Abstract
Background
Intimate partner violence (IPV) is one of the most common causes of posttraumatic stress disorder (PTSD) in women. Victims of IPV are often preoccupied by the anticipation of impending harm. This investigation tested the hypothesis that IPV-related PTSD individuals show exaggerated insula reactivity to the anticipation of aversive stimuli.
Methods
Fifteen women with a history of IPV and consequent PTSD (IPV-PTSD) and 15 non-traumatized control (NTC) women performed a task involving cued anticipation to images of positive and negative events during functional magnetic resonance imaging.
Results
Both groups showed increased activation of bilateral anterior insula during anticipation of negative images minus anticipation of positive images. Activation in right anterior/middle insula was significantly greater in the IPV-PTSD relative to the NTC group. Functional connectivity analysis revealed that changes in activation in right middle insula and bilateral anterior insula were more strongly associated with amygdala activation changes in NTC than in IPV-PTSD subjects.
Conclusions
Increased activation in the anterior/middle insula during negative anticipation in women with IPV-related PTSD. These findings in women with IPV could be a consequence of the IPV exposure, reflect pre-existing differences in insular function, or due to the development of PTSD. Thus, future longitudinal studi4s need to examine these possibilities.
INTRODUCTION
Intimate partner violence (IPV) is a leading cause of injury to women in the US (1), accounting for 20% of non-fatal injuries (2). IPV frequently causes posttraumatic stress disorder (PTSD), which is a severe consequence of extraordinarily stressful events that occurs when the combination of re-experiencing aspects of the stressful event, hyperarousal to one’s surroundings, and avoidance of specific situations significantly impairs daily functioning. PTSD is twice as common in women as men, a difference that is in part due to higher rates of exposure among women to IPV (3). IPV-related PTSD is one of the most prevalent and impairing forms of PTSD among women (4). This impairment is not limited to situations in which individuals are exposed to reminders of traumatic stressors. Individuals with IPV-related PTSD frequently anticipate the occurrence of harmful stimuli, and go to great lengths to avoid situations (and thoughts or feelings) that may cue remembrances associated with prior trauma, in addition anticipation of fear and discomfort (e.g., hyperarousal) contributes substantially to behavioral and emotional avoidance that are hallmarks of PTSD (5).
Despite the public health importance of IPV-related PTSD, its underlying neurobiology is poorly understood. Although a number of studies have used blood-oxygen-level dependent (BOLD) imaging to examine brain function in PTSD patients versus nontraumatized controls (6–26), these studies are highly heterogeneous regarding both the tasks performed, and the analysis techniques employed. Seven studies have utilized tasks involved face viewing/judgment and four studies have used imaging during symptom provocation paradigms. However, there have been no studies to date that have used BOLD imaging to examine the neural correlates of anticipatory processes in individuals with PTSD.
Functional neuroimaging studies of PTSD have focused primarily on amygdala and ventral medial prefrontal gyrus (MPFG)(27–33). A meta-analysis of imaging studies in a variety of anxiety disorders recently published by Etkin and Wager (34) indicated that right middle insula and amygdala are two primary areas with the most robust imaging findings in PTSD. The studies summarized in Table 1 show that individuals with PTSD, when compared to nontraumatized controls (NTC), frequently have increased amygdala activation (supplementary Figure 1). Additionally, although the insula is significantly engaged during anticipatory processing (35–39), this structure has shown differential activity in some but not all functional magnetic resonance imaging (fMRI) studies in PTSD. These seemingly discrepant findings raise the question of whether these tasks activated fear and anticipatory circuits. In fact, in the meta-analysis discussed above the authors highlight the role of the amygdala and anterior/middle insula in fear conditioning, phobias, and anticipatory processing (34). Examining the neural correlates related to anticipation and fear is of specific interest for understanding the neurobiology of a condition like PTSD that is characterized by symptoms that involve hyperarousal (i.e., physiologically activating), as well as numbing and avoidance (i.e., physiologically deactivating) experiences. Hyper-arousal may be expressed in the response of the anterior/middle insula, which has substantial physiological afferents (40-2), and numbing and avoidance may lead to regulation of the amygdala when controlling anticipatory stress (43).
Table 1.
Demographic and Psychological Variables.
| NTC |
IPV |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Demographic Variables | Mean | SD | Range | Mean | SD | Range | t/χ2 | p | |
| Age (yrs) | 37.13 | 7.14 | (25–50) | 34.33 | 7.83 | (24–49) | −1.33 | NS | |
| Education (yrs) | 15.57 | 1.72 | (12–18) | 13.13 | 1.73 | (9–16) | −3.87 | <0.001 | |
| Marital Status | Married/Living w/Partner | 3 | 0 | 10.92 | <0.05 | ||||
| Never Married | 11 | 6 | |||||||
| Separated/Divorced | 1 | 8 | |||||||
| Separated | 0 | 1 | |||||||
| Race | African American | N=2 | N=3 | 5.59 | NS | ||||
| Caucasian | N=8 | N=9 | |||||||
| Hispanic | N=0 | N=2 | |||||||
| Filipino-American | N=1 | N=0 | |||||||
| Asian-American | N=1 | N=0 | |||||||
| Mexican-American | N=1 | N=0 | |||||||
| Other | N=2 | N=1 | |||||||
| Comorbid Diagnosis (Lifetime or Current) | |||||||||
| Major Depressive Disorder | N=8 | ||||||||
| Generalized Anxiety Disorder | N=4 | ||||||||
| Panic Disorder | N=3 | ||||||||
| Psychological Variables | |||||||||
| CAPS | Total | - | - | - | 68.5 | 26.3 | (17–110) | N/A | |
| CTS-2 | Negotiation | 9.4 | 7.2 | (0–20.83) | 3.9 | 3.5 | (0–9.3) | −2.37 | <0.05 |
| Psychological Aggression | 0.5 | 1.0 | (0–3.38) | 9.3 | 6.8 | (0–18.75) | 4.94 | <0.001 | |
| Physical Assault | 0.0 | 0.0 | (0–0.08) | 4.3 | 5.3 | (0–17.83) | 3.14 | <0.005 | |
| Sexual Coercion | 0.0 | 0.0 | (0–0) | 2.7 | 3.9 | (0–12.6) | 2.68 | <0.05 | |
| Injury | 0.0 | 0.1 | (0–0.33) | 2.7 | 3.4 | (0–12.17) | 3.04 | <0.005 | |
| CTQ | Emotional Abuse | 9.2 | 4.4 | (5–21) | 13.6 | 5.7 | (5–20) | 2.37 | <0.05 |
| Physical Abuse | 6.3 | 2.2 | (5–13) | 9.1 | 3.9 | (5–18) | 2.45 | <0.05 | |
| Sexual Abuse | 6.1 | 2.4 | (5–13) | 8.1 | 5.6 | (5–24) | 1.31 | NS | |
| Emotional Neglect | 9.2 | 5.0 | (5–24) | 13.1 | 5.0 | (5–22) | 2.10 | <0.05 | |
| Physical Neglect | 6.4 | 1.8 | (5–10) | 8.2 | 3.9 | (5–20) | 1.62 | NS | |
| DES-T | 1.2 | 3.9 | (0–15) | 6.4 | 8.9 | (0–30) | 2.09 | <0.05 | |
| CES-D | 4.5 | 5.5 | (0–18) | 42.0 | 13.2 | (4–46) | 6.85 | <0.001 | |
| AUDIT | 1.4 | 1.6 | (0–6) | 3.5 | 2.4 | (1–9) | 2.58 | <0.05 | |
| DAST | 0.0 | 0.0 | (0–0) | 0.6 | 1.0 | (0–3) | 2.28 | <0.05 | |
| IES-R | Total | 0.0 | 0.0 | (0–0) | 37.00 | 21.84 | (7–66) | 6.35 | <0.001 |
| Avoidance | 0.0 | 0.0 | (0–0) | 1.75 | 1.05 | (0.13–3.50) | 6.27 | <0.001 | |
| Hyperarousal | 0.0 | 0.0 | (0–0) | 1.59 | 1.02 | (0.13–3.00) | 5.80 | <0.001 | |
| Intrusion | 0.0 | 0.0 | (0–0) | 1.68 | 1.03 | (0.00–3.17) | 6.09 | <0.001 | |
Prior research suggests a conceptual framework in which altered anticipation of aversive events is fundamentally involved in both the initiation and maintenance of symptoms associated with IPV-related PTSD. This conceptualization is supported by a burgeoning functional neuroimaging literature which indicates that some of the same brain structures that are abnormally active in PTSD are also critically involved in anticipatory processing. Neuroimaging studies of various PTSD samples have shown differential activation in the amygdala, medial prefrontal cortex (MPFC), and anterior insula (30). Anticipatory tasks reliably activate the anterior insula (35, 37, 38, 44, 45) and studies examining anticipation of an electric shock or noxious thermal stimuli show increased response in the MPFC (35) and anterior insula (35, 44). Related evidence indicates that the anterior insula is involved not only in anticipatory processing, but also in the integration of motor, sensory and visceral afferent information, as well as in mounting affective (46) and autonomic (47) responses. This evidence converges with elegant anatomical research which describes the connections between the anterior and middle insula and the frontal lobes and limbic system (48). Although neural pain response can usefully probe these systems (44), aversive images may be stimuli that are more tailored toward the understanding of PTSD and related disorders.
