Abstract
Objective
To examine the predictors of histologic confirmation of high-grade squamous intraepithelial lesion (HSIL) cytology occurring in follow-up of young women originally referred into a trial because of less severe cytology.
Methods
We used enrollment HSIL cytology (n = 411) as read by clinical center pathologists for women participating in the Atypical Squamous Cells of Undetermined Significance (ASCUS) and Low-Grade Squamous Intraepithelial Lesion (LSIL) Triage Study (ALTS). The primary outcome was histologic cervical intraepithelial neoplasia grade 3 (CIN3) and early cancer (n = 195; 191 CIN3 and 4 cancers) as diagnosed by the quality control (QC) Pathology group over the 2-year duration of ALTS.
Results
The 2-year absolute risk of CIN3 or worse following a HSIL cytology was 47.4% (95%CI = 42.5%–52.4%). The 2-year absolute risk of CIN3 or worse was lowest (14.3%) for women who were HPV16 negative, had colposcopic impression of less than low-grade, and whose HSIL cytology as called by the clinical center was not also called HSIL or equivocal HSIL cytology by QC Pathology. The 2-year absolute risk of CIN3 or worse was highest (82.4%) for women who were HPV16 positive, had colposcopic impression of low-grade or worse, and whose HSIL cytology was also called HSIL or equivocal HSIL cytology by QC Pathology.
Conclusions
Histologic confirmation of precancer among young women with HSIL cytology was more likely when other risk factors (e.g., HPV16) for cervical precancer were present.
INTRODUCTION
Cytology read as high-grade squamous intraepithelial lesion (HSIL) and equivocal HSIL read as atypical squamous cells “cannot rule out high grade” (ASC-H) are more likely to reflect histologic high-grade precancer (cervical intraepithelial neoplasia grade 2 or 3 [CIN2, CIN3]) than cytology read as atypical squamous cells of undetermined significance (ASC-US) or low-grade squamous intraepithelial lesion (LSIL). In most studies, CIN2/3 is found in about 15% of women referred for the evaluation of ASC-US cytology, about 20% referred for LSIL and about 70% referred for HSIL. However, the total CIN2/3 diagnosed following ASCUS and LSIL cytology is greater than that detected following HSIL cytology because of the numerical majority of these two milder cytologic interpretations (1).
Because of the elevated risk of CIN2/3 for women with HSIL cytology, previous guidelines have generally recommended an excision procedure when a precancerous lesion is not found on the colposcopic biopsy (“unconfirmed” HSIL)(2). However, the 2006 American Society for Colposcopy and Cervical Pathology (ASCCP) guidelines for the management of women having unconfirmed HSIL have increased clinician and patient discretion in post-colposcopy management of young adult women by providing the option of either an excisional procedure or follow-up by repeat cytology and colposcopy at 6-month intervals for up to a year (3). Additionally, it is preferred to manage adolescents with unconfirmed HSIL by follow-up rather than excision. This change in the management of unconfirmed HSIL cytology is the outcome of increasing recognition that cervical excision procedures carry some risk for adverse pregnancy outcome (4) and that cytologic HSIL and histologic CIN2 in young women might represent acute infections that will resolve spontaneously (5).
Unconfirmed HSIL presents clinicians with a management quandary, and it would be useful to better understand why some HSIL are not confirmed. To address these questions, we evaluated the 2-year risk of finding CIN3 and early cancer among women participating in the ASCUS LSIL Triage Study (ALTS) for community cytology of ASCUS or LSIL but found to have HSIL on repeat cytology at enrollment.
MATERIALS AND METHODS
Study Design and Population
ALTS (1997–2001) was a randomized trial comparing three management strategies for 5,060 women with ASCUS (n = 3,488) or LSIL (n = 1,572): (1) immediate colposcopy (referral to colposcopy regardless of enrollment test results); (2) HPV triage (referral to colposcopy if enrollment HPV result by Hybrid Capture 2 (hc2; Digene Corporation, Gaithersburg, MD) was positive or missing or if the enrollment cytology was high-grade squamous intraepithelial lesion [HSIL]); or (3) conservative management (referral to colposcopy at enrollment if cytology was HSIL). ALTS involved four clinical centers: University of Alabama at Birmingham (Birmingham, AL), Magee-Womens Hospital of the University of Pittsburgh Medical Center Health System (Pittsburgh, PA), the University of Oklahoma (Oklahoma City, OK), and the University of Washington (Seattle, WA). The National Cancer Institute and local institutional review boards approved the study and all participants provided written informed consent.
At the enrollment examination all women underwent a pelvic examination with collection of two cervical specimens; the first specimen in PreservCyt for ThinPrep cytology (Cytyc Corporation, Marlborough, MA) and the second in specimen transport medium (STM; Digene Corporation). Women in all 3 arms of the study were re-evaluated by cytology every 6 months for 2 years and sent to colposcopy if cytology was HSIL. An exit examination with colposcopy was scheduled for all women, regardless of study arm or prior procedures, at the completion of the follow-up. We refer readers to other references for details on randomization, examination procedures, patient management, and laboratory and pathology methods (6).
