Abstract
Predator odor fear conditioning involves the use of a natural unconditioned stimulus, as opposed to aversive electric foot-shock, to obtain novel information on the neural circuitry associated with emotional learning and memory. Researchers are beginning to identify brain sites associated with conditioned contextual fear such as the ventral anterior olfactory nucleus, dorsal premammillary nucleus, ventrolateral periaqueductal gray, cuneiform nucleus, and locus coeruleus. In addition, a few studies have reported an involvement of the basolateral and medial nucleus of the amygdala and hippocampus in fear conditioning. However, several important issues concerning the effectiveness of different predator odor unconditioned stimuli to produce fear conditioning, the precise role of brain nuclei in fear conditioning, and the general relation between the current predator odor and the traditional electric foot-shock fear conditioning procedures remain to be satisfactorily addressed. This review discusses the major behavioral results in the current predator odor fear conditioning literature and introduces two novel contextual and auditory fear conditioning models using cat odor. The new models provide critical information on the acquisition of conditioned fear behavior during training and the expression of conditioned responses in the retention test. Future studies adopting fear conditioning procedures that incorporate measures of both unconditioned and conditioned responses during training may lead to broad insights into predator odor fear conditioning and identify specific brain nuclei mediating conditioned stimulus – predator odor unconditioned stimulus associations.
Keywords: predator odor contextual fear conditioning, predator odor auditory fear conditioning, predator odor conditioned fear
1. Introduction
In recent years, several laboratories have begun to use predator odor to investigate the neurobiology of unconditioned fear. In many studies, cat odor, ferret odor, or the synthetic predator odor 2,4,5-trimethythiazoline (TMT) is presented to rodents to assess fear-related behavioral, physiological, and neural processes. Under controlled conditions, rodents exposed to the odor of predators often exhibit a decrease in general exploratory activity in conjunction with increases in freezing, avoidance or hiding, and risk assessment (Blanchard et al., 1990; Dielenberg & McGregor; 2001; Massini et al., 2005; Perrot-Sinal et al., 1996; Wallace & Rosen, 2000; Zangrossi & File, 1992). In some cases, displays of defensive fear-related responses are accompanied by activation of the hypothalamic-pituitary-adrenal stress hormone system leading to increased secretion of adrenocorticotropin hormone and corticosterone (File et al., 1993; Masini, et al., 2006; Thomas et al., 2006). Several studies have also identified brain regions, including the amygdala, the bed nucleus of the stria terminalis, the dorsal premammillary nucleus and implicated their involvement in the expression of predator odor-induced unconditioned fear (reviewed in Blanchard et al., 2005; Dielenberg & McGregor, 2001; Rosen, 2004; Takahashi et al., 2005).
In addition to eliciting unconditioned fear in rodents, exposure to predator odor, i.e., cat odor, is reported to effectively produce fear conditioning (Blanchard et al., 2001; Dielenberg et al., 2001). Presenting a natural predator odor unconditioned stimulus (US) is an attractive psychological stressor beginning to yield insights into the fear conditioning neural circuitry traditionally characterized almost entirely on the basis of applying electric foot-shock US to establish context or auditory conditioned stimulus (CS) – US associations in the brain. For example, studies in rats using predator odor to investigate contextual fear conditioning are reexamining poorly understood sites, such as the medial amygdala that plays an important role in the activation of unconditioned fear (Li et al., 2004; Muller & Fendt, 2006). However, not all predator odors are reported to promote fear conditioning (Blanchard et al., 2003; McGregor et al., 2002; Rosen, 2004), which has stimulated debate on the general efficacy of predator odors to produce a CS – US association.
Therefore, this review highlights some of the key research results related to issues concerning predator odor fear conditioning. In addition, we introduce two recently developed models of predator odor contextual and auditory fear conditioning. Similarities, differences, and their implications will be discussed between the new predator odor fear conditioning models and current predator odor models, as well as their general relation to the traditional foot-shock conditioning models. Notably, this review on predator odor fear conditioning focuses on the CS – predator odor US association and not on diverse olfactory CS – electric foot-shock US conditioned associations (R.L. Davis, 2004; Otto et al., 2000; Sullivan, 2004).
2. Predator odor US and fear conditioning
2.1. General features of predator odor contextual fear conditioning
Contextual fear conditioning is the predominant model currently employed to study predator odor emotional learning and memory. The general features of this model involve acclimating the animal to the test apparatus before delivery of the predator odor. During a period of several minutes of exposure to the predator odor, unconditioned fear-related behavior is measured. At the conclusion of testing, the animal is returned to its home-cage and tested the next day for retention of contextual fear behavior in the conditioning apparatus without further exposure to predator odor.
Under these test conditions, rats exhibit a range of conditioned fear-related behavioral responses similar to those occurring during exposure to predator odor. That is, when returned to the context previously associated with predator odor, rats frequently engage in crouching/freezing, avoidance/hiding, and stretched attention accompanied by directed sniffing towards the previous source of predator odor (Blanchard et al., 2001; Dielenberg et al., 2001). Furthermore, the magnitude of expression of conditioned fear-related acts varies according to the predator odor stimulus intensity presented at the time of contextual fear conditioning (Takahashi et al., 2005). For example, predator odors emanating from a large sized terry cloth (25.4 × 25.4 cm) rubbed on an adult cat will produce higher levels of unconditioned and contextual freezing in comparison to a small sized cloth (2.5 × 2.5 cm). Results showing variable levels of contextual freezing dependent upon prior presentation of predator odor intensity demonstrate predator odor US control of fear conditioning. In addition, the results are consistent with the foot-shock literature reporting that post-shock freezing levels in rats are positively related to US foot-shock intensity (Blanchard & Blanchard, 1969; Fanselow, 1981).
2.2. Neural correlates of contextual fear conditioning
One study investigated the specific brain regions associated with predator odor emotional learning and memory (Staples et al., 2005). This study measured Fos to map specific brain sites activated by contextual fear and reported increased Fos expression primarily in the dorsal premammillary nucleus, ventrolateral periaqueductal gray, cuneiform nucleus, locus coeruleus, and especially the ventral anterior olfactory nucleus. Of particular interest, no reliable alterations in Fos expression were found in the amygdala, the hippocampus, and frontal cortical regions, which were reported to be associated with fear behavior induced by prior contextual foot-shock training (Beck & Fibiger, 1995; Campeau et al., 1991; Stanciu et al., 2001). Differences in Fos expression between predator odor and foot-shock conditioning studies may represent plasticity in neural systems shaped by fear conditioning training using very different US modalities (predator odor stimulus vs. foot-shock somatosensory stimulus). That is, the context (CS) – predator odor (US) association responsible for conditioned fear behavior may be established in brain regions distinct from the CS – foot-shock (US) association. However, only direct neurobiological methods can identify the participation of specific brain nuclei in fear conditioning.
