Abstract
Type II endometrial cancers (uterine serous papillary and clear cell histologies) represent rare but highly aggressive variants of endometrial cancer (EC). HER2 and EGFR may be differentially expressed in type II EC. Here, we evaluate the clinical role of HER2 and EGFR in a large cohort of surgically staged patients with type II (nonendometrioid) EC and compare the findings with those seen in a representative cohort of type I (endometrioid) EC. In this study HER2 gene amplification was studied by fluorescence in situ hybridisation (FISH) and EGFR expression by immunohistochemistry. Tissue microarrays were constructed from 279 patients with EC (145 patients with type I and 134 patients with type II EC). All patients were completely surgically staged and long-term clinical follow up was available for 258 patients. The rate of HER2 gene amplification was significantly higher in type II EC compared with type I EC (17 vs 1%, P<0.001). HER2 gene amplification was detected in 17 and 16% of the cases with uterine serous papillary and clear cell type histology, respectively. In contrast, EGFR expression was significantly lower in type II compared with type I EC (34 vs 46%, P=0.041). EGFR expression but not HER2 gene amplification was significantly associated with poor overall survival in patients with type II EC, (EGFR, median survival 20 vs 33 months, P=0.028; HER2, median survival 18 vs 29 months, P=0.113) and EGFR expression retained prognostic independence when adjusting for histology, stage, grade, and age (EGFR, P=0.0197; HER2, P=0.7855). We conclude that assessment of HER2 gene amplification and/or EGFR expression may help to select type II EC patients who could benefit from therapeutic strategies targeting both HER2 and EGFR.
Keywords: HER-2/neu, EGFR, endometrial cancer, lapatinib, trastuzumab
Endometrial carcinoma (EC) is the most common malignancy of the female reproductive tract. Based on pathological and clinical features, endometrial cancers are classified into two types. Endometrioid or Type I EC, represents the majority of EC cases, is oestrogen-related, usually arises in the setting of endometrial hyperplasia, and tends to be biologically less aggressive (Hecht and Mutter, 2006). Nonendometrioid or Type II EC, predominantly uterine serous papillary carcinoma and clear cell endometrial carcinoma, accounts for approximately 10% of ECs, is not oestrogen-related, arises from atrophic endometrium, and frequently presents in advanced stages with 5-year survival rates, on average, between 30 and 40% (Abeler and Kjorstad, 1991; Goff et al, 1994; Slomovitz et al, 2003). Extra-uterine disease is often found in these patients even in the absence of myometrial invasion (Abeler 1991; Goff et al, 1994; Bristow et al, 2001). Therefore, comprehensive surgical staging is recommended for all patients with type II EC regardless of the depth of myometrial invasion of the tumour (Chan et al, 2003). These different clinical features are paralleled by genetic distinctions. Type II ECs carry mutations of independent sets of genes compared with type I EC. Type I EC is associated with mutations in the PTEN tumour suppressor gene and defects in DNA mismatch repair (Lax et al, 2000, Hecht and Mutter, 2006). In contrast, p53 mutations, which are not usually seen in type I EC have been identified in most cases of type II EC (Acharya et al, 2005). Moreover, HER2 amplification/overexpression has been associated with type II EC (Hetzel et al, 1992; Santin et al, 2005; Morrison et al, 2006; Grushko et al, 2008). However, the exact frequency of HER2 amplification/overexpression in type II EC remains controversial. HER2 gene amplification has been reported to occur in 6 out of 28 (21%), 17 out of 58 (29%), or 11 out of 26 (42%) of patients with uterine serous papillary cancer (Santin et al, 2005; Morrison et al, 2006; Grushko et al, 2008), and HER2 protein overexpression was seen in 12 out of 68 (18%) of cases (Slomovitz et al, 2008). In clear cell endometrial cancer HER2 amplification has been described in two out of nine (22%) and three out of six (50%) of the reported cases (Morrison et al, 2006; Grushko et al, 2007). Similar controversies exist regarding the clinical relevance of the epidermal growth factor receptor (EGFR) in EC in general and more specifically in type II EC. EGFR expression has been demonstrated in 43–67% of patients with endometrial cancer and its association with clinical outcome has been explored with some studies demonstrating an association between EGFR expression and poor clinical outcome (Khalifa et al, 1994; Scambia et al, 1994; Niikura et al, 1995) whereas others show no association (Reinartz et al, 1994). Moreover, the clinical role of EGFR expression specifically in type II EC has not yet been studied. The current study represents a large consecutive series of 134 type II EC cases including 106 patients with uterine serous papillary EC and 28 patients with clear cell EC, who underwent surgery at Mayo Clinic between 1984 and 2004, respectively.