HPV DNA Testing
Two HPV DNA tests were performed on clinical specimens collected at enrollment. Hybrid Capture 2 using probe set B, a pooled probe DNA test for one or more carcinogenic or HR HPV genotypes (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68), was performed on PreservCyt specimens. A positive test does not identify which HR HPV genotype(s) are present. Hybrid Capture 2 is also well-known to cross-react with HPV66, another HR genotype (7), and other LR HPV genotypes (8). Testing for 27 or 38 HPV genotypes was performed using an L1-based PCR assay that employs a primer set designated PGMY09/11 (line blot assay [LBA]) on the STM specimen as previously described (9;10).
We considered HPV genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68 as the primary HR HPV genotypes (11;12). Women were assigned to a HPV risk group based on the results of the two HPV tests and according to a priori established cervical cancer risk: 1) positive for HPV16; 2) positive for any other HR HPV genotypes and negative for HPV16 (HR HPV excluding HPV16); 3) positive for any LR HPV genotypes and negative for all HR genotypes; or 4) HPV negative. In the case of discrepant HPV results between LBA and hc2, we employed the following rules: Women who were negative by hc2 but PCR positive for HR HPV and women who were positive by hc2 but PCR negative for all HPV genotypes were classified as HR HPV positive. Women who were positive by hc2 and PCR negative for HR HPV genotypes but positive for LR HPV genotypes were classified as LR HPV because of the possibility that the hc2 positive result was the consequence of cross-reactivity with LR HPV genotypes (13).
Pathology and Treatment
Clinical management was based on the clinical center pathologists’ cytologic and histologic diagnoses. A large portion of the cytology slides from follow-up were submitted for computer-assisted review (Neopath, TriPath Imaging, Burlington, NC). In addition, all referral smears, ThinPreps, and histology slides were sent to the Pathology Quality Control Group (QC Pathology) based at the Johns Hopkins Hospital for review and secondary diagnoses. Both pathology reviews were required to subcategorize HSIL cytology into more severe HSIL-CIN3 and less severe HSIL-CIN2, which for these analyses included any HSIL in which this distinction was not made (HSIL, not otherwise specified or HSIL, NOS). For cytologic and histologic specimens, a pathology QC diagnosis of CIN3 that had been called less than CIN2 at the center triggered a safety notification sent by fax to the clinical centers. CIN2 or worse (CIN2 or worse) diagnosis based on the clinical center pathology or CIN3 or worse diagnosis based on the QC Pathology review triggered treatment by Loop Electrosurgical Excision Procedure (LEEP). In addition, women with persistent LSIL or HR HPV-positive ASCUS at the time of the exit from the study were offered LEEP.
Statistical Methods
We used CIN3 or worse (CIN3 or worse) diagnosed on biopsy or LEEP by QC Pathology over the 2-year duration of ALTS as our primary outcome. This included 4 cases of cancer, all of which had their enrollment cytology called HSIL by both pathology groups (14). Exclusion of these cases did not appreciably change our results. We focused on 2-year cumulative absolute risk of CIN3 or worse to account for the less than perfect sensitivity of a single colposcopic examination for detection of small, prevalent CIN3 lesions (15;16). Because of the relevance to clinical practice, we also included the clinical center histologic diagnoses of CIN2 or worse as an endpoint in some analyses.
We calculated the 2-year cumulative absolute risk of histologically-confirmed CIN3 or worse diagnosed by QC Pathology or histologically-confirmed CIN2 or worse diagnosed by the clinical center for any HSIL cytology and stratified on whether the HSIL cytology was called HSIL-CIN2 or HSIL-CIN3. For reference, we also calculated the risk for women with atypical squamous cells, cannot rule out HSIL (ASC-H) (viewed as equivocal HSIL). We then evaluated the 2-year cumulative absolute risk of histologically-confirmed CIN3 or worse diagnosed by QC Pathology for HSIL, HSIL-CIN2, and HSIL-CIN3 cytology stratified on HPV risk group status. An extension of the Wilcoxon rank-sum test was used to as a non-parametric test for trend across categories (17). We also examined the 2-year risk of CIN3 or worse in subgroups of women with HSIL cytology stratified by enrollment colposcopic impression (<low-grade or ≥low-grade), whether HPV16 was detected, and/or whether the cytology was confirmed as HSIL or at least ASC-H by QC Pathology.
To determine which factors were primarily responsible for finding CIN3 or worse among women with HSIL cytology, we developed a multivariable model to evaluate the association of independent risk factors with 2-year cumulative CIN3 or worse as diagnosed by QC Pathology. Contingency tables, using Pearson χ2 tests, were used to establish crude associations of risk factors with CIN3 or worse. We then used multinomial logistic regression (18) to calculate multivariable (adjusted) odds ratios (ORs) and 95% confidence intervals (95%CI) for CIN3 or worse, with <CIN2 as the reference histologic outcome and CIN2 kept as a distinct intermediate because it is an equivocal mixture of incipient CIN3 with acute HPV infection by both HR and LR HPV (19). For clarity, we present only the associations with CIN3 (versus <CIN2).
Finally, we examined the crude associations of the timing of the CIN3 (baseline or during follow-up/exit) with the number of biopsies taken at baseline, whether the baseline cytologic impression was HSIL-CIN2 or HSIL-CIN3, HPV risk group, and colposcopic impression.
A P value < 0.05 was considered statistically significant. Stata version 8.2 (Stata Corporation, College Station, TX) was used for all statistical analyses.