Several studies in rats have now shown that lesions of the MeA (Blanchard et al., 2005; Takahashi et al., 2007), BLA (Takahashi et al., 2007) and ventral hippocampus (Pentkowski et al., 2006) occurring prior to exposure to cat odor produces a general impairment in the occurrence of unconditioned and conditioned contextual fear-related behavior. The novelty of the MeA lesion effects on contextual fear conditioning suggests that this nucleus may form a unique context CS – predator odor US association that contributes to the fear conditioning neural circuitry. The BLA and ventral hippocampal contextual fear studies appear consistent with the foot-shock conditioning literature (Bast et al., 2001; Fendt & Fanselow, 1999; LeDoux, 2000, Rudy & Matus-Amat, 2005) and suggest these brain structures are generally responsive to fear conditioning produced by training with different US. These studies examining the effects of permanent lesions on predator odor contextual fear conditioning expand our knowledge of brain regions linked to emotional learning and memory.
Nonetheless, the precise contribution these brain structures play in predator odor fear conditioning requires further research to explain the behavioral deficits. For example, behavioral deficits in both unconditioned and conditioned fear behavior produced by permanent lesions occurring prior to fear conditioning training may result from inactivation of motivational or emotional states that impair the acquisition of contextual fear. Another explanation may involve neuro-cognitive factors, in which brain lesion impairments on unconditioned fear behavior compromised the formation of the CS-US association responsible for emotional memory.
In an attempt to address some of these complex issues, we conducted studies using temporary neural inactivation methods occurring after contextual fear conditioning to evaluate the role of the BLA and MeA in cat odor contextual fear memory (Takahashi et al., 2007). In one study, rats were microinjected with vehicle or the long-lasting GABA agonist muscimol immediately after contextual fear conditioning training and tested the next day for retention of contextual fear. Rats microinjected with muscimol into the BLA subsequently exhibited levels of contextual fear-related behavior such as conditioned avoidance that was significantly lower than levels shown by vehicle-treated rats. In contrast, post-training microinjections of muscimol into the MeA were ineffective in producing a reduction, 24 h later, in contextual fear-related behavior. These results suggest that the BLA, but not the MeA, plays a role in modulating the consolidation of predator odor contextual fear memory.
Although immediate post-training inactivation of the MeA did not impair conditioned fear behavior, rats microinjected with lidocaine to inactivate the MeA immediately prior to the retention test exhibited a significant increase in approaching the region of the test apparatus previously associated with the predator odor cloth (Takahashi et al., 2007). Furthermore, unlike the MeA, microinjections of lidocaine into the BLA produced no reliable alterations in contextual fear behavior.
These post-fear conditioning manipulations in the BLA and MeA provide new information on the emotional learning and memory neurocircuitry. In comparison to the traditional context CS – foot-shock US model, some of the predator odor contextual fear results are similar in showing an involvement of the BLA in fear memory consolidation (Pare, 2003; McGaugh, 2004). However, unlike the robust contextual behavioral impairments reported in foot-shock studies (Davis, 2000; Fendt & Fanselow, 1999; LeDoux, 2000), our predator odor work showed that acute inactivation of the BLA immediately prior to the retention test failed to produce significant alterations in contextual fear-related behavior. Thus, in the BLA, the fear-motivated US including foot-shock and cat odor may form important conditioned associations with the context that modulates fear memory consolidation processes, but the BLA does not appear to exert universal effects in modulating the expression of fear behavior produced by contextual conditioning involving two very different US.
Our predator odor contextual fear conditioning results further showed that MeA manipulations occurring immediately prior to retention testing impair conditioned behavioral responses. Although the MeA receives olfactory information (McDonald, 1998; Scalia & Winans, 1975), deficits on conditioned fear behavior cannot be attributed to olfactory impairments because our retention test does not involve presentation of conditioned olfactory cues. In addition, our results are not based on a single behavioral metric of fear performance, i.e., freezing. Thus, the MeA may play a broad role in predator odor emotionality, perhaps by modulating arousal or fear motivation. Indirect support for a potential motivational role of the MeA is obtained from neuroendocrine studies, which found that MeA lesions decreased stress-induced corticosterone secretion whereas MeA stimulation produced opposite effects (Dunn & Whitener, 1986; Feldman et al., 1990).
2.3. Predator odor US generality in contextual fear conditioning
Although cat odor contextual fear conditioning is an important model that offers insights into the neural basis of emotional learning and memory, several studies reported no significant contextual or cue conditioning effects using TMT (Blanchard et al., 2003; McGregor et al., 2002; Wallace & Rosen, 2000). These results raise issues concerning the general efficacy of different predator odor US to produce fear conditioning. However, some studies recently reported that fear conditioning using TMT is critically dependent on environmental test conditions. One study showed that when rats were exposed to either TMT or cat fur in a large chamber with a hide box, the next day the two groups exhibited high and similar levels of conditioned freezing (Rosen, 2004). However, rats exposed to TMT fear conditioning did not subsequently exhibit conditioned avoidance in the hide box in contrast to rats conditioned to cat odor. Another study using a two-compartment conditioning apparatus found that rats presented to TMT in one compartment subsequently showed conditioned avoidance only to the TMT-paired compartment (Endres & Fendt, 2007). However, contextual fear conditioning involved 7 consecutive days of pairing TMT for 20 min with a specific compartment. This fear conditioning or TMT training period is considerably longer than the typical one day cat odor training period, which produces contextual conditioned fear behavior the next day. Hence, some behavioral and training differences were noted in contextual fear studies using cat odor and TMT.
2.4. Similarities and differences in predator odor and foot-shock fear conditioning models
In addition to some differences between cat odor and TMT in contextual fear conditioning studies, a major issue concerns the relation between the predator odor and foot-shock conditioning models. Both the foot-shock and predator odor contextual fear conditioning models involve an acclimation period, followed by delivery of the US, and subsequent testing for contextual fear behavior. However, important procedural differences are apparent in these two models that extend beyond obvious differences in US modality. First, the traditional foot-shock conditioning model using rodents generally involves delivery of brief foot-shocks, e.g., 1 sec duration, and freezing is measured after each post-shock interval. However, the freezing that is usually measured is a conditioned response and not the unconditioned responses to shock, e.g., jumping, vocalization, or flinching, which are robust but not often measured during fear conditioning training. In the typical predator odor conditioning model, the US delivered over a period of minutes also generates robust unconditioned responses, e.g., freezing, avoidance, which are the primary fear conditioning measures. Second, training or repeated foot-shocks delivered at spaced intervals provide an acquisition index of contextual fear learning, i.e., contextual freezing, whereas the current predator odor conditioning model, which provides an assessment of unconditioned fear, does not provide a critical evaluation of the animal’s magnitude of learning to associate the context with the US due to the absence of measuring a conditioned response. Thus, when an animal exhibits unconditioned fear to predator odor, but subsequently exhibits deficits in contextual fear, the impairment in contextual fear behavior may result from incomplete learning at the time of training. In some studies (Blanchard et al., 2003; McGregor et al., 2002; Wallace & Rosen, 2000), the failure of TMT to produce contextual fear behavior may be due to acquisition deficits in contextual fear. Attempts to overcome this issue using a large test chamber with a hide box (Rosen, 2004) or repeated trials of TMT-paired with a specific test chamber (Endres & Fendt, 2007) were relatively successful in demonstrating an effect of TMT on contextual fear conditioning. However, direct measures of not only unconditioned fear but also acquisition of conditioned fear would be valuable in confirming whether a CS-US association is formed between the context and predator odor.
