Abstract
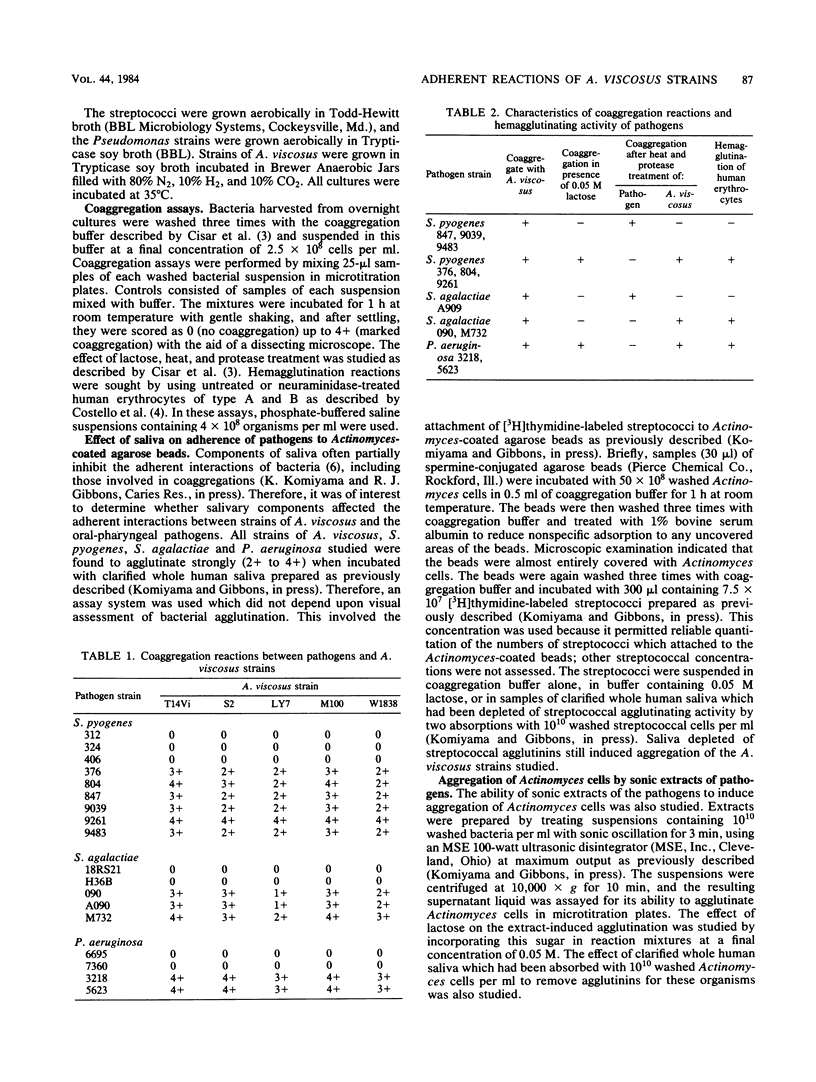
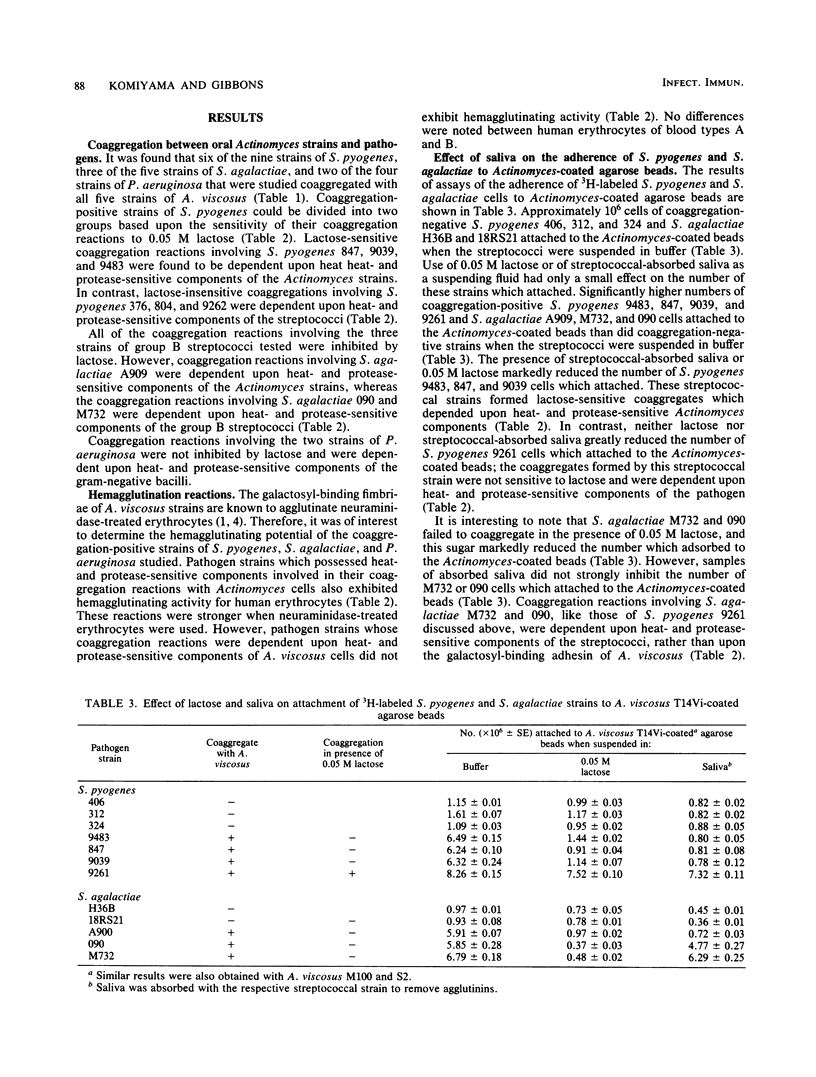
Interbacterial adherence was sought between strains of Actinomyces viscosus indigenous to the human mouth and strains of Streptococcus pyogenes, Streptococcus agalactiae, and Pseudomonas aeruginosa. Six of nine strains of S. pyogenes, three of five strains of S. agalactiae, and two of four strains of P. aeruginosa were found to coaggregate with each of five strains of A. viscosus tested. Some coaggregation reactions were inhibited by 0.05 M lactose and were dependent upon heat- and protease-sensitive Actinomyces components. Such reactions appear to involve the galactosyl-binding adhesin previously described in type 2 fimbriae on A. viscosus. Other coaggregation reactions were dependent upon heat- and protease-sensitive components of the pathogen. That such pathogen strains possessed an adhesin(s) was further suggested by the observation that they agglutinated human erythrocytes. The ability of coaggregation-positive and -negative strains of S. pyogenes and S. agalactiae to adhere to Actinomyces-coated agarose beads was also studied. Coaggregation-positive streptococcal strains attached in higher numbers to the Actinomyces-coated beads than did strains which were coaggregation negative. Lactose (0.05 M) inhibited the attachment of those streptococcal strains which coaggregated with A. viscosus in a lactose-sensitive manner. The adherence of those streptococcal strains whose coaggregation appeared to depend upon the galactosyl-binding adhesin of A. viscosus was also reduced by components of human saliva. Crude sonic extracts of coaggregation-positive streptococci or of P. aeruginosa were also effective in aggregating Actinomyces cells. The effect of lactose and of salivary components on this extract-induced aggregation of Actinomyces cells generally paralleled that observed in other assays. The apparent prevalence and diversity of adherent reactions between the pathogens studied and indigenous strains of A. viscosus suggest that some may affect host susceptibility to these infectious agents.