RESULTS
Four hundred eleven of the 445 women (92.4%) with (repeat) enrollment cytology interpreted as HSIL by the clinical centers had complete disease ascertainment (403 had an exit visit with colposcopy; 7 without an exit visit colposcopy had a clinical center histopathologic diagnosis of CIN2 or worse, and 1 had a QC Pathology Group histopathologic diagnosis of CIN3 or worse, both of which led to a censoring treatment). The median age of the 411 women was 24 years (mean age of 25.5 years). A description of the 411 women can be found in Table 1.
Table 1.
Women with enrollment clinical center cytology of high-grade squamous intraepithelial lesion.
| All (n = 411) | <CIN3 (n = 216) | ≥CIN3 (n = 195) | |||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | p | |
| Study Arm | |||||||
| IC | 155 | 37.7% | 84 | 38.9% | 71 | 36.4% | 0.1 |
| HPV | 101 | 24.6% | 60 | 27.8% | 41 | 21.0% | |
| CM | 155 | 37.7% | 72 | 33.3% | 83 | 42.6% | |
| Clinical Center | |||||||
| 1 | 70 | 17.0% | 23 | 10.6% | 47 | 24.1% | 0.003 |
| 2 | 116 | 28.2% | 70 | 32.4% | 46 | 23.6% | |
| 3 | 49 | 11.9% | 26 | 12.0% | 23 | 11.8% | |
| 4 | 176 | 42.8% | 97 | 44.9% | 79 | 40.5% | |
| Referral Pap | |||||||
| ASCUS | 220 | 53.5% | 110 | 50.9% | 110 | 56.4% | 0.3 |
| LSIL | 191 | 46.5% | 106 | 49.1% | 85 | 43.6% | |
| Age Group (Years) | |||||||
| <20 | 57 | 13.9% | 38 | 17.6% | 19 | 9.7% | 0.01 |
| 20–24 | 173 | 42.1% | 92 | 42.6% | 81 | 41.5% | |
| 25–29 | 106 | 25.8% | 42 | 19.4% | 64 | 32.8% | |
| 30–34 | 37 | 9.0% | 20 | 9.3% | 17 | 8.7% | |
| 35–39 | 13 | 3.2% | 10 | 4.6% | 3 | 1.5% | |
| 40+ | 25 | 6.1% | 14 | 6.5% | 11 | 5.6% | |
| Race | |||||||
| White/Hispanic | 19 | 4.6% | 11 | 5.1% | 8 | 4.1% | 0.5 |
| White/Non-Hispanic | 306 | 74.6% | 155 | 71.8% | 151 | 77.4% | |
| Black | 59 | 14.4% | 32 | 14.8% | 27 | 13.8% | |
| Asian/Pacific Islander | 14 | 3.4% | 10 | 4.6% | 4 | 2.1% | |
| American Indian/Alaskan Native | 12 | 2.9% | 8 | 3.7% | 4 | 2.1% | |
| Missing | 1 | 0.2% | 0 | 0.0% | 1 | 0.5% | |
| Education | |||||||
| Grade 1–6 | 2 | 0.5% | 1 | 0.5% | 1 | 0.5% | 0.07 |
| Grade 7–11 | 85 | 20.7% | 39 | 18.1% | 46 | 23.6% | |
| Completed High School/GED | 129 | 31.4% | 62 | 28.7% | 67 | 34.4% | |
| Cocational/Trade School | 26 | 6.3% | 16 | 7.4% | 10 | 5.1% | |
| Some College | 119 | 29.0% | 63 | 29.2% | 56 | 28.7% | |
| Completed College | 41 | 10.0% | 27 | 12.5% | 14 | 7.2% | |
| Some/Completed Grad School | 9 | 2.2% | 8 | 3.7% | 1 | 0.5% | |
Data are shown for all women, women without histologic confirmation (< cervical intraepithelial neoplasia grade 3 [CIN3]), and women with histologic confirmation (CIN3 or worse). Differences in the distribution of the categories for any variable were tested for significance using Fisher’s exact test.
When comparing those included to those excluded, women referred because of ASCUS (vs. LSIL) were less likely to have complete disease ascertainment (p = 0.01), and there were minor differences between clinical centers (p = 0.02). Study arm, age at enrollment, HPV risk status, and a history of abnormal Paps were unrelated to inclusion in this analysis.
Of the 411 women with HSIL cytology included in the analysis, 354 (86.1%) had a cytologic interpretation of HSIL-CIN2 and 57 (13.9%) had a cytologic interpretation of HSIL-CIN3 (one clinical center read all 52 HSIL cytologic interpretations as HSIL-CIN2). Another 110 women with equivocal HSIL, ASC-H, had complete disease ascertainment as described above. Almost all women (401 of 411, 97.6%) with a clinical center enrollment cytology of HSIL underwent a colposcopic evaluation during the enrollment period.
The 2-year cumulative risk of QC Pathology diagnosed CIN3 or worse following a HSIL cytology was 47.4% (95%CI = 42.5%–52.4%) (Table 2); similar risk estimates were observed when clinical center diagnosed CIN3 or worse was used (data not shown). The 2-year cumulative risk of clinical center-diagnosed CIN2 or worse following a HSIL cytology was 75.9% (95%CI = 71.5%–80.0%). The 2-year risks for CIN3 or worse were greater for cytologic HSIL-CIN3 than for cytologic HSIL-CIN2 (73.7% vs. 43.2%, respectively, p < 0.001); nearly all HSIL-CIN3 had a clinical center diagnosed CIN2 or worse within two years (98%, 95%CI = 90.6%–100%).
Table 2.
