Abstract
Leptin is well known as a hormone important in the central control of appetitive behaviors via receptor-mediated actions in the hypothalamus, where leptin adjusts food intake to maintain homeostasis with the body’s energy stores. Recent evidence has shown that leptin and its receptors are widespread in the central nervous system and may provide neuronal survival signals. This review summarizes our current knowledge of how leptin functions in the brain and then focuses on the ability of leptin to mitigate neuronal damage in experimental models of human neurological disorders. Damage to the brain by acute events such as stroke, or longterm loss of neurons associated with neurodegenerative diseases, including Parkinson’s and Alzheimer’s disease, may be amenable to treatment using leptin to limit death of susceptible cells. Leptin-mediated pro-survival signaling is now known to prevent the death of neurons in these models. The signaling cascades that leptin generates are shared by other neuroprotective molecules including insulin and erythropoietin, and are thus a component of the neurotrophic effects mediated by endogenous hormones. Coupled with evidence that leptin dysregulation in human disease also results in enhanced neuronal susceptibility to damage, development of leptin as a therapeutic methodology is an attractive and viable possibility.
Keywords: Neurodegeneration, stroke, epilepsy, MEK/ERK, PI3-K
Introduction
The hormone leptin was originally discovered in 1994 through its involvement in the homeostatic regulation of body weight (Halaas, et al., 1995). The 16 kDa protein encoded by the obese (ob) gene is primarily synthesized in adipose tissue, and was first linked to obesity by demonstrating its importance in controlling body mass size via inhibition of appetitive behaviors (Halaas, et al., 1995; Pelleymounter, et al., 1995). Leptin is taken into the brain across the blood brain barrier, where its novel and initially only known functional role was in the hypothalamus, inhibiting the arcuate nucleus. Since then, in addition to its roles in feeding and homeostatic energy control, leptin is now known to exert significant effects on reproduction (Fujioka, et al., 1999), thermogenesis (Hwa, et al., 1996), synaptic plasticity (Shanley, et al., 2001) and, more recently, neuroprotective activity in divergent brain regions that impinges on neurodegenerative processes (Weng, et al., 2007; Zhang, et al., 2007).
These recently discovered functions have rekindled research into how the mammalian brain responds to leptin. The potential for using leptin as a therapeutic treatment for brain injury and neurodegenerative conditions has been spurred on by the fact that it has demonstrated anti-apoptotic and neuroprotective effects and that its therapeutic potential and safety has already been established for the treatment of human obesity due to leptin deficiency (Heymsfield, et al., 1999). Here we review the known signaling mechanisms of leptin and the current understanding of how leptin reverses the loss of brain tissue to insults that are relevant to human diseases.
Leptin synthesis and uptake into the Brain
The amount of leptin that crosses the blood brain barrier is much less than what is found in the periphery. After its release by adipose tissue into the blood system, the transport of leptin across the blood brain barrier occurs via specific mechanisms, concentrating it to about 7 nM (12 ng/ml) in the cerebrospinal fluid (Kurrimbux, et al., 2004). The primary system for transport is proposed to be saturable and transfers leptin unidirectionally from the blood and into the brain parenchyma, while any excess leptin is cleared via the cerebral spinal fluid (CSF) (Banks, et al., 1996; Schwartz, et al., 1996a; Banks, et al., 2000a; Maresh, et al., 2001). Based on the high expression of particular leptin receptor isoforms in the choroid plexus, blood vessels and leptomeninges, it is possible that some forms of the leptin receptor itself are involved in binding and transport of leptin across the blood brain barrier (Golden, et al., 1997; Bjorbaek, et al., 1998b; Kastin, et al., 1999). An alternate hypothesis would utilize an altogether non-leptin or leptin receptor related carrier as the primary transporter of circulating leptin (Banks, et al., 2002). One such protein that is believed to have leptin transporter function is megalin, or low-density lipoprotein receptor-mediated protein-2. Megalin is a multi-ligand receptor found on many epithelia and there is evidence that transport of leptin across the proximal convoluted tubules of the kidney is mediated by megalin as part of normal leptin metabolism (Hama, et al., 2004). Megalin is also found in the choroid plexus, and has been proposed to mediate leptin entry into the brain as well as the formation of leptin resistance in neurodegenerative disease (Dietrich, et al., 2007). A non-saturable process has also been proposed, where the increase of leptin in the cerebral spinal fluid is at a relatively constant fraction of the blood concentration, or about 1/400, although no specific carrier system has yet been described for this process (Fujioka, et al., 1999). Overall, the leptin transport system functions at a level on par with other similarly sized proteins such as the adipokine interleukin-1a (Banks, et al., 1991). Transport of leptin is not identical throughout the brain, however. Different regions take up greater or lesser amounts of leptin, with portions of the hypothalamus showing the highest uptake (Faouzi, et al., 2007). Other brain areas with substantial transport include the hippocampus, cortex, thalamus, striatum and midbrain (Banks, et al., 2000b).
In addition to adipose tissue, it is now known that leptin is also synthesized by other tissues and organs. These include the placenta and fetus (Hoggard, et al., 1997), skeletal muscle (Wang, et al., 1998), heart (Purdham, et al., 2004), and stomach (Bado, et al., 1998). There is also evidence for leptin synthesis in the brain itself. In rats, brain regions that contain high levels of leptin receptors also show leptin mRNA and protein, and which colocalize with neurons (Morash, et al., 1999). It is yet to be determined if leptin produced endogenously by the brain is functionally independent from that produced in the periphery. Since the concentration of leptin is considerably low in the CSF even after exogenous supplementation (Fujioka, et al., 1999), an endogenous source of leptin production may provide signaling that is more relevant to brain areas outside of the hypothalamus. Leptin that is generated in the brain itself might conceivably signal in manners analogous to the neurotrophins, including paracrine and autocrine release and signaling. An autocrine function for leptin produced by the heart has been implicated in hypertrophy of myocytes (Rajapurohitam, et al., 2003; Rajapurohitam, et al., 2006). Endogenous synthesis and release of leptin by the brain may help explain how localized leptin production could be involved in promoting the survival of neurons.
Production of leptin in the brain is not universal. Expression of leptin is well conserved phylogenetically, and has been confirmed in amphibians (Boswell, et al., 2006), rat (Morash, et al., 1999), sheep (Ehrhardt, et al., 2002), pig (Smolinska, et al., 2004) and human (Knerr, et al., 2001). A notable exception is that expression of the leptin gene is not found in the adult mouse brain (Zhang, et al., 1994). The species-specific expression of leptin can cause variable or contradictory results when leptin is used in different animal models and humans. These divergent results may argue against the functional significance of brain-produced leptin, or may instead indicate that leptin bioavailability is different between species. This has not yet been fully investigated although, as explained below, the capacity of soluble leptin receptors to bind leptin may decrease available leptin in tissues. Decreased availability of leptin to the brain is now known to be the basis for the paradoxical situation in obesity, wherein obese individuals often have highly elevated blood levels of leptin in response to vastly increased adipocyte mass (Schwartz, et al., 1996a). These high levels would be expected to depress appetite, but fail to do so. Experiments demonstrate that triglycerides can reduce leptin transport across the blood brain barrier (Banks, et al., 2004). Diminished transport of leptin across the blood brain barrier would occur in the presence of higher triglyceride levels in obese individuals, and is therefore thought to be directly responsible for the lack of elevated brain leptin levels in obesity. This change in the leptin transport system may be an adaptive response to fasting, during which the body begins to break down and release triglycerides and where any additional anorexigenic leptin signal would be counterproductive for longterm survival. This also explains the failure of exogenously administered leptin to treat obesity in some individuals, as no matter how much peripheral leptin levels are increased, it is simply prevented from entering the brain. The modulation of leptin availability to the brain is thus more than a conjectural possibility and can alter leptin function by unanticipated means. This may be a rationale behind some of the variable results of experiments between species and in human trials for the treatment of obesity.
Leptin Receptor Signaling
There are six forms of the leptin receptor (ObR, a-f), and splice-variants of the gene were first cloned from mouse choroid plexus, diabetes (db) (Tartaglia, et al., 1995; Lee, et al., 1996; Wang et al., 1996; Guan et al., 1997; Lee, et al., 1997). The Ob receptors are members of the interleukin-6 receptor family of the class I cytokine receptor superfamily (Baumann, et al., 1996). There are three structural groupings that the six Ob receptors are generally classified, the short (ObRa,c-d,f), long (ObRb) and soluble (ObRe) forms (for review, see (Hegyi, et al., 2004). The external leptin-binding N-terminal domains are identical among all variants. All forms except ObRe contain a transmembrane domain, and the short forms contain truncated intracellular domains (34 amino acids long for ObRa) compared to the long form (ObRb, 303 amino acids). The long form, which is 1162 amino acids in total length, contains three additional tyrosine phosphorylation consensus sites on its intracytoplasmic tail compared with the short forms (Tartaglia, 1997). It is thought that many of the physiological actions of leptin, in particular those controlling feeding and energy balance, are due to the long form of the leptin receptor because of its greater ability to activate downstream signaling cascades. Mutation of the long form results in an alternate splicing to a receptor resembling ObRa and that produces the db phenotype (Lee, et al., 1996; Fei, et al., 1997; Tartaglia, 1997). The short forms are less involved in leptin-activated intracellular signaling but instead appear important in mediating the transfer of leptin from the periphery through the blood brain barrier. The best evidence available so far proposes that the soluble form of the receptor, ObRe, is in fact a leptin-binding variety that mediates the bioavailability of leptin in general (Tu, et al., 2008). The ObRa and ObRc short forms are abundantly expressed on the blood brain barrier microvessels and may be involved in the normal transport of leptin into the brain and in the choroid plexus, where they shuttle leptin from the brain to the cerebral spinal fluid (Tartaglia, et al., 1995; Golden, et al., 1997; Bjorbaek, et al., 1998b). See Table 1 for summary of leptin receptor isoform functions.
Table 1. Leptin receptor isoforms in the brain.
