Abstract
Objectives
The aim of this study was to evaluate the effects of 3 commercially available mouth rinses on the color stability of 4 different resin-based composite restorative materials.
Methods
Forty disc-shaped specimens (10x2 mm) were prepared from each of the following materials: A nanofill composite Filtek Supreme XT (3M/Espe, St. Paul, MN, USA); a packable low-shrinkage composite, AeliteLS Packable (BISCO, Inc, Shaumburg, IL, USA); nanoceramic composite resin Ceram-X (Dentsply, Konstanz, Germany); a microhybrid composite, and Aelite All-Purpose Body (BISCO). The specimens were then incubated in distilled water at 37°C for 24 hours. The baseline color values (L*, a*, b*) of each specimen were measured with a colorimeter according to the CIELAB color scale. After baseline color measurements, 10 randomly selected specimens from each group were immersed in 1 of the 3 mouth rinses and distilled water as control. The specimens were stored in 20 mL of each mouth rinse (Oral B Alcohol-free, Listerine Tooth Defense Anti-cavity Fluoride Rinse and Klorhex) for 12 hours. After immersion, the color values of all specimens were remeasured, and the color change value ΔE*ab was calculated. Data were analyzed using a 2-way analysis of variance at a significance level of .05.
Results
All specimens displayed color changes after immersion, and there was a statistically significant difference among restorative materials and mouth rinses (P<.05); however, the change was not visually perceptible (ΔE*ab<3.3). The interaction between the effect of mouth rinses and type of restorative materials was not statistically significant (P>.05).
Conclusions
It may be concluded that although visually nonperceptible, all resin restorative materials tested showed a color difference after immersion in different mouth rinses.
Keywords: Mouth rinse, Resin composites, Color
INTRODUCTION
Tooth-colored restorative materials have been widely used to meet patients’ esthetic demands in dental practice. Various types of composite resins with different physical characteristics are available on the dental market, and they are classified by particle size, shape, and distribution of fillers.1 Since nanotechnology was introduced to dentistry, nanocomposites with filler sizes ranging from 0.01 to 0.04 mm have been developed.2 Nanocomposites have many advantages, such as reduced polymerization shrinkage, increased mechanical properties, improved optical characteristics, and better gloss retention.2–5 Wear resistance of nanocomposites has been shown to be comparable or superior to that of microfill and microhybrid composite resins.6,7
The organically modified, ceramic-based, nanoceramic composite also was developed using the same technology. It contains a methacrylate-modified silicon-dioxide–containing nanofiller and resin matrix that is replaced by a matrix full of highly dispersed methacrylate-modified polysiloxane particles.8 Recently, low-shrinkage composites with reduced polymerization shrinkage were introduced for clinical use. They have a high elastic modulus because of their high filler content.9
Discoloration of tooth-colored, resin-based materials may be caused by several intrinsic and extrinsic factors. Intrinsic factors involve the discoloration of the resin material itself, such as alteration of the resin matrix and changes in the interface of matrix and fillers.10 The resin matrix has been reported as being critical to color stability, and staining may be related to a high resin content and water absorption.11 Color matching plays an important role in achieving good results. However, discoloration of composite resin restorations may occur from time to time, and this unacceptable color change may lead to replacement of these restorations.12–14
Extrinsic factors for discoloration of resin composites include staining by adsorption or absorption of colorants from exogenous sources such as coffee, tea, nicotine, beverages, and mouth rinses.11,15,16
The use of antimicrobial mouth rinses is an approach to limiting the accumulation of dental plaque, with a primary objective of controlling the development and progression of periodontal diseases and dental caries.17,18 However, frequent use of mouth rinses may have detrimental effects on oral and dental tissues.19,20 Despite the increased use of mouth rinses, research comparing resin composite color changes associated with use of mouth rinses is limited.21,22 The effect of alcohol-containing, chlorhexidine-gluconate–containing, and hybrid mouth rinses on the color stability of glass ionomer, compomer, and microhybrid composite resin materials have been evaluated in previous studies.21,22 To the best of our knowledge, however, there has been no study comparing the effect of commercially available mouth rinses on newly developed resin composite materials.
