Abstract
Synapse deterioration underlying severe memory loss in early Alzheimer's disease (AD) is thought to be caused by soluble amyloid beta (Aβ) oligomers. Mechanistically, soluble Aβ oligomers, also referred to as Aβ-derived diffusible ligands (ADDLs), act as highly specific pathogenic ligands, binding to sites localized at particular synapses. This binding triggers oxidative stress, loss of synaptic spines, and ectopic redistribution of receptors critical to plasticity and memory. We report here the existence of a protective mechanism that naturally shields synapses against ADDL-induced deterioration. Synapse pathology was investigated in mature cultures of hippocampal neurons. Before spine loss, ADDLs caused major downregulation of plasma membrane insulin receptors (IRs), via a mechanism sensitive to calcium calmodulin-dependent kinase II (CaMKII) and casein kinase II (CK2) inhibition. Most significantly, this loss of surface IRs, and ADDL-induced oxidative stress and synaptic spine deterioration, could be completely prevented by insulin. At submaximal insulin doses, protection was potentiated by rosiglitazone, an insulin-sensitizing drug used to treat type 2 diabetes. The mechanism of insulin protection entailed a marked reduction in pathogenic ADDL binding. Surprisingly, insulin failed to block ADDL binding when IR tyrosine kinase activity was inhibited; in fact, a significant increase in binding was caused by IR inhibition. The protective role of insulin thus derives from IR signaling-dependent downregulation of ADDL binding sites rather than ligand competition. The finding that synapse vulnerability to ADDLs can be mitigated by insulin suggests that bolstering brain insulin signaling, which can decline with aging and diabetes, could have significant potential to slow or deter AD pathogenesis.
Keywords: therapeutics, diabetes, aging, receptors, plasticity
Alzheimer's disease (AD) is a neurological disorder characterized by profound memory loss and progressively catastrophic dementia. Currently there is no effective treatment for AD, but the pursuit of novel disease-modifying therapeutics is the object of intense investigation. Significant attention focuses on strategies that could neutralize the mechanism(s) initiating memory loss, the major manifestation of early AD (1, 2). Why memory is specifically targeted in AD has long been a fundamental mystery, but it is increasingly evident that the crucial pathogenic event is the functional and morphological deterioration of specific memory center synapses induced by potent neurotoxins that accumulate in AD brain (3–9).
Recent evidence shows that the neurotoxins in AD comprise aggregates of the amyloid-β peptide (Aβ) (10), a molecule generated by proteolytic cleavage of the amyloid precursor protein. While monomeric Aβ is not neurotoxic, the peptide exhibits a marked toxic gain-of-function upon self-association. Fibrillar forms of Aβ found in amyloid plaques were until recently considered responsible for neuronal damage in AD, but the discovery that small Aβ oligomers, also known as Aβ-derived diffusible ligands (ADDLs), are potent central nervous system (CNS) neurotoxins (11), has led to a new view of AD pathogenesis (3). Unlike the insoluble fibrils, ADDLs are diffusible molecules that attach with specificity to particular synapses, acting as pathogenic ligands (1, 12). The attack on synapses inhibits long-term potentiation (LTP) (11, 13, 14), a classic paradigm for memory-related synaptic mechanisms. ADDL binding further induces AD-like pathology including neuronal tau hyperphosphorylation (15), oxidative stress (16), and synapse deterioration and loss (17–21). The pathological relevance of ADDLs has been substantiated by their disease-specific accumulation in human brain and CSF (1, 22) and by the accumulation of structurally equivalent oligomers in transgenic mouse AD models (23). By explaining why AD targets memory and accounting for major features of neuropathology, the ligand-based attack on specific synapses by ADDLs provides a potentially unifying mechanism for AD pathogenesis.
Predictably, therapeutic drugs and antibodies targeting ADDLs have shown promise in preclinical studies and early clinical trials (24, 25). Nonetheless, because no effective approach to AD therapeutics is yet available, the need to identify novel drug targets remains. We have hypothesized that cellular mechanisms exist that physiologically protect synapses against ADDL toxicity. Such active synaptic protection mechanisms could contribute to preserved cognitive function in normal individuals, while impaired mechanisms might serve as drug targets for individuals at early stages of AD or presenting mild cognitive impairment. Recent reports are consistent with the possibility that one such protective mechanism could be provided by CNS insulin signaling. Insulin plays a key role in plasticity mechanisms in the CNS (26, 27) and it recently has been shown that insulin and the insulin-sensitizing drug rosiglitazone improve cognitive performance in mouse models of AD and in patients with early AD (28–30). Conversely, insulin-resistant type 2 diabetes patients show significantly increased risk for developing AD (31). Moreover, experimental induction of diabetes in mouse models of AD results in premature cognitive failure and degeneration of synapse structure (32, 33).