3. Novel predator odor contextual fear conditioning model
To provide new information on the nature of predator odor contextual fear conditioning, we developed a model that incorporates some of the traditional foot-shock conditioning procedures of exposing the rat to several training trials involving application of the US and measuring contextual fear-related behavior to determine whether contextual fear was acquired or learned (Fig. 1). Retention of contextual fear was evaluated by re-exposing the rat to the conditioning apparatus 48 h later in the absence of the US. The general utility of this model is that both unconditioned and conditioned fear-related responses can be assessed during training of contextual learning.
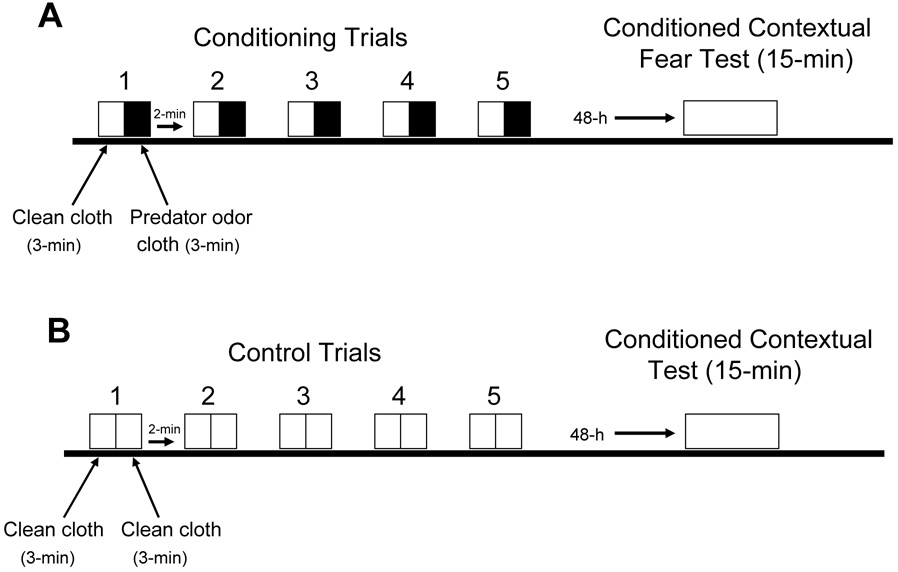
Fig. 1. Predator odor contextual fear conditioning model.
A, Singly housed, naïve, male Long-Evans rats (275 – 325 g, N=8) were exposed to five 6-min contextual fear conditioning trials under red light illumination between 0900 and 1400 h. Conditioning trials began by placing the animal into an elongated rectangular fear conditioning apparatus (100 cm × 12 cm × 50 cm) constructed of three white Plexiglas walls, and a clear Plexiglas front wall to allow videotaping. The test box was positioned on a table divided into three 33.3 cm consecutive floor sections. A white Plexiglas strip projected horizontally 33.3 cm from one end wall of the test apparatus and 13 cm above the floor to form a low ceiling for a sheltered hide box within the remaining open top rectangular apparatus. During the first 3 min a clean white terry cloth (25 cm × 25 cm) is placed over the top end portion the apparatus opposite the sheltered section. This clean cloth is subsequently removed and replaced for 3 min with an identical sized white terry cloth containing the odor of a 13-year-old male domestic cat housed in the animal facility. This cloth was rubbed for 5-min against the cat’s body on the day of testing. Previous studies in rats showed that a 25 cm × 25 cm sized cloth containing cat odor is highly effective in facilitating unconditioned fear, e.g., freezing and avoidance, and the formation of predator-odor memory (Takahashi et al., 2005). After each 6 min trial, the rat is returned to its homecage for a 2 min inter-trial interval, when the apparatus is cleaned with 10% ethanol. B, Control rats (N=8) are tested in the same manner, except in every 6-min trial only clean cloths are presented to rats before and after each 3-min period.
In this study, freezing, avoidance, and approach behavior were measured as in our previous study (Takahashi et al., 2007). Freezing was defined as a stationary posture characterized by cessation of body movements except those required for respiration. Avoidance was scored when the rat’s four paws were in the 33.3 cm hiding segment of the apparatus directly opposite from the location of the cat odor cloth. Approach was measured when the rat’s four paws were in the 33.3 cm floor segment opposite the high box and directly under the end of the apparatus where the cat odor cloth was placed.
3.1. Contextual fear training results
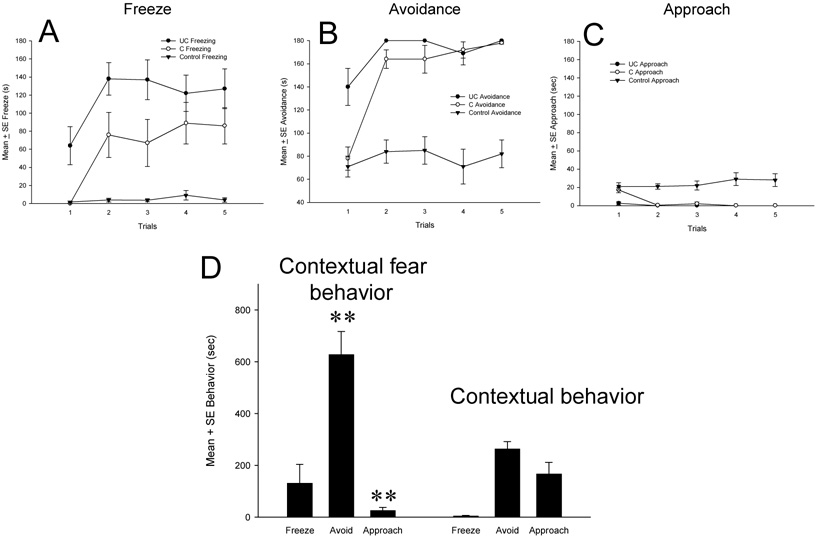
A repeated measures analysis of variance (ANOVA) procedure was used to examine the total duration of conditioned (3-min no odor period) and unconditioned responses (3-min predator odor period) recorded in each of the five conditioning trials. The results for freezing duration showed a significant main effect of trial, F(4,28)=11.93, P<0.001, and odor condition, F(1,7)=28.43, P<0.001. Freezing levels increased significantly from the first to subsequent trials and significantly higher levels of predator odor unconditioned freezing were observed compared to conditioned freezing (Figure 2A, Table 1). The trial × cloth odor interaction effect was not significant.
Fig. 2. Mean ± SE duration of conditioned (C) and unconditioned (UC) fear-related behavior during contextual fear training.
Averaged behavioral durations of freezing (Panel A), avoidance (Panel B), and approach (Panel C) of control rats are shown for comparative purposes to conditioned (C) and unconditioned (UC) responses. D, Mean ± SE duration of fear-related behavior exhibited by rats in the subsequent contextual test. Rats returned to the test context 48-h after training exhibited higher levels of contextual avoidance (**P<0.01) and lower levels of approach (**P<0.01) than control rats with no prior context CS – predator odor US experiences.
Table 1.