Discoloration can be evaluated with different instruments and techniques. In assessing chromatic differences, the Commission Internationale de l’Eclairage (CIE L*, a*, b*) system was chosen for the present study. According to this system, L* represents the lightness of the sample, a* describes green-red axis(−a=green; +a=red) and b* describes blue-yellow axis(−b=blue; +b=yellow).23 It is also possible to calculate the total color change (ΔE*ab), which considers the changes of L*, a* and b*.24 Various studies have different thresholds of color difference values which is perceptible to the human eye. However, the clinically acceptable value for color changes in dental materials is assumed to be ΔE*ab ≤ 3.3.25–28
The aim of this study was to evaluate the effect of alcohol-containing, alcohol-free, and chlorhexidine-gluconate mouth rinses on color stability of a nanofill, a packable low-shrinkage, a nanoceramic, and a microhybrid resin-composite material. The null hypothesis tested in the present study was that daily use of mouth rinses affects the staining ability of resin composites and the color differences will be perceptible.
MATERIALS AND METHODS
The restorative materials used in the present study included a nanofill composite, Filtek Supreme XT; a packable low-shrinkage composite, AeliteLS Packable; nanoceramic composite resin Ceram-X; and a microhybrid composite, Aelite All-Purpose Body of A2 shade (Table 1). Forty disk-shaped specimens from each restorative material, 10 mm in diameter and 2 mm thick, were prepared in a polytetrafluoroethylene ring covered with a celluloid matrix and glass slides. Composite resins were polymerized with an LED unit (Elipar Freelight 2, 3M ESPE, ST Paul, MN, USA) in standard mode (20 seconds) for two cycles with a light intensity of 400 mW/cm2 from the upper and lower surfaces of the specimens. The output of the curing units was checked with a radiometer (Kerr, Demetron, Orange, CA, USA). The distance between the light and the specimen was standardized by using a 1-mm glass slide. After polymerization, the upper surfaces of the specimens were ground with 1200-grit silicone carbide paper under running water.
Table 1.
Compositions of the restorative materials.
| Restorative materials | Manufacturer | Lot Number | Filler weight (%) | Filler volume (%) | Filler Composition |
|---|---|---|---|---|---|
| Aelite All-Purpose Body | BISCO Dental Products, IL, USA | 0600005269 | 73 | 53 | Ethoxylated bisphenol A dimethacrylate
Triethyleneglycol dimethacrylate Glass Filler Amorphous Silica |
| Aelite LS Packable | BISCO Dental Products, IL, USA | 0600005264 | 86 | 74 | Ethoxylated Bisphenol A dimethacrylate
Bisphenol A diglycidylmethacrylate glass frit Amorphous Silica |
| Filtek Supreme XT | 3M, ESPE, St. Paul, MN, USA | 20070410 | 78.5 | 59.5 | Nonagglomerated nanosilica filler (20 nm), Agglomerated Zirconia/silica nanocluster (0.6–1.4 μm) |
| Ceram-X | Dentsply, Konstanz, Germany | 0605001581 | 76 | 57 | Ba-Al-Borosilicate glass filler (1–1.5 μm), Silicone dioxide nanofiller (10 nm) |
The specimens were incubated in distilled water at 37°C for 24 hours. Then, the baseline color values (L*, a*, b*) of each specimen were measured with a colorimeter (Minolta Chroma Meter CR-321, Minolta Co, Osaka, Japan) against a white background. Quality of color was examined using the Commission Internationale de l’Eclairage (CIE Lab) system as tristimulus values and reported as color differences (ΔL*, Δa*, and Δb*) compared with standard conditions.23
Before each group of specimens was measured, the colorimeter was calibrated with a standard white card. Measurements were repeated 3 times in each sample and mean values were calculated.