To test the hypothesis that insulin signaling provides a physiological defense mechanism against ADDLs' synaptotoxicity, we have used highly differentiated hippocampal nerve cell cultures, a preferred model for studies of synapse cell biology (34, 35) and mechanisms of ADDL pathogenicity (12, 18). Results show that insulin blocks ADDL binding to synapses, thereby preventing the ensuing neurotoxicity. Decreased binding is the result of downregulation of ADDL binding sites through a mechanism requiring insulin receptor (IR) tyrosine kinase activity. This downregulation is the converse of ADDL-induced IR downregulation, which we have recently described as a mechanism underlying CNS insulin resistance in AD (9). Thus, physiological insulin and pathological ADDLs negatively regulate the abundance of each other's binding sites, creating a competitive balance between synapse survival and degeneration. Because insulin signaling in the brain is known to decline with age (36), the outcome of this balance represents a unique risk factor for AD well suited for therapeutic intervention. By restoring the balance to favor synapse survival, new drugs designed to specifically enhance CNS insulin signaling would provide a new and potentially significant class of AD therapeutics.
Results
ADDLs Induce Loss of IRs from Neuronal Surfaces.
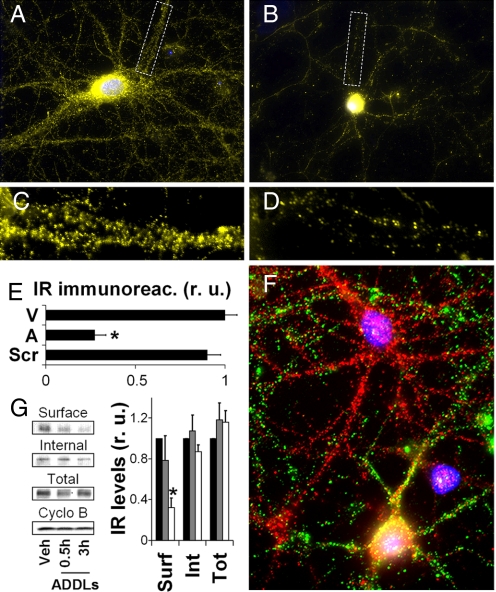
We recently reported that ADDLs cause a major loss of IRs from neuronal dendrites (9), in harmony with the impact of ADDLs on other essential plasticity-related neuronal molecules such as N-methyl-D-aspartate (NMDA) receptors (18, 20). We have now extended those observations using both immunocytochemistry and surface biotinylation. Immunocytochemical analysis showed that ADDLs induced elimination of dendritic IRs (Fig. 1 A–E) and a parallel increase in immunoreactivity in the cell body, indicating IR redistribution (Fig. 1 A and B). Remarkably, neurons attacked by ADDLs (green puncta) showed virtual absence of dendritic IRs and intense somatic labeling whereas neurons devoid of ADDLs exhibited abundant dendritic IR labeling (red puncta) (Fig. 1F). Control experiments showed that a peptide corresponding to a scrambled Aβ sequence and subjected to exactly the same procedure as used for preparation of ADDLs had no effect on insulin receptor levels (Fig. 1E) and was not detected by oligomer-specific antibodies (data not shown). Surface biotinylation results showed that surface-exposed IR levels were decreased by 22% and 68%, respectively, after 30 min or 3 h of exposure to 100 nM ADDLs (Fig. 1G). We did not detect any changes in intracellular IR levels in ADDL-treated neurons, in line with our previous results showing no changes in total IR levels measured by Western blots in total homogenates from ADDL-treated neurons (9). Importantly, surface levels of the membrane protein Na+/K+-ATPase, used as a control, were not affected by ADDLs [supporting information (SI) Fig. S1]. Surface biotinylation results also indicated that ADDLs had no measurable impact on overall surface protein levels (Fig. S1). These results are consistent with the notion that ADDLs have a selective impact in accelerating the endocytosis of particular surface proteins, such as IRs.
Fig. 1.
ADDLs induce the removal of IRs from dendritic plasma membranes. Cultured hippocampal neurons were exposed to 100 nM ADDLs at 37 °C for 3 h followed by immunolabeling with anti-IRα (yellow). Nuclear staining (DAPI) is shown in blue. A and B show representative images from vehicle- and ADDL-treated cultures, respectively. C and D show high-magnification images of dendrites contained in the dotted rectangles indicated in A and B, respectively. (E) Quantification of IR immunofluorescence levels (see SI Methods) for cultures treated with vehicle (V), ADDLs (A), or scrambled Aβ peptide (Scr). (F) A representative image showing double labeling for ADDL binding (NU4 oligomer antibody; green) and IRα (red). (G) Surface abundance of IRs in hippocampal neurons exposed to vehicle or 100 nM ADDLs for 0.5 or 3 h, assessed by surface biotinylation (see SI Methods). Asterisk indicates statistically significant (*, P < 0.0002) decrease compared to vehicle-treated cultures.
Casein Kinase 2 (CK2) and Ca2+/Calmodulin-Dependent Kinase II (CaMKII) Mediate ADDL-Induced Loss of IRs and NMDA subtype glutamate receptors (NMDARs).
