AN IDEAL TREATMENT for several kinds of liver disease would be removal of the diseased organ and orthotopic replacement with a hepatic homograft. Patients with primary carcinoma of the liver, congenital atresia of the bile ducts, and terminal cirrhosis would all be candidates. The application of such therapy depends, first, upon the employment of a satisfactory operative procedure and, second, upon the use of suitable measures to prevent the immunologic rejection of the graft.
Recently, solutions to these problems have evolved which are at least partially satisfactory. The technical requirements for successful canine hepatic transplantation were defined (9). In addition, a regimen of anti-rejection therapy was developed in patients receiving renal homografts which resulted in consistent prolonged survival of the foreign tissue (11, 12).
In the present study, the application of these advances to the problem of clinical hepatic homotransplantation in 3 patients will be described. The first attempt resulted in failure at the operating table. The course of the second 2 patients establishes the feasibility of such an operation in humans, despite the fact that death occurred 22 and 7½ days after transplantation from pulmonary emboli.
METHODS
Recipient patients
Patient 1 was a 3 year old white male with congenital biliary atresia (Fig. 1A). Physical development had been retarded, preoperative weight being 20 pounds. His general condition was poor, with hepatosplenomegaly, jaundice, and ascites. Total bilirubin was 20.7 milligrams per cent with a conjugated fraction of 16.7 milligrams per cent. Alkaline phosphatase was 12.8 Bodansky units. Serum glutamic-oxalacetic acid transaminase (SGOT) was 160 SF units. The hospital course prior to hepatic homotransplantation on 1 March 1963 was uneventful. On 12 February, the patient underwent thymectomy without complication. For 13 days prior to hepatic transplantation, he was given daily doses of azathioprine of 5 to 6 milligrams per kilogram of body weight.
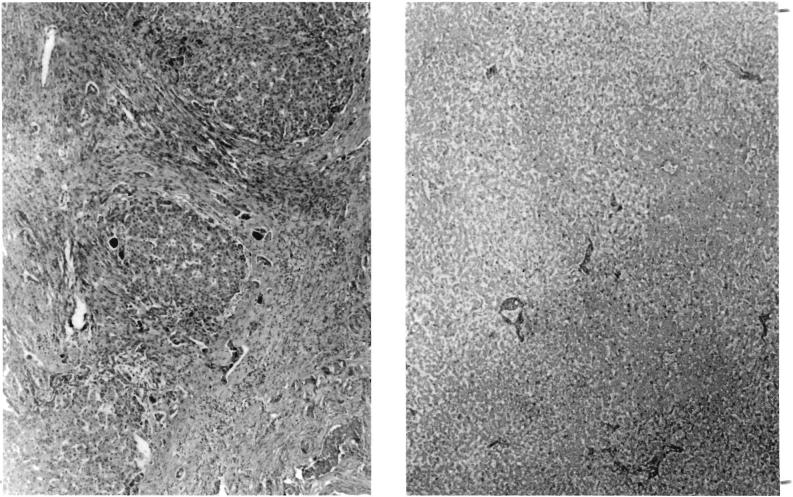
FIG. 1.
Liver tissue in Patient 1. A, left, Patient's own liver, showing advanced biliary cirrhosis. This 3 vear old child had congenital atresia of the bile ducts. B, right, Appearance of homotransplanted liver at autopsy, 12 hours after death. The patient exsanguinated on the operating table, 4 hours after revascularization of the homograft. Note extensive autolysis. Hematoxylin and eosin, X19.
Patient 2 was a 48 year old Negro male with Laennec's cirrhosis and a primary hepatoma (Fig. 2A), proved by operation at another hospital 8 days previous to the transplantation procedure. The tumor and its multiple satellite nodules involved all 4 anatomic segments of the liver and had a localized attachment to the central tendon of the right hemidiaphragm. Except for the diaphragmatic invasion the neoplasm was confined to the liver. His general health had been excellent until 6 weeks previously, when he was admitted to the hospital with weight loss and symptoms suggestive of a duodenal ulcer. He weighed 140 pounds. Blood analyses, during the 48 hours before transplantation on 5 May 1963, were: bilirubin 3.2 milligrams per cent with 1.75 milligrams per cent direct component; alkaline phosphatase 26.9 Bodansky units; SGOT 315 SF units; total proteins 8.3 grams per cent with 2.7 grams per cent albumin. Blood urea nitrogen was 8 milligrams per cent. Serum electrolytes were normal. Complete blood count was within normal limits. Urine was qualitatively positive for bile. The patient had a low grade fever during the preoperative period, which had been present for several weeks during his antecedent hospitalizations elsewhere.
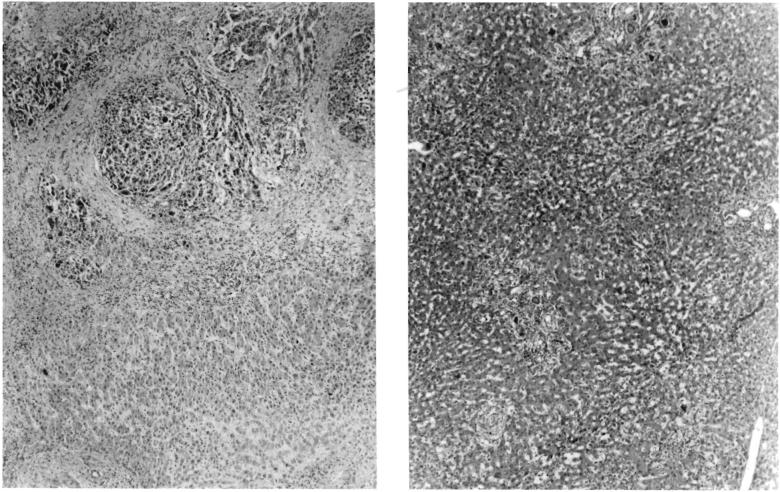
FIG. 2.
Specimens in Patient 2. A, left, Patient's own liver, showing hepatoma, B, right, Liver homograft obtained at autopsy 22 days after operation. Note good preservation of architecture. There was slight periportal fibrosis which is thought to have antedated transplantation. Note mild cholestasis and fatty metamorphosis. A few aggregates of mononuclear cells were present in the periportal areas. Hematoxylin and eosin, X19.
Patient 3 was a 67 year old white male with progressive jaundice. He had received bilateral supracondylar amputations 15 years previously for occlusive peripheral vascular disease. Exploratory operation was performed on 3 June 1963, and an intrahepatic duct cell carcinoma was found (Fig. 3A) which had obstructed both the right and left main hepatic ducts. Two liver biopsies were performed at this time. One week later, the initial phase of the staged hepatic transplantation, to be described, was performed. It was thought that death of a prospective donor was imminent at this time and that the second stage would follow within a few hours. When the abdomen was opened, a massive bile peritonitis was encountered due to leakage from the previous biopsy sites. The preliminary dissection was carried out after lavage of the peritoneal cavity, and the abdomen was closed without drainage. Recovery of the proposed donor necessitated a delay of 14 days before the next suitable cadaver candidate became available. During this interval, it was known that the patient had continuing biliary soilage of the peritoneal cavity. His condition by the time of the definitive transplantation had deteriorated considerably. Prior to the final operation on 24 June, analyses revealed: bilirubin 20.4 milligrams per cent with 10.4 milligrams per cent direct component; alkaline phosphatase 54.9 Bodansky units; SGOT 110 SF units; fasting blood sugar 100 milligrams per cent. Blood urea nitrogen was 69 milligrams per cent. Urine was qualitatively positive for bile. His general condition prior to the definitive transplantation was poor. He weighed 115 pounds.
