Abstract
The pain associated with burn injuries is intense, unremitting and often exacerbated by anxiety, depression and other complicating patient factors. On top of this, modern burn care involves the repetitive performance – often on a daily basis for weeks to months – of painful and anxiety-provoking procedures that create additional treatment-related pain, such as wound care, dressing changes and rehabilitation activities. Pain management in burn patients is primarily achieved by potent pharmacologic analgesics (e.g., opioids), but is necessarily complemented by nonpharmacologic techniques, including distraction or hypnosis. Immersive virtual reality provides a particularly intense form of cognitive distraction during such brief, painful procedures, and has undergone preliminary study by several research groups treating burn patients over the past decade. Initial reports from these groups are consistent in suggesting that immersive virtual reality is logistically feasible, safe and effective in ameliorating the pain and anxiety experienced in various settings of post-burn pain. Furthermore, the technique appears applicable to a wide age range of patients and may be particularly well-adapted for use in children, one of the most challenging populations of burn victims to treat. However, confirmation and extension of these results in larger numbers of patients in various types of burn-related pain is necessary to more clearly define the specific benefits and limitations of virtual reality analgesia in the burn care setting.
Keywords: analgesia, anxiety, burns, distraction, pain, physical therapy, virtual reality
Considered by many to be the pinnacle of human–technology interaction, immersive virtual reality (VR) allows a user to interact with a realistic, computer-generated environment. An immersive VR system differs from conventional television or commercial video games in that immersive VR typically consists of a high-speed microprocessing computer, a helmet or head-mounted display that delivers high-resolution, 3D sights and sounds, head- and/or limb-tracking hardware, and specialized software to render an interactive virtual environment. Accordingly, VR experiences are primarily visual, although recent innovations allow other sensory modalities (such as motion-tracking, sound and tactile sensation). VR also allows for user interaction with the virtual environment. An increasingly common feature of immersive VR systems is the ability to track a user’s head movement, such that when the head moves, the user’s visual environment shifts to account for the movement. The combination of multisensory input and interactivity makes the VR experience more immersive and realistic than conventional television or video games, and can successfully captivate much of the user’s conscious attention.
The most well known applications of immersive VR include its use in various training simulators (e.g., flight simulators), video games or other personal entertainment, and desensitization therapy (such as graded exposure treatment of phobias or post-traumatic stress disorder). Aside from its use in desensitization therapy, there has been limited exploration until recently of the use of VR as an intervention to treat pain and anxiety in the clinical setting. In this review, we will explore the recent use of VR in treating the pain and anxiety that specifically occur during the treatment of cutaneous burn injuries.
The origins of immersive VR in the treatment of burn injuries arose from the recognized need for improved nonpharmacologic analgesia in this challenging acute pain setting. A variety of cognitive–behavioral therapies (including distraction, imagery, biofeedback or hypnotic analgesia provided by trained staff) have previously been employed to help patients escape the brief, yet intense, pain that accompanies burn wound care and post-burn rehabilitation activities. In its simplest forms, distraction can be achieved through audio stimulation (e.g., music), audio–video stimulation (e.g., television) and/or user interaction (e.g., video game), and theoretically helps prevent patients from focusing their attention on the painful procedure, thereby reducing the pain experience associated with the nociceptive signals still generated during the procedure. Two recent reviews of immersive VR analgesia discuss its evolution as a psychological-based analgesic technique (that is essentially a higher form of distraction) and provide a summary of its potential mechanisms of action, including current evidence for such mechanisms [1,2]. The authors of these reviews suggest that while current pharmacologic analgesics (e.g., opioids) act directly on receptors in the nervous system, VR likely manifests its analgesic effects by altering the perception of pain. The most popular theory is that VR effectively competes for a large fraction of a user’s fixed amount of conscious attention, distracting the user’s focus away from simultaneous nociceptive input (e.g., from a painful medical procedure) and replacing it with more pleasant sensory input from the virtual experience. It is hypothesized that descending inhibitory pathways in the CNS may play a role in this process. The precise neurophysiologic mechanisms of this alteration in pain perception are not clearly defined, and are the subject of several ongoing investigations. However, mechanistic investigations of VR analgesia in the setting of controlled, experimental pain suggest that the magnitude of analgesic effect is dependent upon the user’s sense of ‘presence’ (i.e., the sense of being inside) the virtual environment [3], that subjective VR analgesia is accompanied by simultaneous reductions in pain-related brain activity in the cerebral cortex and brainstem [4], and that VR analgesia is of similar magnitude to, and additive with, clinically relevant doses of concurrent systemic opioid analgesics [5].