This aim of this study was to determine whether women with PTSD related to IPV would show altered anticipatory processing and whether this altered processing is reflected in increased activation of the anterior/middle insular cortex. Specifically, we hypothesized that women with IPV-PTSD relative to female NTC would show increased anterior/middle insula activation during anticipatory processing, and that increased insula activity would be functionally connected to increased amygdala activity during the anticipation of aversive stimuli. Given that the insula is thought to play a key role in the physiological experience of PTSD, we also hypothesized that anterior/middle insula activation would relate most strongly to the hyperarousal component of PTSD, and less strongly to the re-experiencing and avoidance components.
METHODS
Subjects
Fifteen women with IPV-PTSD (full or subthreshold; see below) and 15 non-traumatized control (NTC) subjects who had never experienced a PTSD “Criterion A” event (Table 1) completed a cued anticipation task during fMRI. Subjects were excluded if they had: (1) abused substances in the past year, (2) a history of >2 years of alcohol abuse, (3) used psychotropic medication within the last 4 weeks (or fluoxetine within the last 6 weeks), (4) irremovable ferromagnetic material, pregnancy, claustrophobia, bipolar disorder, or schizophrenia. PTSD subjects were included if they had other comorbid affective or mood disorders, such as major depressive disorder, as long as PTSD was the clinically predominant disorder. All participants gave informed written consent to participate in this study, which was approved by the University of California San Diego Human Research Protection Program.
Groups were statistically matched on demographic variables except years of education (i.e., lower in IPV-PTSD subjects), which was used as a covariate in subsequent group contrasts. Twelve of the 15 subjects with IPV exposure met DSM-IV criteria for PTSD and 3 had subthreshold PTSD (i.e., fulfilled Criterion A and the impairment/distress criterion, and had subthreshold Criteria C, and/or D symptoms; CAPS range: 17 to 31). Excluding the 3 subjects with subthreshold PTSD did not change the results in any meaningful way. Therefore, we have reported results from the entire group of 15 subjects with IPV exposure.
Psychological Measures, Stimulus, and Apparatus
Description of the task and the psychological measures are reported in the supplementary section.
Image Acquisition
During the task, an fMRI run sensitive to BOLD contrast was collected for each subject using a Signa EXCITE (GE Healthcare, Milwaukee) 3.0T scanner (T2* weighted echo planar imaging, TR=2000ms, TE=32ms, FOV=250×250 mm3, 64×64 matrix, 30 2.6mm axial slices with 1.4mm gap, 290 scans). During the same experimental session, a high resolution T1-weighted image (SPGR, TI=450ms, TR=8ms, TE=4ms, flip angle=12°, FOV=250×250, ~1 mm3 voxels) was obtained for anatomical reference.
Data were preprocessed and analyzed with the Analysis of Functional NeuroImages (AFNI) software package. Preprocessed time series data for each individual were analyzed using a multiple regression model. Regressors of interest included four regressors: 1) the API, i.e. what activated during anticipation of a positive image, 2) the ANI, i.e. what activated during anticipation of a negative image, 3) the positive image (PI) phase, i.e., what activated during processing of positive stimuli, and 4) the negative image (NI) phase, i.e., what activated during processing of negative stimuli. In addition, six nuisance regressors were entered into the linear regression model: three movement-related regressors used to account for residual motion (roll, pitch, and yaw), a white matter mask to control for physiological noise (58), and regressors for baseline and linear trends used to eliminate slow signal drifts. In this model the CPT task comprises an active baseline. Subsequently, contrasts were constructed on an individual subject level for all anticipation (i.e. ANI+API), differential anticipation of negative and positive (i.e. ANI−API), all images (i.e. NI+PI), and difference between viewing negative and positive images (i.e. NI−PI). A Gaussian filter with full width- half maximum 6 mm was applied to the dataset to account for individual variations in anatomical landmarks. Data of each subject were normalized to Talairach coordinates as defined by AFNI’s built-in atlases.
Voxel-wise percent-signal change data for whole brain were entered into an independent-samples t-test for group differences in activation during anticipation and image presentation between IPV-PTSD and NTC subjects. A threshold adjustment method based on Monte-Carlo simulations was used to guard against identifying false positive areas of activation. A prior voxel-wise probability of p < 0.05 in a cluster of 1024µL resulted in a-posteriori probability of p <0.05. Finally, the average percent-signal difference was extracted from regions of activation that were found to survive this threshold/cluster method (determined by the AFNI program AlphaSim) and the t-values were calculated with and without education as a covariate. All analyses for the behavioral data were carried out with SPSS 12.0.
In addition, a region of interest (ROI) based analysis was performed on several a priori areas of interest: the bilateral anterior/middle insula, bilateral amygdala, ventral medial frontal gyrus, and dorsal medial frontal gyrus. Stereotactic coordinates of the ROIs were based on standardized atlas locations taking from the Talairach atlas (59). To further refine our analyses, we divided the insular cortex into an anterior portion (y >=0), which was of primary interest here, and excluded the posterior insula, which is primarily related to direct processing of ascending c-fibers(48). Using the threshold/clustering method as above, resulted in minimum clusters sizes of 128µL for the amygdala ROIs and 256µL for all remaining ROIs. While the cluster significance is p <.05 for the ROIs, the corrected voxel-wise probabilities are as follows: amygdala p <0.012, anterior/middle insular cortex p <0.000068, ventral medial prefrontal cortex p <0.000145, and dorsal medial prefrontal cortex p <0.000145.