Listed are the six known isoforms for the leptin receptor, ObR, and their general classification as short, long or soluble forms. Location lists the brain tissues and regions with the greatest expression, and does not include areas with extremely low or variable levels. Signaling cascades lists the major pathways activated, with None? signifying that significant signaling in vivo is as yet unproven.
| Leptin Receptor Isoform | Size (aa) | Location | JAK/STAT Activation | Signaling Cascades | Function | |
|---|---|---|---|---|---|---|
| ObRa | Short | 894 | Most abundant short form; high in BBB, choroid plexus, piriform cortex, thalamus, hypothalamus, hippocampus, insular cortex, cerebellar granule cells; lower levels seen in the cerebral cortex | Low | STAT3 MEK/ERK | Leptin transport into endothelial cells; removal and degradation of leptin |
| ObRb | Long | 1162 | Less abundant than short forms, in many tissues of nervous system; greatest concentration in piriform cortex, thalamus, hypothalamus, hippocampus, substantia nigra compacta, cerebellar granule cells; lower levels seen throughout the cerebral cortex | High | STAT3 MEK/ERK CREB PI3-K | Primary signaling isoform |
| ObRc | Short | 892 | Low expression; BBB, choroid plexus, cerebellar granule cells | Low | None? | Leptin transport into endothelial cells, other? |
| ObRd | Short | 901 | Low expression; BBB, choroid plexus | Low | None? | Leptin transport into endothelial cells, other? |
| ObRe | Soluble | 805 | Secreted, blood | None | - | Binds circulating leptin; modulation of leptin bioavailability to BBB |
| ObRf | Short | 896 | Low expression; BBB, choroid plexus, cerebellar granule cells | Low | None? | Leptin transport into endothelial cells, other? |
Abbreviations used: aa = length of receptor in the mouse, in amino acids; BBB = blood brain barrier microvessels.
Intracellular signaling by all forms of the leptin receptor is similar to the class I cytokines, utilizing comparable cascades (Figure 2) (Bjorbaek, et al., 1997). This class of receptors have no intrinsic enzymatic activity of their own. Upon ligand binding to first associate as homodimers, each leptin receptor moiety forms a complex with a cytoplasmic-associated kinase, the Janus tyrosine kinase (JAK) (Taga and Kishimoto, 1997). For the Ob receptors, it is specifically JAK2 that associates with the ObR JAK-binding motifs and responds to leptin binding by undergoing autophosphorylation, thereby becoming activated (Ihle, 1995). The cascade that is initiated upon JAK2 activation is transmitted further downstream by recruitment of a number of other signaling molecules. The major element that JAK2 phosphorylates is the transcription factor signal transducer and activators of transcription (STAT), of which STAT3 seems to be the usual target for ObR mediated signaling (Ghilardi, et al., 1996; Carpenter, et al., 1998), although there is some evidence in cell lines that STAT5B can also be activated (Baumann, et al., 1996). Activation of STAT3 includes its dimerization, which then allows it to translocate to the nucleus and influence the expression of a number of genes, including socs3 (Bjorbaek, et al., 1998a). A negative feedback loop exists wherein SOCS3 inhibits JAK2 signaling (Bjorbaek, et al., 2000). In addition, another comparatively minor phosphorylation target for JAK2 is the SH2/SH3 domain-containing adaptor protein GRB2. The direct activation of GRB2 results in increased p21RAS activity and of the Ras-Ref mitogen activated protein kinase (MEK), which promotes extracellular signal-regulated kinase (ERK, primarily ERK1/2) activity and induction of gene expression such as c-fos (Banks, et al., 2000a). The activation of ERK1/2 by the short forms of ObR via GRB2 recruitment is the only signaling cascades by which they can signal. The long form of the leptin receptor, in addition to the pathways described above, includes two additional indirect cascades by which a much greater recruitment of GRB2 and activation of STAT3 is possible. This is due to the longer intracytoplasmic tail of ObRb containing three tyrosine phosphorylation sites that do not exist in any of the short forms (Bjorbaek, et al., 1997). Phosphorylation of one of these sites (Y1138) by JAK2 allows for the additional recruitment of inactive STAT3 to ObRb, and consequently greater opportunity for phosphorylation by JAK2 (Banks, et al., 2000a). The phosphorylation of a second extra tyrosine (Y985) similarly recruits the protein tyrosine phosphatase SHP2, yet another target for JAK2 phosphorylation. The SHP2 phosphatase complexes with GRB2 and subsequently enhances ERK1/2 activity. Thus, the long form of ObR has two additional pathways with which it can further activate both of the major intracellular leptin cascades that leptin signaling utilizes, resulting in a much greater signaling capacity than any of the short forms.
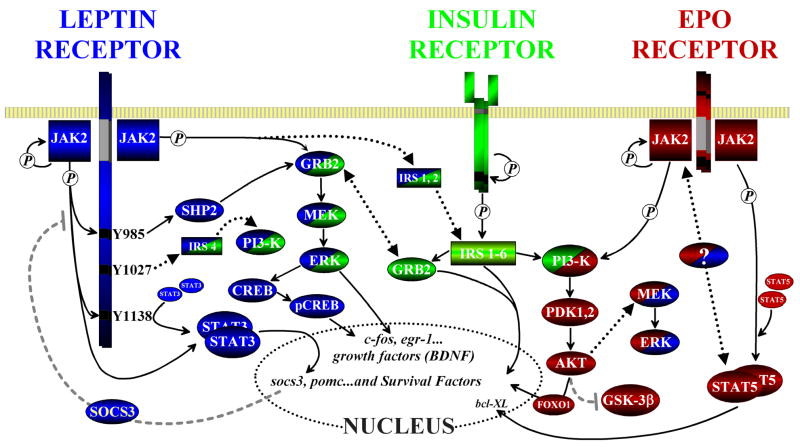
Figure 2. Leptin receptor signaling and crosstalk with the insulin and erythropoietin receptors.
The leptin receptor shares several signaling cascades with other receptors found in the brain. Here the pathways are shown along with known and possible sites of crosstalk between them. Both the leptin and erythropoietin (EPO) receptors become functional dimers upon ligand binding, allowing their associated JAK proteins to undergo activation and autophosphorylation (P). Insulin receptors form a larger heterotetramer of α and β subunits, which undergoes insulin-induced autophosphorylation. For leptin, the second messenger kinase JAK2 contributes to several different cascades. One of its major actions is to phosphorylate the three tyrosine residues on the ObRb intracytoplasmic loop. The Y1138 residue will recruit STAT3 monomers, aiding in STAT3 activation by increasing the rate of phosphorylation and dimerization by JAK2. The phosphorylation of Y985 instead recruits SHP2 (also phosphorylated by JAK2), binds and activates GRB2. Activation of the SHP2/GRB2 complex will then activate the MEK/ERK signaling pathway, a pathway shared by all three receptor cascades shown above. One interesting consequence of activation of this particular pathway that we have demonstrated includes the leptin/ERK-dependent stimulation of BDNF production, which occurs via phosphorylation of the transcription factor CREB. JAK2 also directly activates two molecules, STAT3 and (to a smaller degree) GRB2 by phosphorylation and association with SHP2. STAT3 dimerization allows it to translocate to the nucleus and affect the transcription of a number of factors that mediate neuronal activity, survival and a negative feedback loop on JAK2 activity via socs3. Leptin also has a significant and more direct crosstalk with the insulin receptor via JAK2 mediated activation of IRS1,2 and Y1077 interaction with IRS4. Insulin receptor signaling depends on these molecules to activate effector cascades, including MEK/ERK via GRB2 and PI3-K. Subsequent signaling by these cascades induce the prosurvival factor AKT/PKB and, further downstream, the inhibition of GSK-3β and transcriptional control via FOXO1, which is involved in modulation of gluconeogenic responses. Erythropoietin-mediated activation of JAK2 activates both the MEK/ERK pathways shared by leptin and insulin, and activates STAT5 to alter transcription. There is some evidence from cell lines (see text) that insulin can activate STAT5 in addition to STAT3. Although not shown to occur in bona fide neurons, the ability of JAK2 to signal via other common substrates might represent another avenue of crosstalk between leptin, erythropoietin and other neurotrophic factors.
Abbreviations and terms used: Akt/PKB: AKT kinase/protein kinase B; CREB: cAMP response element-binding; ERK: extracellular signal-regulated kinase; FOXO1: forkhead transcription factor; GRB2: Growth factor receptor-bound protein 2; GSK-3β: Glycogen Synthase Kinase-3β; IRS: insulin receptor substrate; JAK: janus tyrosine kinase; PI3-K: phosphatidylinositol 3-kinase; MEK: mitogen-activated protein kinase kinase; PDK1,2: pyruvate dehydrogenase kinase 1,2; SHP2: SH2 domain-containing protein-tyrosine phosphatase PTPN11; STAT: signal transducer and activators of transcription. The initial phosphorylation events are indicated (circled “P”), while further downstream events are depicted as activating (black arrows) or inhibiting (dashed grey lines) the pathway. The large, black dotted arrows denote crosstalk between the leptin, insulin and erythropoietin receptor cascades. Additionally, the signaling elements with dual coloration further identify the shared pathways.
In addition to the activation of the MAPK/ERK1/2 and STAT3 pathways, leptin receptors are also able to signal via components of the insulin-signaling pathway (Niswender, et al., 2003; Niswender, et al., 2004). This crosstalk is not very surprising, given that leptin and insulin are both involved in energy homeostasis and feeding. For example, in a manner similar to leptin, insulin injected intracerebroventricularly reduces food intake and body weight in monkeys (Woods, et al., 1979). Insulin functions via its receptor by recruiting and phosphorylating insulin receptor substrates (IRS), with downstream targets including the activation of MAPK and the phosphatidylinositol 3-kinase pathways (PI3-K), which subsequently activates the prosurvival factor AKT/PKB (for review, see Plum et al. 2005). A functional link between leptin and PI3-K signaling is indeed known, as leptin-induced anorexia and PI3-K activity in the hypothalamus can be prevented by PI3-K inhibitors (Niswender, et al., 2001). Further experimental evidence shows that the leptin receptor interacts with IRS-1 in microglia (Tang, et al., 2007) and IRS-4 in a hypothalamic cell line (Wauman, et al., 2007). Phosphorylation of the additional intracytoplasmic tyrosine Y1077 on ObRb is required for this latter activity. These interactions are shown in Figure 2. The insulin signaling system is widespread in the brain, and includes brain regions of interest to neurodegenerative diseases. For example, IRS-3 colocalizes with dopaminergic neurons in the ventral tegmental area of the rat brain (Pardini, et al., 2006), a nucleus with a known functioning leptin signaling system (Fulton, et al., 2006). Thus, the ability of leptin receptors to communicate directly with the insulin receptor allows it to expand the signaling cascades to include a third major pathway, the PI3-K.