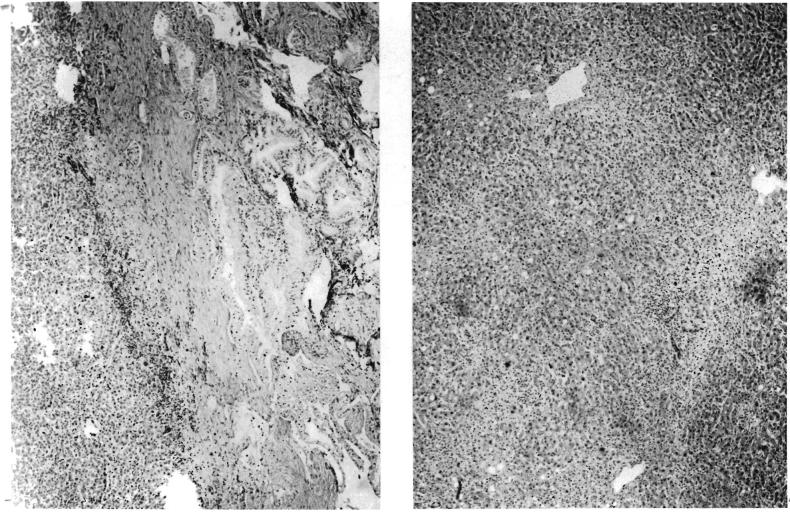
FIG. 3.
Specimens in Patient 3. A, left, Intrahepatic duct cell carcinoma which necessitated operation. B, right, Hepatic homograft 7½ days after transplantation. Note good state of preservation of parenchyma. Periportal accumulations of cells are principally neutrophiles. Hematoxylin and eosin, X19.
Donor patients
The donors were 3, 55, and 69 years old, respectively. The first patient died on the operating table during attempted removal of a third ventricular brain tumor. The second donor died after a protracted terminal illness caused by a cerebral astrocytoma. The third donor died 2 days after massive cerebral hemorrhage.
The circumstances immediately preceding death were different in the first compared with the last 2 cases. The donor for Patient 1 had a cardiac arrest for which open cardiac massage was carried out for 45 minutes before death was acknowledged to have occurred. An additional 15 minutes was required for insertion of the extracorporeal perfusion apparatus. In Donors 2 and 3, respiratory arrest preceded the disappearance of heart action by several minutes. The latter 2 patients maintained blood pressures of 100 millimeters of mercury until a few moments before death. The extracorporeal perfusion was begun 5 and 6 minutes after pronouncement of death.
Donors were selected of the same major blood groups as the recipient patients. In Patients 1 and 2, the blood groups were A+. In the third case; the donor was O- and the recipient O+. Evaluation of liver function in the first donor was not possible. In Donors 2 and 3, complete liver chemistry levels were obtained and found to be essentially normal. The donor liver for Patient 2 was used despite a history of episodic alcoholic excess.
In the last 2 cases, it was possible to maintain a, very close vigil on the donors for the 24 to 48 hours preceding their death. Two measures were followed with particular interest, the blood pressure and the hourly urine output. In both cases the maintenance of an effective blood pressure and the continued excretion of urine were considered to be evidence that good tissue perfusion was present until just before death.
Donor operation
Insertion of the catheters and institution of extracorporeal perfusion were accomplished in 15 minutes, 5 minutes, and 6 minutes after pronouncement of death in the donors used for Patients 1, 2, and 3, respectively. The delay in the first case was caused by the necessity to position and prepare the donor's groin. In the 2 subsequent cases, the operative field was prepared and draped prior to death, after approval had been given by the family.
After certification of death, a longitudinal incision was made over the femoral triangle. The femoral artery and vein were cannulated, and the catheters advanced into inferior vena cava and abdominal aorta (Fig. 4). Extracorporeal perfusion was provided with a circuit consisting of a glucose-primed bubble oxygenator, a single DeBakey pump, and a heat exchanger. The priming solution was 2,000 milliliters of 5 per cent dextrose in water, precooled to 15 degrees C. by passage through the heat exchanger. Each liter of perfusate contained 1 gram of procaine hydrochloride, 10,000,000 units of aqueous penicillin, and the amount of heparin calculated to be 1.5 milligrams per kilogram of the donor patient's weight. In Donor 3, 200 milligrams of prednisolone were also added.
FIG. 4.
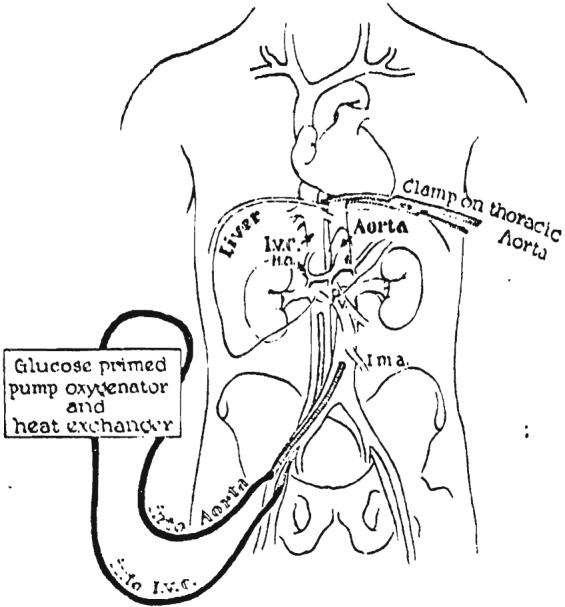
Extracorporcal perfusion of the cadaver donor. The venous drainage is from the inferior vena cava and the arterial inflow is through the aorta, both catheters being inserted through the femoral vessels. Note clamp on thoracic aorta to perfuse the lower half of the corpse selectively. A glucose primed pump oxygenator is used with a heat exchanger.
Initial flow rates of 30 to 50 milliliters per kilogram per minute were obtained in all 3 cadavers. Cooling proceeded evenly so that body temperatures reached 15 degrees C. 45 to 104 minutes after the onset of perfusion. Flow rates were adjusted to 10 to 20 milliliters per kilogram per minute when the temperature reached 20 degrees C. and flow was continued at this rare thereafter. In Donor 1, venous return began to decline after 2 hours and during the next 110 minutes perfusion ceased altogether. In Donors 2 and 3, reduction in venous return was anticipated and successfully prevented by the addition of whole blood, plasma, and 5 per cent dextrose in water to the oxygenator reservoir. Donor 2 required 4 units of whole blood, 500 milliliters of plasma, and 2 liters of additional priming solution. In Donor 3, 4 units of whole blood, 750 milliliters of plasma, and 6 liters of additional dextrose solution were required to maintain adequate venous return. As will be subsequently described, the low thoracic aorta was clamped in order to concentrate perfusion to the lower half of the corpse.