Functional connectivity analysis
Functional connectivity analysis was performed using methods described previously (60). Due to an attempt to maximize power for this event-related design, we selected this technique and examined the entire time series rather than selecting only connectivity within a circumscribed period. Before conducting the functional connectivity analysis, echoplanar signals were corrected for slice-dependent time shifts, spatially filtered using a 6mm FWHM Gaussian filter and bandwidth filtered (0.009 <f<0.08). The resulting echoplanar time series was subsequently transformed to the Talairach atlas. Individual time courses were extracted from these processed echoplanar signals for a seed ROI that showed task dependent activation, specifically in the anterior and anterior/middle insula. Time points in each individual’s time course were censored if they were more than 2 SD from the individual’s average activation for the given seed ROI then entered into a deconvolution analysis as the regressor of interest along with the movement, baseline, and linear regressors. An independent-sample t-test was performed for each seed ROI that contrasted the Fisher Z transforms of the correlation coefficient obtained for IPV and NTC groups to determine differences in functional connectivity between groups.
ROI based connectivity analysis was performed by seeding regions that showed differential group activation (see below) using volume dependent clustering thresholds based on minimum clusters sizes of 256µL. Voxel-wise percent-signal change data were entered into an independent sample t-test to examine group differences in activation during aversive anticipation IPV and NTC. A prior voxel-wise probability of p <0.05 in each ROI cluster resulted in an a-posteriori probability of p <0.05. Finally, the Fisher Z transformed correlations were extracted from each ROI that was found to survive this threshold/cluster method (AFNI program AlphaSim).
RESULTS
Behavioral
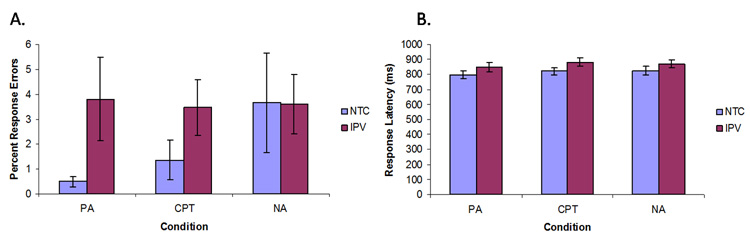
NTC and IPV-PTSD subjects did not differ significantly on response latency (F(1,29)=1.991, p=ns) and accuracy (F(1,29)=2.475, p=ns, Figure 2) during the task. Furthermore, there were no significant task differences in latency (F(1,28)=2.129, p=ns) or accuracy (F(1,28)=0.898, p=ns) when education was used as a covariate.
Figure 2.
Response errors (%) (A) and latency (ms) (B) to the Anticipation Task during positive anticipation (PA), negative anticipation (NA), and continuous performance task (CPT). No statistically significant group differences were found.
Brain Activation
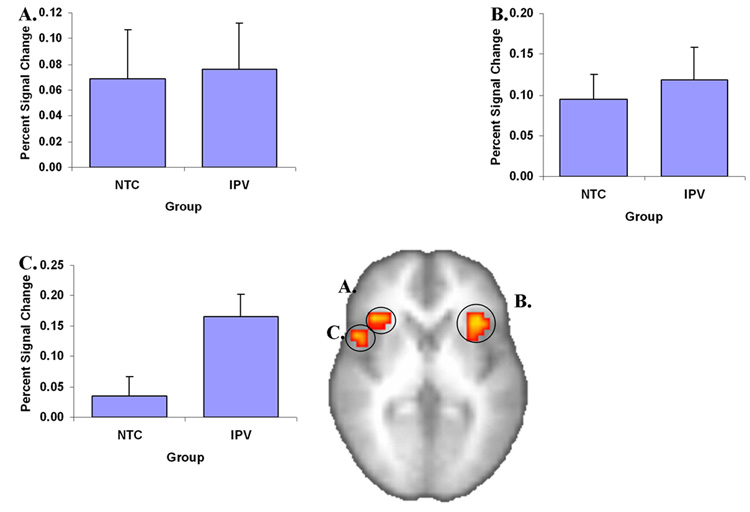
Two primary main effect analyses were performed. First, ROI analysis revealed increased activation related to the differential anticipation contrast (i.e. ANI-API) in bilateral anterior insula, and right anterior/middle insula in both groups (Figure 3, Table 2). ROI findings were corroborated by whole brain results, which showed increased activation related to the differential anticipation contrast in bilateral anterior insula, bilateral prefrontal cortex, bilateral inferior parietal lobule, and right caudate (Table 3). Second, increased activation in IPV-PTSD relative to NTC individuals was observed in right anterior/middle insula. This finding remained significant after covarying for education (Figure 3). As the contrast of interest is between positive and negative image anticipation it should be noted that both conditions ultimately contribute to this contrast.
Figure 3.
Anticipation of images of negative versus positive images leads to increased activation in bilateral anterior insula (A shows right-sided activation and B shows left-sided activation), and (C) right anterior/middle insula, which was significantly more active in IPV relative to NTC subjects.
Table 2.
ROI brain activation differences for negative minus positive anticipation. Increased activation for negative versus positive anticipation. The t-values are shown for the task effect (t-test versus the null hypothesis) and the group effect in these regions (with education covaried out).
| Task Effect |
Group Effect |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Volume | x | y | z | Side | Area | t-value | Sig | t-value | Sig |
| 1024 | −32 | 20 | 3 | Left | Anterior Insula (Ia) | 2.66 | <0.01 | 0.47 | NS |
| 896 | 34 | 22 | 6 | Right | Anterior Insula (Ia) | 4.26 | <0.001 | 0.32 | NS |
| 320 | 47 | 10 | 6 | Right | Anterior/Middle Insula (Ia/Id) | 3.91 | <0.001 | 4.38 | <0.05 |
Table 3.
Whole brain task activation differences for negative minus positive anticipation. The t-values are shown for the task effect (t-test versus the null hypothesis) and the group effect with in these regions (with education covaried out).
| Task Effect |
Group Effect |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Volume | X | y | z | Side | Area | t-value | Sig | t-value | Sig |
| 11264 | 36 | 16 | 35 | Right | Dorsal Lateral Prefrontal | 5.169 | <0.001 | 0.499 | NS |
| 11072 | 42 | −44 | 40 | Right | Inferior Parietal Lobule | 5.476 | <0.001 | 1.915 | NS |
| 8768 | −39 | −40 | 42 | Left | Inferior Parietal Lobule | 5.729 | <0.001 | 1.660 | NS |
| 4352 | 6 | 7 | 16 | Right | Caudate Body | 3.401 | <0.05 | 2.684 | NS |
| Inferior Prefrontal | |||||||||
| 3520 | 32 | 25 | 4 | Right | & Anterior Insula | 4.955 | <0.001 | 0.080 | NS |
| 2816 | 11 | 12 | 46 | Right | Dorsal Medial Frontal Gyrus | 4.663 | <0.001 | 0.446 | NS |
| 2048 | −13 | −3 | 50 | Left | Dorsal Medial Frontal Gyrus | 3.127 | <0.005 | 0.687 | NS |
| 1856 | −31 | 22 | 4 | Left | Anterior Insula | 2.788 | <0.01 | 0.157 | NS |
| 1664 | −41 | 14 | 19 | Left | Inferior Prefrontal | 2.303 | <0.05 | 0.809 | NS |
| 1344 | −15 | 13 | 56 | Left | Superior Frontal Gyrus | 3.299 | <0.001 | 0.945 | NS |
| 1152 | 51 | 13 | 8 | Right | Inferior Prefrontal | 3.788 | <0.001 | 3.819 | <0.05 |
| & Anterior Insula | |||||||||
| 1024 | 10 | 30 | 41 | Right | Medial Frontal Gyrus | 2.573 | <0.05 | 0.582 | NS |
Functional Connectivity
Functional connectivity analyses using bilateral anterior insula and right anterior/middle insula seed regions revealed the following two results: (1) Within each group, significant correlations were observed between activation in bilateral anterior insula and bilateral amygdala and dorsal MPFC, and between right anterior/middle insula and bilateral amygdala and dorsal MPFC, (2) Correlations between activation in bilateral anterior insula and bilateral amygdala, and between right anterior/middle insula and bilateral amygdala were significantly weaker in IPV-PTSD relative to NTC individuals (Table 4 and Figure 4) but dorsal MPFC activations did not differ significantly between groups (supplementary Figure 2). These correlation differences remained significant after including education as a covariate.