Treatment groups were commercially available mouth rinses (Oral B Alcohol-free, Listerine Tooth Defense Anti-cavity Fluoride Rinse, Klorhex) and distilled water as a control (Table 2). Forty specimens of each restorative material group were randomly divided into 4 subgroups (n=10), and each subgroup was stored in 20 mL of one of the mouth rinses for 12 hours, which was reported as the equivalent of 2 mouth rinses per day for 1 year.29 Specimens were kept at 37°C throughout the study, and test solutions were shaken every 3 hours to provide homogeneity. At the end of the test period, the specimens were removed and submerged in distilled water. After the immersion, the color values of each specimen were remeasured, and the color change value ΔE*ab was calculated according to the following formula:24
Table 2.
Chemical compositions of the mouth rinses.
| Mouthrinse | Manufacturer | Chemical composition | pH |
|---|---|---|---|
| Listerine Tooth Defense Anticavity Fluoride Rinse | Pfizer Consumer Healthcare, Morris Plains, NJ 07950, USA | sodium fluoride (0.0221%), water, sorbitol solution, alcohol (21.6%), flavors, poloxamer 407, sodium lauryl sulfate, phosphoric acid, sucralose, dibasic sodium phosphate, D&C red No. 33, FD&C blue No. 1 | 3.7 |
| Oral-B Alcohol-free | Gillette Group Ltd, London, UK | glycerin, polysorbate 20, aroma, methyl paraben, etylpyridinium chloride, sodium fluoride, sodium saccharin, sodium benzoate, propylparaben CI42051 | 5.8 |
| Klorhex | Drogsan, Ankara, Turkey | 0.2% chlorhexidine gluconate | 5.8 |
| Distilled Water | 6.7 |
where L* stands for lightness, a* for green-red (−a=green; +a=red) and b* for blue-yellow (−b=blue; +b=yellow).
Statistical analyses were performed using a 2-way analysis of variance and Tukey’s HSD (Honestly Significant Differences) test at a significance level of 0.05.
RESULTS
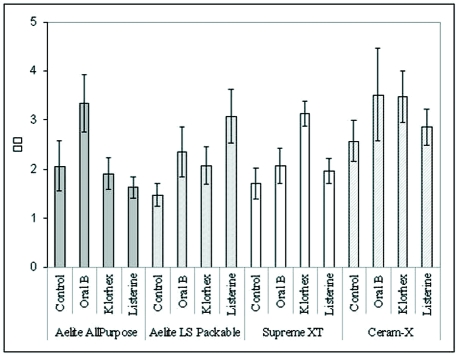
Table 3 and Figure 1 present the mean and standard deviations of color change values ΔE*ab in restorative materials after immersion in 3 different mouth rinses and distilled water as control.
Table 3.
Mean values, standard deviations and standart errors of color change values (ΔE*ab).
| Restorative materials | Mouth rinses | ΔE*ab | SD | SE |
|---|---|---|---|---|
| Aelite All-Purpose Body | Distilled water | 2.06 | 1.61 | 0.510 |
| Oral B | 3.34 | 1.85 | 0.587 | |
| Klorhex | 1.90 | 1.01 | 0.319 | |
| Listerine | 1.62 | 0.70 | 0.221 | |
| Aelite LS Packable | Distilled water | 1.48 | 0.75 | 0.238 |
| Oral B | 2.35 | 1.62 | 0.514 | |
| Klorhex | 2.07 | 1.20 | 0.380 | |
| Listerine | 3.08 | 1.71 | 0.540 | |
| Filtek Supreme XT | Distilled water | 1.71 | 1.00 | 0.316 |
| Oral B | 2.07 | 1.12 | 0.354 | |
| Klorhex | 3.13 | 0.78 | 0.247 | |
| Listerine | 1.97 | 0.80 | 0.252 | |
| Ceram-X | Distilled water | 2.57 | 1.33 | 0.420 |
| Oral B | 3.52 | 2.97 | 0.940 | |
| Klorhex | 3.48 | 1.64 | 0.519 | |
| Listerine | 2.86 | 1.16 | 0.368 | |
Figure 1.
Color parameters of resin composites after immersion period in control and test solutions.