Potential applications of virtual reality to clinical care of patients with burn injuries
Numerous reports have documented the potential analgesic benefit of immersive VR in medical settings ranging from cancer therapy [6–8] to dental care [9] to transurethral prostate ablation [10]. The published research covered in this review will pertain to specific applications in which VR is used to alleviate pain and anxiety in burn patients during wound care or other painful procedures. One of the earliest studies involving VR use during burn wound care [11] provides a brief discussion of the need for nonpharmaco-logic analgesic interventions in this clinical setting. The authors indicate that burn injuries are widely accepted as one of the most painful injuries to treat, and that their presence causes changes in anatomy, neurophysiology and pharmacokinetics that may make standard pharmacologic analgesic therapy less effective than usual. The significant correlation that has been shown between the pain severity experienced during hospitalization and the amount of post-discharge mental and physical dysfunction also suggests that early burn pain experiences might affect long-term recovery [12,13]. Because of the repetitive nature of painful burn wound care, there is also a high risk for developing a physiologic tolerance to (and possibly dependence on) opioid analgesics. Both the economic and psychological costs of using only opioids for burn pain management contribute to the motivation to identify nonpharmacologic analgesic treatments that can improve burn-related pain management and reduce opioid drug use. While nonpharmacologic treatments (including immersive VR) hold promise, their use in this challenging pain setting should remain adjunctive and will likely not replace the use of opioid analgesics, which are the cornerstone of burn pain control.
In the following sections we will review the currently published literature describing the application of immersive VR to burn-related acute pain in two specific clinical settings: burn wound care and post-burn physical therapy.
Published reports of virtual reality analgesia for burn-injured patients: burn wound care
The first published report to document VR as an effective analgesic for burn wound care was authored by Hoffman et al. [11] at the University of Washington Burn Center at Harborview Medical Center in Seattle (WA, USA). It examined the use of adjunctive VR during burn wound care in two adolescent patients, using a within-subjects design (to control for baseline pharmacologic analgesia), comparing distraction with immersive VR to that of a conventional video game. This report demonstrated that immersive VR is an effective adjunctive, nonpharmacologic analgesic. The two patients studied were 16- and 17-year-old males, both with deep flash burns that required extensive wound cleaning and bandaging on a daily basis. Various subjective pain and anxiety ratings were obtained using a 100 mm visual analog scale (VAS), with significant improvements in all ratings during VR compared with video game use. The 17-year-old boy had particular difficulty with pain during wound care, and reported approximately 50% or greater reductions in all pain and anxiety ratings during VR compared with the video game (Figure 1). This study’s results provided the first indication that VR may hold promise in alleviating post-burn procedural pain and anxiety.
Figure 1. Compared with distraction with a 2D video game (dark bars), immersive virtual reality (light bars) significantly reduced various subjective pain and anxiety ratings in a 17-year-old male undergoing bedside burn wound care.
VAS: Visual Analog Scale.
Reproduced with permission from [11].
Since this original report, other groups have reported similar analgesic benefits when immersive VR [14–16] or ‘augmented reality’ distraction [17] is added to standard pharmacologic analgesia for portions of (as opposed to the entirety of) bedside wound care procedures, although generally with limited numbers of patients. Das et al., from Australia, confirmed the previously described findings using a randomized controlled trial (within-subjects design) in which seven hospitalized children with acute burn injuries underwent a series of 11 analgesic comparisons (standard pharmacologic analgesia with/without VR) [15]. In each comparison, subjective pain was assessed using the 0–5 Faces Scale after both the VR and the no VR (control) condition. Results indicated that the mean pain score for pharmacological analgesia was 4.1, compared with 1.3 when VR was added. This study employed a small study population, yet firmly supported the results of the initial case report, suggesting that VR distraction has analgesic benefit this setting. Maani et al. described the use of a novel ‘articulated arm’ that allowed use of a high-resolution, 3D display in two patients whose head/face burns precluded the use of a standard VR delivery helmet [16]. Lastly, Mott etal. reported the use of a small, conventional 2D screen display coupled with an interactive system that allows user manipulation of real world objects to be incorporated into the animated 2D field-of-view [17]. Although not a true immersive VR system, this hardware configuration is potentially attractive to younger pediatric patients for whom a standard, head-mounted display is often inappropriately sized or unfamiliar/frightening. In this controlled study of 42 children aged 3–14 years, both the users of augmented reality and their parents reported significantly lower pain scores than did those in the control group.