The 2-year absolute risk of cervical precancer and/or cancer for women with enrollment clinical center cytology of high-grade squamous intraepithelial lesion.
| Enrollment Cytology (CC) | QC Pathology | CC Pathology | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | N (≥CIN3) | %≥CIN3 | N(CIN3) | %CIN3 | N(Cancer) | %Cancer | N (≥CIN2) | %≥CIN2 | |
| ASC-H | 110 | 24 | 21.8% | 24 | 21.8% | 0 | 0.0% | 40 | 36.4% |
| HSIL | 411 | 195 | 47.4% | 191 | 46.5% | 4 | 1.0% | 312 | 75.9% |
|
| |||||||||
| HSIL:CIN2 | 354 | 153 | 43.2% | 151 | 42.7% | 2 | 0.6% | 256 | 72.3% |
| HSIL:CIN3 | 57 | 42 | 73.7% | 40 | 70.2% | 2 | 3.5% | 56 | 98.2% |
| p < 0.001 | p < 0.001 | p = 0.09 | p < 0.001 | ||||||
Cervical precancer was defined at either quality control (QC) Pathology-diagnosed cervical intraepithelial neoplasia grade 3 (CIN3) or clinical center (CC) pathology-diagnosed CIN2 or CIN3; CIN3 or worse includes CIN3 and cancer and CIN2 or worse includes CIN2, CIN3, and cancer. A Fisher’s exact test was used to test for differences in the 2-year risk between HSIL sub-categories.
Among the 404 of the 411 women (98.3%) with HPV test results, there was a trend of increasing 2-year cumulative risk of QC Pathology diagnosed CIN3 or worse with higher risk HPV risk group (ptrend < 0.001) (Table 3). The risk of CIN3 was low when only an LR genotype was found or when no HPV was found. This trend of increased risk of CIN3 or worse with HPV risk group was also observed when stratified on whether the HSIL cytology was called HSIL-CIN2 (ptrend < 0.001) or HSIL-CIN3 cytology (ptrend = 0.03). Only 1 (1.9%) of 53 cases of HSIL-CIN3 showed no HPV16 or another HR genotype compared with 25 (7.1%) of 351 HSIL-CIN2.
Table 3.
The 2-year absolute risk of quality control pathology-diagnosed cervical intraepithelial neoplasia grade 3 (CIN3) or worse for women with enrollment clinical center cytology of high-grade squamous intraepithelial lesion.*
| HSIL | HSIL:CIN2 | HSIL:CIN3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | N (≥CIN3) | %≥CIN3 | N | N (≥CIN3) | %≥CIN3 | N | N (≥CIN3) | %≥CIN3 | |
| All | 404 | 193 | 47.8% | 351 | 153 | 43.6% | 53 | 40 | 75.5% |
|
| |||||||||
| HPV16 | 188 | 123 | 65.4% | 147 | 90 | 61.2% | 41 | 33 | 80.5% |
| HR HPV | 190 | 67 | 35.3% | 179 | 60 | 33.5% | 11 | 7 | 63.6% |
| LR HPV | 20 | 2 | 10.0% | 19 | 2 | 10.5% | 1 | 0 | 0.0% |
| HPV Negative | 6 | 1 | 16.7% | 6 | 1 | 16.7% | 0 | 0 | |
|
| |||||||||
| ptrend < 0.001 | ptrend < 0.001 | ptrend = 0.03 | |||||||
Stratified by human papillomavirus risk group status (HPV16 > high-risk (HR) HPV > LR HPV > HPV Negative).
The risks are presented for all HSIL and for subcategories of HSIL, HSIL-CIN2 and HSIL-CIN3.
The ptrend in column reflect the trend of risk for higher risk HPV status for a cytologic interpretation.
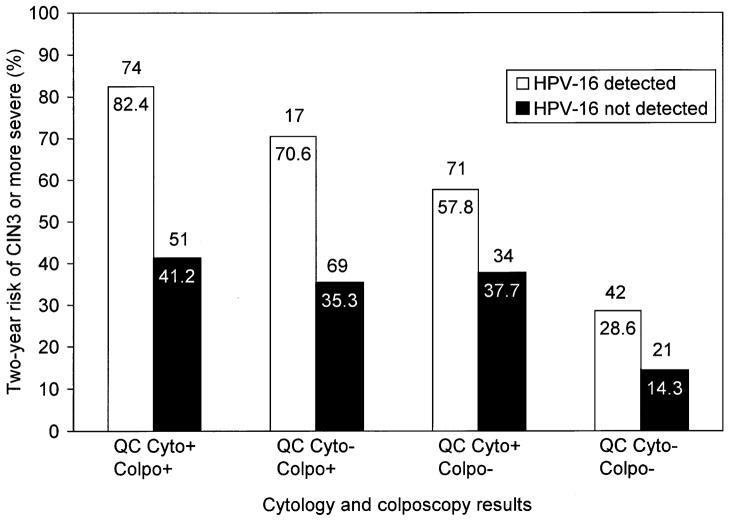
Figure 1 shows the trend of increasing 2-year absolute risk of QC Pathology diagnosed CIN3 or worse among women with HSIL cytology with other indicators of risk. Women at the lowest risk of CIN3 or worse (14.3%) were those who were HPV16 negative, had colposcopic impression of normal or metaplastic (i.e., less than low-grade), and whose HSIL cytology was not confirmed as HSIL or at least ASC-H by QC Pathology (n = 42, 10.2% of total). Women at the highest risk of CIN3 or worse (82.4%) were those who were HPV16 positive, had colposcopic impression of low-grade and more severe, and whose HSIL cytology was read as HSIL or ASC-H by the QC Pathology (n = 74, 18% of the total). Similar patterns were observed using 2-year cumulative risk of clinical center pathology diagnosed CIN2 or worse as the endpoint, with the risk ranging from 52.4% for women with none of the risk factors to 98.7% for women with all three risk factors present (data not shown).