Patients at risk of relapse appear to benefit from adjuvant chemotherapy, and responses to paclitaxel- and platinum-based regimens have been reported in patients with advanced disease (Hoskins et al, 2001; Ramondetta et al, 2001). Importantly, however, target-based treatment approaches have not yet been explored in type II EC. Recently, we were able to demonstrate significant preclinical activity of a dual HER2 and EGFR kinase inhibitor in endometrial cancer cell lines with HER2 amplification or EGFR expression (Konecny et al, 2008). In an effort to obtain a better understanding of the clinical role of HER2 and EGFR in type II EC we studied HER2 gene amplification and EGFR expression in a large cohort of patients with type II EC who were all surgically staged and compared these findings with those seen in a representative cohort of type I endometrioid EC treated during the same time period. These studies were intended to help characterise a subset of type II endometrial cancer patients most likely to benefit from target-based treatment approaches.
Materials and methods
Upon approval from the Institutional Review Board at Mayo Clinic, we identified 137 patients from our database who underwent surgery for type II endometrial cancer at Mayo Clinic between May 1984 and December 2004. Next, we randomly selected 150 patients who underwent surgery for endometrioid endometrial cancer during the same time period. Of the 287 patients included in this study, 279 patients (97%) had archived paraffin-embedded tissue available for analysis of HER2 gene amplification and EGFR expression. All patients were completely surgically staged and long-term clinical follow up was available for 258 patients (125 patients with type I EC and 133 patients with type II EC). Tissue microarrays were created for each histological subtype. All patients had a hysterectomy and removal of existing adnexal structures and no other malignancy was diagnosed within 5 years before or after the diagnosis of endometrial cancer. Staging was defined according to the International Federation of Obstetricians and Gynecologists (FIGO) surgical staging system. For patients treated before 1988, stage was determined retrospectively on the basis of the surgical and pathologic assessments. The histological classification was according to the World Health Organization classification. Architectural grading was based on the degree of glandular differentiation in accordance with the FIGO guidelines. All surgical procedures were the responsibility of a gynaecologic oncologist. Lymphadenectomy was performed in patients considered by the surgeon to be at risk for lymph node metastasis, according to the histological grade and subtype, as well as primary tumour diameter and the depth of myometrial invasion as determined by an intraoperative analysis of frozen tissue sections. Postoperative adjuvant radiotherapy consisted of external pelvic, para-aortic, or abdominal irradiation or vaginal brachytherapy or a combination of these.
Tissue specimens and tissue microarray
For tissue microarray construction, a hematoxylin and eosin (H&E) stained histology slide from each patient's archival tumour block was reviewed to identify and mark the location of tumour and normal components. The markings were transferred to the corresponding tissue block. Marked donor blocks were cored into the recipient master block according to a grid map with 0.8-mm spacing from the center of one core to the center of the next core either manually for the type I endometrial cancer tissue microarrays (TMAs) or by use of an automated Beecher ATA 27 Tissue Arrayer for the type II endometrial cancer TMAs (Beecher Instruments Inc., Silver Spring, MD, USA). The tissue microarray blocks constructed from the study tumours incorporated either one tumour core and one normal tissue core (type I endometrial cancer TMAs) or up to three tumour cores (type II endometrial cancer TMAs) from an archival block for each subject. Each core was 0.6 mm in diameter. Cores were arrayed into grids of either 100 cores (type I endometrial cancer TMAs) or 300 cores (type II endometrial cancer TMAs) in master blocks. After construction, tissue microarray blocks were sealed with paraffin and stored at 4°C. Sections (5 μm thick) were cut from the tissue microarray master blocks, mounted on superfrost slides, and assayed for HER2 gene amplification and EGFR expression. HER2 FISH assays were performed using the PathVysion assay (Abbott-Vysis Inc., Des Plaines, IL, USA) as described elsewhere (Press et al, 2002). A slide was stained with H&E to confirm the presence of invasive tumour. The corresponding area of invasive carcinoma was enumerated on the FISH slides after hybridisation was complete. Slides were evaluated for HER2 gene amplification by determining the HER2/CEP17-signal ratio in at least 20 tumour nuclei as required by the FDA original approval. If the ratio was <2.0, the specimen was considered to lack gene amplification. For ratios near the cutoff value (i.e., 1.8–2.2), an additional 20 nuclei were evaluated by the same analyst and the ratio was recalculated. In these cases a second analyst also scored at least 40 tumour cell nuclei and if the ratios were both in agreement, the case recorded. All assessments of HER2 status were made by a board-certified pathologist with extensive experience in HER2 FISH testing (MFP). EGFR expression was assessed by IHC and EGFR staining was solely assessed in the glandular components of the endometrial tumour tissue by a board-certified pathologist specialised in gynaecologic malignancies (GLK). A polyclonal rabbit antibody to the C-terminus epitope of EGFR was obtained from Santa Cruz Biotechnology, Santa Cruz, CA, USA (cat. no. 1005; dilution 1 : 250). Tissue sections were microwave-heated for 5 min in an 800-W oven in citrate buffer (0.1 mM, pH 6.0). Sections were then incubated at room temperature overnight with primary antibody. Immunostaining was performed with the avidin–biotin complex method (Vector, Burlingame, CA, USA). Negative controls consisted of substituting normal serum for primary antibodies. For tissue microarray analysis images of H&E and EGFR stains were scanned with the Bliss Imaging System (Bacus Laboratories, Inc, Lombard, IL, USA). The x and y coordinates of each core within the grid were determined by the software and included as part of the unique identifier, which was linked to the clinical database. Only staining of the tumour cell membranes was considered positive. Immunoreactivity was qualitatively scored by interpreting the staining intensity (negative; weak, moderate, or strong staining) and the percentage of positive tumour cells per core (⩽25%; >25–50%; >50–75%; and >75%). Tissues were graded positive for EGFR expression with ⩾ moderate staining intensity in >25% of the cells examined.