A recent controlled trial by van Twillert etal. in the Netherlands investigated whether VR can reduce the pain and anxiety during an entire bedside wound care session, and compared VR analgesia effects to those of standard pharmacologic intervention (as well as other forms of distraction) in a population of 19 patients aged 8–65 years [18]. Unlike the reports of VR analgesia for wound care noted above where VR exposures were limited to only a portion of the painful procedure, this study was unique in that it allowed patients to use VR throughout the entire wound care session. The analgesic effects of VR were superior to those of several other distraction techniques (music and non-medical conversation), but were not statistically better than those observed when the patients watched conventional television. The authors pointed out that while the mean time for wound dressing change was 19.2 min, patients did not report any side effects of experiencing VR (e.g., simulator sickness, a nauseating sensation that can occasionally occur with long periods of VR, although less common with modern, high-speed microprocessors). The authors concluded that it is safe to expose patients to longer periods of VR, which is encouraging because analgesic applications of VR can potentially involve procedural pain of longer durations.
Because the procedural pain associated with daily scrubbing of open burn wounds is understood to be one of the most excruciating forms of treatment-induced pain, burn care staff will often partially submerge patients in hydrotanks (sterile bathtubs) to facilitate wound washing and ease bandage changing, especially during the early phases of recovery from burn injuries when such pain is particularly intense. Accordingly, the ability to utilize VR while in the hydrotank may be advantageous. However, electrical dependence of conventional VR technology prohibits its use in or near water due to the risk of electrical shock. To circumvent this issue, a 2004 report by Hoffman et al. described a custom-built, photonic, nonelectrical, ‘water-friendly’ VR delivery system for use in the hydrotank [19]. This case report involved a 40-year-old male with 19% total body surface area deep burns to his extremities. In this VR delivery system (Figure 2), head motion tracking was not possible, so the patient visually navigated in the virtual world using a fingertip-controlled joystick while holding his head still. Wound care was performed both with and without VR, and pain measures (subjective 0–10 VAS ratings) were recorded during a short pause in wound care after each condition. Statistically significant differences were observed in the magnitude of pain experienced. For example, the amount of time the patient spent thinking about his pain during wound care dropped from 10 to 3 on the 0–10 scale. This case report has obvious limitations, but was expanded in a recent case series by the same group [20], in a similarly designed study that was more rigorous in several respects. Care was taken to ensure that patients received pharmacological analgesics shortly before the wound care session, a within-subjects design was used to better attribute the differences in pain ratings to use of VR, a larger study population (n = 11) was analyzed, and the amount of time spent in and out of VR prior to pain assessment was consistent across all subjects and all trials. Pain assessments demonstrated significant improvements with the addition of VR to standard pharmacologic analgesia. For example, mean subjective reports of ‘worst pain’ dropped from 7.6 to 5.1 (0–10 scale). The importance of the immersiveness of the VR experience was demonstrated by the finding that the six patients who reported the strongest illusion of ‘going inside’ the virtual world reported the greatest analgesic effect of VR on ‘worst pain’ ratings, dropping from a mean of 7.2 in the no-VR condition to a mean of 3.7 during VR. These reports of technological adaptation of a VR system to meet a specific medical care need illustrate both the flexibility and utility of VR in an environment where burn care providers are in particular need of improved analgesic tools.
Figure 2. A 40-year-old male undergoes burn wound care (bandage removal and wound cleaning) partially submerged in a hydrotank while experiencing immersive virtual reality delivered by a photonic, non-electric, ‘water-friendly’ virtual reality delivery system.
The patient navigates and interacts with the virtual world using a hand-controlled joystick.
©Hunter Hoffman, University of Washington, DC, USA.
Published reports of virtual reality analgesia for burn-injured patients: post-burn physical therapy
Anticipatory anxiety can be associated with repeated painful procedures and may add to a patient’s experience of pain. Furthermore, the anticipation of procedural pain may interfere with patient cooperation and participation in repetitive rehabilitation activities in which long-term benefits of limb range-of-motion (ROM) or limb function are highly dependent upon the patient’s tolerance to pain and willingness to perform painful physical therapy activities (e.g., passive stretching of burn-injured skin or skin grafts, passive ROM and active ROM). Although the pain intensity associated with such post-burn rehabilitation therapy is of a lesser magnitude than that associated with burn wound care, improved pain control may have an important impact on rehabilitation success. Theoretically, an increase in mood or positive affect associated with VR distraction therapy may prove valuable in increasing patients’ cooperation and compliance – particularly among children – with painful rehabilitation therapy, and ultimately improve their functional outcome.