Table 4.
Insula and amygdala connectivity correlations after Fisher Z transformations for each group. The significance of the correlations is shown for the task effect (t-test versus the null hypothesis) and the group effect with education covaried out
| Seed ROI | Connection ROI | NTC Fz | IPV Fz | Task Effect |
Group Effect |
||
|---|---|---|---|---|---|---|---|
| t-value | Sig | F | Sig | ||||
| Left anterior insula | Right Amygdala | 1.279 | 0.936 | 18.976 | >0.001 | 8.143 | <0.005 |
| Left anterior insula | Left Amygdala | 1.358 | 0.978 | 17.352 | >0.001 | 5.775 | <0.01 |
| Right anterior insula | Right Amygdala | 1.242 | 0.892 | 15.645 | >0.001 | 5.098 | <0.05 |
| Right anterior insula | Left Amygdala | 1.277 | 0.870 | 13.684 | >0.001 | 3.776 | <0.05 |
| Right anterior/middle insula | Right Amygdala | 1.214 | 0.923 | 20.546 | >0.001 | 7.021 | <0.005 |
| Right anterior/middle insula | Left Amygdala | 1.216 | 0.911 | 18.175 | >0.001 | 4.276 | <0.05 |
Figure 4.
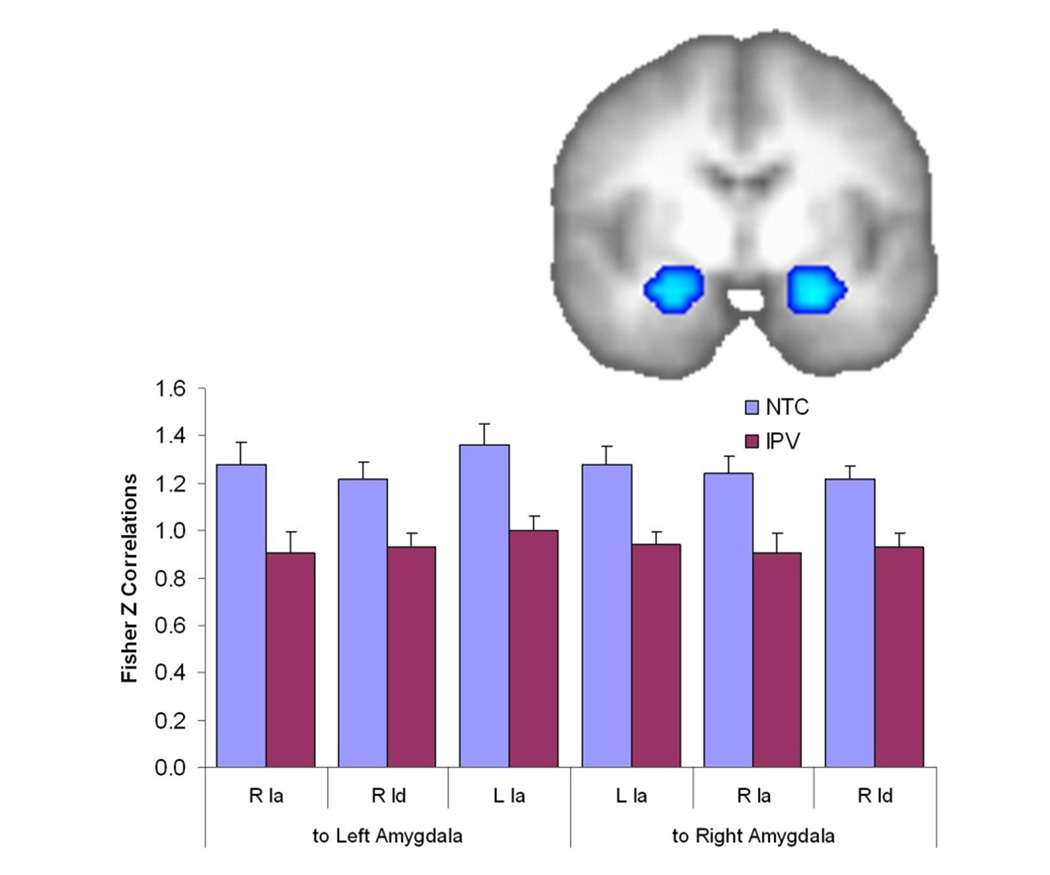
Reduced Functional Connectivity from the anterior insula (Ia), middle insula (Id), and posterior insula (Ig) to the Amygdala in the IPV group. ROI image shows the regions in the amygdala of significantly reduced functional connectivity from a left anterior insula seed; functional connectivity from the other insula regions is very similar to that displayed in the graph.
Brain Behavior Relationships
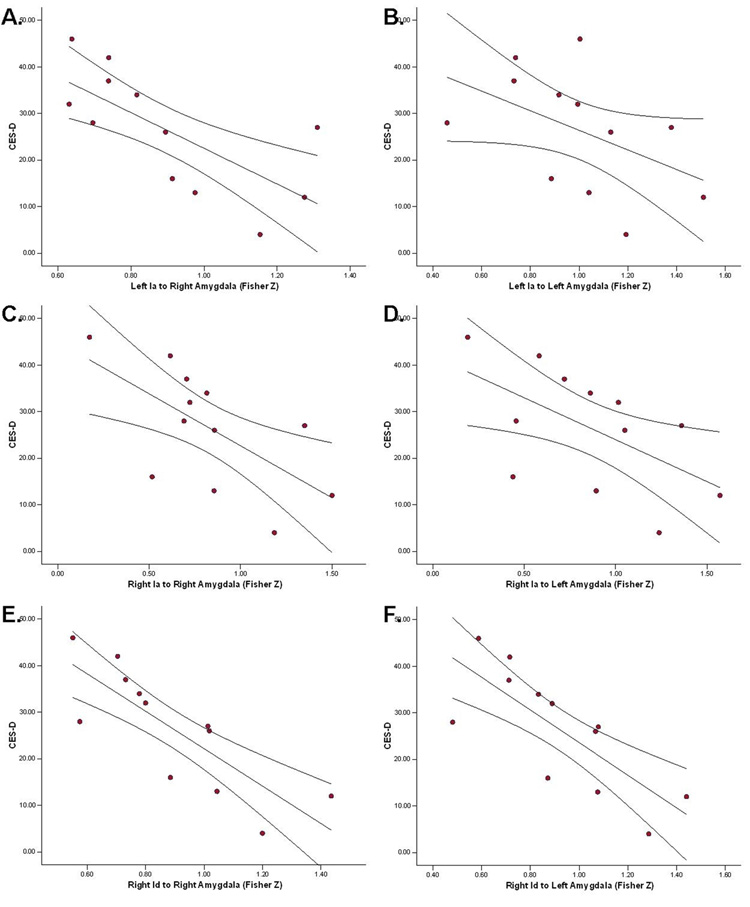
Two main correlative findings were observed. First, controlling for the degree of intrusions and avoidance, we observed partial correlations between subscales of the IES-R (for which there were no missing data) and brain activity in the anterior/middle insula. Within IPV-PTSD individuals, a significant positive correlation between IES-R Hyperarousal scores and activity in the left anterior insula (rp=.647;x=−32, y=20, z=3) was observed. Correlations with IES-R scales were performed that did not reach statistical significance. Second, within the IPV-PTSD group, significant negative correlations were observed between CES-D scores and strength of functional connectivity between (1) left anterior insula and right amygdala, (2) right anterior insula and bilateral amygdala, and (3) right anterior/middle insula and bilateral amygdala (Figure 5 and Table 5). That is, higher levels of depression were associated with weaker connectivity between insula regions and amygdala. If the alpha levels for these correlations are bonferroni-corrected, only three correlations are significant (Table 5). For this reason findings should be considered hypothesis-generating until replicated. There were no significant correlations between task-related (i.e., within or between groups) activation and other symptom measures (e.g., CAPS, DES-T, CES-D, total IES) or behavioral performance (e.g., RT or response accuracy).