Figure 1.
2-year absolute risk of quality control (QC) pathology-diagnosed cervical intraepithelial neoplasia grade 3 (CIN3) or worse (CIN3 or worse) for women with enrollment clinical center cytology of high-grade squamous intraepithelial lesion (HSIL), stratified by other indicators of risk: colposcopic impression of a visible lesion (Colpo− = less than low-grade or Colpo+ = low-grade or more severe), detection of HPV16 (HPV16− or HPV16+), QC Pathology confirmation of HSIL (QC Cyto− = <HSIL/ASC-H or QC Cyto+ = HSIL/ASC-H). ASC-H = atypical squamous cells, cannot rule out HSIL. The actual risk value is shown in white type on the top of the bar and the number of women with those combinations of indicators is shown in black type in a white box within each bar.
We then examined the factors associated with 2-year worst quality control pathology diagnosis of CIN3 or worse (vs. <CIN2) among women with HSIL cytology using a multivariable model (Table 4). The risk of having a CIN3 or worse diagnosis was 2–6 fold less likely for women with a HSIL cytology who 1) tested HPV negative or positive for LR HPV (vs. HR HPV positive); 2) were from Centers 2 or 4 (vs. Center 1); and 3) were randomized into the HPV arm (vs. Immediate Colposcopy arm) of the trial. The risk of having a CIN3 or worse diagnosis was 2–4 fold more likely for women with a HSIL cytology who 1) tested HPV16 positive (vs. HR HPV positive); 2) had HSIL-CIN3 cytology (vs. HSIL-CIN2); 3) were current smokers with 4 or more pack-years of use (vs. those who never smoked); 4) had cytology confirmed as HSIL or ASC-H by QC Pathology (vs. not); or 5) had colposcopic impression of low-grade or worse (vs. less than low-grade).
Table 4.
A multivariable model to examine to factors associated with quality control pathology-diagnosed cervical intraepithelial neoplasia grade 3 (CIN3) or worse for women with enrollment clinical center cytology of high-grade squamous intraepithelial lesion (HSIL).
| N | % | OR | 95%CI | |
|---|---|---|---|---|
| CC Cytology | ||||
| HSIL-CIN2 (ref) | 338 | 86.4% | 1.0 | |
| HSIL-CIN3 | 53 | 13.6% | 3.3 | 1.0–11 |
| HPV Status | ||||
| HPV Neg/LR HPV | 26 | 46.5% | 0.18 | 0.046–0.68 |
| HR HPV (ref) | 182 | 6.6% | 1.0 | |
| HPV16 | 183 | 46.8% | 4.0 | 2.2–7.3 |
| Colposcopic Impression | ||||
| <Low-Grade (ref) | 210 | 53.7% | 1.0 | |
| ≥Low-Grade | 181 | 46.3% | 3.3 | 1.8–6.0 |
| QC Cytology = ASC-H/HSIL | ||||
| No (ref) | 116 | 29.7% | 1.0 | |
| Yes | 275 | 70.3% | 2.5 | 1.3–4.6 |
| Smoking Status | ||||
| Never (ref) | 152 | 38.9% | 1.0 | |
| Former | 39 | 10.0% | 2.3 | 0.85–6.0 |
| Current, <4 pack-years | 90 | 23.0% | 1.4 | 0.67–2.9 |
| Current, ≥4 pack-years | 110 | 28.1% | 2.3 | 1.1–4.7 |
| Center | ||||
| Center 1 (ref) | 67 | 17.1% | 1.0 | |
| Center 2 | 112 | 28.6% | 0.24 | 0.086–0.65 |
| Center 3 | 48 | 12.3% | 0.47 | 0.15–1.5 |
| Center 4 | 164 | 41.9% | 0.28 | 0.11–0.70 |
| Study Arm | ||||
| IC (ref) | 151 | 38.6% | 1.0 | |
| HPV | 93 | 23.8% | 0.43 | 0.21–0.89 |
| CM | 147 | 37.6% | 0.76 | 0.39–1.5 |
OR, Odds ratios; 95%CI, 95% confidence intervals; LSIL, low-grade squamous intraepithelial lesion; HPV, human papillomavirus; HR, high-risk; ASC-H, atypical squamous cells, cannot rule out HSIL
Of the 411 women with HSIL cytology included in this analysis, 391 (95.1%) had complete data and were included in the model.
Finally, we examined the timing of the QC Pathology diagnosis of CIN3 or worse in the 401 (97.6%) of the women with an enrollment colposcopy. One hundred and seventy of the women with enrollment HSIL cytology (42.4%) were diagnosed with CIN3 or worse at baseline (Table 5); 3.0% of women were diagnosed with CIN3 or worse during follow-up visits and 2.5% of women were diagnosed with CIN3 or worse at exit.
Table 5.