Summary of percent response (Mean ± SE) in the training trials based on raw data shown in Fig. 2.
| Trials | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Conditioned response | |||||
| Freeze | 0.0 ± 0.0 | 42.0 ± 13. 7 | 37.3 ± 13.6 | 49.5 ± 12.7 | 48.1 ± 10.8 |
| Avoidance | 43.7 ± 5.9 | 91.1 ± 4.5 | 91.5 ± 6.7 | 95.7 ± 4.0 | 99.0 ± 8.0 |
| Approach | 9.8 ± 2.1 | 0.2 ± 0.1 | 1.1 ± 0. 1 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Unconditioned response | |||||
| Freeze | 37.7 ± 11.6 | 76.6 ± 10.3 | 76.1 ± 12.4 | 67.6 ± 11.3 | 70.5 ± 12.7 |
| Avoidance | 77.7 ± 9.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 94.2 ± 5.6 | 100.0 ± 0.0 |
| Approach | 1.3 ± 1.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Control response | |||||
| Freeze | 0.6 ± 0.6 | 2.1 ± 1.2 | 2.0 ± 0.8 | 5.0 ± 2.8 | 1.9 ± 1.7 |
| Avoidance | 39.6 ± 5.1 | 47.1 ± 5.8 | 47.3 ± 6.7 | 39.8 ± 8.3 | 45.8 ± 7.0 |
| Approach | 11.7 ± 2.5 | 11.7 ± 1.8 | 12.6 ± 2.8 | 16.5 ± 4.2 | 16.0 ± 4.1 |
Similar analysis on avoidance duration revealed a significant main effect of trials, F(4,28)=22.59, P<0.001, odor condition, F(1,7)=33.01, P<0.001, and trial × odor condition interaction, F(4,28)=9.35, P<0.001. Unconditioned and conditioned avoidance duration differed only in Trial 1 and thereafter increased and remained at similar high levels (Fig. 2B, Table 1).
Analysis of approach to the site of the test apparatus directly below the cloth showed a significant main effect of trial, F(4,28)=15.23, P<0.001, odor condition, F(1,7)=12.56, P<0.01, and trial × odor interaction, F(4,28)=11.71, P<.001. The duration of conditioned approach decline significantly after the first trial and remained at low unconditioned approach levels in the next 4 trials (Fig. 2C, Table 1).
Similar repeated measures ANOVA procedures were conducted to determine the effects of repeated clean cloth presentation on behavior. Data showed no significant main or interactions effects of successive exposures to two clean cloths and across trials for freezing, avoidance, or approach (P>0.05). Therefore, behavioral durations measured in the first and second clean cloth exposures were averaged for each trial to represent a control trial score. The averaged control trial scores are presented in relation to conditioned and unconditioned response levels (Fig. 2A–C, Table 1).
3.2. Contextual fear retention results
Forty-eight h after contextual fear or control training, rats were returned to the test apparatus and evaluated for fear-related behavior (Fig. 2D). In comparison to control rats, contextual fear trained rats exhibited significantly higher levels of avoidance, t(14)=3.94, P<.01 (mean ± SE percent avoidance: control group = 31.6 ± 10.6; conditioned group = 69.8 ± 9.8), and low levels of contextual approach, t(14)=3.04, P<.01 (mean ± SE percent approach: control group = 18.2 ± 14.3; conditioned group = 2.8 ± 1.2. No significant group differences were found in freezing (mean ± SE percent freeze: control group = 0.8 ± 1.2; conditioned group = 14.5 ± 8.0).
3.3. Contextual fear conditioning model discussion
Results demonstrate that unconditioned and conditioned fear-related responses can be directly measured during contextual fear conditioning. The significant increase in conditioned freezing and avoidance accompanied by a reliable reduction in approach occurring from the first to subsequent trials suggests that predator odor conditioned fear-related responses may be useful acquisition indices of contextual learning as in foot-shock conditioning trials that assess learning by measuring conditioned freezing. Whether the prior failure of TMT-trained rats to exhibit contextual fear behavior in a subsequent retention test (Blanchard et al., 2003; McGregor et al., 2002; Wallace & Rosen, 2000) was due to deficits in the acquisition of conditioned fear responses during training, irrespective of robust displays of unconditioned fear behavior, remains to be determined.
In the current study, contextual fear trained rats tested 48 h later in the retention test showed high levels of avoidance and a reduction in approach in comparison to control trained rats. Of particular interest, these two conditioned responses – avoidance and approach - showed behavioral levels during training that eventually overlapped in magnitude with their respective unconditioned response levels. In contrast, conditioned and unconditioned freezing levels showed a similar trend across trials but did not overlap in magnitude, and contextual freezing displayed in the retention test did not differ from control levels. Future studies examining the potential relevance in the expression of unconditioned and conditioned responses during training may provide insights into the contextual fear acquisition process.
The assessment of predator odor unconditioned and conditioned fear is also of interest in comparison to differences in unconditioned and conditioned responses elicited by electric foot-shock. As previously noted, in foot-shock fear conditioning, the unconditioned responses of jumping, flinching, vocalizations differ behaviorally from the conditioned freezing response whereas in the current predator odor conditioning study conditioned freezing and avoidance are behaviorally similar to the unconditioned responses. Differences in the specific unconditioned reactions elicited by foot-shock and predator odor unconditioned stimuli may be due, in part, to differences in the rat’s perception of imminent threat or distance from the predator (Blanchard et al., 1986; Fanselow & Lester, 1988, Ratner, 1967). For example, in wild rats, unconditioned jumping and vocalizations begin to increase only when approached within 1 to 2 m by an experimenter. Future studies could examine whether approaching the rat with the predator odor cloth will elicit jumping and vocalization responses similar to unconditioned responses induced by foot-shock and then determine if conditioned freezing is exhibited at prominent levels in the retention test.
4. Auditory fear conditioning
In addition to contextual fear conditioning, auditory fear conditioning is a widely used model to study the neurobiology of emotional learning and memory. Auditory fear conditioning generally involves a few minutes of acclimating the animal to the test apparatus followed by delivery of an auditory stimulus (CS) that is co-terminated following application of a brief foot-shock (US). After several auditory stimulus – foot-shock pairings, the animal is returned to its home-cage and tested the next day for retention of auditory fear conditioning by re-exposing the animal to the auditory stimulus in an unfamiliar test environment.
This auditory fear conditioning model has generated important data suggesting that in the lateral nucleus of the amygdala (LA), auditory stimuli from the auditory cortex converge with foot-shock pain information from the somatosensory cortex to form a CS-US fear memory association (Blair et al., 2001; Maren & Quirk, 2004). In addition, a study reported that stathmin, an inhibitor of microtubule formation, is enriched in the LA and stathmin knockout mice showed deficits in auditory fear conditioning and innate fear as measured in the elevated plus maze and open field (Shumyatsky et al., 2005). However, a significant step to assess the generality of the auditory CS-US fear memory association in the LA is to use predator odor, a distinct US that can be experimentally controlled to elicit unconditioned or innate fear (Takahashi et al., 2005; Wallace & Rosen, 2000). Although lesions or temporary inactivation of the LA do not appear to interfere with the expression of unconditioned fear elicited by TMT (Fendt et al., 2003; Wallace & Rosen, 2001), the role of the LA as a general auditory fear conditioning neural center or specifically involved in the formation of auditory – foot-shock associations remains unknown.