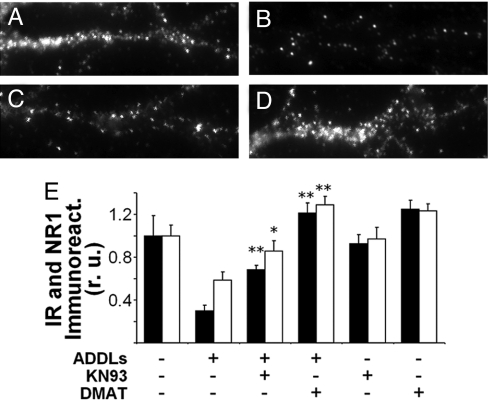
IRs play key roles in important neurological processes including learning and memory (26, 27) and tau phosphorylation (37, 38). Thus, ADDL-induced loss of IRs might represent an important early mechanism underlying memory impairment and other pathological features of AD. As noted above, Aβ oligomers also cause internalization of NMDARs. Physiologically, activity-dependent internalization of NMDARs is mediated by CK2 and CaMKII (39). We therefore tested the hypothesis that ADDL-induced internalization of NMDARs and IRs might share common mechanisms involving CK2 and CaMKII. Consistent with this hypothesis, we found that DMAT, a CK2 inhibitor, completely blocked ADDL-induced loss of both IRs and NMDARs from the dendrites of hippocampal neurons and that KN93, a CaMKII inhibitor, afforded partial protection against ADDL-induced loss of both receptors (Fig. 2 and Fig. S2). Neither DMAT nor KN93 alone had any statistically significant effect on dendritic IR and NMDAR levels (Fig. 2E). These results indicate that CK2 and CaMKII mediate ADDL-induced loss of synaptic receptors germane to plasticity.
Fig. 2.
CK2 and CaMKII mediate ADDL-induced loss of insulin and NMDA receptors. (A–D) Representative high magnification images of IRα labeling in dendrites from hippocampal neurons treated for 3 h with vehicle (A), 100 nM ADDLs (B), 100 nM ADDLs + 5 μM KN93 (C), or 100 nM ADDLs + 10 μM DMAT (D). (E) Quantification of IR (black bars) and NMDAR (white bars) immunofluorescence. Bars correspond to integrated immunofluorescence intensities (see SI Methods) from 3 experiments using independent cultures (30 images analyzed per experimental condition per culture). Asterisks indicate statistically significant (*, P < 0.05; **, P < 0.001) increases compared to ADDL-treated cultures.
Insulin Blocks ADDL-Induced Loss of IRs.
Recent studies have shown that intranasal administration of insulin improves memory in both nondemented humans and in AD patients (30, 40). Moreover, insulin ameliorates Aβ-induced inhibition of LTP in hippocampal slices (41, 42). We therefore asked whether insulin might protect surface IRs in neurons exposed to ADDLs. Significantly, we found that 100 nM insulin partially prevented and 1 μM insulin completely blocked ADDL-induced loss of dendritic IRs in hippocampal cultures (Fig. 3 A–E) and IR accumulation in the cell body (Fig. 3 A–D).
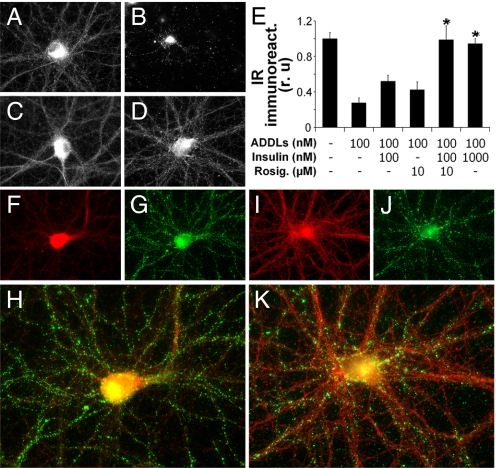
Fig. 3.
Insulin prevents ADDL-induced pathological trafficking of IRs. (A–D) Representative IR immunofluorescence images from hippocampal neurons treated with vehicle (A), 100 nM ADDLs (B), 100 nM ADDLs + 100 nM insulin (C), and 100 nM ADDLs + 1 μM insulin (D). (E) Integrated IR immunofluorescence from 6 experiments using independent cultures (30 images analyzed per experimental condition per culture). Asterisk indicates statistically significant (*, P < 0.005) increase compared to ADDL-treated cultures. (F–K) Neurons treated for 3 h with 100 nM ADDLs alone (F–H) or with 100 nM ADDLs + 100 nM insulin + 10 μM rosiglitazone (I–K) followed by double labeling for IR (red) and ADDLs (green). (H and K) Merged images of IR and ADDL immunolabeling. Note the inverse correlation between ADDL binding and dendritic IRα immunoreactivities on dendritic process.
Rosiglitazone Potentiates the Neuroprotective Action of Insulin.