Figure 5.
CES-D correlated with Fisher-Z transform of the connectivity for the left anterior insula (Ia; A and B), right Ia (C and D), and right anterior/middle insula (Id; E and F) with the right (A, C, and E) and left (B, D, and F) amygdala in IPV subjects.
Table 5.
Correlations of Functional connectivity Fisher Z transforms with depression severity in the IPV subjects.
| Seed ROI | Connection ROI | CES-D |
|
|---|---|---|---|
| r | Sig | ||
| Left anterior insula | Right Amygdala | −0.716 | <0.01 |
| Left anterior insula | Left Amygdala | −0.482 | NS† |
| Right anterior insula | Right Amygdala | −0.664 | <0.05† |
| Right anterior insula | Left Amygdala | −0.598 | <0.05† |
| Right anterior/middle insula | Right Amygdala | −0.808 | <0.001 |
| Right anterior/middle insula | Left Amygdala | −0.763 | <0.005 |
not significant after bonferroni-correction
DISCUSSION
This experiment yielded several findings. First, in both ROI and whole brain analyses, both groups activated bilateral anterior insula during anticipation of negative compared to positive stimuli. Second, IPV-PTSD relative to NTC subjects showed greater activation in right anterior/middle insula during anticipation of negative compared to positive stimuli. Third, functional connectivity between activation in bilateral anterior insula and bilateral amygdala, and between right anterior/middle insula and bilateral amygdala were significantly weaker in IPV-PTSD relative to NTC individuals. We also observed within IPV-PTSD subjects had a significant positive correlation between IES-R Hyperarousal scores and activity in left anterior insula. Taken together, these results support the notion that in women with IPV-related PTSD: (1) the anterior and anterior/middle insula are important in cued anticipation of negative stimuli, (2) subregions of the insula, such as the anterior/middle insula, are hyperactivate during negative anticipation, and (3) anterior/middle insula activity may be most strongly related to symptoms of hyperarousal in IPV-related PTSD.
The insula, a part of the extended limbic system, can be subdivided into anterior agranular (Ia), central/middle dysgranular (Id) and posterior granular (Ig) subregions based on function and cytoarchitectural structure (48,61). Anterior and middle insula have reciprocal connections with ventral frontal brain regions such as the anterior cingulate and orbital frontal gyrus, as well as with the amygdala, and surrounding, areas, regions that comprise a critical emotion processing circuit. Posterior insula also has reciprocal connections with the frontal cortex, as well as the temporopolar cortex and secondary somatosensory area (61). Some investigators have suggested that the anterior aspect of the insula (including Ia and part of Id) is more closely linked to the executive control system, which includes the anterior cingulate and the dorsolateral prefrontal cortex, whereas the posterior insula (Ig and caudal aspect of Id) primarily integrates afferent information from unmyelinated C-fibers to provide a global sense of the physiological condition of the body (42). Further supporting the notion that the insula is critically involved in emotional and interoceptive processing are studies reporting correlations between insula activation and autonomic arousal (62), anxiety, and visceral changes associated with facial emotion processing (63), as well as the evidence that aversive physiological reactions are key in the establishment and maintenance of avoidant behavior in the development of phobias (e.g. agoraphobia) (64). Neuronal measurements in fear conditioning also show strong involvement of the various aspects of the insular cortex (65). These processes take place in full, and sometimes painful, awareness, which is consistent with the role of the middle/posterior insula in mediating self-awareness (66). Lesion studies in both humans and animals also support the notion that the entire insular cortex is important for emotional and interoceptive processing (67). Additionally, although activation across the entire insula has been frequently associated with disgust (68), there is increasing evidence of a broader role for this brain structure in emotion processing (69). Similarly, anterior/middle insula activation is thought to be involved in differential positive versus negative emotion processing (70), in particular fearful face processing (71), pain perception (72), and judgments about emotions (73).
These findings are consistent with a recent model which explains the critical involvement of the anterior insula in anxiety states and anxiety disorders (46), which are characterized by altered emotional and interoceptive processing. Prior studies have shown that phobic individuals relative to non-phobic comparison subjects showed increased anterior/middle insula activation during emotionally evocative paradigms that used both pictures (74) and words (75) of spider-related stimuli. In a related study, we reported that anxiety prone individuals relative to healthy controls showed exaggerated insula responses during the anticipation of images of spiders and snakes – which are among the most commonly reported phobic stimuli. This evidence is in line with research showing that social phobic individuals show increased activation to fearful faces in the right anterior/middle insula (76) and to angry faces in the bilateral anterior/middle insula (77). These, and related studies in which healthy volunteers processed aversive sensory stimuli (37), are consistent with the notion that anterior insular activity may not only underlie the affective process of emotional distress in normal and phobic individuals, but may also be involved in action planning, i.e., mediation of anxiety-related avoidant behavior (46).
Based on this evidence, we speculate that the increased activation in anterior/middle insula observed in IPV subjects with PTSD, in particular on the left side, may represent a neural substrate linking emotional distress, anticipatory processing, and autonomic arousal, which can advance action planning to reduce exposure to the aversive stimuli. Therefore, the anterior/middle insula activation may be interpreted as a “warning signal” that is associated with the anticipation of aversive symptoms such as hyperarousal. This interpretation is supported by the strong functional connectivity between anterior/middle insula and amygdala observed in the current study, and by the strong correlations between activity in the middle insula and the parietal cortex in a prior study (38).
The pathways between the insula and the amygdala have been mapped out in great detail in animal studies. The basomedial and basolateral nuclei of the amygdala, areas that are involved with conditioned fear, show connections to the various regions in the insula (78), particularly the central dysgranular insula (61). The anterior/middle insula shows the greatest differentiation between groups in the current study, suggesting differential connectivity with the amygdala. This interpretation is consistent with a prior study by Lanius et. al. (25), which found differential activation in the dorsal anterior cingulate (dACC) between individuals who had experienced trauma and had developed PTSD versus those who had experienced significant trauma but did not develop PTSD. In this study, connectivity with the dACC differed in these two groups in a mostly lateralized way, with the PTSD subjects showing greater activation in the right side and the non-PTSD subjects showing greater activation in the left side. We found strong connectivity with the dorsal MPFC in the current study but this connectivity strength did not differ significantly between groups. To our knowledge, ours is the first study to identify altered insularamygdala connectivity in PTSD.