All samples displayed color changes after immersion, and there was a statistically significant difference among the restorative materials and mouth rinses (P<.05); however, the interaction between the effect of the mouth rinse and the type of restorative material was not statistically significant (P>.05) (Table 4). The nanoceramic restorative material, Ceram-X specimens had the highest ΔE*ab values among the restorative materials tested, and there was a significant difference between the ΔE*ab values Ceram-X and Filtek Supreme XT, Aelite LS Packable, and Aelite All-Purpose Body (P=.014). The 2-way analysis of variance showed that there also was a significant difference between the ΔE*ab values among the mouth rinses (P=.046). A post hoc Tukey honestly significant difference test revealed that the difference between the ΔE*ab values of control group and the Oral B group was statistically significant (P=.04). There was no statistically significant difference among the mouth rinses (Listerine, Oral-B Alcohol-free, Klorhex) and between the groups Control/Listerine and Control/Klorhex (P>.05).
Table 4.
ANOVA results for color change (ΔE*ab).
| Sum of squares | Mean square | F-value | P value | |
|---|---|---|---|---|
| Material | 22.93 | 7.64 | 3.68 | .014 |
| Solution | 17.04 | 5.68 | 2.73 | .046 |
| Material-Solution | 31.96 | 3.55 | 1.71 | .091 |
Statistically significant, P < .05
The ΔE*ab values ≤ 3.3 were considered visually perceptible and clinically unacceptable in the present study.25–28 In Ceram-X group, the specimens immersed in Oral-B and Klorhex mouth rinses showed higher ΔE*ab values than the other solutions. Although, these results were accepted visually perceptible, the ΔE*ab values were very close to 3.3. In addition, the mean ΔE*ab values were also less than 3.3 in other groups, and the difference was not visually perceptible.
DISCUSSION
The present study evaluated the effects of three commercially available mouth rinses on the color stability of four different resin-based composite restorative materials. According to the results of the current study, the null hypothesis tested was partially accepted since, daily use of mouth rinses increased the staining ability of the resin composites however the color change was not perceivable.
Villalta et al30 have shown that low pH and alcohol concentration of solutions might affect the surface integrity of composite resins and cause staining. In the present study, there was a statistically significant difference regarding color change values between the alcohol-free mouth rinse, Oral-B, and distilled water, but this difference was not visually perceptible. The alcohol concentration (21.6%) and pH value (3.5) of Listerine is very high, but the color stability of resin materials was not affected by this factor, and there was no significant difference among the mouth rinses tested. Asmussen31 reported that mouth rinses with high alcohol content might soften the composite resin material. Ethanol especially has a softening effect on BIS-GMA based polymers. Therefore, Gürgan et al29 showed that irrespective of alcohol concentration, both alcohol-containing and alcohol-free mouth rinses could affect the hardness of resin-restorative materials.
The effect of staining solutions on color changes of composite resins may be material dependent, and the staining susceptibility of a restorative material may be attributed to its resin matrix or filler type. Scotti et al32 showed that the type of material had a significant role on stain resistance. According to the results of the current study, there were statistically significant differences between Ceram-X and the other resin composites. A nanoceramic resin composite, Ceram-X comprises organically modified ceramic (ormocer) nanoparticles and glass fillers (1.1–1.5 mm). Unlike conventional polymers, ormocers have an inorganic backbone based on silicon dioxide and are functionalized with polymerizable organic units to produce 3-dimensional compound polymers.33 The filler concentration of Ceram-X is 76% by weight and 57% by volume. According to manufacturer’s data, these nanoceramic particles are inorganic-organic hybrid particles. Both, nanoceramic particles and nanofillers have methacrylate groups available for polymerization. Additionally, Ceram-X does not contain triethylene glycol dimethacrylate.8 The present study revealed that Ceram-X showed the greatest color change, and the change may be related to these structural differences.