Statistics
Overall survival was defined as the time to death from any cause. Patients were censored on the date of last contact if a treatment failure event had not been observed. Unadjusted survival was assessed by the Kaplan–Meier method. Log-rank statistic was used for outcome comparison in univariate analysis. Cox regression analysis was used to estimate hazard ratios and their 95% confidence intervals in multivariate analysis adjusted for histology type, FIGO stage, tumour grade, and age. All reported P-values and confidence intervals are from two-sided tests. Because well-established and replicated cutoffs for the expression status of EGFR in endometrial cancer were not available, we made the a priori choice to analyse and report the scores as dichotomised values.
Results
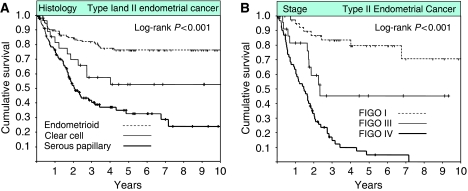
The current study is an observational study in which we compared the pattern of HER2 gene amplification and EGFR expression between a cohort of 134 consecutive patients with type II ECs (106 uterine serous papillary and 28 clear cell type histologies) and a representative group of 145 patients with type I EC treated over the same time period. Patients with clear cell and uterine serous papillary histology had a significantly worse OS when compared with endometrioid EC cases (Figure 1A) and in patients with type II EC FIGO stage was a significant predictor of OS (Figure 1B). Tissue microarrays were constructed from the 279 available tumour specimens for each histological subtype and HER2 as well as EGFR status were assessed by FISH and IHC, respectively. The use of tissue microarrays allowed us to obtain technically evaluable results for HER2 in 275 (99%) and for EGFR in 255 (91%) of the 279 patients, respectively (Table 1). HER2 gene amplification was significantly higher in type II EC when compared with type I EC (17 vs 1.4%, P<0.001). HER2 gene amplification was seen in 18 (17%) of the 105 evaluable uterine serous papillary EC specimens, and in four (16%) of the 25 evaluable clear cell EC specimens. Type I EC demonstrated a lower than expected rate of HER2 gene amplification (1.4%). Furthermore, we were not able to detect an increase in the rate of HER2 gene amplification from grade 1 to grade 3 endometrioid ECs (Table 2). The mean HER2/CEP17 signal ratio was 3.61 (range, 2.00–8.89) in samples with HER2 gene amplification. The mean number of HER2 signals per cell, mean number of CEP17 signals per cell, and the HER2/CEP17 ratios for each HER2-positive case are shown in Table 3.
Figure 1.
(A) Kaplan–Meier survival plots of all type I and type II endometrial cancer patients with available clinical follow-up information (n=258) according to the histology type and (B) Kaplan–Meier survival plots among type II endometrial cancer patients with available follow up (n=133) according to FIGO stage.
