I. Introduction
The vesicular monoamine transporter-2 (VMAT-2) is an important regulator of intraneuronal monoamine concentrations and disposition as this protein sequesters cytoplasmic dopamine (DA), 5-hydroxytryptamine (5HT, serotonin), and norepinephrine within synaptic vesicles thus contributing to subsequent exocytotic release. This review focuses primarily on VMAT-2 and vesicles found in dopaminergic neurons, as the majority of preclinical studies involving the impact of in vivo drug administration on this transporter protein have focused on these neurons.
Historically, many investigators have utilized VMAT-2 levels as a marker of dopaminergic neuronal integrity. The validity of this approach is supported by findings of decreases in VMAT-2 ligand binding site density and/or immunoreactivity in patients with Parkinson’s disease (Wilson et al., 1996b; Frey et al., 1996; Miller et al., 1999), a disorder well established to result from a loss of nigrostriatal dopaminergic neurons. Interestingly and perhaps owing to a lesser severity of insult, VMAT-2 density data following chronic abuse of an agent demonstrated pre-clinically to cause persistent dopaminergic deficits, methamphetamine (METH), have been more equivocal. In particular, a loss of VMAT-2 density has been observed in some (Johanson et al., 2006) but not all (Wilson et al., 1996a) human studies.
In addition to the studies involving human subjects described above, numerous preclinical studies have assessed the long-term impact of administering drug treatments demonstrated to cause long-term dopaminergic deficits, including toxins believed to model some aspects of Parkinson’s disease (e.g., 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), rotenone, or 6-hydroxydopamine) as well as high-doses of METH. In contrast, more recent preclinical studies have focused on the short-term regulation of VMAT-2 and associated vesicles. These investigations of the acute impact of drugs, particular psychostimulants, on VMAT-2 and associated vesicles have extended our understanding that changes in VMAT-2 activity and levels, especially those occurring rapidly after drug treatment, may reflect factors beyond simply loss of dopaminergic nerve terminals. Instead and like other monoamine transporters, these studies have revealed that the VMAT-2 and associated vesicles are dynamic and highly modifiable targets of pharmacological manipulations and have intriguing therapeutic potential. This paper will review the recent research that has led to these conclusions.
II. Differences Among VMAT-2-containing Vesicles
There are likely several populations of VMAT-2-containing vesicles found in neurons, including those of the ready-releasable, recycling, resting, and/or reserve pools (for review, see Rizzoli et al., 2005; Sudhof, 2000). For the purpose of this review, VMAT-2-containing vesicles (obtained principally from dopaminergic neurons from rat striatal tissues) have been divided into two types: those that co-fractionate with synaptosomal membranes after osmotic lysis, and those that do not (for details on the osmotic lysis procedure, see Volz et al., 2007 and references contained therein). The VMAT-2 proteins that co-fractionate with synaptosomal membranes as well as the plasmalemmal membrane marker, Na+/K+-ATPase, the DA transporter (DAT), and the readily releasable/active zone marker, piccolo (Volz et al., 2007), are defined for purposes of this review as VMAT-2M. Those that do not co-fractionate with these membranes and associated markers are referred to as cytoplasmic VMAT-2 (VMAT-2C). Noteworthy, there is no evidence to date suggesting that the VMAT-2C and VMAT-2M are different proteins; however, recent data demonstrate dramatic differences in the kinetics of these transporters and associated vesicles.
Purified VMAT-2-containing vesicles have been studied by several investigators including Dwoskin and co-workers (Teng et al., 1997), who demonstrated using electron microscopy that the predominant structures in their vesicle preparation (presumably containing the VMAT-2C) were plain spheroid or ellipsoid and approximately 50 nm in diameter. Later studies by Volz et al. (2006a) involving rotating disk electrode voltammetry and using a similar subcellular fractionation procedure containing VMAT-2C demonstrated that uptake into these vesicles is ATP- and temperature-dependent, and that initial velocities of DA uptake display Michaelis-Menten kinetics.