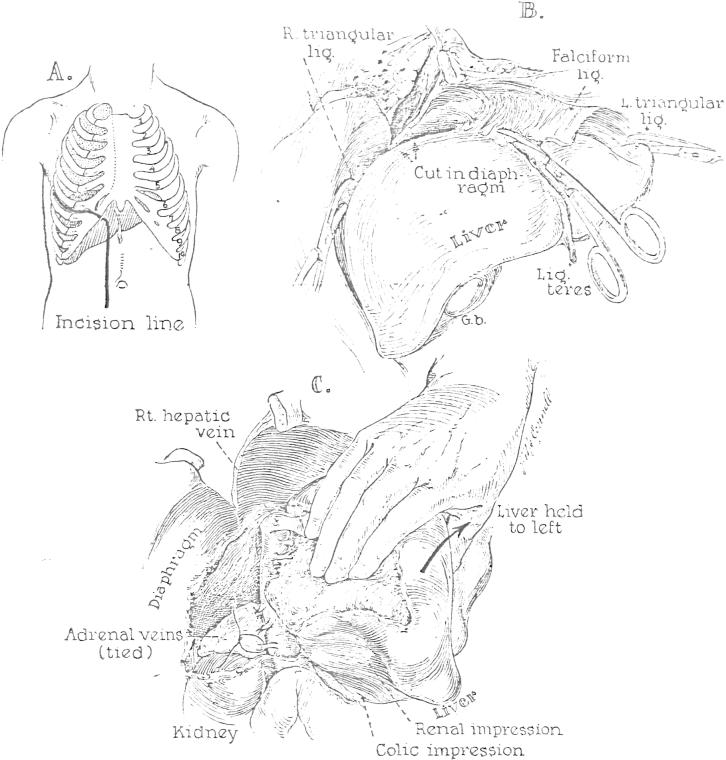
As soon as it was ascertained that perfusion was adequate, the previously prepared chest and abdomen were entered through a generous right thoracoabdominal incision (Fig. 5A). An incision was immediately made in the fundus of the gallbladder at a site which would he suitable for subsequent cholecystoenterostomy, should this type of biliary anastomosis prove to be desirable. All bile was aspirated to prevent autolysis of the extrahepatic biliary structures. The diaphragm was then incised back to the vertebral column. A vascular clamp was placed on the low thoracic aorta (Fig. 4) to provide selective perfusion of the infradiaphragmatic portion of the body. The portal structures were dissected (Fig. 6A), care being taken to obtain suitable lengths of common duct, portal vein and hepatic artery. All extraneous tissue was ligated in continuity before division, including the lesser omentum (Fig. 6A).
FIG. 5.
Preparation for extirpation of the liver. The steps followed are essentially the same for the donor and recipient operations. A, Line of incision used for all the cadavers and in Patient 2. B, Mobilization of the falciform, triangular, and coronary ligaments. C, Dissection of the right lateral and posterior surfaces of the inferior vena cava; ligation of the adrenal veins. After completion of this maneuver, it is possible to sweep the finger from the diaphragm to the renal veins without meeting resistance.
FIG. 6.
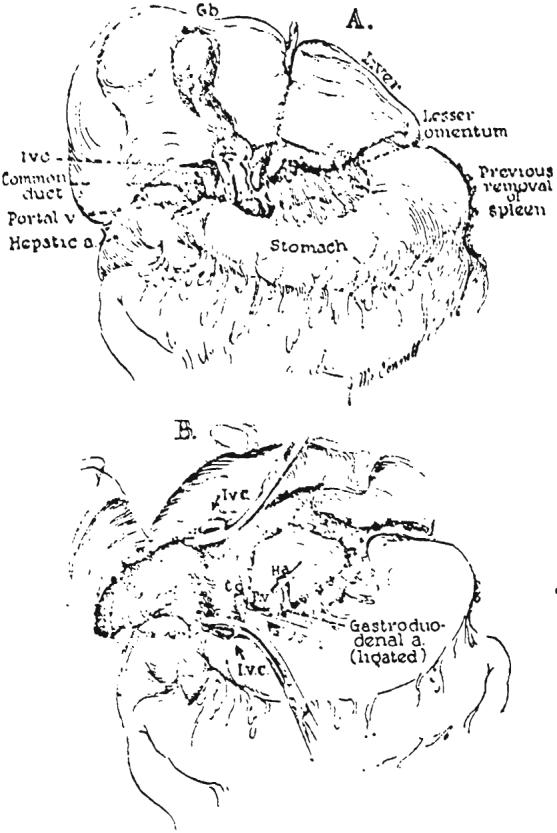
Final steps in removal of the liver. A, Dissection of the structures of the portal triad and division of the lesser omentum. B, Operative field after recipient hepatectomy.
Following isolation of the portal structures, the infrahepatic inferior vena cava was cleaned off as far inferiorly as the renal veins. Next the hepatic ligaments were divided (Fig. 5B). The liver was then gently retracted to the left and the right adrenal gland carefully dissected from the posterior surface of the right lobe (Fig. 5C). The adrenal veins entering the cava were ligated and divided (Fig. 5C). At the conclusion of these measures it was possible to pass the finger behind the cava from diaphragm to renal veins without encountering any obstruction.
Finally, the short segment of suprahepatic inferior vena cava was dissected free. After cutting of the fibrous union of the vessel with the diaphragm, it was possible to provide more length by entering an areolar plane and bluntly dissecting the tendinous diaphragm superiorly. A site for eventual transection was selected above the point of entry of the hepatic veins.
After this preparation of the liver for removal, extracorporeal perfusion was discontinued. The portal vein was cannulated as far inferiorly as possible and infusion begun with lactated Ringer's solution cooled to 15 degrees C. The liver was then removed by transection of the previously isolated structures as the infusion continued (Fig. 7).
FIG. 7.
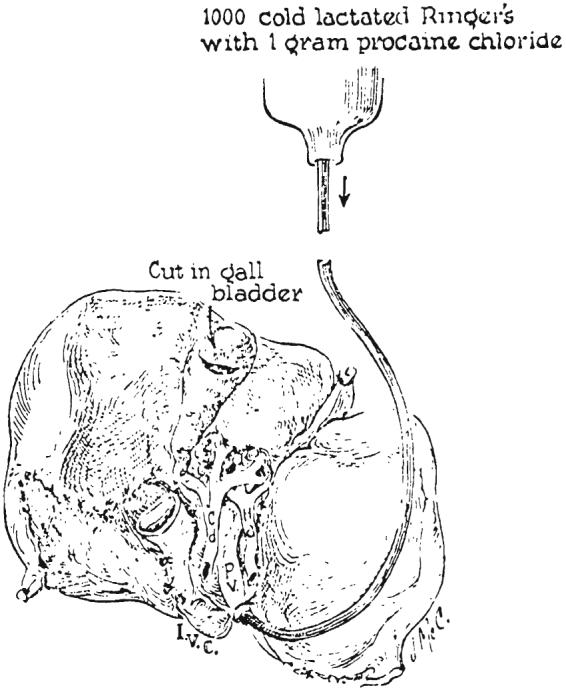
Donor liver after removal from cadaver. The blood is washed from the donor organ by gravity perfusion through the portal vein. Note the incision in the gallbladder, employed to prevent autolysis by entrapped bile during harvesting.
Recipient operation
In Patient 1, the entire procedure to be described was performed at one operation. In Patients 2 and 3, the surgical steps were carried out in two stages. The time interval between the first and second operations was 22 hours in Patient 2 and 14 days in Patient 3. The long delay in the last patient was due to the unexpected recovery of the patient initially proposed for organ donation. Thoracoabdominal incisions were employed in Patients 1 and 2, and a right paramedian abdominal incision in Patient 3.
The first stage operation consisted of preparation for recipient hepatectomy with dissection and skeletonization of those structures to be subsequently anastomosed to the homograft. Consequently, the steps followed were identical to those described in the donor operation (Figs. 5 and 6A). At the conclusion of this stage, the liver was attached inferiorly only by the three constituents of the portal triad and the inferior vena cava (Fig. 6A) and superiorly by the suprahepatic inferior vena cava. The incision was then closed and the patient returned to the recovery room, with provisions for return to the operating room on a moment's notice.