Rosiglitazone, a peroxysome proliferator-activating receptor (PPAR)-γ agonist, is an insulin-sensitizing drug that stimulates IR protein kinase activity. It is used for treating insulin-resistant type II diabetes and is currently in clinical trials for AD (28, 43). We next investigated whether rosiglitazone might potentiate the ability of submaximal insulin doses to protect against ADDL-induced IR loss. In ADDL-treated cultures, neurons exhibiting the characteristic punctate pattern of synaptic ADDL binding showed very low dendritic IR levels (Fig. 3 F–H). In contrast, neurons that were pretreated with 100 nM insulin + 10 μM rosiglitazone showed markedly reduced ADDL binding and abundant IRs in dendrites (Fig. 3 I–K). Quantitative analysis showed that dendritic IR levels in cells exposed to ADDLs in the presence of 100 nM insulin + 10 μM rosiglitazone were similar to the levels found in control cultures and in cultures treated with ADDLs in the presence of 1 μM insulin (Fig. 3E). In the absence of exogenous insulin, rosiglitazone conferred partial protection against ADDL-induced loss of IRs (Fig. 3E). We note, however, that the effects of rosiglitazone and insulin were synergistic rather than additive, indicating potentiation of insulin protection by rosiglitazone.
Insulin Prevents ADDL-Binding to Neurons and Protects Against ADDL-Induced Oxidative Stress.
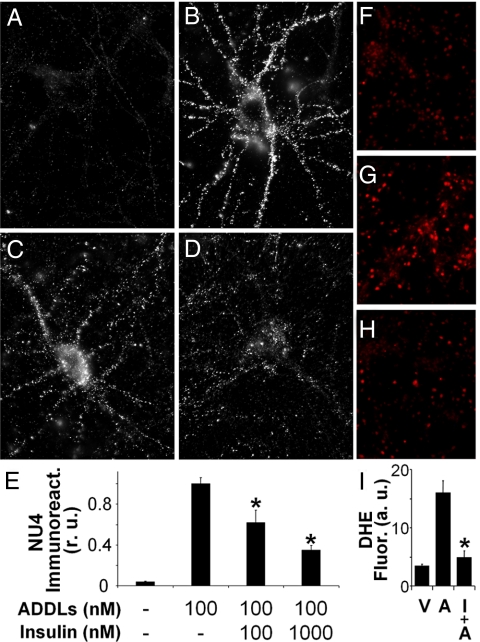
Double-labeling for IRs and ADDL binding (detected by oligomer-selective NU4 antibody) (44) revealed that neurons treated with insulin + rosiglitazone showed markedly reduced ADDL binding (Fig. 3 J and K). This result suggested an insulin-dependent regulation of ADDL-binding sites. We next pretreated hippocampal neurons with either 100 nM or 1 μM insulin for 30 min and then added 100 nM ADDLs for an additional 30 min. Low (100 nM) insulin caused a noticeable reduction and high (1 μM) insulin caused a major (≈70%) blockade of ADDL binding to neurons (Fig. 4 A–E).
Fig. 4.
Insulin blocks neuronal ADDL binding and ADDL-induced oxidative stress. (A–D) Representative images from hippocampal neurons treated with vehicle (A), 100 nM ADDLs (B), 100 nM ADDLs + 100 nM insulin (C), and 100 nM ADDLs + 1 μM insulin (D). ADDL binding was detected using NU4 antibody. (E) Integrated ADDL immunofluorescence intensities from 6 experiments using independent cultures (25 images analyzed per experimental condition per culture). Asterisk indicates statistically significant (*, P < 0.01) decrease compared to ADDL-treated cultures. (F–H) Representative DHE fluorescence images in hippocampal cultures treated with vehicle (F), 1 μM ADDLs (G), or 1 μM insulin + 1 μM ADDLs (H). (I) Integrated DHE fluorescence. Asterisk indicates statistically significant (*, P < 0.007) differences relative to ADDL-treated cultures.
We recently showed that ADDLs induce oxidative stress in hippocampal neurons (16), thus establishing a connection between the neuronal impact of ADDLs and a major AD neuropathology. As might be predicted from the blockade of ADDL binding, we found that insulin effectively inhibited ADDL-induced neuronal oxidative stress (Fig. 4 F–I).
Insulin Prevents ADDL-Induced Synapse Loss.
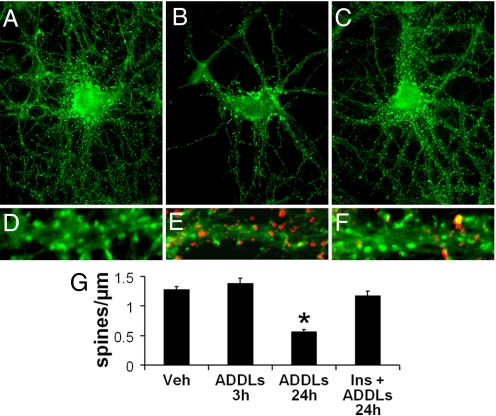
To allow direct comparison of ADDL-induced changes in dendritic spine density relative to changes in IR levels, spines were labeled with phalloidin in tandem with the IR antibody. IRs were eliminated while the number of spines was unaffected in hippocampal neurons exposed to ADDLs for 3 h (Fig. S3). This indicates that loss of insulin receptors precedes and is not a consequence of overall collapse and retraction of synaptic spines. We also note that after a 3-h exposure to ADDLs, a time at which insulin receptors are largely removed from the surface, there is no loss of EphB2 receptors (18). Interestingly, after a 24-h exposure to ADDLs a pronounced loss of spines was detected (Fig. 5), confirming our previous results (18). Remarkably, insulin completely protected against ADDL-induced spine degeneration (Fig. 5). Double labeling using phalloidin and the NU4 anti-ADDLs antibody revealed a marked reduction in spine density in isolated dendrites attacked by ADDLs (Fig. 5E) compared to vehicle-treated neurons (Fig. 5D). In insulin-treated neurons, a marked reduction of ADDL binding was observed along with the presence of abundant, healthy spines (Fig. 5F).