Table 1. Patient and disease characteristics of endometrial cancer patients with endometrioid, uterine serous papillary, and clear cell type histology.
|
Endometrioid
|
Uterine serous papillary
|
Clear cell
|
||||
|---|---|---|---|---|---|---|
| No. | Valid % | No. | Valid % | No. | Valid % | |
| 145 | 106 | 28 | ||||
| Median age (range) | 65 (38–90) | 68 (47–93) | 68 (41–86) | |||
| Median follow up (months) | 83 (0.3–270) | 20 (0.1–162) | 38 (0.2–180) | |||
| Surgery dates (range) | 3/84–3/04 | 2/84–12/04 | 3/88–5/04 | |||
| Stage | ||||||
| I | 97 | (67) | 30 | (29) | 13 | (46) |
| II | 11 | (8) | 0 | (0) | 0 | (0) |
| III | 22 | (15) | 17 | (17) | 7 | (25) |
| IV | 14 | (10) | 56 | (54) | 8 | (29) |
| Grade | ||||||
| 1 | 51 | (35) | 1 | (1) | 2 | (7) |
| 2 | 54 | (37) | 1 | (1) | 4 | (14) |
| 3 | 40 | (28) | 87 | (98) | 22 | (79) |
| HER2 Status | ||||||
| Positive | 2/143 | (1.4) | 18/105 | (17) | 4/25 | (16) |
| EGFR status | ||||||
| Positive | 60/130 | (46) | 36/101 | (36) | 6/24 | (25) |
Table 2. Associations between HER2 gene amplification or EGFR expression and disease characteristics including type of histology, FIGO stage, and grade.
|
Patients
|
HER2-positive
|
EGFR-positive
|
||||
|---|---|---|---|---|---|---|
| No. | Valid % | No. | Valid % | No. | Valid % | |
| Histology* | ||||||
| Endometrioid grade 1 | 51 | (18) | 1/50 | (2) | 27/48 | (56) |
| Endometrioid grade 2 | 54 | (20) | 1/53 | (2) | 22/47 | (47) |
| Endometrioid grade 3 | 40 | (15) | 0/40 | (0) | 11/35 | (31) |
| Clear cell | 28 | (10) | 4/25 | (16) | 6/24 | (25) |
| Serous papillary | 106 | (37) | 18/105 | (17) | 36/101 | (36) |
| FIGO stage** | ||||||
| I | 140 | (52) | 8/140 | (6) | 54/127 | (43) |
| II | 11 | (4) | 0/11 | (0) | 4/9 | (44) |
| III | 46 | (17) | 3/46 | (7) | 14/43 | (33) |
| IV | 77 | (27) | 13/77 | (17) | 27/72 | (38) |
| Grade*** | ||||||
| 1 | 54 | (21) | 1/54 | (2) | 28/51 | (55) |
| 2 | 59 | (23) | 2/59 | (3) | 23/52 | (44) |
| 3 | 148 | (56) | 17/148 | (11) | 45/138 | (33) |
Unknown data: grade (n=17), stage (n=4), HER2 (n=6), EGFR (n=24).
* χ2 test type I versus type II: P<0.001 for HER2, and P=0.041 for EGFR.
** χ2 test: P=0.025 for HER2, and P=0.667 for EGFR.
*** χ2 test: P=0.028 for HER2, and P=0.016 for EGFR.
Table 3. Mean number of HER2 and Cep17 signals per cell, as well as the HER2/Cep17 ratios for each HER2-positive case.
| HER2/Cep17 ratio | HER2 signals/cell | Cep17 signals/cell | Histology |
|---|---|---|---|
| 2.00 | 3.40 | 1.70 | USPC |
| 2.03 | 3.95 | 1.95 | CC |
| 2.05 | 5.73 | 2.80 | USPC |
| 2.06 | 3.40 | 1.65 | USPC |
| 2.06 | 5.57 | 2.70 | CC |
| 2.08 | 5.00 | 2.40 | USPC |
| 2.28 | 4.45 | 1.95 | USPC |
| 2.55 | 6.75 | 2.65 | USPC |
| 2.63 | 5.4 | 1.55 | USPC |
| 2.71 | 3.25 | 1.20 | USPC |
| 2.88 | 4.75 | 1.65 | USPC |
| 2.98 | 5.95 | 2.00 | USPC |
| 3.16 | 8.05 | 2.55 | USPC |
| 3.48 | 5.40 | 1.55 | CC |
| 3.93 | 5.70 | 1.45 | USPC |
| 3.98 | 8.95 | 2.25 | USPC |
| 4.21 | 13.25 | 3.15 | USPC |
| 4.23 | 8.25 | 1.95 | E |
| 4.41 | 7.50 | 1.70 | USPC |
| 4.58 | 13.75 | 3.00 | USPC |
| 6.59 | 10.55 | 1.60 | E |
| 7.15 | 17.55 | 2.45 | CC |
| 8.89 | 15.55 | 1.75 | USPC |
USPC=uterine serous papillary cancer; CC=clear cell cancer; E=endometrioid endometrial cancer.
Missing data: n=1.