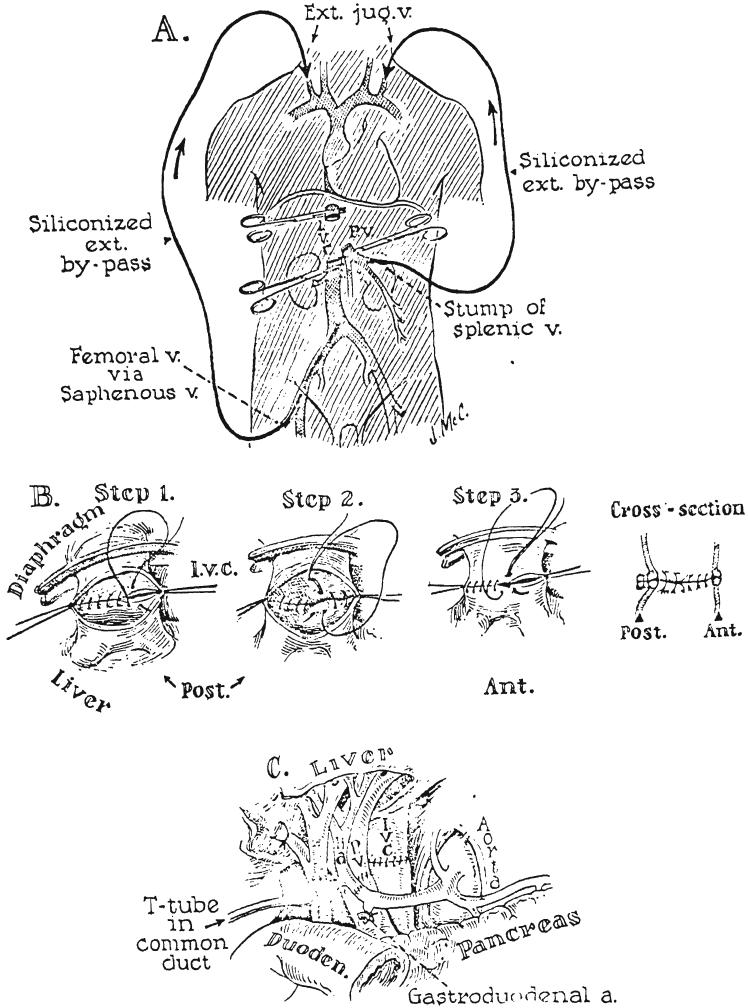
When it was learned that the donor had died, the recipient patient was taken back to the operating room and anesthetized, prepared, and draped. The previous incision was opened. A siliconized plastic tube was inserted into the inferior vena cava, via the femoral vein, and its upper end inserted into the external or internal jugular vein (Fig. 8A). When arrival of the donor liver was imminent, vascular clamps were placed across the suprahepatic and infrahepatic vena cava, the portal vein, and the hepatic artery. The external bypass was then opened, the residual connecting structures transected (Fig. 6B), and the liver removed. In Patient 2, a second external bypass from the splenic to the external jugular veins was used for portal decompression (Fig. 8A), but this clotted after a few minutes.
FIG. 8.
Anastomotic procedures in hepatic transplantation. A, External bypasses for decompression of the inferior vena caval and splanchnic venous beds. Both bypasses were inserted into the cervical jugular system. The splanchnic venous catheter used in Patient 2 was inserted after removal of the spleen. Portal decompression was found to be unnecessary providing the caval bypass functions satisfactorily and is of sufficiently large caliber. B, Anastomosis of suprahepatic inferior vena cava. Note that the cuff of the homograft is actually a confluence of the hepatic veins and the vena cava. The posterior row is performed in 2 everting layers. If considerations of time are not pressing, the anterior row is also doubly sutured. C, Subhepatic operative field at completion of all anastomoses. Note that gallbladder has been removed and that the T tube is inserted through a stab wound in the recipient portion of the composite common duct, with the upper limb passing through the anastomosis.
Reconstitution of the vena cava at the diaphragm was performed first with continuous No. 4-0 silk, after lateral fixation with stay sutures. A 2 layer anastomosis was performed posteriorly, with eversion of both layers (Fig. 8B). Next the vena caval anastomosis below the liver was constructed in 1 layer with No. 4-0 continuous silk, again with an intraluminal everting technique for the posterior row. After completion of these anastomoses, normal flow was restored through the vena cava and the external bypass was removed. The hepatic artery and portal vein were then reconstructed with No. 6-0 silk, and thereby first arterial and then portal venous flow was established (Fig. 8C). In Patient 1, biliary drainage was provided by a loop cholecystojejunostomy, after distal ligation of the common duct, this simple method being selected because of the moribund state of the patient. In Patients 2 and 3, a 2 layer choledocho-choledochostomy was performed with fine catgut and silk. A T tube was placed in the recipient portion of the common duct, with one limb passing through the anastomotic site (Fig. 8C). Both subphrenic spaces were extensively drained.
Because of the fatal multiple pulmonary emboli in Patient 2, a vena caval plication was inserted in the last patient, midway between the renal veins and the caval bifurcation. Three mattress sutures were used to create 4 small channels.
Coagulation studies
The over-all clotting process was monitored by serial thrombelastograms. as described by von Kaulla (13). These provide continuous mechano-optical recordings of the onset and progress of fibrin formation and fibrinolysis, insight into speed and kinetics of coagulation being afforded thereby as well as information on the final firmness of the clot. The thrombelastograrms were supplemented by Quick one stage determinations of the prothrombin complex, plasma fibrinogen analyses by the method of Ratnoff, and measures of the thrombin time by the method of von Kaulla (14).
Fibrinolytic activity was serially measured by the euglobulin lysis time of von Kaulla (15). This method has the advantages of speed and simplicity. In addition, the results are affected neither by heparin nor by the antifibrinolytic drug epsilon-aminocaproic acid (EACA) which was administered to all 3 patients. The technique measures primarily plasminogen activator rather than plasmin (fibrinolysin). Normal lysis times are 120 minutes or longer.
Therapy to prevent rejection
The general scheme of treatment was similar to that previously used for renal homografts (11, 12). In Patient 1, thymectomy was performed 16 days before transplantation. In addition, 5 to 6 milligrams of azathioprine per kilogram were given daily for 13 days preoperatively.
In Patient 2, 4.5 milligrams of azathioprine per kilogram and 30 milligrams of prednisone were given the day preceding definitive transplantation. Splenectomy was performed at the time of the first stage operation. Postoperatively, 1.5 to 6 milligrams of azathioprine per kilogram were given daily, either intravenously or orally. Intermittently actinomycin C was administered intravenously (Fig. 9). Azaserine, 10 milligrams per day, was given intravenously for the first 2 days after operation. A similar regimen was followed for Patient 3, except that neither splenectomy nor thymectomy was performed and azaserine was not used.
FIG. 9.
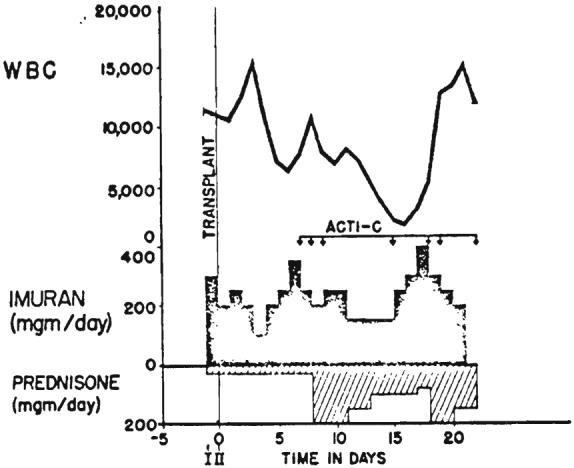
Pharmacologic therapy provided to prevent rejection in Patient 2. Avoidance of leukopenia was attempted by careful selection of doses of azathioprine (imuran). ACTI-C, Each arrow is 200 micrograms of intravenously administered actinomycin C. I, First stage operation; II, Second stage operation.