Immersive VR was first reported by Hoffman et al. as an adjunctive analgesic treatment of physical therapy pain in burn-injured adults in a small, but controlled study using a within-subjects design that controlled for each patient’s pre-procedure pharmacologic analgesic use [21]. Each of the twelve patients (aged 19–47 years) performed 3 min of physical therapy (passive ROM exercises) with no VR and 3 min of physical therapy while in immersive VR (Figure 3). Notably, all patients reported significantly less pain and anxiety when distracted with VR. It was also noted that the three patients who demonstrated the most dramatic analgesic effects also reported the highest presence levels in VR (i.e., the strongest illusion of being inside the computer-generated environment), a finding consistent with that described above for ‘water-friendly VR’. This study widened the scope of the technique’s potential applicability in the setting of brief procedural pain related to burn care.
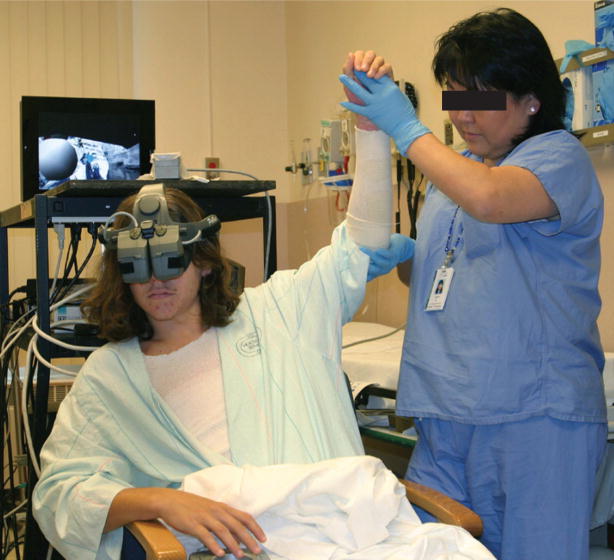
Figure 3. An adolescent male with a left upper extremity burn undergoes passive range-of-motion physical therapy while experiencing immersive virtual reality.
The patient navigates the virtual world using a head position-tracking head-mounted-display and interacts with the virtual world using a fingertip-controlled mouse.
Although this and another case report by Steele et al. in Australia [22] explored the use of VR during painful physical therapy in the nonburns setting, there was no indication of whether VR analgesic effects would be sustained with repeated use (i.e., the novelty effect of the VR experience might extinguish with repeated exposures). A subsequent controlled trial by Hoffman et al. that included seven patients aged 9–32 years explored this question of sustained efficacy of VR analgesia with repeated use [23]. Utilizing a within-subjects design, each patient underwent passive ROM physical therapy on at least three separate and consecutive days, with each session divided into a 3 min VR treatment phase and a 3 min no VR treatment phase. After each treatment condition, pain was assessed using multiple VAS ratings for pain and a potential surrogate for positive mood (‘fun’). As in the previous 1-day study, all pain ratings were significantly lower in the VR condition and, in addition, the patients’ ratings of fun during the physical therapy activities were significantly higher in the VR condition. Furthermore, the analgesic and mood effects of VR were maintained on all 3 study days, indicating that transient novelty of the VR experience was an unlikely explanation of its analgesic effect, at least for this limited number of repetitions.
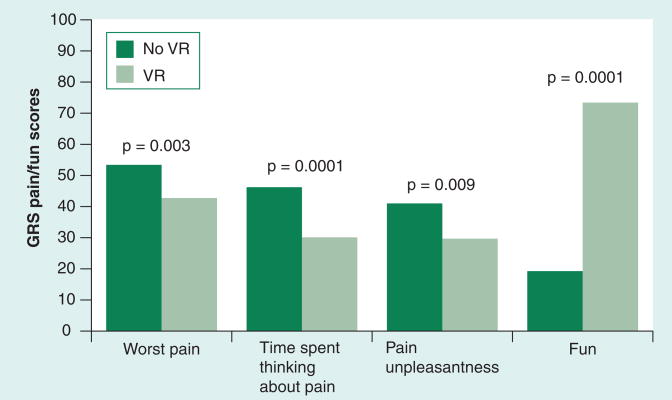
With these initial results suggesting clinical benefit for VR analgesia in post-burn physical therapy, larger studies are necessary to begin exploring specific patient factors that may affect the applicability of VR in the clinical setting. A recent report by Sharar etal. in Seattle compiled results from three ongoing controlled studies to enhance statistical power and investigate such factors as gender, age and ethnicity [24]. This report includes the largest number of subjects published to date – a total of 146 analgesic comparisons in 88 subjects ranging in age from 6 to 65 years – and found that mean subjective pain ratings were reduced 20–37% with immersive VR during passive ROM therapy (Figure 4). Furthermore, none of the pain improvements due to VR distraction varied with differences in gender, ethnicity, initial burn size or duration of the therapy session. Interestingly, the authors found that user assessments of both the realness of the virtual environment, as well as their sense of presence in the virtual environment, differed by age of subjects, with younger subjects (<19 years old) reporting significantly higher ratings for realness and presence than adult subjects (≥19 years old). However, these differences in subjective VR experience did not translate into differences in observed pain ratings while in VR. This study is the first detailed analysis exploring the potential disparities that various clinical cohorts may experience during VR, and is reassuring in suggesting that for the clinical setting of post-burn physical therapy, VR appears applicable to a diverse population of patients.