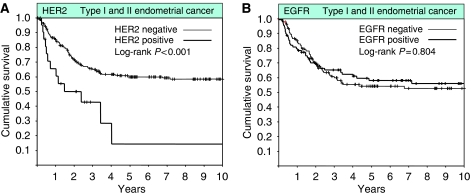
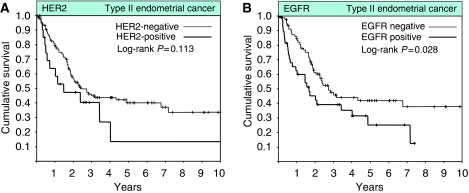
In contrast to the higher rate of HER2 gene amplification in type II EC, EGFR expression was significantly lower in type II EC compared with type I EC (34 vs 46%, P=0.041; Table 1). Moreover, the rate of EGFR expression was significantly lower in grade 3 compared with grade 1/2 endometrioid EC (31 vs 52%, P=0.016). When analysing all 279 patients, there was a significant increase in the rate of HER2 amplification, but not of EGFR expression, from stage I to stage IV disease (HER2, P=0.025; EGFR, P=0.667; Table 2). Similarly, we found a significant increase in the rate of HER2 gene amplification from grade 1 to 3 tumours, and conversely a significant decrease in the rate of EGFR expression from grade 1 to 3 tumours (P=0.028 and P=0.016, respectively; Table 2). HER2 gene amplification was associated with significantly worse OS in univariate analysis (HER2, P<0.001; Figure 2A) but did not retain independent prognostic significance when accounting for grade, stage, age, and histology (HER2, P=0.860). EGFR was not prognostically relevant in the entire cohort of 279 patients including both type I and Type II EC (P=0.804; Figure 2B). When analysing only patients with type II endometrial cancer EGFR expression, but not HER2 gene amplification, was statistically significantly associated with worse OS when compared with those patients with either non-HER2 amplified or non-EGFR expressing EC (HER2, median OS 18 vs 29 months, P=0.113; EGFR, median OS 20 vs 33 months, P=0.028; Figure 3A and B). EGFR-retained independent prognostic significance for OS in type II EC when accounting for age and stage in multivariate analysis (EGFR, risk ratio 1.81, 95% CI 1.10 – 2.99, P=0.0197; Table 4).
Figure 2.
Kaplan–Meier survival plots of all type I and type II endometrial cancer patients with available clinical follow-up information (n=258) according to HER2 status (A), and EGFR status (B).
Figure 3.
Kaplan–Meier survival plots of all type II endometrial cancer patients with available clinical follow-up information (n=133) according to HER2 status (A), and EGFR status (B).
Table 4. Prognostic significance of EGFR status in type II endometrial cancer patients using multivariate analysis.
| Parameter | Risk ratio (95% CI) | Wald test |
|---|---|---|
| Age | 1.06 (1.03, 1.09) | P=0.0002 |
| Stage | ||
| 1–2 (reference) | 1.0 | |
| 3 | 3.23 (1.24, 8.40) | P=0.0160 |
| 4 | 11.8 (5.33, 26.2) | P<0.0001 |
| EGFR | ||
| Negative (reference) | 1.0 | |
| Positive | 1.81 (1.10, 2.99) | P=0.0197 |
A stepwise method was used for variable selection. EGFR expression, stage, and age were selected as significant prognostic factors.
Discussion
Our understanding of the pathogenesis or the optimal treatment of uterine serous papillary and clear cell EC is limited. The existing lack of prospective clinical trials assessing adjuvant therapy in these aggressive variants of EC and the absence of targeted treatment approaches reflects at least in part the low incidence of type II EC with the accompanying limited single institutional experiences. However, a reasonable estimate would predict that 3000–4000 women in the United States alone will be diagnosed with type II EC during 2008 and an estimated 55–65% will die, accounting for approximately 18–24% of all endometrial cancer-related deaths (Podratz and Mariani, 2003). Thus the absence of randomised clinical trials for one of the most aggressive gynaecologic malignancies appears unacceptable.