RESULTS
Times from death to revascularization of the homografts
The total intervals from donor death to revascularization of the homograft in the new bed were 465, 152, and 192 minutes. Fifteen, 5, and 6 minutes elapsed, respectively, from pronouncement of death to the institution of extracorporeal perfusion. The 3 perfusions, including the brief final infusions, lasted 375, 98, and 126 minutes. The time lapses from removal of the donor liver to rearterialization were 75, 49, and 60 minutes, an additional 10 to 26 minutes being required for the subsequent reconstruction of the portal vein.
Survival
The first patient bled to death on the operating table, 4 hours after revascularization of the homograft. The second and third patients lived for 22 and 7½ days, respectively.
Clinical course
Respiratory insufficiency was the most prominent feature of the postoperative course in the last 2 patients. With Patient 2, air hunger was evident immediately after recovery from anesthesia. At this time, blood sugar was 350 milligrams per cent; serum lactic acid 33 milligrams per cent, normal 6 to 16 milligrams per cent; serum pyruvates 2 milligrams per cent, normal 0.7 to 1.2 milligrams per cent; arterial oxygen tension 54 millimeters of mercury; arterial carbon dioxide tension 25 millimeters of mercury; and plasma pH 7.28. Spontaneous ventilation was 25 liters per minute. The data were interpreted as being consistent with an alveolar-capillary diffusion defect. Eighteen hours postoperatively, tracheostomy was performed, and the patient was maintained on a closed system respirator for the next 30 hours. After withdrawal from the respirator, mild dyspnea persisted, but this was not progressive until the last 48 hours when respirator support again became necessary. Four days after operation, evidence of extensive thrombophlebitis developed in the right lower extremity. Intravenous and intramuscular heparin therapy was provided from the eleventh to the fifteenth postoperative days with decrease in the swelling of the leg. Oral diet was taken from the fourth to the nineteenth postoperative days. Terminally, high fever developed. The roentgenogram of the chest, which had previously been clear, showed the appearance of extensive consolidation in the left lung during the last 3 days of life.
The third patient had normal respiration for the first 72 postoperative hours. Acute dyspnea then developed, necessitating tracheostomy and mechanical ventilation for the rest of his life. One day later, radio-graphic evidence was noted of extensive consolidation of both lower lung fields. Despite the previous performance of a vena caval plication, pulmonary emboli from the lower extremities were suspected because of the appearance of edema in the leg amputation stumps. Intravenous heparin therapy was instituted from the third to fifth postoperative days and then discontinued because of gastrointestinal hemorrhage. His condition progressively deteriorated, with increasingly difficult ventilation and with continuing gastrointestinal hemorrhage, until his death 7½ days after transplantation. Oral dietary intake could never be resumed.
Alterations in the coagulation mechanisms
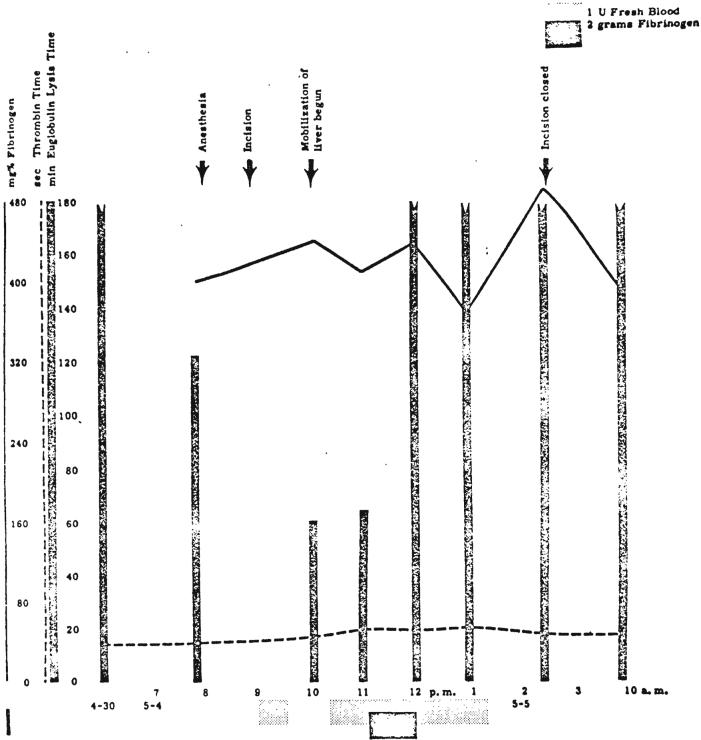
Essentially normal preoperative thrombelastograms were obtained in Patient 1, despite a moderate reduction of the prothrombin complex—prothrombin time 53 per cent. After implantation of the liver there was an extreme activation of the fibrinolytic system as demonstrated by almost immediate dissolution of the clot on the thrombelastogram (Fig. 10) and by a euglobulin lysis time of 5 minutes. Intrarvenous infusion of 0.1 gram of EACA per kilogram abolished completely the fibrinolytic activity (Fig. 10). The clot obtained after EACA administration was, however, structurally poor (Fig. 10) because of the low plasma fibrinogen content of 95 milligrams per cent, and the hemorrhagic diathesis proceeded to a fatal termination. After administration of EACA, the euglobulin lysis time remained unaltered, which indicated that the mechanism causing excessive levels of plasminogen activator substance had not been corrected.
FIG. 10.

Thrombelastograms of Patient 1. A, B, Two hours after revascularization of homograft. Bleeding diathesis was evident clinically. Note onset of clot formation occurs at normal time, since “r” value—time from start of record to clot deposition—is normal. However, the clot is tiny and is lysed as fast as it is formed. C, D, Fifty and 20 per cent of normal fresh blood added to specimen A delays but does not prevent clot lysis. E, Recordings after 0.1 gram per kilogram intravenous EACA. Fibrinolytic activity is abolished. The abnormally small thrombelastogram is due to low fibrinogen. Hemostasis could never be obtained.
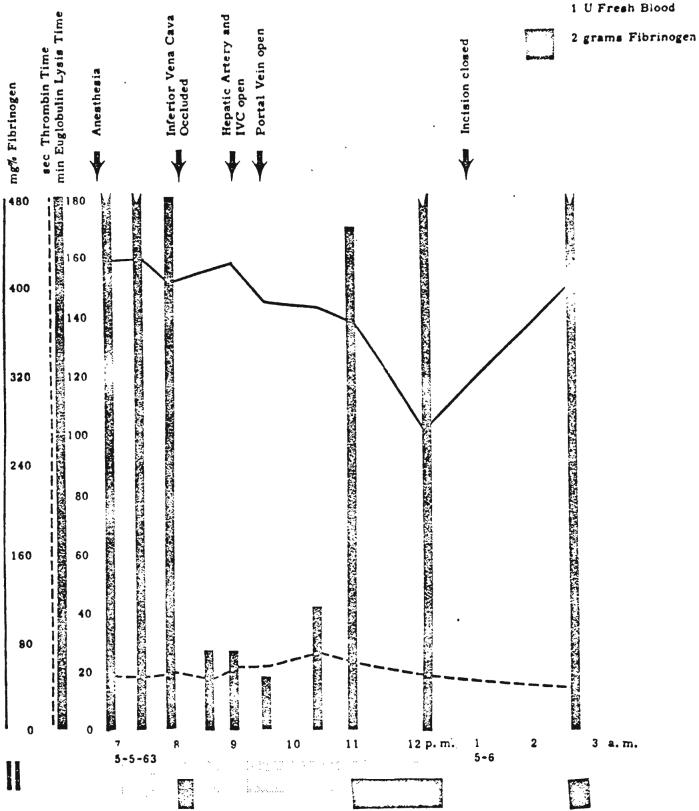
Patient 2 had a normal preoperative prothrombin complex and thrombelastogram. Plasma fibrinogen averaged 400 milligrams per cent, and the euglobulin lysis time was 3 hours. At the first stage operation, mobilization of the liver evoked a marked increase of fibrinolytic activity (Fig. 11). This change returned to normal near the end of the procedure at a time when therapeutic infusions of fibrinogen and fresh blood had been started. None of the other clotting parameters were markedly affected (Fig. 11). During the actual liver transplantation at a second stage the fibrinolytic activity increased drastically. Occlusion of the hepatic circulation as the last preparatory step for hepatectomy was followed by a very pronounced shortening of the euglobulin lysis time despite the preceding prophylactic infusion of fresh blood and fibrinogen (Fig. 12). The thrombelastogram showed progressive dissolution of the clot obtained at this time. Twenty-eight minutes after beginning recipient hepatectomy, 0.1 gram of EACA per kilogram was administered intravenously, abolishing the fibrinolytic activity as measured with the thrombelastogram. The euglobulin lysis time was again unaffected and remained pathologically short for more than 2 hours (Fig. 12), which indicated that high levels of plasminogen activator persisted during this period. During the latter part of this interval, the new liver was in place with a blood supply, which suggested that the pathologic mechanism was not immediately corrected with the provision of the homograft. Approximately 1 hour after revascularization of the homograft, the euglobulin lysis time had returned to normal values. There were no clinical signs of bleeding during the operative and the postoperative period at either stage of the procedure. Other clotting measures remained within normal limits except for a moderate rise of the thrombin time during the actual transplantation.