Figure 4. Mean subjective pain and mood (fun) ratings during post-burn, passive range-of-motion physical therapy in a population of 88 subjects (aged 6–65 years) receiving standard pharmacologic analgesia (dark bars) and standard pharmacologic analgesia plus immersive (light bars).
GRS: Graphic rating scale; VR: Virtual reality.
Expert commentary
Since its first published description in 2000, the use of immersive VR for the treatment of acute, burn-related pain has been reported in at least the ten peer-reviewed reports described above. These reports have in common the use of a standard VR system (consisting of a high-resolution, 3D, audio–video display, customized video game-like software, and user interaction through head position-tracking hardware and/or peripheral devices) and clinical application to one of the two burn care settings commonly associated with significant procedural pain (burn wound care or post-burn rehabilitation therapy). The reports to date are consistent in suggesting that the adjunctive use of this nonpharmacologic analgesic technique:
Is technically and logistically feasible;
Is acceptable to patients across much of the age spectrum;
Reduces patients’ reports of their pain experience in several subjective pain domains;
Is not associated with significant side effects.
However, as preliminary or exploratory studies, these reports have several shortcomings that limit their generalizability to the larger population of burn-injured patients. For example:
Small subject numbers in the majority of these studies limit their statistical power;
The absence of a suitable control condition (to account for the demand characteristics associated with the VR experience) introduces a potential subject bias;
The common use of within-subject study designs allows for comparison to a control condition and minimizes some nuisance variables, yet limits assessment of certain critically important clinical outcomes (e.g., long-term pain control, functional outcome and procedure duration).
Thus, future clinical research must consist of larger study populations, employ single- and double-blind between-subject study designs, and utilize clinically relevant and long-term outcomes, in addition to the assessment of mere periprocedural pain.
The current understanding of the mechanism(s) by which immersive VR reduces subjective pain is skeletal, and largely based on the assumption that the multisensory VR experience is distracting to the user and thereby reduces the amount of conscious attention he/she can utilize to process and interpret noci-ceptive input arising from painful procedures. In the setting of burn wound care, the head-mounted display also obscures the patient’s view of his/her injured and often frightening deformity, and is thought to also contribute to the analgesic effect of VR. However, a better understanding of the analgesic mechanisms (e.g., relative roles of attentional demand, mood enhancement, affect and endogenous opiates) of immersive VR is necessary in order to utilize the technique in the most clinically efficient and cost-effective way. For example, it is reported that modifying VR hardware and software to enhance the VR experience by making the user feel more ‘present’ in the virtual environment is associated with a greater analgesic effect [3]. However, such hardware modifications may come at significant economic cost, with head-mounted displays being commercially available in a wide range of display resolutions (e.g., pixel densities), sizes (e.g., field-of-view), comfort (e.g., weight) and connectivity options (e.g., wireless) that are reflected in an approximately 100-fold difference in acquisition costs (e.g., US$200–US$20,000) for this component alone. A more sophisticated knowledge of the most important determinants of an effective immersive VR system, as well as the most important patient factors associated with VR analgesic efficacy (e.g., coping style), will enhance the application of immersive VR to pain management in burns and other clinical settings.
Given the current challenge of providing adequate pain relief to burn-injured patients who must endure a variety of necessary, but painful therapeutic procedures on a repeated basis, it appears that the addition of immersive VR to standard analgesic regimens has provisionally proven of benefit. Therefore, centers caring for these patients should be encouraged to explore such technologies, to consider their implementation (with the caveats noted above) and to be attentive to ongoing clinical research that will help determine their most appropriate use.