Leptin receptor distribution and function in brain
Functional leptin receptors have been found extant in many regions of the brain. The ventral hypothalamus, in particular the arcuate nucleus, has the greatest density of leptin receptors (Schwartz, et al., 1996b). The high numbers of leptin receptors in the arcuate nucleus are expected, which correlate with the functional role leptin has in modulating feeding. Leptin also plays a role in the development of the hypothalamic brain circuits involved in feeding and in several other brain regions including the neocortex (Udagawa and Otani, 2007). Of further interest are the substantial numbers of ObR found in extra-hypothalamic nuclei.
The distribution of leptin receptors in other brain regions includes the piriform cortex, thalamus, cerebellum, midbrain, hippocampus, brainstem and diffuse signals in the neocortex (Elmquist, et al., 1998; Figlewicz, et al., 2003; Mutze, et al., 2006). The midbrain contains two dopaminergic nuclei that have very different functional roles but neurons in both contain significant leptin receptor expression (Figlewicz et al. 2003). One nucleus, the ventral tegmental area, is well known for its involvement in the reward system via the mesolimbic and mesocortical pathways, and is critical for reward behaviors (see Fields et al. 2007 for review). Leptin receptors found in the ventral tegmental area are also involved in the central control of feeding and hedonic responses to food (Hommel, et al., 2006). This finding not only confirms and expands leptin’s involvement for appetitive behaviors, it also demonstrates that leptin is biologically active in extra-hypothalamic regions and can influence behaviors specifically associated with that brain area. The dopaminergic neurons in the substantia nigra pars compacta, the lateral group of midbrain dopamine producing cells, are also immunopositive for leptin receptors (Figlewicz et al. 2003). The nigral dopaminergic neurons are well known for their involvement in the control of movement (Fisone, et al., 2007). Curr.ly, no known normal homeostatic functions have yet been ascribed to the leptin receptors found there. Leptin is also involved in the processing of olfactory signals, in particular during the fasting state (Julliard, et al., 2007). In both the neocortex and hippocampus, leptin is involved in the acquisition of plasticity (Oomura, et al., 2006; Harvey, 2007). The list of brain areas and normal functions that leptin modulates is being expanded to include other extra-hypothalamic areas, and is likely to increase further with continued studies.
Neuroprotection by leptin
The search for an effective therapy that slows down or even reverses neuronal damage from brain injuries, such as the acute damage from stroke or slowly accumulated impairment from longterm neurodegenerative processes, is ongoing. Dysregulation of growth factors, cytokines and related prosurvival signaling molecules may be a contributing factor in the development of neurodegeneration in Parkinson’s disease (PD) and other diseases associated most often with aging (Enwere, et al., 2004; Levy, et al., 2005; Mattson and Magnus, 2006). The evidence that leptin is involved in influencing the viability of cells is well documented. Leptin can inhibit apoptotic cell death that is part of the normal life cycle of several peripheral cell types including lymphocytes (Howard, et al., 1999; Fujita, et al., 2002), pancreatic β-cells (Shimabukuro, et al., 1998) and hepatic stellate cells (Saxena, et al., 2004). The very high levels of leptin seen in obesity have been linked to pathological conditions, including prosurvival effects on colon, breast and prostate cancer (Rouet-Benzineb, et al., 2004; Schaffler, et al., 2007) and inflammation (Gil, et al., 2007).
Experiments performed using cancer cell lines shows that leptin has direct cell death-suppressing effects (Somasundar, et al., 2003; Hoda, et al., 2007). Neurons in culture require the presence of neurotrophic factors or serum for survival, and the abrupt withdrawal of either results in neuronal death. Leptin has been reported to reduce cell death caused by serum withdrawal in neuroblastoma cells (Russo, et al., 2004) or removal of neurotrophic factor from hippocampal neurons (Guo, et al., 2008). The neuroprotective mechanisms of leptin appear to involve the activation of JAK2-STAT3, AKT and MEK/ERK signaling pathways (Russo, et al., 2004; Guo, et al., 2008).
There is also growing experimental evidence that leptin has neuroprotective properties in the central nervous system. The first studies to demonstrate that leptin has protective effects on neuronal-like cells were on cell lines (Dicou, et al., 2001; Lu, et al., 2006). Since then, a number of studies have extended these initial findings to include protective effects to bona fide neurons in both in vitro and in vivo models of human disease. Leptin has been tested clinically for use in treating leptin-deficiency, and appears to be tolerated well with minimal side effects (Heymsfield, et al., 1999). Augmenting endogenous leptin signaling may be a viable therapeutic strategy for the treatment of conditions or disorders that damage the brain, including cerebral ischemia and epilepsy. Unfortunately, although neurotrophins can indeed rescue dopaminergic neurons from neurotoxins in relevance to PD, their usefulness as a treatment methodology for PD and other neurodegenerative diseases have shown only limited and variable efficacy in clinical trials (Tomac, et al., 1995; Lang, et al., 2006). Since leptin, as both a peripherally-derived and a CNS-endogenously synthesized molecule, is involved in the normal function of the brain, exogenous manipulation of leptin signaling has the potential to be both efficacious and well accepted by the brain. Figure 1 summarizes the factors influencing neurodegeneration including Parkinson’s disease, stroke and epilepsy and the relevance of leptin signaling in the brain.
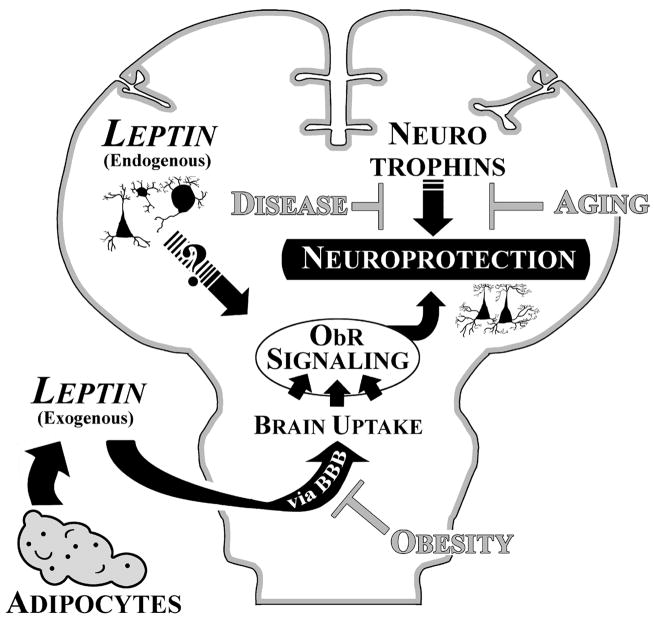
Figure 1. Leptin neuroprotection in the brain.
Schematic illustration of the two possible sources of brain leptin and how leptin may foster neuroprotection against ageing and disease. Adipocytes are the major leptin-producing organ in the periphery (“Exogenous”). Peripheral leptin can be bound in the serum by soluble leptin receptor (ObRe) and or taken up across the blood brain barrier (BBB). High levels of triglycerides, as found with obesity, can create a form of leptin resistance by diminishing its BBB transport, and causing very high levels of leptin to accumulate in the periphery because of increased adipocyte production. Leptin can also be synthesized by neuronal elements in the brain itself (“Endogenous”), although the signaling contribution made by this source of leptin is still unknown. Signaling via leptin receptors (ObR), along with the homeostatic contribution by the brain’s host of neurotrophins and other endogenous trophic molecules, act to maintain longterm neuronal viability. A decrease in the neuroprotective or increase in the harmful influences may tip the scales against susceptible neuronal populations.
Parkinson’s Disease
Halting or preventing the progression of neurodegenerative diseases by treatment with exogenous factors has been the target of many studies, including Parkinson’s disease (PD). This debilitating disorder is the second most prevalent neurodegenerative disease, next to Alzheimer’s disease. A large component of PD pathogenesis, which is responsible for the primary clinical manifestations of PD including motor disturbances, is caused by the loss of a restricted population of dopaminergic neurons. These cells reside in the substantia nigra, which form the nigrostriatal pathway to the striatum and play a role in the control of movement. Leptin itself may be involved in the homeostatic regulation of the nigrostriatal pathway. Leptin-deficient mice have lowered overall dopamine stores in midbrain dopamine neurons, resulting in diminished neurotransmission capacity (Roseberry, et al., 2007). Likewise, a decline in the number of D2 type dopamine receptors was found in the striatum of obese individuals (Wang, et al., 2001). Higher body mass index is associated with diminished brain leptin availability, and there may be an interaction between leptin and dopamine function in the Parkinsonian brain.
Leptin can reverse the loss of dopaminergic neurons in a commonly used model of PD that employs the dopamine cell specific neurotoxin, 6-hydroxydopamine (6-OHDA) (Weng, et al., 2007). In both in vitro and in vivo experimental paradigms, dopaminergic neurons and dopamine-mediated behavior were protected from 6-OHDA induced toxicity by exogenously administered leptin. Thus, not only were leptin-treated dopaminergic neurons less susceptible to 6-OHDA toxicity, but increased leptin preserved the functionality of the nigrostriatal tract for two months after treatment. Critical signaling mechanisms involved in leptin-mediated neuroprotection of dopaminergic cells were found to be via the activation of JAK-STAT, MEK/ERK and GRB2. Further downstream, activation of ERK1/2 and enhanced nuclear localization of the transcription factor phospho-CREB were vital effectors. Leptin decreased the activity of the pro-apoptotic caspases-3 and -9, as well as other markers of apoptosis. Evidence was also shown that these neuroprotective effects occurred via ObR receptor activation. Use of an Ob-R receptor antagonist or knockdown of the leptin adaptor signaling proteins JAK2 or GRB2 resulted in loss of protection and loss of activation of ERK1/2. Therefore, leptin-mediated neuroprotection in the 6-OHDA PD model did indeed occur via the specific activation of the leptin receptor and its second messenger signaling systems (Weng, et al., 2007).