FIG. 11.
Changes in coagulation during first stage operation in Patient 2, when liver was prepared for subsequent removal. Note striking decrease in euglobulin lysis time during mobilization of liver.
FIG. 12.
Changes in coagulation during definitive transplantation at second stage in Patient 2. Note drastic decrease in euglobulin lysis time and moderate delayed fall in fibrinogen level. EACA, 0.1 gram per kilogram, was given intravenously at 8:47 p.m.
For several days after operation, the thrombelastograms indicated a progressive tendency to hypercoagulability. There was shortening of the “r” values of the thrombelastogram and a concomitant shortening of prothrombin time. Starting on the fifth postoperative day, a progressive fall of plasma fibrinogen level was noted. The euglobulin lysis time was frequently unusually long—many hours. Retrospectively, these later observations are best interpreted as indications of intravascular clotting.
The changes in coagulation in Patient 3 were comparable to those of Patient 2, both quantitatively and qualitatively, except that the shortening of euglobulin lysis time persisted for several hours longer. Therapy with fresh blood, EACA, and fibrinogen was similar to that employed in Patient 2.
Liver function
Serial postoperative liver functions were obtained in the last 2 patients. The presence of hepatic function was evident from the facts that blood sugars could be maintained at normal levels without intravenous glucose, that bile in quantities of 150 to 600 cubic centimeters per day was collected from the T tube the bilirubin content of which was 65 per cent or more conjugated, that no evidence of hepatic coma was observed, and that secondary bleeding did not occur except when heparin therapy was being administered.
There was evidence that moderately severe parenchymal injury occurred at the time of transplantation. The serum glutamic-oxalacetic acid transaminase values rose to 1.150 and 990 units within a few hours in Patients 2 and 3. respectively, but these were returning toward normal in 24 hours (Fig. 13). Lactic acid dehydrogenase and serum glutamic pyruvic acid transaminase values followed similar curves. Bilirubin rose to 12.8 milligrams per cent after 9 days in Patient 2 and then progressively improved (Fig. 14). In Patient 3, the bilirubin dropped from 20.4 milligrams per cent preoperatively to 7.2 milligrams per cent after transplantation. During the last 3 days of life, concomitant with the gastrointestinal bleeding, bilirubin again rose, to 15.4 milligrams per cent.
FIG. 13.
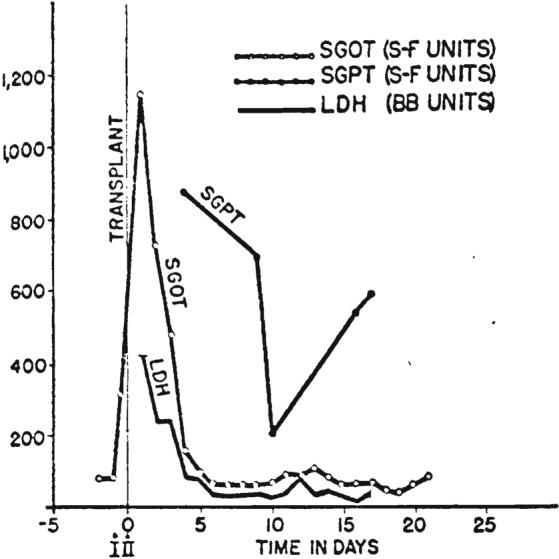
Graphic depiction of serum enzyme alterations following hepatic transplantation in Patient 2. Note immediate rise in SGOT with subsequent decline. LDH and SGPT were similarly affected.
FIG. 14.
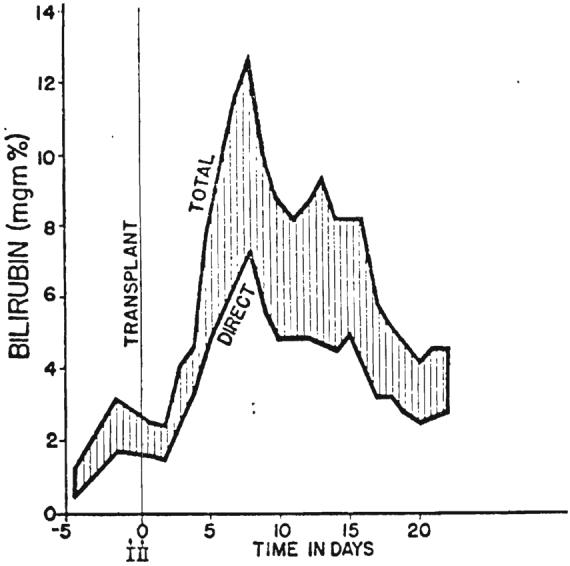
Graph showing rise in serum bilirubin to 12.8 milligrams per cent in Patient 2 nine days after transplantation. After transient deepening of jaundice there was progressive improvement.
Alkaline phosphatase began to fall immediately after operation in both the second and third patients. The lowest postoperative prothrombin time in Patient 2 was 43 per cent and in Patient 3, 26 per cent. The latter value was obtained just before death. Total proteins were maintained at 5 grams per cent or more, although terminally the albumin dropped to 1.6 grams per cent in Patient 2.
The improvement in hepatic function of Patient 2 continued for the entire course. In Patient 3, there was a secondary deterioration of liver function terminally, coincident with gastrointestinal bleeding.
Autopsy findings
The anastomoses in Patient 1 were all patent. The liver weighed 220 grams, and on cut surface had a prominent lobular pattern. Histologically, the lobules of the transplanted liver were preserved in general outline only. A few scattered clusters of liver cells were partially preserved, but the remainder were severely autolyzed (Fig. 1B). The cytoplasm was smudged and many nuclei were absent. Portal triads were intact. The sinusoids and central veins were dilated.
The homograft in Patient 2 weighed 1,700 grams. All vascular anastomoses were intact, although there was a thin layer of circumferential adherent thrombus at the portal vein anastomosis. The right iliac vein and terminal inferior vena cava were occluded with old and fresh clot, above which was a 4 by 1.5 centimeter free-floating, nonocclusive thrombus. Multiplc large and small emboli occupied both pulmonary arteries. There was massive infarction of the left lung as well as the right lower lobe. Histologic sections of the liver revealed good preservation of lobular architecture (Fig. 2B). There was considerable bile stasis and a mild degree of fatty metamorphosis and periportal fibrosis. A few aggregates of mixed neutrophiles and mononuclear cells were in the periportal areas (Fig. 2B). Some hepatic cells had finely granular cytoplasm with shrunken nuclei.