Five-year view
The use of VR to treat acute, procedural pain associated with burn injuries has steadily increased over the last decade. The pioneering preliminary studies discussed above demonstrate the potential analgesia efficacy of VR in this clinical setting, but are significantly limited by their small sample sizes and their focus on short-term outcomes of subjective pain reports. As such, these studies demand extension and elaboration to better determine the precise role for VR analgesia, not only in this clinical setting, but in the many other procedural pain settings encountered in modern medical care. In the coming years, one should anticipate performance of large-scale, randomized, controlled trials that assess not only pain, but also other clinically important outcomes such as procedure duration, patient compliance with procedures, long-term functional outcomes, procedural duration and procedural cost. Such detailed assessments of VR analgesia efficacy and related economics should also include comparisons to other, potentially less costly nonpharmacologic analgesic techniques, so that the most appropriate use of immersive VR can be identified. Also, in addition to its uses for acute, procedural burn pain as described in this review, VR will likely be investigated for potential use in preventing and/or treating chronic pain that is often associated with burns, such as itching and burning sensations on scar tissue. Lastly, most current applications of VR analgesia capitalize on the highly distracting nature of the VR experience to effect a cognitive–behavioral modification of the pain experience. Other applications of immersive VR, such as VR-assisted hypnosis to facilitate hypnotic analgesia, are currently under development [25,26] and may offer valuable alternative techniques for nonpharmacologic pain relief for burn patients.
Key issues
Procedural burn pain (e.g., burn wound care and post-burn rehabilitation activities) is not adequately managed in many patients, despite the availability and use of potent pharmacologic analgesics and adjunctive nonpharmacologic therapies.
Immersive virtual reality (VR) is a cognitive–behavioral therapy, ideally suited for short-term pain relief in the procedural pain setting.
The psychological and neurophysiologic mechanisms of VR analgesia are undefined, but likely involve cognitive distraction and/or enhancement of affect or mood.
Immersive VR appears to be most effective when users feel highly ‘present’ in the computer-generated virtual environment; therefore, specialized hardware/software and user interaction with the virtual environment are key elements of VR analgesia systems.
When added to standard pharmacologic analgesia, immersive VR has been shown to significantly reduce patients’ subjective pain ratings compared with standard pharmacologic analgesia alone in a limited number of randomized, controlled, small-scale studies of post-burn patients undergoing bedside wound care, wound care in the hydrotank and passive range-of-motion physical therapy.
Immersive VR analgesia does not appear to carry significant safety risks or unpleasant side effects.
Large-scale clinical trials of VR analgesia, including long-term outcomes, are necessary to determine its most appropriate use and potential benefit in settings of acute, procedural burn care.
VR may have analgesic applications in other forms (e.g., VR-assisted hypnosis) and other clinical populations (e.g., chronic pain) in the burn-injured population.
Footnotes
No writing assistance was utilized in the production of this manuscript.
The authors have no financial relationships with commercial interests related to virtual reality hardware or software. The authors receive research funding and other support from the National Institutes of Health (AR054115, CA103728 and GM042725), the International Anesthesia Research Society, ScanDesign by Inger and Jens Bruun Foundation, the Paul G Allen Family Foundation, and the Gustavas and Louise Pfeiffer Research Foundation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Contributor Information
Sam R Sharar, Professor, Department of Anesthesiology, Harborview Medical Center, #359724, 325 Ninth Avenue, Seattle, WA 98104, USA, Tel.: +1 206 744 5765, Fax: +1 206 744 8090, sharar@u.washington.edu.
William Miller, Research Assistant, Department of, Rehabilitation Medicine, Harborview, Medical Center, #359796, 325 Ninth, Avenue, Seattle, WA 98104, USA, Tel.: +1 206 744 4811, Fax: +1 206 744 8580, ano327@u.washington.edu.
Aubriana Teeley, Research Coordinator, Department of, Rehabilitation Medicine, Harborview, Medical Center, #359796, 325 Ninth, Avenue, Seattle, WA 98104, USA, Tel.: +1 206 744 4811, Fax: +1 206 744 8580, aubriana@u.washington.edu.
Maryam Soltani, Research Coordinator, Department of, Rehabilitation Medicine, Harborview, Medical Center, #359796, 325 Ninth, Avenue, Seattle, WA 98104, USA, Tel.: +1 206 744 2172, Fax: +1 206 744 8580, soltani@u.washington.edu.
Hunter G Hoffman, Research Scientist, Human Interface, Technology Laboratory, University of Washington, #352142, Seattle, WA 98195, USA Tel.: +1 206 616 1496 Fax: +1 206 543 5380 hunter@hitl.washington.edu.