In contrast to the effects presented for VMAT2C, DA transport via the VMAT-2M does not obey Michaelis-Menten kinetics as is common for monoaminergic transporters. Instead, the uptake profile for VMAT-2M is sigmoidal, with a Hill coefficient of approximately 4.5 (Volz et al., 2007). The sigmoidal nature of this profile has the unique potential of permitting substantial adjustment in transport rate over the concentration range spanning its “steepest” portion. Noteworthy, DA sequestration into VMAT-2M-associated vesicles in the presence of high concentrations of DA is greater in total capacity than in VMAT-2C-associated vesicles. This capacity permits speculation that at least a subpopulation of the VMAT-2M-associated vesicles may serve as a “DA sink” to capture DA and thus prevent its cytoplasmic accumulation. This is relevant, as aberrant DA sequestration leads to reactive species generation and likely contributes to the long-term damage caused by high-dose METH treatment (see Discussion below). Thus, the differential kinetic profiles of these two populations of VMAT-2 and related vesicles not only suggest unique physiological roles for these systems, but that these transporters might also provide distinct therapeutic opportunities.
III. VMAT-2 as an Index of Neurotoxicity Following Psychostimulant Treatment
It is well established in preclinical models that multiple high-dose injections of METH, administered in patterns that mimics “bingeing” in humans, causes long-lasting decreases in the activity of the rate-limiting enzyme in DA synthesis, tyrosine hydroxylase (Kogan et al., 1976; Hotchkiss et al., 1979; Hotchkiss and Gibb, 1980; Morgan and Gibb, 1980), the function and binding of the DAT (Wagner et al., 1980; Eisch et al., 1992; Guilarte et al., 2003) and levels of associated transmitters (Seiden et al., 1976; Wagner et al., 1980). As these changes can persist for months, these deficits likely reflect destruction of corresponding DA axons and/or terminals.
In addition to the persistent alterations noted above, preclinical studies have focused on the long-term impact of METH on VMAT-2. For example, Guilarte et al. (2003) have demonstrated that METH administration decreases striatal VMAT-2 ligand binding, as assessed 14 d after treatment. Similarly and as noted above, Johanson and colleagues (2006) demonstrated modest decreases in VMAT-2 binding in positron emission tomography studies. In contrast, Kish and co-workers (Wilson et al., 1996a) found diminished levels of three DA terminal markers (DA, tyrosine hydroxylase and the DAT) in post-mortem striatum of chronic METH users with total VMAT-2 levels being normal. While there may be multiple explanations for this discrepancy, it is possible that total VMAT-2 levels, as detected in this latter study, may not necessarily reflect the integrity of the system and that other factors may contribute to unexpected alterations in its expression. Although the specific explanation for this finding is unclear, the ability to pharmacologically manipulate the state of VMAT-2 and its associated function, as described below, may contribute.
IV. Acute Effects of DA releasers on VMAT-2C
Early evidence that alterations in VMAT-2 levels may reflect processes unrelated to DA terminal loss comes from studies wherein VMAT-2-containing vesicles are separated by subcellular fractionation (vs. assessing the total content or density of VMAT-2 as illustrated in studies cited above). In particular, Sonsalla and co-workers (Hogan et al., 2000) reported that METH treatment decreased both binding of the VMAT-2 ligand, dihydrotetrabenazine (DHTBZ), and DA uptake, as assessed in a purified cytoplasmic vesicle preparation 24 h after drug treatment. However and importantly, no significant loss of DHTBZ binding was observed in whole striatal homogenates at this time point. These data were the first to highlight the “disparity between homogenates and vesicle preparations” with regard to VMAT-2 after METH treatment.