Evidence for a role of HER2 and EGFR in the pathogenesis of various cancers has led to the rational design and development of agents that selectively target HER2 and EGFR. In unselected patients with endometrial cancer, HER2 amplification/overexpression represents a rare event. However, the findings of our study confirm and extend previous reports that indicate HER2 amplification/overexpression is seen more commonly in well-defined subtypes of EC such as uterine serous papillary cancer or clear cell cancer. Trastuzumab (Herceptin) a humanised anti-HER2 antibody has recently been approved for the adjuvant treatment of HER2-overexpressing (3+ IHC) or FISH-positive primary breast cancers based on a highly significant 52% reduction in the risk of recurrence in node-positive HER2-positive primary breast cancer (Romond et al, 2005). More recent advances in biotechnology have led to the development of the oral dual tyrosine kinase inhibitor lapatinib, which has been shown to have significant activity in trastuzumab-refractory breast cancer (Geyer et al, 2006). Encouraged by these clinical response data which were generated in breast cancer patients with HER2 amplification/overexpression, we comprehensively assessed the rate of HER2 gene amplification in a large consecutive series of patients with uterine serous papillary and clear cell endometrial cancers. These studies were intended to define a subset of type II EC patients who may benefit from a selective HER2 inhibitor or a dual kinase inhibitor, which targets both HER2 and EGFR. The aggressive nature of type II EC is confirmed in our study by the observation that two out of three patients diagnosed with uterine serous papillary and one of two diagnosed with clear cell cancer had extra-uterine disease at the time of primary surgery. HER2 gene amplification was found in 17 and 16% of the serous papillary and clear cell EC cases, respectively. Earlier studies have reported higher rates of HER2 gene amplification in uterine serous papillary EC. The most recent study conducted by Grushko et al. detected HER2 gene amplification in 6 out of 28 (21%) patients with serous papillary and 3 out of 6 (50%) of clear cell EC (Grushko et al, 2008). This study cohort of that report, however, differed considerably from the consecutive series in our study, as it included patients with measurable stage III, stage IV, or recurrent endometrial cancer that were enrolled in GOG study no. 177 evaluating the role of doxorubicin and cisplatin with or without paclitaxel in advanced endometrial cancer. Santin et al. reported HER2 gene amplification in 14 out of 30 (47%) patients with uterine serous papillary cancer. Importantly, however, of the 30 patients included in his study, 12 were African-American patients of whom eight (67%) showed amplification by FISH compared with six (33%) of the remaining 18 Caucasian patients. Information on the patient's race was not collected in our current study, yet possible differences in patient populations may account for the reported discrepancy in the rate of HER2 gene amplification between both studies. Importantly, previous studies have reported a higher incidence of serous papillary endometrial cancer and a higher rate of HER2 gene amplification in African-American patients when compared with Caucasian patients (Maxwell and Risinger, 2006; Morrison et al, 2006). When combining the results of the aforementioned studies, which have all used FISH for assessment of HER2 status, HER2 gene amplification, at average, was detected in 54 out of 222 (24%) patients with serous papillary EC. In contrast to our low rate of HER2 gene amplification in type I EC, other groups have been able to demonstrate HER2 gene amplification in 9 out of 363 (4%) of unselected type I EC and in 5 out of 63 (8%) grade 3 endometrioid endometrial cancers (Morrison et al, 2006). We may not have been able to confirm these rates possibly because of differences in the patient populations or because of the smaller sample size of type I ECs in our study. Previous data on the rate of HER2 gene amplification in clear cell EC (22–50%) are unquestionably limited by the small number of samples investigated so far. The actual rate of HER2 gene amplification in clear cell EC may thus be somewhat lower according to the findings of our study.
The clinical role of EGFR has not been studied well in EC, moreover this is the first study to evaluate the incidence and prognostic relevance of EGFR expression in type II EC. Importantly, EGFR may have a dual role in EC, such that high EGFR expression in type I EC was associated with low grade and favourable outcome. In contrast, EGFR expression in type II EC was associated with high grade and adverse clinical outcome. Therefore, EGFR expression did not appear to impact disease progression in well-differentiated endometrioid endometrial cancer, but did seem to affect disease progression in undifferentiated nonendometrioid endometrial cancer. To date EGFR inhibitors have not been clinically tested in type II EC. Importantly, the clinical benefit observed with anti-EGFR tyrosine kinase inhibitors (TKIs) across different disease entities has been variable. For example, EGFR TKIs are largely inactive in colorectal cancer and breast cancer (Tan et al, 2004; Baselga and Arteaga, 2005). Nevertheless, two of these drugs, gefitinib and erlotinib, have demonstrated clinical activity in non small cell lung cancer and responses have been observed in patients with advanced pancreatic cancer and in head-and-neck cancer (Baselga, 2006). Preclinical data suggest that EGFR inhibitors may be clinically active in well-defined subgroups of endometrial cancer patients with HER2 gene amplification or high levels of EGFR expression (Konecny et al, 2008). Importantly, however, EGFR receptor expression levels when assessed by IHC have not been able to predict a response to EGFR inhibitors in other tumour types. Earlier clinical studies in other disease entities show that potential markers of sensitivity to EGFR TKIs include the presence of EGFR gene amplification, mutations of the EGFR gene, and increased expression of EGFR ligands (Baselga and Arteaga, 2005). Earlier studies have demonstrated significantly higher expression levels of the EGFR ligands TGF-α and amphiregulin in EC compared with normal endometrium (Pfeiffer et al, 1997; Ejskjaer et al, 2007). The roles of EGFR gene amplification or mutations in EC, however, have not yet been studied.