There are several mechanisms which may explain the functional connectivity in the current study. Due to the varying directionality of amygdala activation in PTSD fMRI studies (34) the connectivity with the insula may be highly dependent on the stimulus, task, and population. Therefore, it is not expected that this finding will be generalizable to other situation and should be interpreted with great caution. In the current study, this somewhat paradoxical relationship may imply that insula hyperactivation results in compensatory functional weakening with other brain areas, similar to what has been reported elsewhere (38). Alternatively, the amygdala may receive input from other regions such as the MPFC (19,24,25), which in turn could attenuate the functional connectivity to interoceptive stimuli provided by the anterior/middle insula cortex. These possibilities need to be further investigated with a greater number of subjects that exhibit different insula activation patterns or show significant differences in MPFC. The prevalence of PTSD in females is much higher than in males, which is related to differences in the rates and types of assault (79) and that the accumulation of traumas makes females more vulnerable to retraumatization (80). Structural equation modeling has suggested that the symptom cluster of hyperarousal may relate more to the incidence of retraumatization in females (81). This may suggest that the anterior/middle insula, and subsequent functional correlation findings, may be more pronounced in this population. Combat-related PTSD has received greater investigation in the literature and appears to have higher rates of PTSD subsequent to trauma and potentially reporting compared to other forms of PTSD (82). However, there is no research to date on the neural differences between IPV and combat -related PTSD.
This study has several limitations. First, this group was comprised of women who had experienced relatively recent IPV. These findings cannot be presumed to extend to males, to those with more chronic PTSD, or to those with PTSD stemming from exposure to other types of trauma (e.g., combat). Second, we cannot determine whether the current findings reflect pre-existing processing differences in individuals who are more susceptible to developing PTSD (e.g., it should be noted that the IPV-PTSD group has slightly lower education levels, which might reflect subtle group differences in pre-trauma cognitive ability, thought to be a risk factor for PTSD development (83,84), or are a consequence of the trauma and the post-trauma alterations. Third, future longitudinal studies with individuals exposed to IPV but failing to develop PTSD – a “resilient” comparison group that was lacking from the current study – would help distinguish neural systems involved in trauma exposure from those associated with PTSD, per se. Fourth, the lack of behavioral differences on the CPT task both within and between groups may indicate that the images have only a modest emotional impact. However, due to concerns about distressing subjects we did not include images we deemed too violent. We expect that we may see behavioral differences if stronger images were utilized. Fifth, we used the time series of the entire task as subjects to inspect connectivity rather than just during a single condition. This was done to maximize the within subject power of the connectivity analysis and to see how these networks behaved across anticipatory conditions, thus we do not assert that the functional relationships in this network are highly state dependant.
Anticipation of impending adverse events is potentially a major aspect of the negative effects of PTSD in IPV and may reflect the relationship between hyperarousal and avoidance. Pathological trauma reactions may be perpetuated via increased involvement of the anterior/middle insular cortex in processing negative anticipation, leading to avoidance of affective processing (which may be experienced as “numbing”), as indicated by decreased connectivity with the bilateral amygdala. Important follow up work should determine the heritability (e.g., through twin studies) of this pattern of connectivity and the degree to which treatment may effect this neuronal behavior in individuals with PTSD.
Supplementary Material
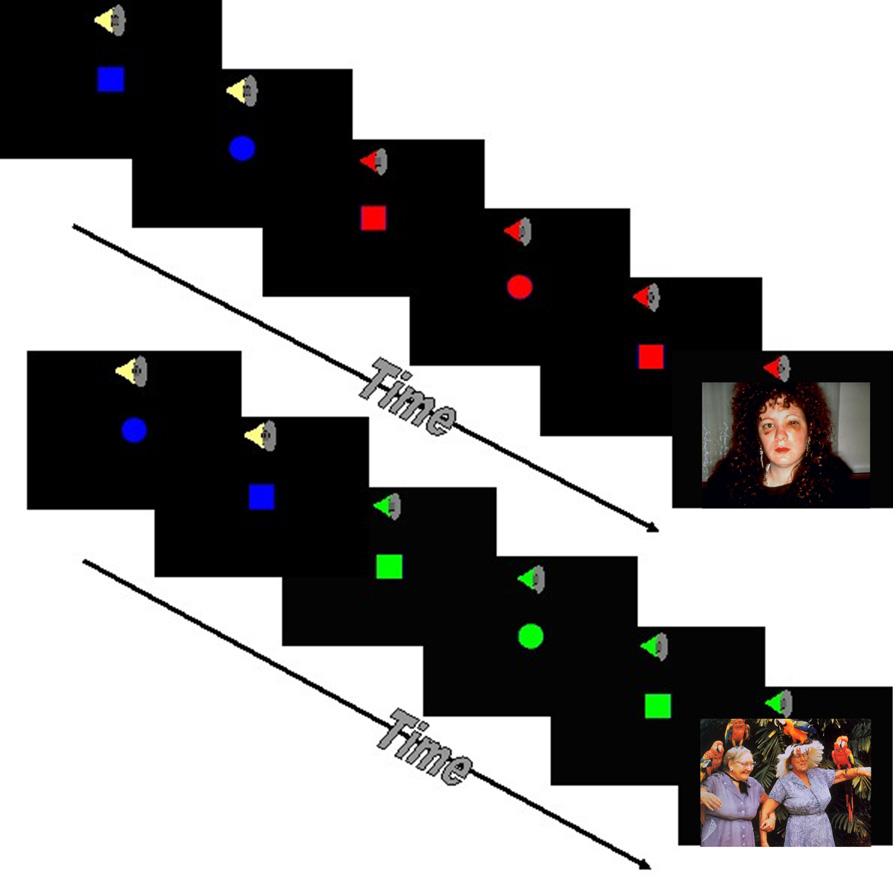
Figure 1.
Anticipation Task. The fMRI task combined a continuous performance task with the interspersed presentation of affective stimuli. Subjects were asked to press the left or right button on a touch pad based on the shape on the screen. Subjects were instructed prior to the task that a switch from a blue to a green shape accompanied by a low tone would indicate that a positive image was going to appear on the screen. In contrast, a switch from a blue to a red shape accompanied by a high tone signaled an impending negative image.