One possible explanation for this disparity reported by Hogan et al. (2000) is that VMAT-2C was redistributed within nerve terminals after treatment with the METH. This possibility is supported by the work of Brown et al. (2000) who observed that repeated, high-dose administrations of METH to rats rapidly (within 1 h) decreased vesicular DA uptake, as assessed in VMAT-2C-associated vesicles purified from striata of treated rats. This effect on uptake was largely attributable to effects on dopaminergic (vs. serotonergic) nerve terminals, because destruction of the serotonergic projections to the striatum did not alter the effect on vesicular DA transport in this study. Later, Riddle et al. (2002) reported that repeated, high-dose METH injections rapidly redistribute rat striatal VMAT-2C immunoreactivity, and presumably associated vesicles, to a location not retained in the preparation of the synaptosomes. This decrease occurs concurrent with a METH-induced reduction in vesicular DA content in the VMAT-2C-associated fraction (Sandoval et al., 2003). Subsequently, Yamamoto and co-workers (Eyerman and Yamamoto, 2005; Tata et al., 2007) also demonstrated that METH administration decreased VMAT-2 immunoreactivity and 1 and 24 h after treatment in a similar preparation.
Of interest are findings that exposure to chronic unpredictable stress can impact both VMAT-2 levels and the persistent dopaminergic deficits caused by METH treatment (Tata et al., 2007). In particular, pre-exposure to the stressors augmented METH-induced decreases in VMAT-2 immunoreactivity, as assessed in synaptosomal, purified vesicular (presumably cytoplasmic) and membrane-associated tissue fractions prepared from rat striata. Unpredictable stress also worsened both METH-induced hyperthermia and the persistent decreases in DA content caused by METH. Noteworthy, both the acute effects on VMAT-2 and the persistent dopaminergic deficits were attenuated in rats in which METH-induced hyperthermia was attenuated, thus suggesting an association among the phenomena (Tata et al., 2007).
Of further interest are findings that multiple administrations of METH cause a rapid (within 1 h) nitrosylation of the VMAT-2 in the purified vesicular (presumably cytoplasmic) fraction from striatal synaptosomes concurrent with a decrease in VMAT-2 immunoreactivity. Both the acute decrease and the characteristic long-term (e.g., 7 d) METH-induced decrease in VMAT-2 immunoreactivity were attenuated by pretreatment with the neuronal nitric oxide synthase inhibitor, S-methyl-l-thiocitrulline (Eyerman and Yamamamoto, 2007), again suggesting an association between the acute and long-term impact of METH on VMAT-2.
One functional consequence of the rapid impact of METH on VMAT-2 function is likely its contribution to the persistent DA deficits caused by the stimulant. This hypothesis is based on the fact that, as noted above, the VMAT-2 is a critical regulator of intraneuronal DA levels as it regulates DA sequestration within neuronal vesicles. Further, amphetamine, and presumably METH, causes DA release from synaptic vesicles into the cytoplasm (for review, see Sulzer et al., 2005). In 1994, Cubells et al. suggested this redistribution of DA from the reducing environment inside synaptic vesicles to oxidizing environments outside of vesicles promoted formation of reactive species within DA neurons that contribute to DA terminal loss. In particular, DA likely contributes to these deficits as it can promote the generation of highly toxic reactive species including superoxide radicals, hydroxyl radicals and DA quinones (for review, see Stokes et al. (1999) and references cited therein). Noteworthy, the work by Cubells et al. (1994) built on previous in vivo studies demonstrating that DA is a major contributor to METH-induced dopaminergic deficits as its depletion consequent to treatment with alpha-methyl-p-tyrosine attenuates the decreases in TH activity and DA levels caused by the stimulant (Gibb and Kogan, 1979; Wagner et al., 1983; Schmidt et al., 1985). Not surprisingly, METH treatment in vivo causes formation of reactive oxygen species and other damaging moieties (e.g., Giovanni et al., 1995; Fleckenstein et al., 1997; Yamamoto and Zhu, 1998; Kita et al., 1999; LaVoie and Hastings, 1999).