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cisar J. O., Barsumian E. L., Curl S. H., Vatter A. E., Sandberg A. L., Siraganian R. P. Detection and localization of a lectin on Actinomyces viscosus T14V by monoclonal antibodies. J Immunol. 1981 Oct;127(4):1318–1322. [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello A. H., Cisar J. O., Kolenbrander P. E., Gabriel O. Neuraminidase-dependent hamagglutination of human erythrocytes by human strains of Actinomyces viscosus and Actinomyces naeslundii. Infect Immun. 1979 Nov;26(2):563–572. doi: 10.1128/iai.26.2.563-572.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Interbacterial aggregation of plaque bacteria. Arch Oral Biol. 1970 Dec;15(12):1397–1400. doi: 10.1016/0003-9969(70)90031-2. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E., Inouye Y., Holdeman L. V. New Actinomyces and Streptococcus coaggregation groups among human oral isolates from the same site. Infect Immun. 1983 Aug;41(2):501–506. doi: 10.1128/iai.41.2.501-506.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Williams B. L. Lactose-reversible coaggregation between oral actinomycetes and Streptococcus sanguis. Infect Immun. 1981 Jul;33(1):95–102. doi: 10.1128/iai.33.1.95-102.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride B. C., Van der Hoeven J. S. Role of interbacterial adherence in colonization of the oral cavities of gnotobiotic rats infected with Streptococcus mutans and Veillonella alcalescens. Infect Immun. 1981 Aug;33(2):467–472. doi: 10.1128/iai.33.2.467-472.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Crosby L. K., Vatter A. E. Inhibitors of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34: beta-galactosides, related sugars, and anionic amphipathic compounds. Infect Immun. 1982 Apr;36(1):371–378. doi: 10.1128/iai.36.1.371-378.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Gibbons R. J. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978 Jan;19(1):254–264. doi: 10.1128/iai.19.1.254-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