Acknowledgments
This work was supported by the Veterans Administration via Merit Grants (to MPP and MBS) and by grants from NIMH (MH65413 and MH64122 to MBS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors reported no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Eisenstat SA, Bancroft L. Domestic violence. The New England journal of medicine. 1999;341:886–892. doi: 10.1056/NEJM199909163411206. [DOI] [PubMed] [Google Scholar]
- 2.Rennison C. (US) DoJ, editor. Washington (DC): Bureau of Justice Statistics; 2003. Intimate partner violence, 1993–2001. [Google Scholar]
- 3.Stein MB, Kennedy C. Major depressive and post-traumatic stress disorder comorbidity in female victims of intimate partner violence. JAffectDisord. 2001;66:133–138. doi: 10.1016/s0165-0327(00)00301-3. [DOI] [PubMed] [Google Scholar]
- 4.Stein MB, Walker JR, Forde DR. Gender differences in susceptibility to posttraumatic stress disorder. Behaviour research and therapy. 2000;38:619–628. doi: 10.1016/s0005-7967(99)00098-4. [DOI] [PubMed] [Google Scholar]
- 5.Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. AnnNYAcadSci. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- 6.Geuze E, Vermetten E, Ruf M, de Kloet CS, Westenberg HG. Neural correlates of associative learning and memory in veterans with posttraumatic stress disorder. J Psychiatr Res. 2007 doi: 10.1016/j.jpsychires.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Kemp AH, Felmingham K, Das P, Hughes G, Peduto AS, Bryant RA, et al. Influence of comorbid depression on fear in posttraumatic stress disorder: An fMRI study. Psychiatry research. 2007;155:265–269. doi: 10.1016/j.pscychresns.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Bryant RA, Kemp AH, Felmingham KL, Liddell B, Olivieri G, Peduto A, et al. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: An fMRI study. Hum Brain Mapp. 2007 doi: 10.1002/hbm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganzel B, Casey BJ, Glover G, Voss HU, Temple E. The aftermath of 9/11: effect of intensity and recency of trauma on outcome. Emotion (Washington, DC. 2007;7:227–238. doi: 10.1037/1528-3542.7.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanius RA, Frewen PA, Girotti M, Neufeld RW, Stevens TK, Densmore M. Neural correlates of trauma script-imagery in posttraumatic stress disorder with and without comorbid major depression: A functional MRI investigation. Psychiatry research. 2007 doi: 10.1016/j.pscychresns.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Kim MJ, Chey J, Chung A, Bae S, Khang H, Ham B, et al. Diminished rostral anterior cingulate activity in response to threat-related events in posttraumatic stress disorder. J Psychiatr Res. 2007 doi: 10.1016/j.jpsychires.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Hou C, Liu J, Wang K, Li L, Liang M, He Z, et al. Brain responses to symptom provocation and trauma-related short-term memory recall in coal mining accident survivors with acute severe PTSD. Brain Res. 2007;1144:165–174. doi: 10.1016/j.brainres.2007.01.089. [DOI] [PubMed] [Google Scholar]
- 13.Geuze E, Westenberg HG, Jochims A, de Kloet CS, Bohus M, Vermetten E, et al. Altered pain processing in veterans with posttraumatic stress disorder. Archives of general psychiatry. 2007;64:76–85. doi: 10.1001/archpsyc.64.1.76. [DOI] [PubMed] [Google Scholar]
- 14.Astur RS, St Germain SA, Tolin D, Ford J, Russell D, Stevens M. Hippocampus function predicts severity of post-traumatic stress disorder. Cyberpsychol Behav. 2006;9:234–240. doi: 10.1089/cpb.2006.9.234. [DOI] [PubMed] [Google Scholar]
- 15.Williams LM, Kemp AH, Felmingham K, Barton M, Olivieri G, Peduto A, et al. Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. NeuroImage. 2006;29:347–357. doi: 10.1016/j.neuroimage.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 16.Jatzko A, Schmitt A, Demirakca T, Weimer E, Braus DF. Disturbance in the neural circuitry underlying positive emotional processing in post-traumatic stress disorder (PTSD). An fMRI study. European archives of psychiatry and clinical neuroscience. 2006;256:112–114. doi: 10.1007/s00406-005-0617-3. [DOI] [PubMed] [Google Scholar]
- 17.Bryant RA, Felmingham KL, Kemp AH, Barton M, Peduto AS, Rennie C, et al. Neural networks of information processing in posttraumatic stress disorder: a functional magnetic resonance imaging study. BiolPsychiatry. 2005;58:111–118. doi: 10.1016/j.biopsych.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 18.Sakamoto H, Fukuda R, Okuaki T, Rogers M, Kasai K, Machida T, et al. Parahippocampal activation evoked by masked traumatic images in posttraumatic stress disorder: a functional MRI study. NeuroImage. 2005;26:813–821. doi: 10.1016/j.neuroimage.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 19.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. ArchGenPsychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 20.Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien W, et al. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biol Psychiatry. 2005;57:464–473. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 21.Yang P, Wu MT, Hsu CC, Ker JH. Evidence of early neurobiological alternations in adolescents with posttraumatic stress disorder: a functional MRI study. Neuroscience letters. 2004;370:13–18. doi: 10.1016/j.neulet.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 22.Lanius RA, Williamson PC, Hopper J, Densmore M, Boksman K, Gupta MA, et al. Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. BiolPsychiatry. 2003;53:204–210. doi: 10.1016/s0006-3223(02)01466-x. [DOI] [PubMed] [Google Scholar]
- 23.Lanius RA, Williamson PC, Boksman K, Densmore M, Gupta M, Neufeld RW, et al. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. BiolPsychiatry. 2002;52:305–311. doi: 10.1016/s0006-3223(02)01367-7. [DOI] [PubMed] [Google Scholar]
- 24.Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, et al. An fMRI study of anterior cingulate function in posttraumatic stress disorder. BiolPsychiatry. 2001;50:932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- 25.Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA, Neufeld RW, et al. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. AmJPsychiatry. 2001;158:1920–1922. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- 26.Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. BiolPsychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 27.Elzinga BM, Bremner JD. Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? JAffectDisord. 2002;70:1–17. doi: 10.1016/s0165-0327(01)00351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grossman R, Buchsbaum MS, Yehuda R. Neuroimaging studies in post-traumatic stress disorder. PsychiatrClinNorth Am. 2002;25:317–340. doi: 10.1016/s0193-953x(01)00011-9. vi. [DOI] [PubMed] [Google Scholar]
- 29.Hull AM. Neuroimaging findings in post-traumatic stress disorder. Systematic review. BrJPsychiatry. 2002;181:102–110. [PubMed] [Google Scholar]
- 30.Liberzon I, Phan KL. Brain-imaging studies of posttraumatic stress disorder. CNSSpectr. 2003;8:641–650. doi: 10.1017/s109285290000883x. [DOI] [PubMed] [Google Scholar]
- 31.Nemeroff CB, Bremner JD, Foa EB, Mayberg HS, North CS, Stein MB. Posttraumatic stress disorder: a state-of-the-science review. JPsychiatrRes. 2006;40:1–21. doi: 10.1016/j.jpsychires.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Vermetten E, Bremner JD. Circuits and systems in stress. II. Applications to neurobiology and treatment in posttraumatic stress disorder. DepressAnxiety. 2002;16:14–38. doi: 10.1002/da.10017. [DOI] [PubMed] [Google Scholar]
- 34.Etkin A, Wager TD. Functional Neuroimaging of Anxiety: A Meta-Analysis of Emotional Processing in PTSD, Social Anxiety Disorder, and Specific Phobia. The American journal of psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chua P, Krams M, Toni I, Passingham R, Dolan R. A functional anatomy of anticipatory anxiety. Neuroimage. 1999;9:563–571. doi: 10.1006/nimg.1999.0407. [DOI] [PubMed] [Google Scholar]
- 36.Porro CA, Baraldi P, Pagnoni G, Serafini M, Facchin P, Maieron M, et al. Does anticipation of pain affect cortical nociceptive systems? JNeurosci. 2002;22:3206–3214. doi: 10.1523/JNEUROSCI.22-08-03206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simmons A, Matthews SC, Stein MB, Paulus MP. Anticipation of emotionally aversive visual stimuli activates right insula. Neuroreport. 2004;15:2261–2265. doi: 10.1097/00001756-200410050-00024. [DOI] [PubMed] [Google Scholar]
- 38.Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol Psychiatry. 2006;60:402–409. doi: 10.1016/j.biopsych.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 39.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, et al. Placebo-induced changes in fMRI in the anticipation and experience of pain. Science (New York, NY. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 40.Craig AD. Interoception: the sense of the physiological condition of the body. CurrOpinNeurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 41.Craig AD. A new view of pain as a homeostatic emotion. Trends Neurosci. 2003;26:303–307. doi: 10.1016/s0166-2236(03)00123-1. [DOI] [PubMed] [Google Scholar]