5. Novel predator odor auditory fear conditioning model
To address these important neurobiological issues in the future, we developed an auditory fear conditioning model (Fig. 3A) involving a variant of the forward conditioning procedure by exposing rats to three training trials consisting of an audible clicking stimulus (CS) prior to and briefly overlapping with delivery of cat odor (US). Rats were trained to associate the clicks with predator odor in a clean home-cage sized box and 48 h after training evaluated for auditory conditioned behavior in a different test box that we have used in foot-shock conditioning studies (Hubbard et al., 2007, Fig. 3A). CS control (Fig. 3B, N=8) and US control (Fig. 3C, N=8) training groups were also assessed for fear behavior.
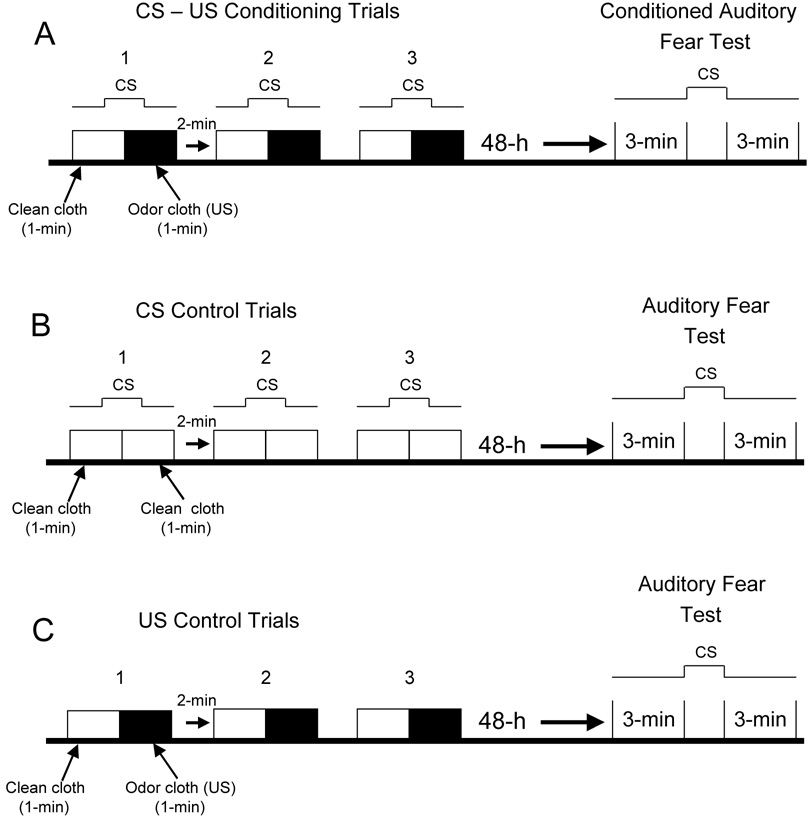
Fig. 3. Predator auditory fear conditioning model.
A, Singly housed, naïve, male Long-Evans rats (275 – 325 g, N=8) were exposed to three auditory fear conditioning trials between 0900 and 1400 h. Each trial began by placing the rat into a clear polycarbonate cage box (48 cm × 27 cm × 20 cm) and a clean cloth (25 × 25 cm) was immediately spread on the secured wire cage top. After 1-min the first of 10 successive clicks (2 msec, 70 db) spaced approximately 1 sec apart was delivered using a hand clicker (Gary Wikes Mega Click, J & J Dog Supplies, Galesburg, IL). After 5 clicks, the clean cloth was simultaneously replaced with a 25 cm × 25 cm cat odor cloth (US) for the remaining 5 clicks. The rat remained in the cage box with the predator odor cloth for 1 min after the last click and then returned to its homecage for a 2 min inter-trial interval, when the test box was cleaned with 10% ethanol. The rat was exposed to a total of three auditory conditioning trials and videotaped for analysis of conditioned freezing occurring during the first minute of each trial and unconditioned freezing during the last minute when the predator odor cloth was present.
Forty-eight hour after training, the rat was tested for retention of auditory conditioned fear. The rat was placed in a novel box (25.3 cm × 20.3 cm × 22.6 cm) constructed of three white Plexiglas sides and top and a clear front wall for video recording. The floor consisted of stainless steel rods. After 3 min, a train of 10 successive CS (i.e., clicks) spaced 1 sec apart was introduced. The rat remained in the test box for another 3 min after the last CS. The total duration of freezing was measured 3 min before and 3 min after the last click.
In addition to exposing rats to the auditory fear conditioning procedure, two control groups of rats (N=8 per group) were tested and assessed for conditioned and unconditioned freezing. B, CS control group was tested in the same manner, except only clean cloths were presented to rats before and after exposure to the 10 successive clicks. C, US control group was tested with clean and cat odor cloths but without delivery of the auditory clicks during each trial. Both CS and US control groups were tested 48 h later in the same manner as CS-US conditioned rats.
5.1. Auditory fear training results
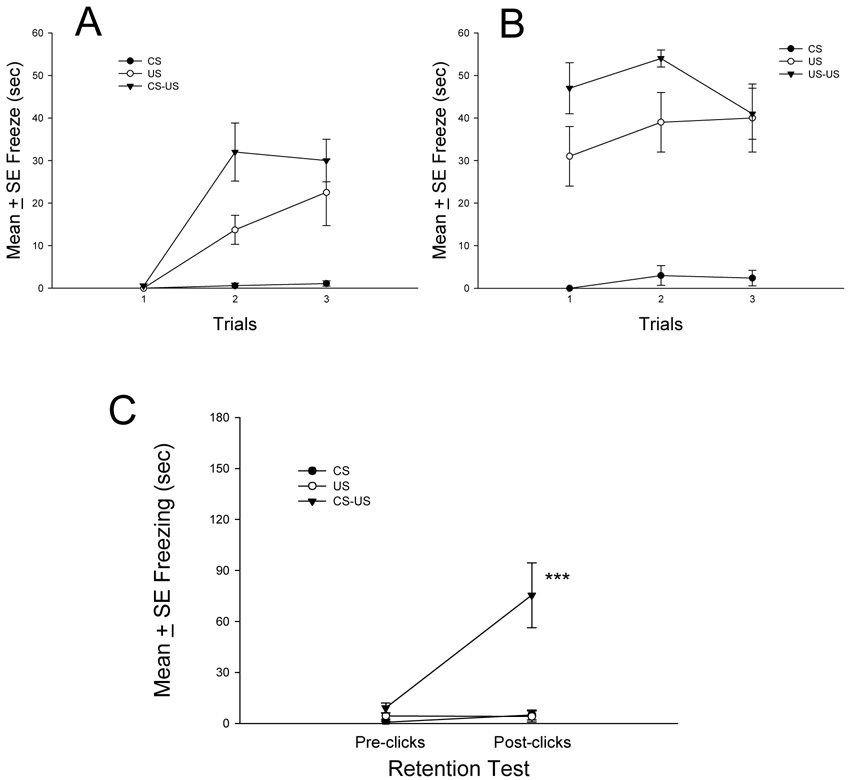
The total duration of freezing measured prior to the first click or during the first minute of each trial was analyzed using a repeated measures ANOVA, which revealed a significant group, F(2,21)=11.79, P<0.001, trial, F(2,42)=25.57, P<0.001, and group × trial interaction, F(4,42)=7.57, P<0.001 (Fig. 4A). Freezing levels were similarly elevated and significantly higher in CS-US and US control groups in comparison to the rats exposed to clicks and presented only to clean cloths (CS control group).