Mark P Jensen, Professor, Department of Rehabilitation, Medicine, University of Washington, #356490, Seattle, WA 98195, USA, Tel.: +1 206 543 3185, Fax: +1 206 685 3244, mjensen@u.washington.edu.
David R Patterson, Professor, Department of Rehabilitation, Medicine, Harborview Medical Center, #359740, 325 Ninth Avenue, Seattle, WA 98104, USA, Tel.: +1 206 744 3418, Fax: +1 206 744 8580, davepatt@u.washington.edu.
References
Papers of special note have been highlighted as:
•of interest
••of considerable interest
- 1••.Gold JI, Belmont KA, Thomas DA. The neurobiology of virtual reality pain attenuation. Cyberpsychol Behav. 2007;10(4):536–544. doi: 10.1089/cpb.2007.9993. Concise review of the potential psychological and neurophysiological mechanisms of virtual reality analgesia. [DOI] [PubMed] [Google Scholar]
- 2.Wismeijer AAJ, Vingerhoets JJM. The use of virtual reality and audiovisual eyeglass systems as adjunct analgesic techniques: a review of the literature. Ann Behav Med. 2005;30(3):268–278. doi: 10.1207/s15324796abm3003_11. [DOI] [PubMed] [Google Scholar]
- 3••.Hoffman HG, Sharar SR, Coda B, et al. Manipulating presence influences the magnitude of virtual reality analgesia. Pain. 2004;111(1–2):162–168. doi: 10.1016/j.pain.2004.06.013. Evidence demonstrating that changes in virtual reality system hardware and software can affect both the user’s sense of presence in the virtual environment, as well as the as analgesic response. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman HG, Richards TL, Coda B, et al. Modulation of thermal pain-related brain activity with virtual reality: evidence from fMRI. Neuroreport. 2004;15(8):1245–1248. doi: 10.1097/01.wnr.0000127826.73576.91. [DOI] [PubMed] [Google Scholar]
- 5••.Hoffman HG, Richards TL, Van Oostrom T, et al. The analgesic effects of opioids and immersive virtual reality distraction: evidence from subjective and functional brain imaging assessments. Anesth Analg. 2007;105(6):1776–1783. doi: 10.1213/01.ane.0000270205.45146.db. Demonstration of the comparative independent and additive effects of immersive virtual reality and/or systemic opioid administration on the subjective pain experience and concurrent pain-related brain activity. [DOI] [PubMed] [Google Scholar]
- 6.Gershon J, Zimand E, Pickering M, Rothbaum BO, Hodges L. A pilot and feasibility study of virtual reality as a distraction for children with cancer. J Am Acad Child Adolesc Psychiatry. 2004;43(10):1243–1249. doi: 10.1097/01.chi.0000135621.23145.05. [DOI] [PubMed] [Google Scholar]
- 7.Gershon J, Zimand E, Lemos R, Rothbaum BO, Hodges L. Use of virtual reality as a distractor for painful procedures in a patient with pediatric cancer: a case study. Cyberpsychol Behav. 2003;6(6):657–661. doi: 10.1089/109493103322725450. [DOI] [PubMed] [Google Scholar]
- 8.Windich-Biermeier A, Sjoberg I, Dale JC, Eshelman D, Guzzetta CE. Effects of distraction on pain, fear, and distress during venous port access and venipuncture in children and adolescents with cancer. J Pediatr Oncol Nurs. 2007;24(1):8–19. doi: 10.1177/1043454206296018. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman HG, Garcia-Palacios A, Patterson DR, et al. The effectiveness of virtual reality for dental pain control: a case study. Cyberpsychol Behav. 2001;4(4):527–535. doi: 10.1089/109493101750527088. [DOI] [PubMed] [Google Scholar]
- 10.Wright JL, Hoffman HG, Sweet RM. Virtual reality as an adjunctive pain control during transurethral microwave thermotherapy. Urology. 2005;66(6):1320. doi: 10.1016/j.urology.2005.06.123. [DOI] [PubMed] [Google Scholar]
- 11••.Hoffman HG, Doctor JN, Patterson DR, Carrougher GJ, Furness TA., 3rd Virtual reality as an adjunctive pain control during burn wound care in adolescent patients. Pain. 2000;85(1–2):305–309. doi: 10.1016/s0304-3959(99)00275-4. First published report of virtual reality as an analgesic in the clinical setting of burn care, specifically bedside wound care. [DOI] [PubMed] [Google Scholar]