The neurotrophin-activated signaling mechanisms are often quite complex, and the opportunities for crosstalk to occur between multiple neurotrophin receptor-mediated signaling pathways are common (Levy, et al., 2005; Tardito, et al., 2006). As discussed above and shown in Figure 2, leptin can act in concert with portions of the insulin receptor signaling cascade, in particular to increase the activity of the PI3-K pathway (Plum, et al., 2005). Diminished expression of brain insulin receptor is found in PD and Alzheimer’s disease (Moroo, et al., 1994; de la Monte and Wands, 2005). Therefore, reductions in the signaling system of both leptin and insulin may have a synergistic effect on the aged brain and contribute to the pathophysiology of neurodegenerative diseases such as PD. Leptin can increase the protein expression levels of brain-derived neurotrophic factor (BDNF) following leptin receptor activation (Komori, et al., 2006; Weng, et al., 2007). BDNF is a well-known survival factor for dopaminergic neurons and is also diminished in PD (Nagatsu, et al., 2000). BDNF functions by binding to the TrkB receptor kinase and subsequent activation of signaling cascades that include PI3-K and MAPK/ERK; thus it utilizes many of the same intermediary signaling molecules as leptin, including SH2 and GRB2 (Kaplan and Miller, 2000). Since both leptin and BDNF activate common signaling cascades, leptin may induce a form of positive feedback by increasing BDNF expression that may in turn continue to activate those same signals. Likewise, the hematopoietic hormone erythropoietin has also been shown to be neuroprotective in models of PD, primarily via the activation of the PI3-K/AKT pathway (Signore, et al., 2006). The parallel activation of these two pathways, the MAPK/ERK1/2 by leptin and PI3-K/AKT by erythropoietin, could act in concert to produce significantly greater neuroprotection together than either activate individually. In the periphery, leptin acts in concert with and increases the secretion of erythropoietin to stimulate immature erythroid development (Axelsson, et al., 2005). There is currently no direct evidence that leptin and erythropoietin can function synergistically in the nervous system; however, the transcriptional regulator that induces erythropoietin production, hypoxia-inducible factor-1 (Sasaki, et al., 2000), can also transactivate the leptin gene in a trophoblast-derived cell line (Grosfeld, et al., 2002) and in non-adipose tissue (Meissner, et al., 2005). Since the induction of erythropoietin in dopaminergic cultures by hypoxia results in greatly enhanced dopamine-cell survival (Studer, et al., 2000), there could well be a functional interaction between leptin- and erythropoietin-mediated neuroprotection.
Ischemic stroke
Ischemic stroke is a medical emergency triggered by a rapid reduction in blood supply to localized portions of the brain, usually due to thrombosis or embolism, which leads to neuronal dysfunction and death in the affected brain areas. Stroke is the third leading cause of death and the leading cause of long-term disability in the United States and Europe. The major mechanisms of neuronal death after stroke include necrosis by deprivation of oxygen and glucose, glutamate-mediated excitotoxicity, oxidative stress and the subsequent triggering of apoptosis (Lipton, 1999).
A successful cure for stroke has been particularly difficult to develop due in large part to the extreme vulnerability of neurons to deprivation of oxygen and nutrition. Currently, the only FDA-approved treatment of medication for stroke is the clot dissolving tissue plasminogen activator (tPA), which must be administrated within three hours after the onset of ischemia. Unfortunately, only about 5% of stroke patients have the opportunity to receive effective tPA treatment due to the short time window for treatment and other complications of the stroke itself. Therefore, other strategies including neuroprotection are being explored for the treatment of stroke. Leptin may be a realistic candidate to treat stroke as it has demonstrated neuroprotection against ischemic neuronal injury in both in vitro and in animal models of stroke (Zhang et al. 2007).
Stroke produces significant alterations in brain tissue that survives the initial insult. Many normal biochemical processes are altered and even become pathophysiological, producing damage that continues to occur after the stroke event itself. Of primary concern is the reperfusion-induced changes that accompany the resumption of blood flow into the formerly occluded brain regions, as occurs in cardiac arrest or after tPA treatment restores blood flow. Inhibiting these responses would potentially significantly reduce stroke-induced damage. Several lines of evidence show that leptin can indeed target several of these processes and diminish ischemic cell death, including excitotoxicity, oxidative stress, and apoptosis.
Neuronal excitotoxicity plays a key role in the neuronal necrosis after cerebral ischemia, which results from excessive accumulation of glutamate around neurons. The subsequent excessive stimulation of glutamate receptors results in cytosolic calcium overload that indiscriminately activates calcium-dependent processes. The protective effects of leptin against glutamatergic excitoxicity was first reported in mouse neurons by Dicou, et al (Dicou, et al., 2001). They found that the pretreatment of primary neuronal cultures with leptin for 20 hours rescues neurons from cell death induced by NMDA, and that the JAK2 inhibitor AG490 antagonized the neuroprotective effects of leptin. They also showed that the co-injection of leptin intracerebrally in postnatal day 5 mouse pups reduced cortical lesion size and white matter cysts by 50% induced by the injection of ibotenate, a glutamate analogue. This neuroprotective effect of leptin was confirmed later by Guo, et al., who reported that pretreatment of rat hippocampal neurons with leptin for 24 hours increased the number of surviving neurons after NMDA treatment (Guo, et al., 2008).
Oxidative stress is a significant cause of ischemic reperfusion injury and neuronal apoptosis following cerebral ischemia. Neuronal exposure to ferrous (Fe2+) iron can result in oxidative stress and membrane lipid peroxidation through hydroxyl radical production. The report by Guo, et al. (Guo, et al., 2008) demonstrated that leptin significantly improves neuronal survival after exposure of rat hippocampal neurons to ferrous iron, and that neuroprotection conferred by leptin is long-term.
Apoptosis is the predominant mode of neuronal death occurring due to hypoxia-ischemia in neonate rats and in global ischemia; apoptosis also plays an important role in the enlargement of the infarct induced by focal cerebral ischemia, especially in the so-called penumbral region (Zhang, et al., 2004). An anti-apoptotic role of leptin has been reported recently in neuroblastoma cells following growth factor withdrawal (Russo, et al., 2004; Guo, et al., 2008). In these studies, the anti-apoptotic effects of leptin required the activation of JAK2-STAT3, MEK/ERK and PI3K/AKT signaling pathways. The down-stream of anti-apoptotic mechanisms also includes the up-regulation of Mn-SOD and Bcl-xL (Guo, et al., 2008) as well as down-regulation of caspase-10 and TNF-related apoptosis-inducing ligand (Russo, et al., 2004).
All of the above-discussed studies were carried out in vitro and only examined one or two of the many parallel mechanisms that contribute to ischemic neuronal injury in humans. The next critical step is to determine whether leptin is efficacious in reducing damage caused by ischemic stroke in an animal model. Our own data demonstrate that intraperitoneal administration of leptin decreased infarct volume following middle cerebral artery occlusion in mice (Zhang et al. 2007). Leptin protection was dose-dependent, and remained effective even when leptin administration was delayed up to 90 min after the onset of reperfusion. Ischemia-induced behavioral changes were also significantly reversed by leptin. Activation of the ERK1/2 signal pathway by leptin was found to be the major protective mechanism, and the activation of CREB and STAT3 signaling pathways were also involved.
Leptin has demonstrated neuroprotective effects against ischemic stroke, with evidence that this protection is mediated by leptin receptors in the brain. This finding makes leptin a potential neuroprotective agent in the treatment of human stroke events. A promising approach might be to use leptin in tandem with tPA by administering it following tPA treatment. This dual treatment may extend the time window of efficacy for tPA treatment, and subsequently reduce reperfusion injury.
Epilepsy
Epilepsy is a relatively common neurological problem, and seizure activity is often associated with neuronal cell death. There is increasing evidence that leptin is both neuroprotective against seizures and has anticonvulsant properties in most seizure models. The hippocampus, regarded as the brain region most susceptible to seizure activity, expresses leptin-receptors functionally coupled to STAT3 activation (Shanley, et al., 2002a; Guo, et al., 2008). Leptin can inhibit the firing of hippocampal neurons via activation of large conductance calcium-activated potassium channels (BK) (Shanley, et al., 2002b). These channels are important in determining the excitability of these neurons, and may contribute to aberrant firing such as during seizure activity. One study has revealed, however, that leptin can also be a proconvulsant. Using the penicillin-induced seizure model, leptin increased epileptiform-like spike activity in rat brain (Ayyildiz, et al., 2006).
Leptin itself has in fact been implicated in epilepsy, although the mechanisms by which it acts are not yet understood. Obesity, where high plasma leptin levels but diminished leptin transport occurs, is a risk factor for epilepsy. The induction of leptin, however, may be one of the benefits from epileptic patients who have been treated by being placed on the ketogenic diet. This high fat, low carbohydrate diet can increase leptin plasma serum levels in rats (Kinzig, et al., 2005; Thio, et al., 2006) and is anti-epileptogenic.