Although HER2 gene amplification or EGFR expression each can only be detected in small subsets of patients with type II EC, collectively, 46% of the patients with type II EC demonstrated HER2 gene amplification and/or EGFR expression in our study. The pooling of national or global patient resources should allow the realisation of prospective clinical trials (that stratify for HER2 gene amplification or EGFR expression) for patients with type II EC that may involve HER2 and EGFR tyrosine kinase inhibitors in well-defined subsets with HER2 gene amplification or EGFR expression.
References
- Abeler VM, Kjorstad KE (1991) Clear cell carcinoma of the endometrium: a histopathological and clinical study of 97 cases. Gynecol Oncol 40(3): 207–217 [DOI] [PubMed] [Google Scholar]
- Acharya S, Hensley ML, Montag AC, Fleming GF (2005) Rare uterine cancers. Lancet Oncol 6(12): 961–971 [DOI] [PubMed] [Google Scholar]
- Baselga J (2006) Targeting tyrosine kinases in cancer: the second wave. Science 312(5777): 1175–1178 [DOI] [PubMed] [Google Scholar]
- Baselga J, Arteaga CL (2005) Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. J Clin Oncol 23: 2445–2459 [DOI] [PubMed] [Google Scholar]
- Bristow RE, Asrari F, Trimble EL, Montz FJ (2001) Extended surgical staging for uterine papillary serous carcinoma: survival outcome of locoregional (Stage I–III) disease. Gynecol Oncol 81(2): 279–286 [DOI] [PubMed] [Google Scholar]
- Chan JK, Loizzi V, Youssef M, Osann K, Rutgers J, Vasilev SA, Berman ML (2003) Significance of comprehensive surgical staging in noninvasive papillary serous carcinoma of the endometrium. Gynecol Oncol 90(1): 181–185 [DOI] [PubMed] [Google Scholar]
- Ejskjaer K, Sorensen BS, Poulsen SS, Forman A, Nexo E, Mogensen O (2007) Expression of the epidermal growth factor system in endometrioid endometrial cancer. Gynecol Oncol 104(1): 158–167 [DOI] [PubMed] [Google Scholar]
- Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, Skarlos D, Campone M, Davidson N, Berger M, Oliva C, Rubin SD, Stein S, Cameron D (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355(26): 2733–2743 [DOI] [PubMed] [Google Scholar]
- Goff BA, Kato D, Schmidt RA, Ek M, Ferry JA, Muntz HG, Cain JM, Tamimi HK, Figge DC, Greer BE (1994) Uterine papillary serous carcinoma: patterns of metastatic spread. Gynecol Oncol 54(3): 264–268 [DOI] [PubMed] [Google Scholar]
- Grushko TA, Filiaci VL, Mundt AJ, Ridderstråle K, Olopade OI, Fleming GF, Gynecologic Oncology Group (2008) An exploratory analysis of HER-2 amplification and overexpression in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 108(1): 3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht JL, Mutter GL (2006) Molecular and pathologic aspects of endometrial carcinogenesis. J Clin Oncol 24(29): 4783–4791 [DOI] [PubMed] [Google Scholar]
- Hetzel DJ, Wilson TO, Keeney GL, Roche PC, Cha SS, Podratz KC (1992) HER-2/neu expression: a major prognostic factor in endometrial cancer. Gynecol Oncol 47(2): 179–185 [DOI] [PubMed] [Google Scholar]
- Hoskins PJ, Swenerton KD, Pike JA, Wong F, Lim P, Acquino-Parsons C, Lee N (2001) Paclitaxel and carboplatin, alone or with irradiation, in advanced or recurrent endometrial cancer: a phase II study. J Clin Oncol 19(20): 4048–4053 [DOI] [PubMed] [Google Scholar]
- Khalifa MA, Mannel RS, Haraway SD, Walker J, Min KW (1994) Expression of EGFR, HER-2/neu, P53, and PCNA in endometrioid, serous papillary, and clear cell endometrial adenocarcinomas. Gynecol Oncol 53(1): 84–92 [DOI] [PubMed] [Google Scholar]
- Konecny GE, Venkatesan N, Yang G, Dering J, Ginther C, Finn R, Rahmeh M, Fejzo MS, Toft D, Jiang SW, Slamon DJ, Podratz KC (2008) Activity of lapatinib a novel HER2 and EGFR dual kinase inhibitor in human endometrial cancer cells. Br J Cancer 98(6): 1076–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax SF, Kendall B, Tashiro H, Slebos RJ, Hedrick L (2000) The frequency of p53, K-ras mutations, and microsatellite instability differs in uterine endometrioid and serous carcinoma: evidence of distinct molecular genetic pathways. Cancer 88(4): 814–824 [PubMed] [Google Scholar]