The transplanted liver in Patient 3 weighed 2,070 grams. The vascular and common duct anastomoses were patent. No thrombi were found anywhere except for 2 small adherent clots just above the site of the vena caval plication. Two pulmonary emboli were found in each lung, 6 to 9 milli-meters in diameter. Necrosis of lung had not occurred. There was extensive pulmonary edema. Microscopically, the liver cells were well preserved, although moderate fatty metamorphosis was present (Fig. 3B). Aggregates of periportal cells (Fig. 3B) were chiefly neutrophiles.
DISCUSSION
Few experimental studies are available concerning whole organ liver homotransplantation, and all of these involve the use of dogs. Goodrich and his colleagues published the first extensive experiments on hepatic transplantation in 1956. The value of these investigations was limited by the fact that the organs were transplanted to the pelvis, without removal of the dog's own liver, Since then, Moore (4, 5) and Starzl (9, 10) and their colleagues have reported succcssful hepatic transplantation in dogs with anatomically normal placement of the homograft after removal of the recipient's own liver. These studies clarified the physiological and biochemical events which transpire during rejection of hepatic grafts. In addition. the investigations defined the specific difficulties which must be surmounted if operative failure is to be avoided.
The provision of a viable and minimally damaged homograft is undoubtedly the most important single factor in the determination of success. It is necessary to obtain the donated tissue from a cadaver. Yet the extraordinary sensitivity of the tissue to anoxia requires that an adequate hepatic circulation be present before death and that some form of hepatic preservation be instituted immediately after death before the onset of irreversible cellular injury. For the latter purpose, the hypothermic perfusion technique recently developed by Marchioro and his colleagues was used. It is possible with this method simultaneously to cool and perfuse the liver within a few minutes after death. before beginning its operative removal. The most effective means of postmortem preservation are futile, however, if the moribund state has been protracted. In Patient 1, the selection of a donor after prolonged cardiac massage was unwise. In contrast, the circumstances of death of the donors for Patients 2 and 3 were highly favorable.
Although the technique of hypothermic perfusion has made liver transplantation feasible, the method has definite limitations in extending the postmortem viability of hepatic tissue. Marchioro and his associates have shown that severe hepatocellular injury almost invariably occurs with perfusions of more than 2 hours. The policy of staging the operation in the recipients, which was followed in the last 2 patients, allows reduction of this time to a minimum. After preliminary mobilization of the diseased liver and preparation of the structures for subsequent anastomoses, the definitive second operation can be performed after the incision has been quickly reopened with a minimum of tedious time-consuming dissection.
The surgical details of implantation involve, for the most part. utilization of well standardized surgical methods. The vascular anastomoses frequently must be performed with short cuffs and with limited exposure, necessitating intraluminal suturing techniques (Fig. 8B). The greatest hazard is in performance of the inferior vena caval anastomosis at the diaphragm. Both the donor and recipient segments of the vena cava often have orifices of small posterior phrenic tributaries which are unknowingly severed when the respective livers are removed. Such open orifices are located a short distance from the cut edge of and can only be seen from the inside of the vessel. Failure to suture the openings during the caval-caval anastomosis results in later hemorrhage at a time when the presence of the homograft makes secondary exposure of this area almost impossible. The double suture method developed in dogs for this anastomosis has virtually eliminated this problem (Fig. 8).
Reconstitution of internal biliary drainage in infants will probably be most effectively accomplished with a Roux-en-Y cholecystojejunostomy. In the 2 adults, choledochocholedochostomy was simple to perform by the stenting of the anastomosis with a T tube inserted through the recipient portion of the common duct ( Fig. 8C). Although the principal arterial supply to the common duct comes from retroduodenal sources, Parke, Michels, and Ghosh have demonstrated that vascular contributions, upon which viability of the donor common duct depends, also come from the hepatic arteries in the hilum of the liver.
One unique technical requirement is for decompression of the venous systems which must be temporarily occluded during the transplantation. In the dog, failure to obtain satisfactory drainage from these venous pools results in certain failure, particularly when any degree of venous hypertension is allowed to develop in the splanchnic bed. In man, the necessity for providing drainage from at least the portal system is probably not so important as in the dog. Child has demonstrated that acute occlusion of the portal vein in monkeys and man is usually well tolerated, presumably because collateral channels are more abundant in primates than in lower forms. Indeed, it was demonstrated in the presently reported 3 cases that drainage of the portal system was not necessary. In 2 of the 3 patients, external bypasses were not used, and in the third, flow ceased after a few minutes. The conclusion seems justified that a single effective bypass from the inferior vena cava to the superior vena cava is all that is required for adequate venous return to the heart from both the inferior vena caval and portal systems.
Experience with these 3 patients stresses the need for close control of the coagulation processes during and after transplantation. Profound clotting defects were demonstrated in all at the time of transplantation resulting in fatal hemorrhage in the first patient. The hemorrhagic tendency did not result from an acute deficiency of those clotting factors which are synthesized in the liver. Analysis of plasma fibrinogen during the liverless phase in Patients 2 and 3 did not reveal a clinically significant drop, a finding in accord with many experimental studies which show that a substantial decrease in clotting factors after total hepatectomy requires several hours to develop. Instead, the important finding appeared to be an explosive increase in the plasma fibrinolytic activity which developed within minutes, both during manipulation of the liver (Fig. 11) and after its removal (Fig. 12). The exact cause of this change is open to some speculation. As a working hypothesis, it might be assumed that the liver normally elaborates a substance which inhibits conversion of plasminogen to plasmin (fibrinolysin) presumably by a deterrent action on a plasminogen activator. Absence of the liver or severe hepatic injury during operative manipulation could conceivably permit uncontrolled conversion of plasminogen to plasmin by removal of such a restraining influence on the activator system.
Whatever the explanation for the increased fibrinolytic activity, it is imperative to anticipate this tendency and to provide prophylactic treatment before a frank hemorrhagic diathesis develops. In the second and third patients, EACA was administered prophylactically, within a few minutes after removal of the recipient's liver. This drug, which prevents activation of plasminogen to plasmin by inhibiting plasminogen activators, apparently prevented the fibrinolytic crisis in these last 2 patients despite profound decreases in euglobulin lysis time. The administration of fibrinogen and fresh blood at the time of transplantation are probably also of significant value in reducing the danger of a hemorrhagic diathesis.
In view of the effort expended to define and prevent the hemorrhagic diathesis which led to the death of the first patient, it is ironic that Patients 2 and 3 should have died because of the late consequences of intravascular clotting. In both the latter paticnts, the immediate cause of death was multiple pulmonary emboli. In both, the characteristic clotting deficiency which was observed at the time of operation was sueceeded by a hypcrcoagulable state several days later, which may have been an important contributing factor in the formation and propagation of the emboli.
The source of the pulmonary emboli in Patients 2 and 3 is a matter of interest. It is encouraging that the vascular suture lines of the transplant itself did not have a selective tendency to develop thrombosis. Rather, the peripheral thrombosis in Patient 2 was in the right iliac vein and the terminal inferior vena cava, which led to the belief that protection from this complication would be afforded by the use of Spencer's vena caval plication procedure. In Patient 3, however, in which vena caval plication was performed, a peripheral focus of thrombus could not be found at autopsy. It is possible in this paticnt and in the second one as well that semi-fluid clots were originally formed in and passed through the external bypass system from the inferior to the superior vena cava during the transplantation. The early devcloprncnt of respiratory distress in both patients is compatible with such a sequence of events. In future attempts at hepatic homotransplantation, it is probable that these embolic complications can be prevented with well timed and accurately controlled heparin therapy, either at the time when the external bypass is in use or in the postoperative period.