The timing of a quality control pathology-diagnosed cervical intraepithelial neoplasia grade 3 (CIN3) or worse or a clinical center-diagnosed CIN2 or worse for women with an enrollment clinical center cytology of high-grade squamous intraepithelial lesion (HSIL).
| HSIL | ||||
|---|---|---|---|---|
| N(≥CIN3) | %≥CIN3 | N(≥CIN2) | %≥CIN2 | |
| Enrollment | 170 | 42.4% | 273 | 68.1% |
| Follow-Up | 12 | 3.0% | 25 | 6.2% |
| Exit | 10 | 2.5% | 14 | 3.5% |
|
| ||||
| total | 192 | 47.9% | 312 | 77.8% |
|
| ||||
| HSIL-CIN2 | ||||
|
| ||||
| N(≥CIN3) | %≥CIN3 | N(≥CIN2) | %≥CIN2 | |
| Enrollment | 128 | 37.2% | 219 | 63.7% |
| Follow-Up | 12 | 3.5% | 24 | 7.0% |
| Exit | 10 | 2.9% | 13 | 3.8% |
|
| ||||
| total | 150 | 43.6% | 256 | 74.4% |
|
| ||||
| HSIL-CIN3 | ||||
|
| ||||
| N(≥CIN3) | %≥CIN3 | N(≥CIN2) | %≥CIN2 | |
| Enrollment | 42 | 73.7% | 54 | 94.7% |
| Follow-Up | 0 | 0.0% | 1 | 1.8% |
| Exit | 0 | 0.0% | 1 | 1.8% |
|
| ||||
| total | 42 | 73.7% | 56 | 98.2% |
The analysis was restricted to 401 of 411 women (97.6%) who had colposcopy during the enrollment time period.
All 42 women with HSIL-CIN3 cytology and QC Pathology-diagnosed CIN3 or worse had their lesions detected at baseline whereas only 83.3% with HSIL-CIN2 and QC Pathology-diagnosed CIN3 or worse had their lesions detected at baseline (p = 0.003). Greater HPV risk groups were also associated with detection at baseline (ptrend = 0.02). More than one biopsy (vs. one biopsy) (p = 0.2) and a colposcopic impression of low-grade or worse (vs. less than low-grade) (p = 0.2) were not associated with the timing of diagnosis.
Women with HSIL-CIN3 cytology had more biopsies taken during enrollment colposcopy than women with HSIL-CIN2 cytology (ptrend < 0.001). This tendency remained even when restricted to women who had at least one biopsy taken (p < 0.001). Women with HSIL-CIN3 cytology were more likely to have a colposcopic impression of low-grade or worse (p = 0.001), which in turn was associated with having multiple (vs. one) biopsy taken (p < 0.001). HPV risk group status was associated with HSIL-CIN3 cytology versus HSIL-CIN2 (p < 0.001) but was not associated with number of biopsies taken in women with HSIL cytology (p = 0.6) or colposcopic impression (p = 0.5).
DISCUSSION
We evaluated cytologic interpretations of HSIL and the predictors of histologic confirmation in young women (mean age of 25.5 years) participating in ALTS. The goal of the analysis was not only to quantify the risk of precancer associated with HSIL cytology but also to better understand predictors of “false positive” or “unconfirmed” HSIL cytology, i.e., HSIL cytology in the absence of histologically-confirmed precancer. On the other hand, there is the possibility of “false negative” histology, in which very small CIN2 or even CIN3 lesions are not found despite cytologic and virologic evidence.
As expected, there was a greater likelihood of histological CIN3 with greater certainty (e.g., confirmation) and severity of cytologic HSIL interpretation and/or the presence of other risk factors for cervical cancer. We observed a gradation of increasing 2-year cumulative risk of histological CIN3 (increasing likelihood of histologic confirmation) from cytological ASC-H to HSIL-CIN2 to HSIL-CIN3. While the distinction (made for research purposes only by the ALTS pathologists) is not required by The Bethesda System (20) for classification of cytology and has not been demonstrated to be particularly reproducible (only 54.1% of the HSIL-CIN3 interpreted by clinical center was also called HSIL-CIN3 by QC Pathology, and 33.7% of the HSIL-CIN3 interpreted by QC Pathology was also called HSIL-CIN3 by the clinical center) the data nevertheless raises the question as to whether such differentiation might be clinically useful. The new ASCCP Guidelines provide greater leeway for women having unconfirmed HSIL. If additional supporting clinical validation were to emerge, new guidelines might encourage differentiating HSIL, when possible, into HSIL-CIN2 and HSIL-CIN3 to provide a greater margin of safety in determining which women with unconfirmed HSIL can be followed and which would be better managed by having an excisional procedure.
HPV16 detection was strongly associated with a CIN3 or worse diagnosis among women with HSIL cytology. We infer from our data that when HPV genotype-specific testing becomes available, further delineation of risk will be significantly advanced. Women with unconfirmed HSIL cytology who test negative for HPV16 would appear to have the safest margin for post-colposcopy management by close follow-up, whereas those testing HPV16 positive are at significantly higher risk and may be best managed by having an excisional procedure.
We also anticipate based on these data that in HPV-vaccinated populations, primarily because HPV16 infection will be prevented, HSIL cytology will be less common and what remains will be less predictive of CIN3. Given that the remaining precancerous lesions are caused by HR HPV genotypes that are less carcinogenic and therefore less likely to invade (21), watchful waiting for the management of HSIL cytology in younger, reproductive age women who have been vaccinated may be even more appropriate.