Recent laboratory data do support a more explicit role for leptin in preventing seizures and neuronal toxicity. Leptin protects hippocampal neurons against excitotoxicity in leptin deficient ob/ob mice, which are more prone to seizures (Erbayat-Altay, et al., 2006). This model induced seizure activity by glutamate receptor activation using either glutamate in vitro or kainate infused intracerebroventricularly (Guo, et al., 2008). Likewise, seizure activity induced by different chemical models, in the rat neocortex with intracerebral injections of 4-aminopyridine (a voltage-gated potassium channel inhibitor) or intraperitoneal injections of pentylenetetrazole (a non-competitive γ-aminobutyric acid antagonist) into mice, was significantly diminished when the animals were pretreated with leptin (Xu, et al., 2008). Of particular interest in this latter study is the methodology used to deliver leptin. The hormone was delivered to mice via intranasal injection, demonstrating that a non-invasive delivery of leptin can produce significant physiological responses in the brain including neuroprotection. Leptin levels in both serum and brain were also measured in this study; both increased above background levels 30 min after injection. Injections of radiolabeled leptin have also demonstrated the feasibility of the intranasal route for leptin delivery (Fliedner, et al., 2006). Thus, there is proof of principle that intranasal injection can be a viable administration route for the treatment of pathophysiological and perhaps neurodegenerative brain conditions.
Hippocampal plasticity and diabetes
In addition to its neuroprotective characteristics, leptin can modulate synaptic plasticity in the brain. The induction of longterm potentiation and longterm depression (LTP and LTD) has been implicated in the cellular and molecular basis of memory formation. Leptin can alter learning and memory in the rodent hippocampus by facilitating or inhibiting both LTP and LTD (Shanley, et al., 2001; Li, et al., 2002; Durakoglugil, et al., 2005), and similarly, in the retention of behavioral tasks (Farr, et al., 2006). Several direct mechanisms are known to contribute to these effects. One is leptin-induced functional enhancement of NMDA receptors, which are critically involved in most models of learning and memory (Oomura, et al., 2006). Another is hippocampal neuron hyperpolarization by leptin-activation of the BK potassium channels (Shanley, et al., 2002b). In addition to these signaling effects, structural remodeling of hippocampal dendritic processes, also associated with synaptic plasticity, is sensitive to leptin via NMDA receptors and MEK/ERK signaling pathways (O'Malley, et al., 2007).
If leptin can induce hippocampal plasticity, then the converse situation, where deficient leptin signaling contributes to pathophysiology in neuronal plasticity, might also be true. A lack of proper leptin signaling results in memory impairment and neuronal excitability and has been well characterized in the leptin receptor deficient db/db mice and Zucker fa/fa rats (Li, et al., 2002). These animal lines show deficiencies in neuronal and behavioral plasticity comparable to those found in rats with streptozotocin-induced diabetes (Biessels, et al., 1996; Biessels, et al., 1998) as well as in both type I (insulin deficiency) and type II (insulin resistance) diabetes in humans (Desrocher and Rovet, 2004; Greenwood and Winocur, 2005; Messier, 2005). Since the deficiencies in hippocampal plasticity in these cases are independent of insulin levels, per se, they may instead be due to the loss of leptin and insulin functioning in concert, as insulin also modulates activity-dependent hippocampal NMDA receptor function (van der Heide, et al., 2005).
Other neurophysiological changes occurring in both animal models of diabetes and diabetics contribute to altered neuronal plasticity. In particular, the hypothalamic pituitary adrenal (HPA) axis is very sensitive and is overactive in diabetes (Tsigos, et al., 1993; see Convit, 2005 for review). Because of increased HPA axis tone, glucocorticoid and, specifically, cortisol levels are elevated, which are negatively correlated with learning and memory during stress and disease states (Oei, et al., 2006; Bruehl, et al., 2007; and see Leonard, 2007 for review). The hippocampus is most sensitive to the harmful effects of high glucocorticoids, and in diabetes, the hippocampus is even more vulnerable to these injurious effects (Magarinos and McEwen, 2000). The susceptibility of neurons to damage by enhanced glucocorticoid levels has been well documented in the brain, where longterm stressors increase the predisposition to many pathological conditions, including diabetes, and to impair neuronal plasticity (see Sapolsky, 2003 for review). The combination of enhanced stress response compounded by disease such as diabetes may then act in synergy, and over time producing a positive feedback cycle that progressively interferes with and degrades the normal functioning of the nervous system (Akiray, et al., 2004). Finally, neurogenesis in the dentate gyrus, another more recently recognized hallmark of neuronal plasticity in the adult hippocampus, is compromised in diabetic rodents (Zhang, et al., 2008). Learning and memory deficits in diabetic rodents were restored by reinstatement of normal physiological levels of cortisol, which also prevented the decrease in adult neurogenesis (Stranahan, et al., 2008).
Potential involvement of leptin in other neurodegenerative diseases
Continuing research is recognizing an increased number of roles that leptin plays in various other neurodegenerative diseases. Although leptin itself may not be a component in the primary causative mechanism of these diseases, dysregulation of leptin function in the brain contributes to aspects of the disease that are just now being realized. In Alzheimer’s disease, leptin can decrease the load of Aβ protein by inhibiting β-secretase activity (Fewlass, et al., 2004). In Huntington’s disease, patients have diminished circulating leptin levels (Popovic, et al., 2004). When dietary restriction is used in a mouse model of Huntington’s disease, there is decreased weight loss and an increase in brain BDNF levels, while leptin levels are slightly above normal (Duan, et al., 2003). Not all effects of leptin are beneficial, however. In multiple sclerosis and other inflammatory states, adipokines including leptin have emerged as important proinflammatory hormones that can influence the progression of the disease state (Matarese, et al., 2005; Frisullo, et al., 2007); for review, see Lago et al. (Lago, et al., 2007). Intracerebroventricular injection of leptin can induce cyclooxygenase-2 expression in the hypothalamus, a major proinflammatory signal (Inoue, et al., 2006). As discussed earlier, leptin is also an antiapoptotic signal for cancer cells, and the very high levels of leptin in obesity and metabolic syndrome may contribute to the increased risk of developing cancer under these altered metabolic states (Hsing, et al., 2007).
Conclusions
The evidence that leptin has beneficial effects in a variety of neurodegenerative diseases is now firmly established. The widespread distribution of leptin, its receptors and leptin-mediated functional cascades found in many parts of the brain demonstrate that leptin has functional properties beyond its original role in appetitive behaviors and energy homeostasis. Leptin receptors can activate several divergent signaling cascades that promote neuroprotective effects, namely the MAPK/ERK, STAT3 transcription factor and, via crosstalk with insulin and erythropoietin receptor components, PI3-K/AKT activity. Of particular interest to neurodegenerative diseases associated with aging is the demonstration that leptin can induce neurotrophic signals. Leptin treatment may be a workable therapeutic methodology that can reverse aging or disease associated deficiency in growth factors and helps prevent such detrimental processes as oxidative damage occurring in the aging nervous system. The fact that leptin has already been tested and is well tolerated for the treatment of some forms of obesity bodes well for its use in neurodegenerative disease states.
Acknowledgments
Research for the work in this paper was by: NIH Grants NS36736, NS44178, NS43802, NS56118 and NS45048, as well the V.A. Merit Review.
References
- Akiray EM, Chan O, Inouye K, Riddell MC, Matthews SG, Vranic M. Partial leptin restoration increases hypothalamic-pituitary-adrenal activity while diminishing weight loss and hyperphagia in streptozotocin diabetic rats. Metabolism. 2004;53:1558–1564. doi: 10.1016/j.metabol.2004.06.024. [DOI] [PubMed] [Google Scholar]
- Axelsson J, Qureshi AR, Heimburger O, Lindholm B, Stenvinkel P, Barany P. Body fat mass and serum leptin levels influence epoetin sensitivity in patients with ESRD. Am J Kidney Dis. 2005;46:628–634. doi: 10.1053/j.ajkd.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Ayyildiz M, Yildirim M, Agar E, Baltaci AK. The effect of leptin on penicillin-induced epileptiform activity in rats. Br Res Bull. 2006;68:374–378. doi: 10.1016/j.brainresbull.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, Moizo L, Lehy T, Guerre-Millo M, Le Marchand-Brustel Y, Lewin MJM. The stomach is a source of leptin. Nature. 1998;394:790–793. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- Banks AS, Davis SM, Bates SH, Myers MG. Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275:14563–14572. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- Banks WA, Clever CM, Farrell CL. Partial saturation and regional variation in the blood-to-brain transport of leptin in normal weight mice. Am J Physiol-Endoc M. 2000;278:E1158–E1165. doi: 10.1152/ajpendo.2000.278.6.E1158. [DOI] [PubMed] [Google Scholar]
- Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaoke R, Morley JE. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004;53:1253–1260. doi: 10.2337/diabetes.53.5.1253. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Huang WT, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- Banks WA, Niehoff ML, Martin D, Farrell CL. Leptin transport across the blood-brain barrier of the Koletsky rat is not mediated by a product of the leptin receptor gene. Br Res. 2002;950:130–136. doi: 10.1016/s0006-8993(02)03013-5. [DOI] [PubMed] [Google Scholar]
- Banks WA, Ortiz L, Plotkin SR, Kastin AJ. Human Interleukin (Il) 1 Alpha, Murine Il-1 Alpha and Murine Il-1 Beta Are Transported from Blood to Br. in the Mouse by a Shared Saturable Mechanism. J Pharm and Exp Ther. 1991;259:988–996. [PubMed] [Google Scholar]
- Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim HK, Lai CF, Tartaglia LA. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci USA. 1996;93:8374–8378. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessels GJ, Kamal A, Ramakers GM, Urban IJ, Spruijt BM, Erkelens DW, Gispen WH. Place learning and hippocampal synaptic plasticity in streptozotocin-Induced diabetic rats. Diabetes. 1996;45:1259–1266. doi: 10.2337/diab.45.9.1259. [DOI] [PubMed] [Google Scholar]
- Biessels GJ, Kamal A, Urban IJA, Spruijt BM, Erkelens DW, Gispen WH. Water maze learning and hippocampal synaptic plasticity in streptozotocin-diabetic rats: effects of insulin treatment . Br Res. 1998;800:125–135. doi: 10.1016/s0006-8993(98)00510-1. [DOI] [PubMed] [Google Scholar]
- Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998a;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- Bjorbaek C, Elmquist JK, Michl P, Ahima RS, van Bueren A, McCall AL, Flier JS. Expression of leptin receptor isoforms in rat brain microvessels. Endocrinology. 1998b;139:3485–3491. doi: 10.1210/endo.139.8.6154. [DOI] [PubMed] [Google Scholar]
- Bjorbaek C, Lavery HJ, Bates SH, Olson RK, Davis SM, Flier JS, Myers MG. SOCS3 mediates feedback inhibition of the leptin receptor via Tyr(985) J Biol Chem. 2000;275:40649–40657. doi: 10.1074/jbc.M007577200. [DOI] [PubMed] [Google Scholar]
- Bjorbaek C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem. 1997;272:32686–32695. doi: 10.1074/jbc.272.51.32686. [DOI] [PubMed] [Google Scholar]
- Boswell T, Dunn IC, Wilson PW, Joseph N, Burt DW, Sharp PJ. Identification of a non-mammalian leptin-like gene: Characterization and expression in the tiger salamander (Am. bystoma tigrinum) Gen Comp Endocr. 2006;146:157–166. doi: 10.1016/j.ygcen.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Bruehl H, Rueger M, Dziobek I, Sweat V, Tirsi A, Javier E, Arentoft A, Wolf OT, Convit A. Hypothalamic-pituitary-adrenal axis dysregulation and memory impairments in type 2 diabetes . J Clin Endocr Metab. 2007;92:2439–2445. doi: 10.1210/jc.2006-2540. [DOI] [PubMed] [Google Scholar]
- Carpenter LR, Farruggella TJ, Symes A, Karow ML, Yancopoulos GD, Stahl N. Enhancing leptin response by preventing SH2-containing phosphatase 2 interaction with Ob receptor. Proc Natl Acad Sci USA. 1998;95:6061–6066. doi: 10.1073/pnas.95.11.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convit A. Links between cognitive impairment in insulin resistance: An explanatory model . Neurobiol Aging. 2005;26:S31–S35. doi: 10.1016/j.neurobiolaging.2005.09.018. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Wands JR. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: Relevance to Alzheimer's disease. J Alzheimers Dis. 2005;7:45–61. doi: 10.3233/jad-2005-7106. [DOI] [PubMed] [Google Scholar]
- Desrocher M, Rovet J. Neurocognitive correlates of type 1 diabetes mellitus in childhood. Child Neuropsychol. 2004;10:36–52. doi: 10.1076/chin.10.1.36.26241. [DOI] [PubMed] [Google Scholar]
- Dicou E, Attoub S, Gressens P. Neuroprotective effects of leptin in vivo and in vitro. Neuroreport. 2001;12:3947–3951. doi: 10.1097/00001756-200112210-00019. [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Spuch C, Antequera D, Rodal I, de Yébenesd JG, Molina JA, Bermejo F, Eva Carro E. Megalin mediates the transport of leptin across the blood-CSF barrier. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Duan WZ, Guo ZH, Jiang HY, Ware M, Li XJ, Mattson MP. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc Natl Acad Sci USA. 2003;100:2911–2916. doi: 10.1073/pnas.0536856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durakoglugil M, Irving AJ, Harvey J. Leptin induces a novel form of NMDA receptor-dependent long-term depression. J Neurochem. 2005;95:396–405. doi: 10.1111/j.1471-4159.2005.03375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt RA, Bell AW, Boisclair YR. Spatial and developmental regulation of leptin in fetal sheep. Am J Physiol-Reg I. 2002;282:R1628–R1635. doi: 10.1152/ajpregu.00750.2001. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
- Enwere E, Shingo T, Gregg C, Fujikawa H, Ohta S, Weiss S. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci. 2004;24:8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbayat-Altay E, Yamada KA, Wong M, Thio LL. Increased severity of pentylentetrazol-induced seizures in leptin deficient ob/ob mice. Epilepsia. 2006;47:303–304. [Google Scholar]
- Faouzi M, Leshan R, Bjornholm M, Hennessey T, Jones J, Munzberg H. Differential accessibility of circulating leptin to individual hypothalamic sites. Endocrinology. 2007;148:5414–5423. doi: 10.1210/en.2007-0655. [DOI] [PubMed] [Google Scholar]
- Farr SA, Banks WA, Morley JE. Effects of leptin on memory processing. Peptides. 2006;27:1420–1425. doi: 10.1016/j.peptides.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Fei H, Okano HJ, Li C, Lee GH, Zhao C, Darnell R, Friedman JM. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues . Proc Natl Acad Sci USA. 1997;94:7001–7005. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewlass DC, Noboa K, Pi-Sunyer FX, Johnston JM, Yan SD, Tezapsidis N. Obesity-related leptin regulates Alzheimer's A beta. Faseb J. 2004;18:1870–1878. doi: 10.1096/fj.04-2572com. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Br Res. 2003;964:107–115. doi: 10.1016/s0006-8993(02)04087-8. [DOI] [PubMed] [Google Scholar]
- Fisone G, Hakansson K, Borgkvist A, Santini E. Signaling in the basal ganglia: Postsynaptic and presynaptic mechanisms. Physiol Behav. 2007;92:8–14. doi: 10.1016/j.physbeh.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Fliedner S, Schulz C, Lehnert H. Br. uptake of intranasally applied radioiodinated leptin in Wistar rats. Endocrinology. 2006;147:2088–2094. doi: 10.1210/en.2005-1016. [DOI] [PubMed] [Google Scholar]
- Frisullo G, Mirabella M, Angelucci F, Caggiula M, Morosetti R, Sancricca C, Patanella AK, Nociti V, Lorio R, Bianco A, Tomassini V, Pozzilli C, Tonali PA, Matarese G, Batocchi AP. The effect of disease activity on leptin, leptin receptor and suppressor of cytokine signalling-3 expression in relapsing-remitting multiple sclerosis. J Neuroimmunol. 2007;192:174–183. doi: 10.1016/j.jneuroim.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Fujioka K, Patane J, Lubina J, Lau D. CSF leptin levels after exogenous administration of recombinant methionyl human leptin. Jama. 1999;282:1517–1518. doi: 10.1001/jama.282.16.1517. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Murakami M, Ogawa Y, Masuzaki H, Tanaka M, Ozaki S, Nakao K, Mimori T. Leptin inhibits stress-induced apoptosis of T lymphocytes. Clin Exp Immunol. 2002;128:21–26. doi: 10.1046/j.1365-2249.2002.01797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Ghilardi N, Ziegler S, Wiestner A, Stoffel R, Heim MH, Skoda RC. Defective STAT signaling by the leptin receptor in diabetic mice. Proc Natl Acad Sci USA. 1996;93:6231–6235. doi: 10.1073/pnas.93.13.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil A, Aguilera CM, Gil-Campos M, Canete R. Altered signalling and gene expression associated with the immune system and the inflammatory response in obesity. Brit J Nutr. 2007;98:S121–S126. doi: 10.1017/S0007114507838050. [DOI] [PubMed] [Google Scholar]
- Golden PL, Maccagnan TJ, Pardridge WM. Human blood-brain barrier leptin receptor - Binding and endocytosis in isolated human brain microvessels. J Clin Invest. 1997;99:14–18. doi: 10.1172/JCI119125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood CE, Winocur G. High-fat diets, insulin resistance and declining cognitive function . Neurobiol Aging. 2005;26:S42–S45. doi: 10.1016/j.neurobiolaging.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Grosfeld A, Andre J, Hauguel-de-Mouzon S, Berra E, Pouyssegur J, Guerre-Millo M. Hypoxia-inducible factor 1 transactivates the human leptin gene promoter. J Biol Chem. 2002;277:42953–42957. doi: 10.1074/jbc.M206775200. [DOI] [PubMed] [Google Scholar]
- Guan XM, Hess JF, Yu H, Hey PJ, van der Ploeg LHT. Differential expression of mRNA for leptin receptor isoforms in the rat brain. Mol Cell Endocrin. 1997;133:1–7. doi: 10.1016/s0303-7207(97)00138-x. [DOI] [PubMed] [Google Scholar]
- Guo ZH, Jiang HY, Xu XR, Duan WZ, Mattson MP. Leptin-mediated cell survival signaling in hippocampal neurons mediated by JAK STAT3 and mitochondrial stabilization. J Biol Chem. 2008;283:1754–1763. doi: 10.1074/jbc.M703753200. [DOI] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-Reducing Effects of the Plasma-Protein Encoded by the Obese. Gene Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Hama H, Saito A, Takeda T, Tanuma A, Xie YS, Sato K, Kazama JJ, Gejyo F. Evidence indicating that renal tubular metabolism of leptin is mediated by megalin but not by the leptin receptors. Endocrinology. 2004;145:3935–3940. doi: 10.1210/en.2004-0074. [DOI] [PubMed] [Google Scholar]