- 12.Ptacek JT, Patterson DR, Montgomery BK, Heimbach DM. Pain, coping, and adjustment in patients with burns: preliminary findings from a prospective study. J Pain Symptom Manage. 1995;10(6):446–455. doi: 10.1016/0885-3924(95)00083-b. [DOI] [PubMed] [Google Scholar]
- 13.Patterson DR, Tininenko J, Ptacek JT. Pain during burn hospitalization predicts long-term outcome. J Burn Care Res. 2006;27(5):719–726. doi: 10.1097/01.BCR.0000238080.77388.FE. [DOI] [PubMed] [Google Scholar]
- 14.Chan EA, Chung JW, Wong TK, Lien AS, Yang JY. Application of a virtual reality prototype for pain relief of pediatric burn in Taiwan. J Clin Nurs. 2007;16(4):786–793. doi: 10.1111/j.1365-2702.2006.01719.x. [DOI] [PubMed] [Google Scholar]
- 15.Das DA, Grimmer KA, Sparnon AL, McRae SE, Thomas BH. The efficacy of playing a virtual reality game in modulating pain for children with acute burn injuries: a randomized controlled trial [ISRCTN87413556] BMC Pediatr. 2005;5(1):1. doi: 10.1186/1471-2431-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maani C, Hoffman HG, DeSocio PA, et al. Pain control during wound care for combat-related burn injuries using custom articulated arm mounted virtual reality goggles. J Cybertherapy Rehabil. 2008;1(2):193–198. [Google Scholar]
- 17.Mott J, Bucolo S, Cuttle L, et al. The efficacy of an augmented virtual reality system to alleviate pain in children undergoing burns dressing changes: a randomized controlled trial. Burns. 2008;34:803–808. doi: 10.1016/j.burns.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 18.van Twillert B, Bremer M, Faber AW. Computer-generated virtual reality to control pain and anxiety in pediatric and adult burn patients during wound dressing changes. J Burn Care Res. 2007;28(5):694–702. doi: 10.1097/BCR.0B013E318148C96F. [DOI] [PubMed] [Google Scholar]
- 19••.Hoffman HG, Patterson DR, Magula J, et al. Water-friendly virtual reality pain control during wound care. J Clin Psychol. 2004;60(2):189–195. doi: 10.1002/jclp.10244. First published report of a photonic, nonelectric virtual reality system for burn wound care performed on a patient submerged in a hydrotank. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman HG, Patterson DR, Seibel E, et al. Virtual reality pain control during burn wound debridement in the hydrotank. Clin J Pain. 2008;24(4):299–304. doi: 10.1097/AJP.0b013e318164d2cc. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman HG, Patterson DR, Carrougher GJ. Use of virtual reality for adjunctive treatment of adult burn pain during physical therapy: a controlled study. Clin J Pain. 2000;16(3):244–250. doi: 10.1097/00002508-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Steele E, Grimmer K, Thomas B, et al. Virtual reality as a pediatric pain modulation technique: a case study. Cyberpsychol Behav. 2003;6(6):633–638. doi: 10.1089/109493103322725405. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman HG, Patterson DR, Carrougher GJ, Sharar SR. Effectiveness of virtual reality-based pain control with multiple treatments. Clin J Pain. 2001;17(3):229–235. doi: 10.1097/00002508-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 24••.Sharar SR, Carrougher GJ, Nakamura D, et al. Factors influencing the efficacy of virtual reality distraction analgesia during postburn physical therapy: preliminary results from 3 ongoing studies. Arch Phys Med Rehabil. 2007;88(12 Suppl 2):S43–S49. doi: 10.1016/j.apmr.2007.09.004. Largest series of patients reported to date using immersive virtual reality to reduce post-burn pain, specifically passive range-of-motion physical therapy. [DOI] [PubMed] [Google Scholar]
- 25••.Patterson DR, Tininenko JR, Schmidt AE, Sharar SR. Virtual reality hypnosis: a case report. Int J Clin Exp Hyp. 2004;52:27–38. doi: 10.1076/iceh.52.1.27.23925. First published report of virtual reality-assisted hypnosis to facilitate hypnotic analgesia for bedside burn wound care. [DOI] [PubMed] [Google Scholar]
- 26.Patterson DR, Wiechman SA, Jensen M, Sharar SR. Hypnosis delivered through immersive virtual reality for burn pain: a clinical case series. Int J Clin Exp Hypn. 2006;54(2):130–142. doi: 10.1080/00207140500528182. [DOI] [PubMed] [Google Scholar]