In a previous study, Jung et al34 demonstrated that Ceram-X did not yield better surface quality than did the other nanofill composites, Filtek Supreme and Tetric Evoceram. This difference was explained with the low volumetric filler content of the material and the porosities that were detected on the Ceram-X specimens. Rough surfaces have been shown to mechanically retain stains more than smooth surfaces.35,36 In many studies,25–28 discoloration will be referred to as acceptable up to the value ΔE*ab=3.3, which is considered to be the upper limit of acceptability in visual evaluations. The staining potentials of various mouth rinses have been already established for dental hard and soft tissues.37-41 Also, the staining potentials of the mouth rinses were evaluated for many kinds of restorative materials. Gürdal et al21 have shown that the effects of the mouth rinses on the color stability are no different from those of distilled water. Similarly, Lee et al22 have found that although visually nonperceptible, mouth rinses affect color stability. In the current study, none of the restorative materials showed insufficient color stability and also presented visually perceptible discoloration after the immersion period.
In their study, because the effects of the mouth rinses were not different from those of distilled water, Geurtsen et al42 stated that the water component of the mouth rinses might affect the color shift and microhardness changes. In the current study, there were no statistically significant differences between the mouth rinses and distilled water except for Oral-B.
In clinical situations, how the effects of mouth rinses differ on esthetic restorative materials depends on many factors that cannot be simulated in vitro. Saliva, salivary pellicle, foods, and beverages may affect the color stability of resin restorative materials. Further in vivo studies are necessary to determine the staining potential of different types of mouth rinses.
CONCLUSIONS
According to the results of the present study, effects of the mouth rinses on the color change of the materials were not different from that of control solution. All resin restorative materials showed color difference after immersion in tested solutions but these differences were not visually perceptible. However, future studies should consider longer periods of immersion. Within the limitations of the current study, it may be concluded that aging of tooth-colored restoratives in different solutions may exert detrimental effects on these materials.
ACKNOWLEDGMENTS
The authors would like to thank the BISCO, 3M ESPE, and Denstply companies for supporting this study by supplying restorative materials.
REFERENCES
- 1.Lutz F, Phillips RW. A classification and evaluation of composite resin systems. J Prosthet Dent. 1983;50:480–488. doi: 10.1016/0022-3913(83)90566-8. [DOI] [PubMed] [Google Scholar]
- 2.Moszner N, Klapdohr S. Nanotechnology for dental composites. Int J Nanotechnology. 2004;1:130–156. [Google Scholar]
- 3.Moszner N, Salz U. New developments of polymeric dental composites. Prog Polym Sci. 2001;26:535–576. [Google Scholar]
- 4.Mitra SB, Wu D, Holmes BN. An application of nanotechnology in advanced dental materials. JADA. 2003;134:1382–1390. doi: 10.14219/jada.archive.2003.0054. [DOI] [PubMed] [Google Scholar]
- 5.Terry DA. Direct applications of a nanocomposite resin system: Part 1-The evolution of contemporary composite materials. Pract Proced Aesthet Dent. 2004;16:417–422. [PubMed] [Google Scholar]
- 6.Yap AU, Tan CH, Chung SM. Wear behavior of new composite restoratives. Oper Dent. 2004;29:269–274. [PubMed] [Google Scholar]