Fig. 5.
Insulin blocks ADDL-induced synapse loss. (A–C) Representative images from hippocampal neurons treated with vehicle (A), 100 nM ADDLs (B), or 100 nM ADDLs + 1 μM insulin (C) for 24 h. Spines were labeled using phalloidin (green). (D–F) Double-labeling high-magnification images of dendrites from neurons treated with vehicle (D), ADDLs (E), or ADDLs + insulin (F). Spines were labeled by phalloidin (green) and ADDLs were detected using the NU4 antibody (red). (G) Quantification of spine number per unit dendrite length. Asterisk indicates statistically significant difference (*, P < 0.001) relative to vehicle-treated neurons.
Protection by Insulin Requires IR Activity.
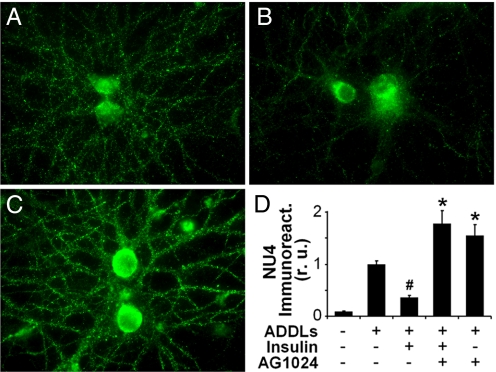
Previous work from our group has shown that ADDLs colocalize with PSD-95 and synaptic spines labeled with CaMKII (12). We recently suggested that ADDLs interact with a receptor complex that includes IRs (9). This raised the possibility that blockade of neuronal ADDL binding by insulin could be the result of direct competition between ADDLs and insulin for a common binding site on neuronal surfaces, as also recently suggested (41). Surprisingly, however, inhibition of IR protein tyrosine kinase (PTK) activity by AG1024 completely abolished the ability of insulin to block ADDL binding (Fig. 5). In fact, AG1024 caused significant increases in neuronal ADDL binding both when AG1024 was added alone and in the presence of exogenous insulin. These results suggest that inhibition of ADDL binding by insulin involves an IR signaling-dependent downregulation of ADDL binding sites, consistent with known effects of neuronal IRs on synaptic receptor trafficking (45, 46).
Fig. 6.
Protection by insulin requires IR tyrosine kinase activity. (A–C) Representative images from hippocampal neurons treated with 100 nM ADDLs (A), 100 nM ADDLs + 1 μM insulin (B), or 100 nM ADDLs + 1 μM insulin + 5 μM AG1024 (C). ADDL binding was detected using the NU4 anti-ADDL antibody. (D) Integrated ADDL immunofluorescence from 3 experiments using independent neuronal cultures (25 images analyzed per experimental condition per culture). Pound sign indicates statistically significant (#, P < 0.001) difference relative to ADDL-treated cultures. Asterisk indicates statistically significant (*, P < 0.007) differences relative to cultures treated with ADDLs + insulin.
Discussion
We have identified a unique molecular mechanism that protects CNS neurons against ADDLs, soluble neurotoxins putatively responsible for the synaptic deterioration underlying Alzheimer's memory failure. ADDLs are known to initiate deterioration by acting as highly specific pathogenic ligands. We have found that ADDL binding to particular synaptic sites and the resulting neuronal oxidative stress, IR downregulation, and synapse loss are markedly decreased by the presence of insulin. Interestingly, neuroprotection by insulin requires IR activity. Thus the mechanism of protection does not involve simple competition between ADDLs and insulin for a common binding site on the neuronal surface, but rather is a signaling-dependent downregulation of ADDL binding sites. The insulin-sensitizing drug rosiglitazone, a PPARγ agonist, potentiated the ability of insulin to protect synapses against ADDLs. Results strongly support the hypothesis that insulin signaling plays a role in defending CNS neurons against AD and provide a disease-specific basis for treatments based on stimulating CNS insulin pathways.
Although the brain once was considered insulin insensitive, it is now known that CNS insulin signaling is important for many aspects of neuronal function, including plasticity and memory formation (26, 27, 47). Recently, human subjects have been found to respond to CNS insulin signaling stimulation with enhanced verbal memory performance (40). When given to subjects with early AD, intranasal insulin also improves performance (30) but only at higher doses, suggesting involvement of additional mechanisms other than stimulation of plasticity. Results here support the hypothesis that the beneficial effect of insulin in AD derives at least in part from an acute decrease in ADDL synaptotoxicity. In harmony with this possibility, it recently was shown that insulin ameliorates Aβ oligomer-induced inhibition of LTP (41, 42), a standard paradigm for plasticity and memory mechanisms. Long-term CNS insulin stimulation potentially could increase cognitive benefits to AD patients further by reducing ADDL-induced neuronal deterioration. Insulin, besides decreasing ADDL binding, ADDL-induced spine degeneration, oxidative stress, and insulin receptor loss (as shown here), also protects against accumulation of hyperphosphorylated tau (48), a pathological hallmark of AD that is induced by ADDLs (15).