Fig. 4. Conditioned and unconditioned freezing exhibited during auditory fear acquisition and retention testing.
A, Mean ± SE conditioned freezing during acquisition training in CS control, US control, and CS-US association groups. B, Mean ± SE unconditioned freezing during acquisition training in CS control, US control, and CS-US association groups. C, Mean ± SE conditioned freezing 3 min prior to (pre-clicks) and 3 min after exposure to the auditory stimulus (post-clicks). ***P<0.001, post-clicks freezing duration increased significantly in CS-US association group compared to auditory CS control and predator odor US control groups.
Freezing duration scored after delivery of the last click or the last minute of each trial was also analyzed using a repeated measures ANOVA. This analysis revealed a highly significant group main effect, F(2,21)=27.98, P<0.001 (Fig. 4B). Main effect of the trial and group × trial interaction was not significant.
5.2. Auditory fear retention results
The total duration of freezing measured 3-min before the first click (Pre-clicks) and 3-min after the last click (Post-clicks) was analyzed in the retention test using a repeated measures ANOVA (Fig. 4C, Table 2). Results revealed a significant main effect of group, F(2,21)=14.35, P<0.001, test, F(1,21)=12.63, P<0.01, and group × test interaction, F(2,21)=10.60, P<0.001. After re-exposure to the clicks in a novel test environment, freezing levels increased significantly only in the CS – US association group in comparison to CS and US control groups, which showed similar low levels of freezing prior to and after clicks.
Table 2.
Summary of percent freezing (Mean ± SE) in the auditory fear acquisition and retention test based on raw data shown in Fig. 4.
| Acquisition test | ||||
|---|---|---|---|---|
| Trials | ||||
| 1 | 2 | 3 | ||
| 1st min | ||||
| CS control | 0.0 ± 0.0 | 1.0 ± 1.0 | 1.8 ± 1.0 | |
| US control | 0.0 ± 0.0 | 22.8 ± 5.7 | 37.5 ± 13.0 | |
| CS-US association | 0.8 ± 0.8 | 53.3 ± 11.3 | 50.0 ± 8.3 | |
| 2nd min | ||||
| CS control | 0.0 ± 0.0 | 5.0 ± 3.8 | 4.0 ± 3.0 | |
| US control | 51.6 ± 11.7 | 65.0 ± 11.7 | 66.7 ± 13.3 | |
| CS-US association | 78.3 ± 10.0 | 90.0 ± 3.3 | 68.3 ± 10.0 | |
| Retention test | ||||
| Pre-clicks | Post-clicks | |||
| CS control | 0.4 ± 0.4 | 2.7 ± 1.6 | ||
| US control | 2.3 ± 1.1 | 2.2 ± 1.8 | ||
| CS-US association | 5.0 ± 1.7 | 41.9 ± 10.7*** | ||
P<0.001, significantly different from CS and US control groups.
5.3. Effects of predator odor auditory fear conditioning on general fear-motivated behavior
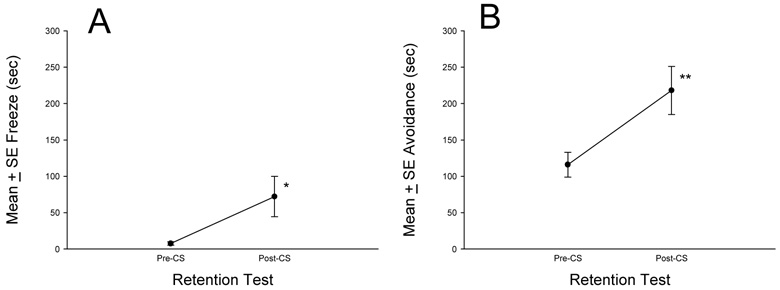
To determine whether the audible click CS – predator odor US association acquired during training is specific to the acquisition of conditioned freezing, we exposed another group of naïve male Long-Evans rats (N=8) to the auditory fear conditioning training procedure (Fig. 3A), but 48 h later, the retention test was conducted using our elongated test apparatus with a hide box (see Fig. 1 for apparatus and behavioral description). The retention test began by placing rats into the novel test apparatus and the total duration of conditioned freezing and spontaneous avoidance or hiding in the small chamber was measure 5 min prior to the first of 10 successive clicks and 5 min after the last click.
Results showed that auditory fear conditioned rats increased their duration of freezing after re-exposure to the clicks, t(7)=2.4, P<0.05 (Fig. 5A, mean ± SE percent Pre-CS freezing = 3.2 ± 0.8; Post-CS freezing = 24.8 ± 9.0). In addition, time spent hiding or avoiding the open top region of the apparatus increased significantly in the 5 min block after delivery of the clicks, t(7)=3.93, P<0.01 (Fig. 5B, mean ± SE percent Pre-CS avoidance = 38.7 ± 5.7; Post-CS avoidance = 72.6 ± 11.1).
Fig. 5. Effects of auditory fear conditioning on pre- and post-CS fear-related behavior.
Auditory fear conditioning training was conducted in a box without shelter and subsequent auditory fear testing was conducted in an apparatus with shelter. A, Mean ± SE conditioned freezing 5 min prior to and 5 min after exposure to conditioned clicks, *P<0.05, post-CS conditioned freezing duration significantly higher than pre-CS freezing level. B, Mean ± SE avoidance or hiding 5 min prior to and 5 min after exposure to conditioned clicks, **P<0.01, post-CS avoidance duration significantly higher than pre-CS avoidance levels.
5.4. Predator odor auditory fear conditioning model discussion
Results showed that cat odor auditory fear conditioning is acquired in rats. Administration of audible clicks and cat odor cloths during training increased conditioned and unconditioned freezing in a home-cage sized training box. Importantly, when click CS – predator odor US trained groups were subsequently placed in a small novel environment, re-exposure to the clicks elicited a robust increase in freezing. In contrast, control rats that received prior training exposures to only clicks or predator odor showed no significant increase in freezing when exposed to clicks in a novel test box. These results suggest that: 1) the auditory clicks do not elicit unconditioned or anxiety-like responses; and 2) prior freezing to the predator odor US is not readily displayed in a novel environment. Therefore, an audible click – predator odor emotional association appears to be learned and reactivated by the CS to trigger fear behavior, i.e., freezing, beyond the confines of the training context.
Our results further demonstrate that the click – predator odor association is not limited to the expression or performance of the acquired freezing response. When CS-US trained rats were subsequently tested in an environment that provided shelter, re-exposure to the CS facilitated retreat or hiding and freezing. Results suggest the auditory (CS) – predator odor (US) association establishes a general fear-motivated state that can be reactivated by the CS to generate appropriate behavioral strategies dependent on options available in the environment.
6. Conclusions
Predator odor fear conditioning offers new insights into the neurobiological basis of emotional learning and memory. In particular, the controlled use of a natural unconditioned stimulus has facilitated interest into both well studied and less understood brain sites that participate not only in the expression of unconditioned fear but also in fear conditioning. In addition, our new predator odor contextual and auditory fear conditioning models may significantly advance our understanding of the nature of the CS – predator odor US association by assessing the expression of unconditioned fear and the acquisition of conditioned fear behavior, the standard measure in well-established foot-shock fear conditioning models. Relating the emergence and acquisition of conditioned fear behavior produced during training to the subsequent behavioral expression of conditioned fear in a retention test may offer insights into the fear conditioning process using different predator odors, i.e., cat odor, TMT, ferret odor, etc.