Some cases of apparently false-positive HSIL cytology were not in fact false-positives but rather CIN3 missed at initial colposcopy and found during intensive follow-up. Colposcopy is a visual tool that was not originally designed to detect very small CIN3 lesions that can underlie minor cytologic changes in well-screened populations (22). We found that colposcopists took more biopsies in those women with more definitive HSIL cytology, HSIL-CIN3, and this resulted in early detection of the precancer. The increase in the number of biopsies taken could have been prompted by the cytologic interpretation of HSIL-CIN3 that raised clinician concern or because HSIL-CIN3 cytology was an indicator of a larger cervical lesion visualized by colposcopy.
However, we note that among women with any enrollment HSIL cytology, colposcopic evaluation was more sensitive for the detection of precancerous lesions than the approximately 70% sensitivity reported for all women referred to colposcopy in the ALTS population (12). Almost 90% of all >CIN3 diagnosed by QC Pathology (88.5%) and all >CIN2 diagnosed by the clinical centers (87.5%) were detected at enrollment as the result of colposcopic evaluation, likely as a result of these lesions being more colposcopically apparent and heightened concern on the part of the colposcopist.
Women were referred into ALTS with a community ASCUS or LSIL cytology result and had a repeat cytology taken at enrollment. This study design increased the finding of HSIL cytology at enrollment in comparison to that found in a routine screening population (8.8% in ALTS versus 0.7% in a recent U.S. review of screening cytology results)(23). The ALTS sub-population of women with enrollment HSIL cytology was also young, with 75% of women under the age of 28 (which is near the median age of CIN3 diagnosis) and 95% under the age of 41 (approximate median age of early cancer). Thus, we were also analyzing earlier-detected, less severe HSIL cytology than is typically found in a routine screening population. This undoubtedly led to a bias, possibly modifying risks typically observed for HSIL cytology found in routine screening of the entire general population. However, HSIL cytology results are often preceded by cytology interpreted as ASC-US or LSIL. Therefore we do not believe our findings to be at odds with the findings for referral of HSIL cytology in routine clinical practice. We anticipate that the relative patterns of risk observed in this analysis are unlikely to be affected by the bias.
In conclusion, HSIL cytology is a highly specific indicator of histologic CIN3 or at least CIN2, especially in the context of a HPV16 infection. Forty-two percent of women with HSIL cytology had an immediate QC Pathology diagnosis of CIN3 or worse and 68% had an immediate clinical center diagnosis of CIN2 or worse; during follow-up, another 6% of women had a QC Pathology diagnosis of CIN3 or worse and almost 10% had a clinical center diagnosis of CIN2 or worse. Some false-positive HSIL does occur, and when it occurs, it is found to be associated with testing HPV negative or positive for LR HPV genotypes with greater uncertainty (unconfirmed as HSIL or ASC-H upon review), and/or less specific use (HSIL-CIN2 vs. HSIL-CIN3) of the HSIL cytologic interpretation. More studies are needed to confirm the possible clinical utility of distinguishing HSIL:CIN2 from HSIL:CIN3 and HPV16-negative from HPV16-positive HSIL cytology to guide management decisions. The distinction between HSIL:CIN2 and HSIL:CIN3 needs further validation. It will be important to show that watchful waiting of those with HSIL:CIN2 or HPV16-negative HSIL is sufficiently safe against invasive cancer, which might be accomplished by large, retrospective studies of women who do not return immediately for colposcopy following HSIL cytology. Finally, with the advent of prophylactic vaccination against HPV16, the clinical meaning of HSIL cytology will need to be reconsidered as it will be less predictive of imminent precancer risk.
Acknowledgments
Supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute. ALTS was supported by the National Cancer Institute, National Institutes of Health Department of Health and Human Services contracts CN-55153, CN-55154, CN-55155, CN-55156, CN-55157, CN-55158, CN-55159 and CN-55105. Some of the equipment and supplies used in these studies were donated or provided at reduced cost by Digene Corporation, Gaithersburg, MD; Cytyc Corporation, Marlborough, MA; National Testing Laboratories, Fenton, MO; DenVu, Tucson, AZ; and TriPath Imaging, Inc., Burlington, NC, and Roche Molecular Systems Inc., Alameda, CA.
The authors thank the ALTS Group Investigators for their help in planning and conducting the trial and Information Management Services, Inc., Rockville, MD for data management and programming support.
Footnotes
Financial Disclosure: Dr. Cox holds stock in Tigris Pharmaceuticals (city, state), serves on the Scientific Advisory Board of Gen-Probe (city, state) and Diamics (city, state), serves as a consultant to Graceway Pharmaceuticals, Takeda Pharmaceuticals (city, state), TriPath (city, state), Digene Corporation (Gaithersburg, MD), and JM Pharmaceuticals (city, state), serves on the HPV Vaccine Data and Safety Monitoring Board (DSMB) for Merck (Whitehouse Station, NJ), and serves on the speakers bureau for Digene and JM Pharmaceuticals. The other authors have no potential conflicts of interest to disclose.
