 100K and 116K CFCS proteins suggests that one or both of these proteins may have potential for vaccine development, either by themselves or covalently coupled to Hib capsular polysaccharide.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. Antibody responses to Haemophilus influenzae type b and diphtheria toxin induced by conjugates of oligosaccharides of the type b capsule with the nontoxic protein CRM197. Infect Immun. 1983 Jan;39(1):233–238. doi: 10.1128/iai.39.1.233-238.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Pitt J., Smith D. H. Synthesis and release of polyribophosphate by Haemophilus influenzae type b in vitro. Infect Immun. 1976 Feb;13(2):581–589. doi: 10.1128/iai.13.2.581-589.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deich R. A., Hoyer L. C. Generation and release of DNA-binding vesicles by Haemophilus influenzae during induction and loss of competence. J Bacteriol. 1982 Nov;152(2):855–864. doi: 10.1128/jb.152.2.855-864.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigliotti F., Insel R. A. Protection from infection with Haemophilus influenzae type b by monoclonal antibody to the capsule. J Infect Dis. 1982 Aug;146(2):249–254. doi: 10.1093/infdis/146.2.249. [DOI] [PubMed] [Google Scholar]
- Gulig P. A., Frisch C. F., Hansen E. J. A set of two monoclonal antibodies specific for the cell surface-exposed 39K major outer membrane protein of Haemophilus influenzae type b defines all strains of this pathogen. Infect Immun. 1983 Nov;42(2):516–524. doi: 10.1128/iai.42.2.516-524.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., McCracken G. H., Jr, Frisch C. F., Johnston K. H., Hansen E. J. Antibody response of infants to cell surface-exposed outer membrane proteins of Haemophilus influenzae type b after systemic Haemophilus disease. Infect Immun. 1982 Jul;37(1):82–88. doi: 10.1128/iai.37.1.82-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Haemophilus influenzae type b: disease and immunity in humans. Ann Intern Med. 1973 Feb;78(2):259–269. doi: 10.7326/0003-4819-78-2-259. [DOI] [PubMed] [Google Scholar]
- Hansen E. J., Frisch C. F., Johnston K. H. Detection of antibody-accessible proteins on the cell surface of Haemophilus influenzae type b. Infect Immun. 1981 Sep;33(3):950–953. doi: 10.1128/iai.33.3.950-953.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Frisch C. F., McDade R. L., Jr, Johnston K. H. Identification of immunogenic outer membrane proteins of Haemophilus influenzae type b in the infant rat model system. Infect Immun. 1981 Jun;32(3):1084–1092. doi: 10.1128/iai.32.3.1084-1092.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Robertson S. M., Gulig P. A., Frisch C. F., Haanes E. J. Immunoprotection of rats against Haemophilus influenzae type B disease mediated by monoclonal antibody against a haemophilus outer-membrane protein. Lancet. 1982 Feb 13;1(8268):366–368. doi: 10.1016/s0140-6736(82)91394-0. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Cell membrane antigen isolation with the staphylococcal protein A-antibody adsorbent. J Immunol. 1976 Nov;117(5 Pt 1):1482–1490. [PubMed] [Google Scholar]
- McDade R. L., Jr, Johnston K. H. Characterization of serologically dominant outer membrane proteins of Neisseria gonorrhoeae. J Bacteriol. 1980 Mar;141(3):1183–1191. doi: 10.1128/jb.141.3.1183-1191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R. S., Jr, Shenep J. L., Barenkamp S. J., Granoff D. M. Purification and comparison of outer membrane protein P2 from Haemophilus influenzae type b isolates. J Clin Invest. 1983 Aug;72(2):677–684. doi: 10.1172/JCI111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä P. H., Peltola H., Käyhty H., Jousimies H., Pettay O., Ruoslahti E., Sivonen A., Renkonen O. V. Polysaccharide vaccines of group A Neisseria meningtitidis and Haemophilus influenzae type b: a field trial in Finland. J Infect Dis. 1977 Aug;136 (Suppl):S43–S50. doi: 10.1093/infdis/136.supplement.s43. [DOI] [PubMed] [Google Scholar]
- Robertson S. M., Frisch C. F., Gulig P. A., Kettman J. R., Johnston K. H., Hansen E. J. Monoclonal antibodies directed against a cell surface-exposed outer membrane protein of Haemophilus influenzae type b. Infect Immun. 1982 Apr;36(1):80–88. doi: 10.1128/iai.36.1.80-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneerson R., Barrera O., Sutton A., Robbins J. B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980 Aug 1;152(2):361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller P. F., Smith A. L., Smith D. H., Anderson P. Role of immunity in the clearance of bacteremia due to Haemophilus influenzae. J Infect Dis. 1978 Oct;138(4):427–436. doi: 10.1093/infdis/138.4.427. [DOI] [PubMed] [Google Scholar]