This experience with humans has confirmed many previous experimental impressions of the functional behavior of the hepatic homograft. In dogs receiving liver homografts under optimal circumstances of cooling and minimal donor organ ischemia, there is a prompt resumption of hepatic metabolism with minimal immediate derangcment of function. Biochemical evidence of graft repudiation begins on the fourth or fifth day in the untreated animal, but this can be mitigated or prevented by the use of a therapeutic regimen similar to that employed in the clinical cases. The most useful measurements to follow the course of the rejection process are serum bilirubin, serum alkaline phosphatase, and serum glutamic oxalacetic acid transaminase. Under more adverse experimental conditions, simulating those necessary in a clinical setting, Marchioro and his colleagues have shown that there is a moderately severe ischemic injury to the canine liver, manifested by sharp rises in bilirubin, alkaline phosphatase and SGOT within the first 24 hours. Differentiation of the latter nonspecific changes from those due to rejection is an important aspect of the postoperative care.
Both of the patients who survived the operative procedure exhibited a functional pattern of acute parenchymal injury. Rises in SGOT to 950 to 1150 SF units occurred within the first day and then rapidly receded. Jaundice temporarily deepened. The subsequent curves of various function tests were in the direction of improvement until immediately before death, demonstrating the reversibility of these early changes.
The experiences in these cases have demonstrated that the immediate problems of clinical hepatic homotransplantation are subject to practical solution. They provide little information, however, concerning the feasibility of long term maintenance of liver homografts, although the progressive improvement of liver function and the degree of histologic preservation of the transplants after 7½ and 22 days are encouraging notations. In unpublished observations from our laborntorics, it has been found possible to obtain prolongation of survival of pharmacologically altered dogs with liver homografts, which has been comparable to that obtained in treated animals receiving renal homografts. Ultimately, it may be necessary to conclude that the treatment required to prevent rejection is significantly different with livers than with kidneys. At present, however, there is no evidence to support such a belief, and the further acquisition of experience in the treatment of these otherwise doomed patients appears to be justified.
SUMMARY
A number of problems are described which must be surmounted for the clinical use of liver homotransplantation, based upon experience with 3 patients. The first patient died of hemorrhage during conclusion of the operation. The second and third patients lived for 22 and 7½ days, respectively, both ultimately dying from multiple pulmonary emboli.
The operative requirements for successful liver transplantation appear to be subject to practical solution. Of the utmost importance is the procurement of a viable and relatively undamaged donor organ. This has been accomplished with the use of an extracorporeal circuit which perfuses and cools the liver immediately after death. In addition, the time interval between death of the donor and restoration of a hepatic blood supply in the transplanted site has been shortened by operating on the recipient patient in 2 stages. At the preliminary operation, all structures are skeletonized above and below the liver with facilitation of the recipient hepatectomy and multiple anastomoses which are performed at the second and definitive procedure. While the transplantation is being performed, the venous return from the splanchnic and inferior vena caval systems is temporarily occluded. It has been found necessary to decompress only the inferior vena cava during this time with an external bypass from the inferior to the superior vena caval systems.
Changes in the coagulation mechanisms constitute a serious deterrent to success. During operation, a bleeding diathesis is regularly detectable by laboratory examination. Postoperatively, a state of hypercoagulability has developed, which probably contributed to the lethal complication of multiple pulmonary embolization in 2 patients. It is also possible that the use of the external bypass contributed to the formation of the emboli.
After operation, hepatic functions were immediately deranged, probably as the result of injury incurred during the transplantation, with progressive improvement thereafter. Later, biochemical evidence of homograft rejection was not observed, and at autopsy in the last 2 patients there was surprisingly good gross and histologic preservation of graft structure. It is thought that the therapy with azathioprine, prednisone, and actinomycin C had forestalled the rejection process.
Acknowledgments
Aided by Grants A-6283, A-6344, AI-04152, HE-00735-01, and AM-07772-01.
REFERENCES
- 1.CHILD CG. W. B. Saunders Co.; Philadelphia: 1954. The Hepatic Circulation and Portal Hypertension; p. 285. [Google Scholar]
- 2.GOODRICH EO, WELCH HF, NELSON JA, BEECHER TS, WELCH CS. homotransplantation of the canine liver. Surgery. 1956;39:244. [PubMed] [Google Scholar]
- 3.MARCHIORO TL, HUNTLEY RT, WADDELL WR, STARZL TE. The use of extracorporeal perfusion for obtaining postmortem grafts. Surgery. In press. [PMC free article] [PubMed] [Google Scholar]
- 4.McBRIDE RA, WHEELER HB, SMITH LL, MOORE FD, DAMMIN GJ. homotransplantation of the canine liver as an orthotopic vascularized graft; histologic and functional correlations during residence in the new host. Am. J. Path. 1962;41:501. [PMC free article] [PubMed] [Google Scholar]
- 5.MOORE FD, WHEELER HB, DEMISSIANOS HV, SMITH LL, BALANKURA O, ABEL K, GREENBERG JB, DAMMIN GJ. Experimental whole organ transplantation of the liver and of the spleen. Ann. Surg. 1960;152:374. [PMC free article] [PubMed] [Google Scholar]
- 6.PARKE WW, MICHELS NA, GHOSH CM. Blood supply of the common bile duct. Surg. Gyn. Obst. 1963;117:47. [PubMed] [Google Scholar]
- 7.RAINOFF OD, MENZIE C. New method for determination of fibrinogen in small samples of plasma. J. Laborat. Clin. M. 1951;37:316. [PubMed] [Google Scholar]
- 8.SPENGER FC, QUATTLEBAUM JK, QUATTLEBAUM JK, Jr., SHARP EH, JUDE JR. Plication of the inferior vena cava for pulmonary embolism; report of 20 cases. Ann. Surg. 1962;155:827. doi: 10.1097/00000658-196215560-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.STARZL TE, KAUPP HA, JR., BROCK DR, LAZARUS RE, JOHNSON RV. Reconstructive problems in canine liver homotransplantation with special reference to the postoperative role of hepatic venous flow. Surg. Gyn. Obst. 1960;111:733. [PMC free article] [PubMed] [Google Scholar]
- 10.STARZL TE, KAUPP HA, JR., BROCK DR, LINMAN JW. Studies on the rejection of the transplanted homologous dog liver. Surg. Gyn. Obst. 1961;112:135. [PMC free article] [PubMed] [Google Scholar]
- 11.STARZL TE, MARCHIORO TL, HOLMES JH, BRITTAIN RS, HERMAN G, STONINOTON OG, KNIGHT ICS, TALMADGE DW, WADDELL WR. Clinical problems in renal homotransplantation. J. AM. M. ASS. in press. [Google Scholar]
- 12.STARZL TE, MARCHIORO TL, WADDELL WR. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg. Gyn. Obst. in press. [PMC free article] [PubMed] [Google Scholar]
- 13.VON KAULLA KN. Continuous automatic recording of fibrin formation and fibrinolysis; a valuable tool for coagulation reserch. J. Laborat. clin. M. 1957;49:304. [PubMed] [Google Scholar]
- 14.The Coagulation of Blood; Methods of Study. Grunc & Stratton, Inc.; New York: Estimation of the thrombin time of plasma. Idem. in press. [Google Scholar]
- 15.VON KAULLA KN, SCHULTZ RL. Methods for evaluation of human fibrinolysis; studies with two combined techniques. Am. J. Clin. Path. 1958;29:104. doi: 10.1093/ajcp/29.2.104. [DOI] [PubMed] [Google Scholar]