- 7.Turssi CP, Ferracane JL, Serra MC. Abrasive wear of resin composites as related to finishing and polishing procedures. Dent Mater. 2005;21:641–648. doi: 10.1016/j.dental.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Schirrmeister JF, Huber K, Hellwig E, Hahn P. Two-year evaluation of a new nanoceramic restorative material. Clin Oral Investig. 2006;10:181–186. doi: 10.1007/s00784-006-0048-1. [DOI] [PubMed] [Google Scholar]
- 9.Calheiros FC, Sadek FT, Braga RR, Cardoso PE. Polymerization contraction stress of low-shrinkage composites and its correlation with microleakage in class V restorations. J Dent. 2004;32:407–412. doi: 10.1016/j.jdent.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Um CM, Ruyter IE. Staining of resin-based veneering materials with coffee and tea. Quint International. 1991;22:377–386. [PubMed] [Google Scholar]
- 11.Dietschi D, Campanile G, Holz J, Meyer JM. Comparison of the color stability of ten new-generation composites: An in vitro study. Dent Mater. 1994;10:353–362. doi: 10.1016/0109-5641(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 12.Kroeze HJ, Plasschaert AJ, van’t Hof MA, Truin GJ. Prevalence and need for replacement of amalgam and composite restorations in Dutch adults. J Dent Res. 1990;69:1270–1274. doi: 10.1177/00220345900690060901. [DOI] [PubMed] [Google Scholar]
- 13.Wilson NH, Burke FJ, Mjör IA. Reasons for placement and replacement of restorations of direct restorative materials by a selected group of practitioners in the United Kingdom. Quint International. 1997;28:245–248. [PubMed] [Google Scholar]
- 14.Mjör IA, Moorhead JE, Dahl JE. Reasons for replacement of restorations in permanent teeth in general practice. Int Dent J. 2000;50:361–366. doi: 10.1111/j.1875-595x.2000.tb00569.x. [DOI] [PubMed] [Google Scholar]
- 15.Asmussen E, Hansen EK. Surface discoloration of restorative resins in relation to surface softening and oral hygiene. Scand J Dent Res. 1986;94:174–177. doi: 10.1111/j.1600-0722.1986.tb01382.x. [DOI] [PubMed] [Google Scholar]
- 16.Noie F, O’Keefe KL, Powers JM. Color stability of resin cements after accelerated aging. Int J Prosthodont. 1995;8:51–55. [PubMed] [Google Scholar]
- 17.Lamster IB. Antimicrobial mouthrinses and the management of periodontal diseases. JADA. 2006;137(Suppl):5–9. doi: 10.14219/jada.archive.2006.0407. [DOI] [PubMed] [Google Scholar]
- 18.Scheie AA. The role of antimicrobials. In: Kidd E, Fejerskov O, editors. Dental Caries: The Disease and Its Clinical Management. Blackwell Munskaard; Iowa: 2003. pp. 179–188. [Google Scholar]
- 19.Gagari E, Kabani S. Adverse effects of mouthwash use. A review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;80:432–439. doi: 10.1016/s1079-2104(05)80337-3. [DOI] [PubMed] [Google Scholar]
- 20.Winn DM, Blot WJ, McLaughlin JK, Austin DF, Greenberg RS, Preston-Martin S, Schoenberg JB, Fraumeni JF., Jr Mouthwash use and oral conditions in the risk of oral and pharyngeal cancer. Cancer Res. 1991;1:3044–3047. [PubMed] [Google Scholar]
- 21.Gürdal P, Akdeniz BG, Sen BH. The effects of mouthrinses on microhardness and color stability of aesthetic restorative materials. J Oral Rehabil. 2002;29:895–901. doi: 10.1046/j.1365-2842.2002.00924.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee YK, El Zawahry M, Noaman KM, Powers JM. Effect of mouthwash and accelerated aging on the color stability of esthetic restorative materials. Am J Dent. 2000;13:159–161. [PubMed] [Google Scholar]
- 23.Bayne SC, Thompson JY, Taylor DF. Dental materials. In: Robenson TM, Heymann HO, Swift EJ, editors. ‘Sturdevant’s, the art and science of operative dentistry. Mosby Inc; Missouri: 2002. pp. 133–234. [Google Scholar]
- 24.Wyszecki G, Siles WS. Color science: concepts and methods, quantitative data and formula. John Wiley & Sons; New York: 1982. pp. 166–169. [Google Scholar]
- 25.Ruyter IE, Nilner K, Moller B. Color stability of dental composite resin materials for crown and bridge veneers. Dent Mater. 1987;3:246–251. doi: 10.1016/S0109-5641(87)80081-7. [DOI] [PubMed] [Google Scholar]