The marked decrease in ADDL binding caused by insulin ostensibly could be a simple competitive interaction at the cell surface, which would be consistent with coimmunoprecipitation data (9, 41). However, this mechanism is not supported by the current experiments using AG1024, an inhibitor of IR PTK. When receptor activity is blocked, insulin no longer prevents ADDL binding. Furthermore, we note that addition of rosiglitazone markedly enhanced the ability of submaximal insulin to block ADDL binding (Fig. 3 E–J). These findings indicate that the mechanism of protection involves insulin signaling-dependent downregulation of ADDL binding sites from the neuronal surface. Future identification of proteins lost from the membrane in response to insulin could provide insight into the nature of the ADDL binding sites, which have yet to be determined.
It is intriguing that insulin and ADDLs have reciprocal effects on their neuronal binding sites. Insulin signaling downregulates ADDL binding, while ADDLs downregulate IRs. Given that insulin protects neurons against AD-causing neurotoxins, it follows that dysfunctional CNS insulin signaling would be an AD risk factor. In fact, brain insulin signaling declines with age (36), the primary risk factor for AD. An elevated risk for AD also exists in type 2 diabetes patients, who manifest deficient CNS insulin signaling. This deficiency is likely a consequence of decreased insulin uptake into the brain following sustained peripheral hyperinsulinemia (31). Experimentally induced diabetes moreover causes AD mice models to exhibit accelerated cognitive failure (32, 33). Significantly, the marked loss of IRs from synaptic plasma membranes seen in response to ADDLs (9) has been confirmed in neuropathology studies showing that IRs are lost from dendrites in AD brain (49).
Recent clinical trials indicate that insulin-sensitizing drugs such as rosiglitazone improve cognition and memory in both AD and type 2 diabetes patients (28, 43, 50, 51). The basis for the cognitive benefits of rosiglitazone has not been established, although reductions in inflammation and amyloid plaque burden have been hypothesized. However, rosiglitazone attenuates deficits in learning and memory in AD animal models without affecting amyloid deposition (29). A plausible mechanism of protection is provided by the current finding that rosiglitazone potentiates the ability of insulin to protect synapses against ADDLs. This effect is particularly salient given the relevance of synaptotoxic Aβ oligomers to AD memory impairment (7, 8). Overall, an appealing strategy to protect synaptic memory mechanisms would be to increase the inherent synaptic defense against ADDLs while, in tandem, reducing ADDL abundance. Improved insulin-signaling sensitizing drugs may serve the first function, while therapeutic antibodies may serve the latter, as suggested by promising results from recent clinical trials (25).
Materials and Methods
Materials.
Synthetic Aβ1–42 peptide was from American Peptides (Sunnyvale, California). Scrambled Aβ1–42 peptide was from Anaspec (San Jose, California). Bovine and human insulin, 1,1,1,3,3,3,-hexafluoro-2-propanol (HFIP), DMSO, papain and poly-L-lysine were from Sigma (St. Louis, Missouri). Culture medium/reagents were from Invitrogen (Carlsbad, California). Precast electrophoresis gels, Alexa-labeled secondary antibodies, ProLong, Alexa 488-conjugated phalloidin, and DHE were from Invitrogen. Electrophoresis buffers were from BioRad (Hercules, California). SuperSignal chemiluminescence reagents, Sulfo-NHS-SS-biotin, neutravidin, and the BCA protein assay kit were from Pierce (Deerfield, Illinois). Streptavidin was from Fluka (Sigma, Buchs, Switzerland). Antibodies against the α subunit of insulin receptors (IRα), Na+,K+-ATPase and NR1 subunit of NMDA receptors were from Santa Cruz Biotechnology (Santa Cruz, California).
ADDL and Scrambled Aβ1–42 Preparation.
These were prepared from Aβ1–42 or scrambled Aβ as previously described (1, 11) and detailed in SI Methods.
Mature Hippocampal Cultures.
Primary hippocampal neuronal cultures were prepared according to established procedures (15, 16) and were used after 21 DIV. Cultures were treated at 37 °C for 3 h with 100 nM ADDLs or an equivalent volume of F12 vehicle. Insulin (100 nM or 1 μM) or rosiglitazone (10 μM), when present, were added 30 min before ADDLs. AG1024 (5 μM) was added to cultures 30 min before insulin.
Surface Biotinylation-Based Western Blot Assay.
Surface proteins from neuronal cultures treated with vehicle or ADDLs were biotinylated and analyzed by Western immunoblotting as detailed in SI Methods.
Immunocytochemistry and Phalloidin Labeling.