Future predator odor research evaluating the expression of both conditioned and unconditioned fear behavior has the potential to yield important fear conditioning information. For example, brain regions previously shown to be involved in predator odor fear may be reexamined to determine whether the nucleus is specifically linked to the production of only unconditioned responses or both learned and unlearned fear-related behavior. Future studies may also consider the extent to which distinct brain nuclei have general fear conditioning effects produced by exposure to different US (e.g., predator odor vs. foot-shock). However, the brain sites listed in the extensive foot-shock fear conditioning literature (Charney & Deutch, 1996; M. Davis, 2004) do not consistently overlap with the currently reported predator odor-induced unconditioned and conditioned brain regions as assessed by Fos mapping work (Canteras, 2002; Dielenberg et al., 2001b; Staples et al., 2005) and the limited number of functional predator odor fear conditioning studies (Blanchard et al, 2005; Takahashi et al., 2007). Thus, new investigations will be required to chart the general brain circuitry that participates in fear conditioning as well as characterize the brain sites uniquely involved in predator odor fear conditioning.
Finally, identifying brain nuclei mediating unconditioned fear behavior provides, at the very least, an important starting point to obtain fundamental information on where the emotion of predator odor fear is generated in the brain and potentially where CS-US associations are acquired and stored. Uncovering these unconditioned fear mediating brain sites using a predator odor fear-conditioning model may contribute broadly to research on the neurobiology of individual differences in fear predispositions or sensitivity to unconditioned psychological stressors that contribute to abnormal emotional learning and resistance to extinction (Field, 2006; Öhman & Mineka, 2001).
Acknowledgments
Preparation of this review and research conducted in our laboratory were supported by grants National Institutes of Health grants NS39406 and GM07684-27.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bast T, Zhang W-N, Feldon J. The ventral hippocampus and fear conditioning in rats. Experimental Brain Research. 2001;139:39–52. doi: 10.1007/s002210100746. [DOI] [PubMed] [Google Scholar]
- Beck CHM, Fibiger HC. Conditioned fear-induced changes in behavior and in expression of the immediate early gene c-fos: with and without diazepam pretreatment. Journal of Neuroscience. 1995;15:709–720. doi: 10.1523/JNEUROSCI.15-01-00709.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE. Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learning & Memory. 2001;8:229–242. doi: 10.1101/lm.30901. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Canteras NS, Markham CM, Pentkowski NS, Blanchard RJ. Lesions of structures showing Fos expression to cat presentation: effects on responsivity to a cat, cat odor, and nonpredator threat. Neuroscience and Biobehavioral Reviews. 2005;29:1243–1253. doi: 10.1016/j.neubiorev.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Markham C, Yang M, Hubbard D, Madarang E, Blanchard RJ. Failure to produce conditioning with low-dose trimethylthiazoline or cat feces as unconditioned stimuli. Behavioral Neuroscience. 2003;117:360–368. doi: 10.1037/0735-7044.117.2.360. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. Journal of Comparative and Physiological Psychology. 1969;67:370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, Rodgers J, Weiss SM. The effects of ethanol and diazepam on reactions to predatory odors. Pharmacology, Biochemistry, and Behavior. 1990;35:775–780. doi: 10.1016/0091-3057(90)90357-n. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Flannelly KJ, Blanchard DC. Defensive behaviors of laboratory and wild Rattus norvegicus. Journal of Comparative Psychology. 1986;100:101–107. [PubMed] [Google Scholar]
- Blanchard RJ, Yang M, Li C-I, Gervacio A, Blanchard DC. Cue and context conditioning of defensive behaviors to cat odor stimuli. Neuroscience and Biobehavioral Reviews. 2001;25:587–595. doi: 10.1016/s0149-7634(01)00043-4. [DOI] [PubMed] [Google Scholar]
- Campeau S, Hayward MD, Hope BT, Rosen JB, Nestler EJ, Davis M. Induction of the c-fos proto-oncogene in the rat amygdala during conditioned and unconditioned fear. Brain Research. 1991;565:349–352. doi: 10.1016/0006-8993(91)91669-r. [DOI] [PubMed] [Google Scholar]
- Canteras NS. The medial hypothalamic defensive system: hodological organization and functional implications. Pharmacology, Biochemistry, and Behavior. 2002;71:481–491. doi: 10.1016/s0091-3057(01)00685-2. [DOI] [PubMed] [Google Scholar]
- Charney DS, Deutch A. A functional neuroanatomy of anxiety and fear: implications for the pathophysiology and treatment of anxiety disorders. Critical Reviews in Neurobiology. 1996;10:419–446. doi: 10.1615/critrevneurobiol.v10.i3-4.70. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in conditioned and unconditioned fear and anxiety. In: Aggleton JP, editor. The Amygdala: A Functional Analysis. 2nd ed. New York: Oxford University Press; 2000. pp. 213–287. [Google Scholar]
- Davis M. Functional neuroanatomy of anxiety and fear. In: Charney DS, Nestler EJ, editors. Neurobiology of Mental Illness. 2nd ed. New York: Oxford University Press; 2004. pp. 584–604. [Google Scholar]
- Davis RL. Olfactory learning. Neuron. 2004;44:31–48. doi: 10.1016/j.neuron.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, Carrive P, McGregor IS. The cardiovascular and behavioral response to cat odor in rats: unconditioned and conditioned effects. Brain Research. 2001a;897:228–237. doi: 10.1016/s0006-8993(01)02227-2. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, Hunt GE, McGregor IS. ‘When a rat smells a cat’: distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience. 2001b;104:1085–1097. doi: 10.1016/s0306-4522(01)00150-6. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, McGregor IS. Defensive behavior in rats towards predatory odors: a review. Neuroscience and Biobehavioral Reviews. 2001;25:597–609. doi: 10.1016/s0149-7634(01)00044-6. [DOI] [PubMed] [Google Scholar]
- Dunn JD, Whitener J. Plasma corticosterone responses to electrical stimulation of the amygdala complex: cytoarchitectural specificity. Neuroendocrinology. 1986;42:211–217. doi: 10.1159/000124442. [DOI] [PubMed] [Google Scholar]
- Endres T, Fendt M. Conditioned behavioral responses to a context paired with the predator odor trimethylthiazoline. Behavioral Neuroscience. 2007;121:594–601. doi: 10.1037/0735-7044.121.3.594. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Naloxone and Pavlovian fear conditioning. Learning and Motivation. 1981;12:398–419. [Google Scholar]
- Fanselow MS, Lester LS. A functional behavioristic approach to aversively motivated behavior: predatory imminence as a determinant of the topography of defensive behavior. In: Bolles RC, Beecher MD, editors. Evolution and Learning. New Jersey: Erlbaum; 1988. pp. 185–212. [Google Scholar]
- Feldman S, Conforti N, Saphier D. The preoptic and bed nucleus of the stria terminalis are involved in the effects of amygdala on adrenocortical secretion. Neuroscience. 1990;37:775–779. doi: 10.1016/0306-4522(90)90107-f. [DOI] [PubMed] [Google Scholar]