- 42.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. NatRevNeurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 43.Stam R. PTSD and stress sensitisation: A tale of brain and body Part 1: Human studies. Neuroscience and biobehavioral reviews. 2007;(31):530–557. doi: 10.1016/j.neubiorev.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 44.Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, et al. Dissociating pain from its anticipation in the human brain. Science (New York, NY. 1999;284:1979–1981. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- 45.Porro CA. Functional imaging and pain: behavior, perception, and modulation. Neuroscientist. 2003;9:354–369. doi: 10.1177/1073858403253660. [DOI] [PubMed] [Google Scholar]
- 46.Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 47.Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. JComp Neurol. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- 48.Augustine JR. Circuitary and functional aspects of the insular lobe in primates including humans. Brain ResRev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- 49.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 50.Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 51.Straus MA, Hamby SL, BoneyMcCoy S, Sugarman DB. The revised Conflict Tactics Scales (CTS2) - Development and preliminary psychometric data. Journal of Family Issues. 1996;17:283–316. [Google Scholar]
- 52.Carlson EB, Putnam FW, Ross CA, Torem M, Coons P, Dill DL, et al. Validity of the Dissociative Experiences Scale in screening for multiple personality disorder: a multicenter study. AmJPsychiatry. 1993;150:1030–1036. doi: 10.1176/ajp.150.7.1030. [DOI] [PubMed] [Google Scholar]
- 53.Weathers FW, Litz BT, Huska JA, Keane TM. PTSD Checklist—Civilian Version. Boston: National Center for PTSD, Behavioral Science Division; 1994. [Google Scholar]
- 54.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosom Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 55.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a Clinician-Administered PTSD Scale. JTrauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 56.Bohn MJ, Babor TF, Kranzler HR. The Alcohol-Use Disorders Identification Test (Audit) - Validation of A Screening Instrument for Use in Medical Settings. Journal of Studies on Alcohol. 1995;56:423–432. doi: 10.15288/jsa.1995.56.423. [DOI] [PubMed] [Google Scholar]
- 57.Cocco KM, Carey KB. Psychometric properties of the Drug Abuse Screening Test in psychiatric outpatients. Psychological Assessment. 1998;10:408–414. [Google Scholar]
- 58.Strigo I, Simmons A, Craig AD, Paulus MP. Society for Neuroscience. Atlanta, GA: 2006. Breathing and BOLD fMRI: watch out. [Google Scholar]
- 59.Talairach J, Tournoux P. New York: Thieme Medical Publishers, Inc.; 1998. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. [Google Scholar]
- 60.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. ProcNatlAcadSciUSA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dupont S, Bouilleret V, Hasboun D, Semah F, Baulac M. Functional anatomy of the insula: new insights from imaging. Surg Radiol Anat. 2003;25:113–119. doi: 10.1007/s00276-003-0103-4. [DOI] [PubMed] [Google Scholar]
- 62.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. NatNeurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 63.Critchley HD, Rotshtein P, Nagai Y, O'Doherty J, Mathias CJ, Dolan RJ. Activity in the human brain predicting differential heart rate responses to emotional facial expressions. NeuroImage. 2005;24:751–762. doi: 10.1016/j.neuroimage.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 64.Starcevic V, Kellner R, Uhlenhuth EH, Pathak D. The phenomenology of panic attacks in panic disorder with and without agoraphobia. ComprPsychiatry. 1993;34:36–41. doi: 10.1016/0010-440x(93)90033-z. [DOI] [PubMed] [Google Scholar]
- 65.LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. JNeurosci. 1990;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karnath HO, Baier B, Nagele T. Awareness of the functioning of one's own limbs mediated by the insular cortex? JNeurosci. 2005;25:7134–7138. doi: 10.1523/JNEUROSCI.1590-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adolphs R, Damasio H, Tranel D, Cooper G, Damasio AR. A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. JNeurosci. 2000;20:2683–2690. doi: 10.1523/JNEUROSCI.20-07-02683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Phillips ML, Young AW, Scott SK, Calder AJ, Andrew C, Giampietro V, et al. Neural responses to facial and vocal expressions of fear and disgust. ProcBiolSci. 1998;265:1809–1817. doi: 10.1098/rspb.1998.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 70.Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- 71.Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, et al. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121(Pt 1):47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- 72.Gelnar PA, Krauss BR, Sheehe PR, Szeverenyi NM, Apkarian AV. A comparative fMRI study of cortical representations for thermal painful, vibrotactile, and motor performance tasks. NeuroImage. 1999;10:460–482. doi: 10.1006/nimg.1999.0482. [DOI] [PubMed] [Google Scholar]
- 73.Gorno-Tempini ML, Pradelli S, Serafini M, Pagnoni G, Baraldi P, Porro C, et al. Explicit and incidental facial expression processing: an fMRI study. NeuroImage. 2001;14:465–473. doi: 10.1006/nimg.2001.0811. [DOI] [PubMed] [Google Scholar]
- 74.Dilger S, Straube T, Mentzel HJ, Fitzek C, Reichenbach JR, Hecht H, et al. Brain activation to phobia-related pictures in spider phobic humans: an event-related functional magnetic resonance imaging study. NeurosciLett. 2003;348:29–32. doi: 10.1016/s0304-3940(03)00647-5. [DOI] [PubMed] [Google Scholar]
- 75.Straube T, Mentzel HJ, Glauer M, Miltner WH. Brain activation to phobia-related words in phobic subjects. NeurosciLett. 2004;372:204–208. doi: 10.1016/j.neulet.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 76.Wright CI, Martis B, McMullin K, Shin LM, Rauch SL. Amygdala and insular responses to emotionally valenced human faces in small animal specific phobia. BiolPsychiatry. 2003;54:1067–1076. doi: 10.1016/s0006-3223(03)00548-1. [DOI] [PubMed] [Google Scholar]
- 77.Straube T, Kolassa IT, Glauer M, Mentzel HJ, Miltner WH. Effect of task conditions on brain responses to threatening faces in social phobics: an event-related functional magnetic resonance imaging study. BiolPsychiatry. 2004;56:921–930. doi: 10.1016/j.biopsych.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 78.Pare D, Smith Y, Pare JF. Intra-amygdaloid projections of the basolateral and basomedial nuclei in the cat: Phaseolus vulgaris-leucoagglutinin anterograde tracing at the light and electron microscopic level. Neuroscience. 1995;69:567–583. doi: 10.1016/0306-4522(95)00272-k. [DOI] [PubMed] [Google Scholar]
- 79.Breslau N. Gender differences in trauma and posttraumatic stress disorder. JGendSpecifMed. 2002;5:34–40. [PubMed] [Google Scholar]
- 80.Breslau N, Anthony JC. Gender differences in the sensitivity to posttraumatic stress disorder: An epidemiological study of urban young adults. Journal of abnormal psychology. 2007;116:607–611. doi: 10.1037/0021-843X.116.3.607. [DOI] [PubMed] [Google Scholar]
- 81.Risser HJ, Hetzel-Riggin MD, Thomsen CJ, McCanne TR. PTSD as a mediator of sexual revictimization: the role of reexperiencing, avoidance, and arousal symptoms. Journal of traumatic stress. 2006;19:687–698. doi: 10.1002/jts.20156. [DOI] [PubMed] [Google Scholar]
- 82.Brinker M, Westermeyer J, Thuras P, Canive J. Severity of combat-related posttraumatic stress disorder versus noncombat-related posttraumatic stress disorder: a community-based study in American Indian and Hispanic veterans. The Journal of nervous and mental disease. 2007;195:655–661. doi: 10.1097/NMD.0b013e31811f4076. [DOI] [PubMed] [Google Scholar]
- 83.Gilbertson MW, Paulus LA, Williston SK, Gurvits TV, Lasko NB, Pitman RK, et al. Neurocognitive function in monozygotic twins discordant for combat exposure: relationship to posttraumatic stress disorder. Journal of abnormal psychology. 2006;115:484–495. doi: 10.1037/0021-843X.115.3.484. [DOI] [PubMed] [Google Scholar]
- 84.Kremen WS, Koenen KC, Boake C, Purcell S, Eisen SA, Franz CE, et al. Pretrauma cognitive ability and risk for posttraumatic stress disorder: a twin study. Archives of general psychiatry. 2007;64:361–368. doi: 10.1001/archpsyc.64.3.361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.