Cells were fixed and blocked as previously described (15), incubated with both ADDL-selective NU4 mouse monoclonal antibody (44; 1 μg/mL) and IR-α rabbit polyclonal antibody (1:500) overnight at 4 °C and then incubated for 3 h at 23 °C with Alexa-conjugated secondary antibodies. Spines were labeled with Alexa 488-conjugated phalloidin (which binds to spine-localized dense bundles of F-actin) for 20 min at 23 °C, according to manufacturer's instructions. Coverslips were mounted with Prolong and imaged on a Nikon Eclipse TE 2000-U microscope.
Oxidative Stress.
Formation of reactive oxygen species (ROS) was evaluated in live neurons using dihydroethidium (DHE) as detailed in SI Methods.
Data Analysis.
IR and ADDL binding immunofluorescence intensities were analyzed using NIH Image J as described in SI Methods.
Supplementary Material
Acknowledgments.
This work was supported by grants from the American Health Assistance Foundation, Alzheimer's Association, National Institutes of Health-National Institute on Aging Grants RO1-AG18877 and RO1-AG22547 (to W.L.K.); Howard Hughes Medical Institute (S.T.F.), Conselho Nacional de Desenvolvimento Cientifico e Tecnologico/Brazil (F.G.F. and S.T.F.) and Fundacao de Amparo à Pesquisa do Estado do Rio de Janeiro/Brazil (F.G.F. and S.T.F.). F.G.F. is supported by Human Frontier Science Program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809158106/DCSupplemental.
References
- 1.Gong Y, et al. Alzheimer's disease-affected brain: presence of oligomeric A beta ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc Natl Acad Sci USA. 2003;100:10417–10422. doi: 10.1073/pnas.1834302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheibel ME, Lindsay RD, Tomiyasu U, Scheibel AB. Progressive dendritic changes in aging human cortex. Exp Neurol. 1975:47392–47403. doi: 10.1016/0014-4886(75)90072-2. [DOI] [PubMed] [Google Scholar]
- 4.Terry RD, et al. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 5.Klein WL, Krafft GA, Finch CE. Targeting small A beta oligomers: the solution to an Alzheimer's disease conundrum? Trends Neurosci. 2001;24:219–224. doi: 10.1016/s0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- 6.Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 7.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 8.Ferreira ST, Vieira MN, De Felice FG. Soluble protein oligomers as emerging toxins in Alzheimer's and other amyloid diseases. IUBMB Life. 2007;59:332–345. doi: 10.1080/15216540701283882. [DOI] [PubMed] [Google Scholar]
- 9.Zhao WQ, et al. Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J. 2008;22:246–260. doi: 10.1096/fj.06-7703com. [DOI] [PubMed] [Google Scholar]
- 10.Klein WL, Stine WB, Jr, Teplow DB. Small assemblies of unmodified amyloid beta-protein are the proximate neurotoxin in Alzheimer's disease. Neurobiol Aging. 2004;25:569–580. doi: 10.1016/j.neurobiolaging.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Lambert MP, et al. Diffusible, nonfibrillar ligands derived from A beta1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacor PN, et al. Synaptic targeting by Alzheimer's-related amyloid beta oligomers. J Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh DM, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 14.Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Felice FG, et al. Alzheimer's disease-type neuronal tau hyperphosphorylation induced by A beta oligomers. Neurobiol Aging. 2008;29:1334–1347. doi: 10.1016/j.neurobiolaging.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Felice FG, et al. A beta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- 17.Roselli F, et al. Soluble beta-amyloid1–40 induces NMDA-dependent degradation of postsynaptic density-95 at glutamatergic synapses. J Neurosci. 2005;25:11061–11070. doi: 10.1523/JNEUROSCI.3034-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lacor PN, et al. A beta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shankar GM, et al. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snyder EM, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh H, et al. AMPAR removal underlies A beta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Georganopoulou DG, et al. Nanoparticle-based detection in cerebral spinal fluid of a soluble pathogenic biomarker for Alzheimer's disease. Proc Natl Acad Sci USA. 2005;102:2273–2276. doi: 10.1073/pnas.0409336102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lesné S, et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 24.Klyubin I, et al. Amyloid beta protein immunotherapy neutralizes A beta oligomers that disrupt synaptic plasticity in vivo. Nat Med. 2005;11:556–561. doi: 10.1038/nm1234. [DOI] [PubMed] [Google Scholar]
- 25.Relkin NR, et al. 18-Month study of intravenous immunoglobulin for treatment of mild Alzheimer disease. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2007.12.021. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Zhao W, et al. Brain insulin receptors and spatial memory. Correlated changes in gene expression, tyrosine phosphorylation, and signaling molecules in the hippocampus of water maze trained rats. J Biol Chem. 1999;274:34893–34902. doi: 10.1074/jbc.274.49.34893. [DOI] [PubMed] [Google Scholar]