- 26.Vichi A, Ferrari M, Davidson CL. Color and opacity variations in three different resin-based composite products after water aging. Dent Mater. 2004;20:530–534. doi: 10.1016/j.dental.2002.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Um CM, Ruyter IE. Staining of resin-based veneering materials with coffee and tea. Quint International. 1991;22:377–386. [PubMed] [Google Scholar]
- 28.Ferracane JL, Moser JB, Greener EH. Ultraviolet light-induced yellowing of dental restorative resins. J Prosthet Dent. 1985;54:483–487. doi: 10.1016/0022-3913(85)90418-4. [DOI] [PubMed] [Google Scholar]
- 29.Gürgan S, Önen A, Köprülü H. In vitro effects of alcohol-containing and alcohol-free mouthrinses on microhardness of some restorative materials. J Oral Rehabil. 1997;24:244–246. [PubMed] [Google Scholar]
- 30.Villalta P, Lu H, Okte Z, Garcia-Godoy F, Powers JM. Effects of staining and bleaching on color change of dental composite resins. J Prosthet Dent. 2006;95:137–142. doi: 10.1016/j.prosdent.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Asmussen E. Softening of BISGMA-based polymers by ethanol and by organic acids of plaque. Scand J Dent Res. 1984;92:257–261. doi: 10.1111/j.1600-0722.1984.tb00889.x. [DOI] [PubMed] [Google Scholar]
- 32.Scotti R, Mascellani SC, Forniti F. The in vitro color stability of acrylic resins for provisional restorations. Int J Prosthodont. 1997;10:164–168. [PubMed] [Google Scholar]
- 33.Manhart J, Hollwich B, Mehl A, Kunzelmann KH, Hickel R. Randqualität von Ormocer-und Kompositfüllungen in Klass-II-Kavitäten nach künstlicher Alterung. Deutsche Zahnärztliche Zeitschrift. 1999;54:89–95. [Google Scholar]
- 34.Jung M, Sehr K, Klimek J. Surface texture of four nanofilled and one hybrid composite after finishing. Oper Dent. 2007;32:45–52. doi: 10.2341/06-9. [DOI] [PubMed] [Google Scholar]
- 35.Hachiya Y, Iwaku M, Hosoda H, Fusayama T. Relation of finish to discoloration of composite resins. J Prosthet Dent. 1984;52:811–814. doi: 10.1016/s0022-3913(84)80010-4. [DOI] [PubMed] [Google Scholar]
- 36.Shintani H, Satou J, Satou N, Hayashihara H, Inoue T. Effects of various finishing methods on staining and accumulation of Streptococcus mutans HS-6 on composite resins. Dent Mater. 1985;1:225–227. doi: 10.1016/S0109-5641(85)80046-4. [DOI] [PubMed] [Google Scholar]
- 37.Addy M, Mahdavi SA, Loyn T. Dietary staining in vitro by mouthrinses as a comparative measure of antiseptic activity and predictor of staining in vivo. J Dent. 1995;23:95–99. doi: 10.1016/0300-5712(95)98974-8. [DOI] [PubMed] [Google Scholar]
- 38.Sheen S, Addy M. An in vitro evaluation of the availability of cetylpyridinium chloride and chlorhexidine in some commercially available mouthrinse products. Br Dent J. 2003;194:207–210. doi: 10.1038/sj.bdj.4809913. [DOI] [PubMed] [Google Scholar]
- 39.Addy M, Wade WG, Jenkins S, Goodfield S. Comparison of two commercially available chlorhexidine mouthrinses: I. Staining and antimicrobial effects in vitro. Clin Prev Dent. 1989;11:10–14. [PubMed] [Google Scholar]
- 40.Carpenter GH, Pramanik R, Proctor GB. An in vitro model of chlorhexidine-induced tooth staining. J Periodontal Res. 2005;40:225–230. doi: 10.1111/j.1600-0765.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- 41.Addy M, Wade W, Goodfield S. Staining and antimicrobial properties in vitro of some chlorhexidine formulations. Clin Prev Dent. 1991;13:13–17. [PubMed] [Google Scholar]
- 42.Geurtsen W, Leyhausen G, Garcia-Godoy F. Effect of storage media on the fluoride release and surface microhardness of four polyacid-modified composite resins (compomers) Dent Mater. 1999;15:196–201. doi: 10.1016/s0109-5641(99)00034-2. [DOI] [PubMed] [Google Scholar]