- Harvey J. Leptin regulation of neuronal excitability and cognitive function. Curr Opin Pharmacol. 2007;7:643–647. doi: 10.1016/j.coph.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegyi K, Fulop K, Kovacs K, Toth S, Falus A. Leptin-induced signal transduction pathways. Cell Biol Int. 2004;28:159–169. doi: 10.1016/j.cellbi.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, McCamish M. Recombinant leptin for weight loss in obese and lean adults - A randomized, controlled, dose-escalation trial. Jama. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- Hoda MR, Keely SJ, Bertelsen LS, Junger WG, Dharmasena D, Barrett KE. Leptin acts as a mitogenic and antiapoptotic factor for colonic cancer cells. Brit J Surg. 2007;94:346–354. doi: 10.1002/bjs.5530. [DOI] [PubMed] [Google Scholar]
- Hoggard N, Hunter L, Duncan JS, Williams LM, Trayhurn P, Mercer JG. Leptin and leptin receptor mRNA and protein expression in the murine fetus and placenta. Proc Natl Acad Sci USA. 1997;94:11073–11078. doi: 10.1073/pnas.94.20.11073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Howard JK, Lord GM, Matarese G, Vendetti S, Ghatei MA, Ritter MA, Lechler RI, Bloom SR. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest. 1999;104:1051–1059. doi: 10.1172/JCI6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsing AW, Sakoda LC, Chua SC. Obesity, metabolic syndrome, and prostate cancer . Am J Clin Nutr. 2007;86:843s–857s. doi: 10.1093/ajcn/86.3.843S. [DOI] [PubMed] [Google Scholar]
- Hwa JJ, Ghibaudi L, Compton D, Fawzi AB, Strader CD. Intracerebroventricular injection of leptin increases thermogenesis and mobilizes fat metabolism in ob/ob mice. Horm Metab Res. 1996;28:659–663. doi: 10.1055/s-2007-979873. [DOI] [PubMed] [Google Scholar]
- Ihle JN. Cytokine Receptor Signaling. Nature. 1995;377:591–594. doi: 10.1038/377591a0. [DOI] [PubMed] [Google Scholar]
- Inoue W, Poole S, Bristow AF, Luheshi GN. Leptin induces cyclooxygenase-2 via an interaction with interleukin-1 beta in the rat brain. Euro J Neurosci. 2006;24:2233–2245. doi: 10.1111/j.1460-9568.2006.05105.x. [DOI] [PubMed] [Google Scholar]
- Julliard AK, Chaput MA, Apelbaum A, Aime P, Mahfouz M, Duchamp-Viret P. Changes in rat olfactory detection performance induced by orexin and leptin mimicking fasting and satiation. Behav Br Res. 2007;183:123–129. doi: 10.1016/j.bbr.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Op in Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Pan WH, Maness LM, Koletsky RJ, Ernsberger P. Decreased transport of leptin across the blood-brain barrier in rats lacking the short form of the leptin receptor. Peptides. 1999;20:1449–1453. doi: 10.1016/s0196-9781(99)00156-4. [DOI] [PubMed] [Google Scholar]
- Kinzig KP, Scott KA, Hyun J, Bi S, Moran TH. Altered hypothalamic signaling and responses to food deprivation in rats fed a low-carbohydrate diet. Obes Res. 2005;13:1672–1682. doi: 10.1038/oby.2005.205. [DOI] [PubMed] [Google Scholar]
- Knerr I, Schuster S, Nomikos P, Buchfelder M, Dotsch J, Schoof E, Fahlbusch R, Rascher W. Gene expression of adrenomedullin, leptin, their receptors and neuropeptide Y in hormone-secreting and non-functioning pituitary adenomas, meningiomas and malignant intracranial tumours in humans. Neuropath Appl Neuro. 2001;27:215–222. doi: 10.1046/j.0305-1846.2001.00324.x. [DOI] [PubMed] [Google Scholar]
- Komori T, Morikawa Y, Nanjo K, Senba E. Induction of brain-derived neurotrophic factor by leptin in the ventromedial hypothalamus. Neurosci. 2006;139:1107–1115. doi: 10.1016/j.neuroscience.2005.12.066. [DOI] [PubMed] [Google Scholar]
- Kurrimbux D, Gaffen Z, Farrell CL, Martin D, Thomas SA. The involvement of the blood-brain and the blood-cerebrospinal fluid barriers in the distribution of leptin into and out of the rat brain. Neurosci. 2004;123:527–536. doi: 10.1016/j.neuroscience.2003.08.061. [DOI] [PubMed] [Google Scholar]
- Lago F, Dieguez C, Gomez-Reino J, Gualillo O. Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheum. 2007;3:716–724. doi: 10.1038/ncprheum0674. [DOI] [PubMed] [Google Scholar]
- Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P, Brodsky MA, Burchiel K, Kelly P, Dalvi A, Scott B, Stacy M, Turner D, Wooten VGF, Elias WJ, Laws ER, Dhawan V, Stoessl AJ, Matcham J, Coffey RJ, Traub M. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol. 2006;59:459–466. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- Lee GH, Li C, Montez J, Halaas J, Darvishzadeh J, Friedman JM. Leptin receptor mutations in 129 db(3J)/db(3J) mice and NIH fa(cp)/fa(cp) rats. Mamm Genome. 1997;8:445–447. doi: 10.1007/s003359900466. [DOI] [PubMed] [Google Scholar]
- Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- Levy YS, Gilgun-Sherki Y, Melamed E, Offen D. Therapeutic potential of neurotrophic factors in neurodegenerative diseases. Biodrugs. 2005;19:97–127. doi: 10.2165/00063030-200519020-00003. [DOI] [PubMed] [Google Scholar]
- Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience. 2002;113:607–615. doi: 10.1016/s0306-4522(02)00162-8. [DOI] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Lu JN, Park CS, Lee SK, Shin DW, Kang JH. Leptin inhibits 1-methyl-4-phenylpyridinium-induced cell death in SH-SY5Y cells . Neurosci Lett. 2006;407:240–243. doi: 10.1016/j.neulet.2006.08.053. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Experimental diabetes in rats causes hippocampal dendritic and synaptic reorganization and increased glucocorticoid reactivity to stress . Proc Natl Acad Sci USA. 2000;97:11056–11061. doi: 10.1073/pnas.97.20.11056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresh GA, Maness LM, Zadina JE, Kastin AJ. In vitro demonstration of a saturable transport system for leptin across the blood-brain barrier. Life Sci. 2001;69:67–73. doi: 10.1016/s0024-3205(01)01093-1. [DOI] [PubMed] [Google Scholar]
- Matarese G, Carrieri PB, La Cava A, Perna F, Sanna V, De Rosa V, Aufiero D, Fontana S, Zappacosta S. Leptin increase in multiple sclerosis associates with reduced number of CD4(+)CD25(+) regulatory T cells. Proc Natl Acad Sci USA. 2005;102:5150–5155. doi: 10.1073/pnas.0408995102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner U, Hanisch C, Ostreicher I, Knerr I, Hofbauer KH, Blum WF, Allabauer I, Rascher W, Dotsch J. Differential regulation of leptin synthesis in rats during short-term hypoxia and short-term carbon monoxide inhalation. Endocrinology. 2005;146:215–220. doi: 10.1210/en.2004-0782. [DOI] [PubMed] [Google Scholar]
- Messier C. Impact of impaired glucose tolerance and type 2 diabetes on cognitive aging . Neurobiol Aging. 2005;26:S26–S30. doi: 10.1016/j.neurobiolaging.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Morash B, Li A, Murphy PR, Wilkinson M, Ur E. Leptin gene expression in the brain and pituitary gland. Endocrinology. 1999;140:5995–5998. doi: 10.1210/endo.140.12.7288. [DOI] [PubMed] [Google Scholar]
- Moroo I, Yamada T, Makino H, Tooyama I, Mcgeer PL, Mcgeer EG, Hirayama K. Loss of Insulin-Receptor Immunoreactivity from the Substantia-Nigra Pars-Compacta Neurons in Parkinson's-Disease. Acta Neuropath. 1994;87:343–348. doi: 10.1007/BF00313602. [DOI] [PubMed] [Google Scholar]
- Mutze J, Roth J, Gerstberger M, Matsumura K, Hubschle T. Immunohistochemical evidence of functional leptin receptor expression in neuronal and endothelial cells of the rat brain. Neurosci Lett. 2006;394:105–110. doi: 10.1016/j.neulet.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Mogi M, Ichinose H, Togari A. Cytokines in Parkinson's disease. J Neural Transm-Supp. 2000:143–151. [PubMed] [Google Scholar]
- Niswender KD, Baskin DG, Schwartz MW. Insulin and its evolving partnership with leptin in the hypothalamic control of energy homeostasis. Trends Endocrin Met. 2004;15:362–369. doi: 10.1016/j.tem.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Niswender KD, Morrison CD, Clegg DJ, Olson R, Baskin DG, Myers MG, Seeley RJ, Schwartz MW. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus - A key mediator of insulin-induced anorexia. Diabetes. 2003;52:227–231. doi: 10.2337/diabetes.52.2.227. [DOI] [PubMed] [Google Scholar]
- Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Schwartz MW. Intracellular signalling - Key enzyme in leptin-induced anorexia. Nature. 2001;413:794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- Oei NYL, Everaerd WTAM, Elzinga BM, Van Well S, Bermond B. Psychosocial stress impairs working memory at high loads: An association with cortisol levels and memory retrieval. Stress. 2006;9:133–141. doi: 10.1080/10253890600965773. [DOI] [PubMed] [Google Scholar]
- O'Malley D, MacDonald N, Mizielinska S, Connolly CN, Irving AJ, Harvey J. Leptin promotes rapid dynamic changes in hippocampal dendritic morphology . Mol Cell Neurosci. 2007;35:559–572. doi: 10.1016/j.mcn.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomura Y, Hori N, Shiraishi T, Fukunaga K, Takeda H, Tsuji M, Matsurihiya T, Ishibashi M, Aou S, Li XL, Kohno D, Uramura K, Sougawa H, Yada T, Wayner MJ, Sasaki K. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides. 2006;27:2738–2749. doi: 10.1016/j.peptides.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Pardini AW, Nguyen HT, Figlewicz DP, Baskin DG, Williams DL, Kim F, Schwartz MW. Distribution of insulin receptor substrate-2 in brain areas involved in energy homeostasis. Br Res. 2006;1112:169–178. doi: 10.1016/j.brainres.2006.06.109. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the Obese Gene-Product on Body-Weight Regulation in Ob/Ob. Mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- Plum L, Schubert M, Bruning JC. The role of insulin receptor signaling in the brain. Trends Endocrin Met. 2005;16:59–65. doi: 10.1016/j.tem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Popovic V, Svetel M, Djurovic M, Petrovic S, Doknic M, Pekic S, Miljic D, Milic N, Glodic J, Dieguez C, Casanueva FF, Kostic V. Circulating and cerebrospinal fluid ghrelin and leptin: potential role in altered body weight in Huntington's disease. Eur J Endocrinol. 2004;151:451–455. doi: 10.1530/eje.0.1510451. [DOI] [PubMed] [Google Scholar]