- Maxwell GL, Risinger JI (2006) Racial disparities research: it's not just black and white. Gynecol Oncol 101(2): 194–197 [DOI] [PubMed] [Google Scholar]
- Morrison C, Zanagnolo V, Ramirez N, Cohn DE, Kelbick N, Copeland L, Maxwell GL, Fowler JM (2006) HER-2 is an independent prognostic factor in endometrial cancer: association with outcome in a large cohort of surgically staged patients. J Clin Oncol 24(15): 2376–2385 [DOI] [PubMed] [Google Scholar]
- Niikura H, Sasano H, Matsunaga G, Watanabe K, Ito K, Sato S, Yajima A (1995) Prognostic value of epidermal growth factor receptor expression in endometrioid endometrial carcinoma. Hum Pathol 26(8): 892–896 [DOI] [PubMed] [Google Scholar]
- Pfeiffer D, Spranger J, Al-Deiri M, Kimmig R, Fisseler-Eckhoff A, Scheidel P, Schatz H, Jensen A, Pfeiffer A (1997) mRNA expression of ligands of the epidermal-growth-factor-receptor in the uterus. Int J Cancer 72(4): 581–586 [DOI] [PubMed] [Google Scholar]
- Podratz KC, Mariani A (2003) Uterine papillary serous carcinomas: the exigency for clinical trials. Gynecol Oncol 91(3): 461–462 [DOI] [PubMed] [Google Scholar]
- Press MF, Slamon DJ, Flom KJ, Park J, Zhou J-Y, Bernstein L (2002) Evaluation of HER-2/neu gene amplification and overexpression: comparison of frequently used assay methods in a molecularly characterized cohort of breast cancer specimens. J Clin Oncol 20: 3095–3105 [DOI] [PubMed] [Google Scholar]
- Ramondetta L, Burke TW, Levenback C, Bevers M, Bodurka-Bevers D, Gershenson DM (2001) Treatment of uterine papillary serous carcinoma with paclitaxel. Gynecol Oncol 82(1): 156–161 [DOI] [PubMed] [Google Scholar]
- Reinartz JJ, George E, Lindgren BR, Niehans GA (1994) Expression of p53, transforming growth factor alpha, epidermal growth factor receptor, and c-erbB-2 in endometrial carcinoma and correlation with survival and known predictors of survival. Hum Path 25(10): 1075–1083 [DOI] [PubMed] [Google Scholar]
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer Jr CE, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353(16): 1673–1684 [DOI] [PubMed] [Google Scholar]
- Santin AD, Bellone S, Van Stedum S, Bushen W, Palmieri M, Siegel ER, De Las Casas LE, Roman JJ, Burnett A, Pecorelli S (2005) Amplification of c-erbB2 oncogene: a major prognostic indicator in uterine serous papillary carcinoma. Cancer 104(7): 1391–1397 [DOI] [PubMed] [Google Scholar]
- Scambia G, Benedetti Panici P, Ferrandina G, Battaglia F, Distefano M, D'Andrea G, De Vincenzo R, Maneschi F, Ranelletti FO, Mancuso S (1994) Significance of epidermal growth factor receptor expression in primary human endometrial cancer. Int J Cancer 56(1): 26–30 [DOI] [PubMed] [Google Scholar]
- Slomovitz BM, Broaddus RR, Burke TW, Sneige N, Soliman PT, Wu W, Sun CC, Munsell MF, Gershenson DM, Lu KH (2008) Her-2/neu overexpression and amplification in uterine papillary serous carcinoma. J Clin Oncol 22(15): 3126–3132 [DOI] [PubMed] [Google Scholar]
- Slomovitz BM, Burke TW, Eifel PJ, Ramondetta LM, Silva EG, Jhingran A, Oh JC, Atkinson EN, Broaddus RR, Gershenson DM, Lu KH (2003) Uterine papillary serous carcinoma (UPSC): a single institution review of 129 cases. Gynecol Oncol 91(3): 463–469 [DOI] [PubMed] [Google Scholar]
- Tan AR, Yang X, Hewitt SM, Berman A, Lepper ER, Sparreboom A, Parr AL, Figg WD, Chow C, Steinberg SM, Bacharach SL, Whatley M, Carrasquillo JA, Brahim JS, Ettenberg SA, Lipkowitz S, Swain SM (2004) Evaluation of biologic end points and pharmacokinetics in patients with metastatic breast cancer after treatment with erlotinib, an epidermal growth factor receptor tyrosine kinase inhibitor. J Clin Oncol 22: 3080–3090 [DOI] [PubMed] [Google Scholar]