- Fendt M, Endres T, Apfelbach R. Temporary inactivation of the bed nucleus of the stria terminalis but not the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. Journal of Neuroscience. 2003;23:23–28. doi: 10.1523/JNEUROSCI.23-01-00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neuroscience and Biobehavioral Reviews. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Field AP. Is conditioning a useful framework for understanding the development and treatment of phobia? Clinical Psychology Review. 2006;26:857–875. doi: 10.1016/j.cpr.2005.05.010. [DOI] [PubMed] [Google Scholar]
- File SE, Zangrossi H, Jr, Sanders FL, Mabbutt PS. Dissociation between behavioral and corticosterone responses on repeated exposures to cat odor. Physiology & Behavior. 1993;54:1109–1111. doi: 10.1016/0031-9384(93)90333-b. [DOI] [PubMed] [Google Scholar]
- Hubbard DT, Nakashima BR, Lee I, Takahashi LK. Activation of basolateral amygdala corticotropin-releasing factor 1 receptors modulates the consolidation of contextual fear. Neuroscience. 2007;150:818–828. doi: 10.1016/j.neuroscience.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Li C-I, Maglinao TL, Takahashi LK. Medial amygdala modulation of predator odor-induced unconditioned fear in the rat. Behavioral Neuroscience. 2004;118:324–332. doi: 10.1037/0735-7044.118.2.324. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signaling of fear memory. Nature Reviews Neuroscience. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Masini CV, Sauer S, White J, Day HEW, Campeau S. Non-associative defensive responses of rats to ferret odor. Physiology & Behavior. 2006;87:72–81. doi: 10.1016/j.physbeh.2005.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways in the mammalian amygdala. Progress in Neurobiology. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Schrama L, Ambermoon P, Dielenberg RA. Not all ‘predator odours’ are equal: cat odour but not 2,4,5 trimethylthiazoline (TMT; fox odor) elicits specific defensive behaviours in rats. Behavioural Brain Research. 2002;129:1–16. doi: 10.1016/s0166-4328(01)00324-2. [DOI] [PubMed] [Google Scholar]
- Müller M, Fendt M. Temporary inactivation of the medial and basolateral amygdala differentially affects TMT-induced fear behavior in rat. Behavioural Brain Research. 2006;167:57–62. doi: 10.1016/j.bbr.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Öhman A, Mineka S. Fear, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychological Review. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Otto T, Cousens G, Herzog C. Behavioral and neuropsychological foundations of olfactory fear conditioning. Behavioural Brain Research. 2000;110:119–128. doi: 10.1016/s0166-4328(99)00190-4. [DOI] [PubMed] [Google Scholar]
- Pare D. Role of the basolateral amygdala in memory consolidation. Progress in Neurobiology. 2003;70:409–420. doi: 10.1016/s0301-0082(03)00104-7. [DOI] [PubMed] [Google Scholar]
- Pentkowski NS, Blanchard DC, Lever C, Litvin Y, Blanchard RJ. Effects of lesions to the dorsal and ventral hippocampus on defensive behaviors in rats. European Journal of Neuroscience. 2006;23:2185–2196. doi: 10.1111/j.1460-9568.2006.04754.x. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Heale VR, Ossenkopp K-P, Kavaliers M. Sexually dimorphic aspects of spontaneous activity in meadow voles (Microtus pennsylvanicus): effects of exposure to predator odor. Behavioral Neuroscience. 1996;110:1126–1132. doi: 10.1037//0735-7044.110.5.1126. [DOI] [PubMed] [Google Scholar]
- Ratner SC. Comparative aspects of hypnosis. In: Gordon JE, editor. Handbook of Clinical and Experimental Hypnosis. New York: Macmillan; 1967. pp. 550–587. [Google Scholar]
- Rosen JB. The neurobiology of conditioned and unconditioned fear: a behavioral system analysis of the amygdala. Behavioral and Cognitive Neuroscience Reviews. 2004;3:23–41. doi: 10.1177/1534582304265945. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Fanselow MS, Young SL, Sitcoske M, Maren S. Immediate-early gene expression in the amygdala following footshock stress and contextual fear conditioning. Brain Research. 1998;796:132–142. doi: 10.1016/s0006-8993(98)00294-7. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Matus-Amat P. The ventral hippocampus supports a memory representation of context and contextual fear conditioning: implications for a unitary function of the hippocampus. Behavioral Neuroscience. 2005;119:154–163. doi: 10.1037/0735-7044.119.1.154. [DOI] [PubMed] [Google Scholar]
- Scalia F, Winans SS. The differential projections of the olfactory bulb and accessory bulb in mammals. Journal of Comparative Neurology. 1975;161:31–55. doi: 10.1002/cne.901610105. [DOI] [PubMed] [Google Scholar]
- Shumyatsky GP, Malleret G, Shin R-M, Takizawa S, Tully K, Tsvetkov E, Zakharenko SS, Joseph J, Vronskaya S, Yin D, Schubart UK, Kandel ER, Bolshakov VY. stathmin, a gene enriched in the amygdala, controls both learned and innate fear. Cell. 2005;123:697–709. doi: 10.1016/j.cell.2005.08.038. [DOI] [PubMed] [Google Scholar]
- Stanciu M, Radulovic J, Spiess J. Phosphorylation cAMP response element binding protein in the mouse brain after fear conditioning: relationship to Fos production. Molecular Brain Research. 2001;94:15–24. doi: 10.1016/s0169-328x(01)00174-7. [DOI] [PubMed] [Google Scholar]
- Staples LG, Hunt GE, Cornish JL, McGregor IS. Neural activation during cat odor-induced conditioned fear and ‘trial 2’ fear in rats. Neuroscience and Biobehavioral Reviews. 2005;29:1265–1277. doi: 10.1016/j.neubiorev.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Sullivan RM. Unique characteristics of neonatal classical conditioning: the role of the amygdala and locus coeruleus. Integrative Physiological and Behavioral Science. 2001;36:293–307. doi: 10.1007/bf02688797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi LK, Hubbard DT, Lee I, Dar Y, Sipes SM. Predator odor-induced conditioned fear involves the basolateral and medial amygdala. Behavioral Neuroscience. 2007;121:100–110. doi: 10.1037/0735-7044.121.1.100. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Nakashima BR, Hong H, Watanabe K. The smell of danger: a behavioral and neural analysis of predator odor-induced fear. Neuroscience and Biobehavioral Reviews. 2005;29:1157–1167. doi: 10.1016/j.neubiorev.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Thomas RM, Urban JH, Peterson DA. Acute exposure to predator odor elicits a robust increase in corticosterone and a decrease in activity without altering proliferation in the adult rat hippocampus. Experimental Neurology. 2006;201:308–315. doi: 10.1016/j.expneurol.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Wallace KJ, Rosen JB. Predator odor as an unconditioned fear stimulus in rats: elicitation of freezing by trimethylthiazoline, a component of fox feces. Behavioral Neuroscience. 2000;114:912–922. doi: 10.1037//0735-7044.114.5.912. [DOI] [PubMed] [Google Scholar]
- Zangrossi H, Jr, File SE. Behavioral consequences in animal tests of anxiety and exploration of exposure to cat odor. Brain Research Bulletin. 1992;29:381–388. doi: 10.1016/0361-9230(92)90072-6. [DOI] [PubMed] [Google Scholar]