- 27.Zhao WQ, Alkon DL. Role of insulin and insulin receptor in learning and memory. Mol Cell Endocrinol. 2001;177:125–134. doi: 10.1016/s0303-7207(01)00455-5. [DOI] [PubMed] [Google Scholar]
- 28.Watson GS, et al. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry. 2005;13:950–958. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- 29.Pedersen WA, et al. Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Exp Neurol. 2006;199:265–273. doi: 10.1016/j.expneurol.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 30.Reger MA, et al. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;70:440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- 31.Craft S. Insulin resistance and Alzheimer's disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res. 2007;4:147–152. doi: 10.2174/156720507780362137. [DOI] [PubMed] [Google Scholar]
- 32.Ho L, et al. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer's disease. FASEB J. 2004;18:902–904. doi: 10.1096/fj.03-0978fje. [DOI] [PubMed] [Google Scholar]
- 33.Cao D, Lu H, Lewis TL, Li L. Intake of sucrose-sweetened water induces insulin resistance and exacerbates memory deficits and amyloidosis in a transgenic mouse model of Alzheimer disease. J Biol Chem. 2007;282:36275–36282. doi: 10.1074/jbc.M703561200. [DOI] [PubMed] [Google Scholar]
- 34.Crump FT, Dillman KS, Craig AM. cAMP-dependent protein kinase mediates activity-regulated synaptic targeting of NMDA receptors. J Neurosci. 2001;21:5079–5088. doi: 10.1523/JNEUROSCI.21-14-05079.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyer C, Schikorski T, Stevens CF. Comparison of hippocampal dendritic spines in culture and in brain. J Neurosci. 1998;18:5294–5300. doi: 10.1523/JNEUROSCI.18-14-05294.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cole GM, Frautschy SA. The role of insulin and neurotrophic factor signaling in brain aging and Alzheimer's Disease. Exp Gerontol. 2007;42:10–21. doi: 10.1016/j.exger.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 37.Clodfelder-Miller BJ, Zmijewska AA, Johnson GV, Jope RS. Tau is hyperphosphorylated at multiple sites in mouse brain in vivo after streptozotocin-induced insulin deficiency. Diabetes. 2006;55:3320–3325. doi: 10.2337/db06-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grünblatt E, Salkovic-Petrisic M, Osmanovic J, Riederer P, Hoyer S. Brain insulin system dysfunction in streptozotocin intracerebroventricularly treated rats generates hyperphosphorylated tau protein. J Neurochem. 2007;101:757–770. doi: 10.1111/j.1471-4159.2006.04368.x. [DOI] [PubMed] [Google Scholar]
- 39.Chung HJ, Huang YH, Lau LF, Huganir RL. Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. 2004;i24:10248–10259. doi: 10.1523/JNEUROSCI.0546-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benedict C, et al. Intranasal insulin improves memory in humans: superiority of insulin aspart. Neuropsychopharmacology. 2007b;32:239–243. doi: 10.1038/sj.npp.1301193. [DOI] [PubMed] [Google Scholar]
- 41.Townsend M, Mehta T, Selkoe DJ. Soluble A beta inhibits specific signal transduction cascades common to the insulin receptor pathway. J Biol Chem. 2007;282:33305–33312. doi: 10.1074/jbc.M610390200. [DOI] [PubMed] [Google Scholar]
- 42.Lee CC, Kuo YM, Huang CC, Hsu KS. Insulin rescues amyloid beta-induced impairment of hippocampal long-term potentiation. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.06.014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 43.Risner ME, et al. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer's disease. Pharmacogenomics J. 2006;6:246–254. doi: 10.1038/sj.tpj.6500369. [DOI] [PubMed] [Google Scholar]
- 44.Lambert MP, et al. Monoclonal antibodies that target pathological assemblies of A beta. J Neurochem. 2007;100:23–35. doi: 10.1111/j.1471-4159.2006.04157.x. [DOI] [PubMed] [Google Scholar]
- 45.Skeberdis VA, Lan J, Zheng X, Zukin RS, Bennett MV. Insulin promotes rapid delivery of N-methyl-D- aspartate receptors to the cell surface by exocytosis. Proc Natl Acad Sci USA. 2001;98:3561–3566. doi: 10.1073/pnas.051634698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang CC, You JL, Lee CC, Hsu KS. Insulin induces a novel form of postsynaptic mossy fiber long-term depression in the hippocampus. Mol Cell Neurosci. 2003;24:831–841. doi: 10.1016/s1044-7431(03)00238-0. [DOI] [PubMed] [Google Scholar]
- 47.Gispen WH, Biessels GJ. Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci. 2000;23:542–549. doi: 10.1016/s0166-2236(00)01656-8. [DOI] [PubMed] [Google Scholar]
- 48.Spillantini MG, Goedert M. Tau protein pathology in neurodegenerative diseases. Trends Neurosci. 2008;21:428–433. doi: 10.1016/s0166-2236(98)01337-x. [DOI] [PubMed] [Google Scholar]
- 49.Moloney AM, et al. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer's disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.04.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 50.Landreth G. Therapeutic use of agonists of the nuclear receptor PPARgamma in Alzheimer's disease. Curr Alzheimer Res. 2007;4:159–164. doi: 10.2174/156720507780362092. [DOI] [PubMed] [Google Scholar]
- 51.Ryan CM, et al. Improving metabolic control leads to better working memory in adults with type 2 diabetes. Diabetes Care. 2006;29:345–351. doi: 10.2337/diacare.29.02.06.dc05-1626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.