Reference List
- 1.Kinney WK, Manos MM, Hurley LB, Ransley JE. Where’s the high-grade cervical neoplasia? The importance of minimally abnormal Papanicolaou diagnoses. Obstet Gynecol. 1998 Jun;91(6):973–6. doi: 10.1016/s0029-7844(98)00080-5. [DOI] [PubMed] [Google Scholar]
- 2.Wright TC, Jr, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ. 2001 Consensus Guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002 Apr 24;287(16):2120–9. doi: 10.1001/jama.287.16.2120. [DOI] [PubMed] [Google Scholar]
- 3.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007 Oct;197(4):346–55. doi: 10.1016/j.ajog.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 4.Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet. 2006 Feb 11;367(9509):489–98. doi: 10.1016/S0140-6736(06)68181-6. [DOI] [PubMed] [Google Scholar]
- 5.Guido R, Schiffman M, Solomon D, Burke L. Postcolposcopy management strategies for women referred with low-grade squamous intraepithelial lesions or human papillomavirus DNA-positive atypical squamous cells of undetermined significance: a two-year prospective study. Am J Obstet Gynecol. 2003 Jun;188(6):1401–5. doi: 10.1067/mob.2003.456. [DOI] [PubMed] [Google Scholar]
- 6.Schiffman M, Adrianza ME. ASCUS-LSIL Triage Study. Design, methods and characteristics of trial participants. Acta Cytol. 2000 Sep;44(5):726–42. doi: 10.1159/000328554. [DOI] [PubMed] [Google Scholar]
- 7.Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005 Apr;6(4):204. doi: 10.1016/s1470-2045(05)70086-3. [DOI] [PubMed] [Google Scholar]
- 8.Castle PE, Schiffman M, Burk RD, Wacholder S, Hildesheim A, Herrero R, et al. Restricted cross-reactivity of hybrid capture 2 with nononcogenic human papillomavirus types. Cancer Epidemiol Biomarkers Prev. 2002 Nov;11(11):1394–9. [PubMed] [Google Scholar]
- 9.Gravitt PE, Peyton CL, Alessi TQ, Wheeler CM, Coutlee F, Hildesheim A, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000 Jan;38(1):357–61. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiffman M, Wheeler CM, Dasgupta A, Solomon D, Castle PE. A comparison of a prototype PCR assay and hybrid capture 2 for detection of carcinogenic human papillomavirus DNA in women with equivocal or mildly abnormal papanicolaou smears. Am J Clin Pathol. 2005 Nov;124(5):722–32. doi: 10.1309/E067-X0L1-U3CY-37NW. [DOI] [PubMed] [Google Scholar]
- 11.Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995 Jun 7;87(11):796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 12.Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005 Apr;6(4):204. doi: 10.1016/s1470-2045(05)70086-3. [DOI] [PubMed] [Google Scholar]
- 13.Schiffman M, Herrero R, Desalle R, Hildesheim A, Wacholder S, Cecilia RA, et al. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology. 2005 Jun:20;337(1):76–84. doi: 10.1016/j.virol.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Atkins KA, Jeronimo J, Stoler MH. Description of patients with squamous cell carcinoma in the atypical squamous cells of undetermined significance/low-grade squamous intraepithelial lesion triage study. Cancer. 2006 Aug 25;108(4):212–21. doi: 10.1002/cncr.21940. [DOI] [PubMed] [Google Scholar]
- 15.Guido R, Schiffman M, Solomon D, Burke L. Postcolposcopy management strategies for women referred with low-grade squamous intraepithelial lesions or human papillomavirus DNA-positive atypical squamous cells of undetermined significance: a two-year prospective study. Am J Obstet Gynecol. 2003 Jun;188(6):1401–5. doi: 10.1067/mob.2003.456. [DOI] [PubMed] [Google Scholar]
- 16.Gage JC, Hanson VW, Abbey K, Dippery S, Gardner S, Kubota J, et al. Number of cervical biopsies and sensitivity of colposcopy. Obstet Gynecol. 2006 Aug;108(2):264–72. doi: 10.1097/01.AOG.0000220505.18525.85. [DOI] [PubMed] [Google Scholar]
- 17.Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985 Jan;4(1):87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 18.Long JS. Regression models for categorical and limited dependent variables. Thousand Oaks, CA: Sage Publications; 1997. Nominal outcomes:multinomial logit and related models; pp. 148–86. [Google Scholar]
- 19.Castle PE, Stoler MH, Solomon D, Schiffman M. The Relationship of Community Biopsy-Diagnosed Cervical Intraepithelial Neoplasia Grade 2 to the Quality Control Pathology-Reviewed Diagnoses:An ALTS Report. Am J Clin Pathol. 2007 May;127(5):805–15. doi: 10.1309/PT3PNC1QL2F4D2VL. [DOI] [PubMed] [Google Scholar]
- 20.Solomon D, Davey D, Kurman R, Moriarty A, O’Connor D, Prey M, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002 Apr 24;287(16):2114–9. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 21.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007 Aug 1;121(3):621–32. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 22.Jeronimo J, Schiffman M. Colposcopy at a crossroads. Am J Obstet Gynecol. 2006 Aug;195(2):349–53. doi: 10.1016/j.ajog.2006.01.091. [DOI] [PubMed] [Google Scholar]
- 23.Davey DD, Neal MH, Wilbur DC, Colgan TJ, Styer PE, Mody DR. Bethesda 2001 implementation and reporting rates: 2003 practices of participants in the College of American Pathologists Interlaboratory Comparison Program in Cervicovaginal Cytology. Arch Pathol Lab Med. 2004 Nov;128(11):1224–9. doi: 10.5858/2004-128-1224-BIARRP. [DOI] [PubMed] [Google Scholar]