- Purdham DM, Zou MX, Rajapurohitam V, Karmazyn M. Rat heart is a site of leptin production and action. Am J Physiol-Heart C. 2004;287:H2877–H2884. doi: 10.1152/ajpheart.00499.2004. [DOI] [PubMed] [Google Scholar]
- Rajapurohitam V, Gan XHT, Kirshenbaum LA, Karmazyn M. The obesity-associated peptide leptin induces hypertrophy in neonatal rat ventricular myocytes. Circ Res. 2003;93:277–279. doi: 10.1161/01.RES.0000089255.37804.72. [DOI] [PubMed] [Google Scholar]
- Rajapurohitam V, Javadov S, Purdham DM, Kirshenbaum LA, Karmazyn M. An autocrine role for leptin in mediating the cardiomyocyte hypertrophic effects of angiotensin II and endothelin-1. J Mol Cell Cardiol. 2006;41:265–274. doi: 10.1016/j.yjmcc.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Roseberry AG, Painter T, Mark GP, Williams JT. Decreased vesicular somatodendritic dopamine stores in leptin-deficient mice. J Neurosci. 2007;27:7021–7027. doi: 10.1523/JNEUROSCI.1235-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouet-Benzineb R, Aparicio T, Guilmeau S, Pouzet C, Descatoire V, Buyse M, Bado A. Leptin counteracts sodium butyrate-induced apoptosis in human colon cancer HT-29 cells via NF-kappa B signaling. J Biol Chem. 2004;279:16495–16502. doi: 10.1074/jbc.M312999200. [DOI] [PubMed] [Google Scholar]
- Russo VC, Metaxas S, Kobayashi K, Harris M, Werther GA. Antiapoptotic effects of leptin in human neuroblastoma cells. Endocrinology. 2004;145:4103–4112. doi: 10.1210/en.2003-1767. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Stress and plasticity in the limbic system . Neurochem Res. 2003;28:1735–1742. doi: 10.1023/a:1026021307833. [DOI] [PubMed] [Google Scholar]
- Sasaki R, Masuda S, Nagao M. Erythropoietin: Multiple physiological functions and regulation of biosynthesis. Biosci Biotech Bioch. 2000;64:1775–1793. doi: 10.1271/bbb.64.1775. [DOI] [PubMed] [Google Scholar]
- Saxena NK, Titus MA, Ding XK, Floyd J, Srinivasan S, Sitaraman SV, Anania FA. Leptin as a novel profibrogenic cytokine in hepatic stellate cells: mitogenesis and inhibition of apoptosis mediated by extracellular regulated kinase (Erk) and Akt phosphorylation. Faseb J. 2004;18:1612. doi: 10.1096/fj.04-1847fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffler A, Scholmerich J, Buechler C. Mechanisms of Dis.: adipokines and breast cancer - endocrine and paracrine mechanisms that connect adiposity and breast cancer. Nat Clin Pract Endoc. 2007;3:345–354. doi: 10.1038/ncpendmet0456. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte D. Cerebrospinal fluid leptin levels: Relationship to plasma levels and to adiposity in humans. Nature Med. 1996a;2:589–593. doi: 10.1038/nm0596-589. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest. 1996b;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley LJ, Irving AJ, Harvey J. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. J Neurosci. 2001;21:art. no.-RC186. doi: 10.1523/JNEUROSCI.21-24-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley LJ, Irving AJ, Rae MG, Ashford MLJ, Harvey J. Leptin inhibits rat hippocampal neurons via activation of large conductance calcium-activated K+ channels. Nat Neurosci. 2002a;5:299–300. doi: 10.1038/nn824. [DOI] [PubMed] [Google Scholar]
- Shanley LJ, O'Malley D, Irving AJ, Ashford ML, Harvey J. Leptin inhibits epileptiform-like activity in rat hippocampal neurones via PI 3-kinase-driven activation of BK channels. J Physiol London. 2002b;545:933–944. doi: 10.1113/jphysiol.2002.029488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimabukuro M, Wang MY, Zhou YT, Newgard CB, Unger RH. Protection against lipoapoptosis of beta cells through leptin-dependent maintenance of Bcl-2 expression. Proc Natl Acad Sci USA. 1998;95:9558–9561. doi: 10.1073/pnas.95.16.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signore AP, Weng ZF, Hastings T, Van Laar AD, Liang QH, Lee YJ, Chen J. Erythropoietin protects against 6-hydroxydopamine-induced dopaminergic cell death. J Neurochem. 2006;96:428–443. doi: 10.1111/j.1471-4159.2005.03587.x. [DOI] [PubMed] [Google Scholar]
- Smolinska N, Przala J, Kaminski T, Siawrys G, Gajewska A, Kochman K, Okrasa S. Leptin gene expression in the hypothalamus and pituitary of pregnant pigs. Neuroendocrin Lett. 2004;25:191–195. [PubMed] [Google Scholar]
- Somasundar P, Yu AK, Vona-Davis L, McFadden DW. Differential effects of leptin on cancer in vitro. J Surg Res. 2003;113:50–55. doi: 10.1016/s0022-4804(03)00166-5. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, VArumugam T, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons . Nat Neurosci. 2008;11:309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B, McKay R. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci. 2000;20:7377–7383. doi: 10.1523/JNEUROSCI.20-19-07377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga T, Kishimoto T. gp130 and the interleukin-6 family of cytokines. Annual Rev Immunology. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- Tang CH, Lu DY, Yang RS, Tsai HY, Kao MC, Fu WM, Chen YF. Leptin-induced IL-6 production is mediated by leptin receptor, insulin receptor substrate-1, phosphatidylinositol 3-kinase, Akt, NF-kappa B, and p300 pathway in microglia. J Imm. 2007;179:1292–1302. doi: 10.4049/jimmunol.179.2.1292. [DOI] [PubMed] [Google Scholar]
- Tardito D, Perez J, Tiraboschi E, Musazzi L, Racagni G, Popoli M. Signaling pathways regulating gene expression, neuroplasticity, and neurotrophic mechanisms in the action of antidepressants: A critical overview. Pharmacol Rev. 2006;58:115–134. doi: 10.1124/pr.58.1.7. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA. The leptin receptor. J Biol Chem. 1997;272:6093–6096. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, Dembski M, Weng X, Deng NH, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Woolf EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Thio LL, Erbayat-Altay E, Rensing N, Yamada KA. Leptin contributes to slower weight gain in juvenile rodents on a ketogenic diet. Pediatric Res. 2006;60:413–417. doi: 10.1203/01.pdr.0000238244.54610.27. [DOI] [PubMed] [Google Scholar]
- Tomac A, Lindqvist E, Lin LFH, Ogren SO, Young D, Hoffer BJ, Olson L. Protection and Repair of the Nigrostriatal Dopaminergic System by Gdnf in-Vivo. Nature. 1995;373:335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Young RJ, White A. Diabetic Neuropathy Is Associated with Increased Activity of the Hypothalamic-Pituitary-Adrenal Axis . J Clin Endocr Metab. 1993;76:554–558. doi: 10.1210/jcem.76.3.8383141. [DOI] [PubMed] [Google Scholar]
- Tu H, Kastin AJ, Hsuchou H, Pan WH. Soluble receptor inhibits leptin transport. J Cell Physiol. 2008;14:301–305. doi: 10.1002/jcp.21195. [DOI] [PubMed] [Google Scholar]
- Udagawa J, Otani H. The role of leptin in fetal brain development. Early Hum Dev. 2007;83:S45–S45. [Google Scholar]
- van der Heide LP, Kamal A, Artola A, Gispen WH, Ramakers GMJ. Insulin modulates hippocampal activity-dependent synaptic plasticity in a N-methyl-D-aspartate receptor and phosphatidyl-inositol-3-kinase-dependent manner. J Neurochem. 2005;94:1158–1166. doi: 10.1111/j.1471-4159.2005.03269.x. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Br. dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- Wang JL, Liu R, Hawkins M, Barzilai N, Rossetti L. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature. 1998;393:684–688. doi: 10.1038/31474. [DOI] [PubMed] [Google Scholar]
- Wang MY, Zhou YT, Newgard CB, Unger RH. A novel leptin receptor in rat. FEBS Lett. 1996;392:87–90. doi: 10.1016/0014-5793(96)00790-9. [DOI] [PubMed] [Google Scholar]
- Wauman J, De Smet A-S, Catteeuw D, Belsham D, Jan Tavernier J. Insulin Receptor Substrate 4 couples the leptin receptor to multiple signalling pathways. Mol Endocrin. 2007 doi: 10.1210/me.2007–0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng Z, Signore AP, Gao Y, Wang S, Zhang F, Hastings T, Yin XM, Chen J. Leptin protects against 6-hydroxydopamine-induced dopaminergic cell death via mitogen-activated protein kinase signaling. J Biol Chem. 2007;282:34479–34491. doi: 10.1074/jbc.M705426200. [DOI] [PubMed] [Google Scholar]
- Woods SC, Lotter EC, McKay LD, Porte D. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282:503–505. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- Xu L, Rensing N, Yang XF, Zhang HX, Thio LL, Rothman SM, Weisenfeld AE, Wong M, Yamada KA. Leptin inhibits 4-aminopyridine- and pentylenetetrazole-induced seizures and AMPAR-mediated synaptic transmission in rodents. J Clin Invest. 2008;118:272–280. doi: 10.1172/JCI33009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang SP, Signore AP, Chen J. Neuroprotective effects of leptin against ischemic injury induced by oxygen-glucose deprivation and transient cerebral ischemia. Stroke. 2007;38:2329–2336. doi: 10.1161/STROKEAHA.107.482786. [DOI] [PubMed] [Google Scholar]
- Zhang F, Yin W, Chen J. Apoptosis in cerebral ischemia: executional and regulatory signaling mechanisms. Neurological Res. 2004;26:835–845. doi: 10.1179/016164104X3824. [DOI] [PubMed] [Google Scholar]
- Zhang WJ, Tan YF, Yue JTY, Vranic M, Wojtowicz JM. Impairment of hippocampal neurogenesis in streptozotocin-treated diabetic rats . Acta Neurol Scand. 2008;117:205–210. doi: 10.1111/j.1600-0404.2007.00928.x. [DOI] [PubMed] [Google Scholar]
- Zhang YY, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional Cloning of the Mouse Obese Gene and Its Human Homolog. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]