Direct evidence of a role for VMAT-2 as contributing to METH-induced dopaminergic deficits comes from findings that pretreatment with the VMAT-2 inhibitor, reserpine, worsens METH-induced dopaminergic deficits (Wagner et al., 1983; Thomas et al., 2008). Further, in a postnatal ventral midbrain neuronal culture preparation, METH-induced accumulation of oxygen radicals and damage to DA neurites varied inversely with VMAT-2 expression levels (Larsen et al., 2002). VMAT2 transfection into PC12 cells attenuates cell death induced by METH (Vergo et al., 2008). In addition, heterozygotic VMAT-2 knockout mice are more susceptible to METH-induced DA deficits than wild-type controls (Fumagalli et al., 1999). Finally, important evidence suggesting that aberrant VMAT-2 function contributes to the persistent DA deficits caused by METH includes findings that post-treatment with the pharmacologically distinct agents, lobeline (Eyerman and Yamamoto, 2005) and methylphenidate (MPD; Sandoval et al., 2003) both reverse METH-induced alterations in VMAT-2 and prevent the persistent DA deficits caused by METH treatment (i.e., “rescue” DA neurons).
Additional recent evidence of an association between VMAT-2 expression and the susceptibility to METH toxicity comes from findings by Miller and coworkers (Guillot et al., 2008) that pretreatment of mice with pituitary adenylyl cyclase activating polypeptide, 38 amino acids (PACAP38), increases VMAT-2 expression and function. Further, PACAP38 treatment both reduced the formation of oxidative products consequent to METH treatment, and protected against the long-term DA deficits caused by the stimulant.
Noteworthy, administration of several agents demonstrated to cause DA release (vs. those that inhibit DA reuptake; see discussion below) decrease VMAT-2C activity and/or immunoreactivity in a manner resembling the METH-induced decreases in these parameters; these include a single administration of AMPH (Riddle et al., 2007), and repeated injections of methylenedioxymethamphetamine (MDMA; Hansen et al., 2002). However, unlike the effects of repeated METH administrations, the decreases in VMAT-2C activity caused by repeated MDMA injections recovers, at least in part, by 24 h after treatment and may contribute to its dissimilar neurotoxic profile including the relatively lack of DA deficits caused by MDMA.
Not all brain areas are vulnerable to METH-induced persistent dopaminergic deficits. For example, the nucleus accumbens and hypothalamus are relatively resistant to the long-term damage caused by the stimulant (Chu et al., in press). Noteworthy and in contrast to effects on VMAT-2C activity in the striatum that persists at least 48 h after treatment, METH reversibly decreases VMAT-2C function in the accumbens, and does not alter VMAT-2 activity in the hypothalamus (Chu et al., in press). These findings support the suggestion that persistent deficits in striatal VMAT-2C function contribute to, or are a part of, the long-term deficits caused by METH.
Finally, it is noteworthy that the impact of METH on VMAT-2C is not restricted to dopaminergic neurons. For example, repeated high-dose METH administrations rapidly reduce hippocampal vesicular DA uptake, as assessed in VMAT-2C-containing vesicles purified from METH-treated rats. This decrease is likely associated with serotonergic nerve terminals. Since pretreatment with the 5HT reuptake inhibitor, fluoxetine, attenuates both the rapid effect on VMAT-2 and the decrease in 5HT content observed 7 d after METH treatment, the data suggest an association between METH-induced alterations in 5HT hippocampal vesicular uptake and the long-term hippocampal 5HT damage caused by the stimulant (Rau et al., 2006).
V. Effects of DA reuptake inhibitors on VMAT-2C and VMAT-2M
In contrast to the ability of DA-releasing agents to rapidly decrease VMAT-2C levels and function, a property common to many DA reuptake inhibitors is an ability to rapidly increase VMAT-2C levels and function. More specifically, these agents appear to redistribute VMAT-2-containing vesicles from the membrane-associated to the non-membrane-associated (cytoplasmic) subcellular fraction, thus resulting in increased vesicular uptake, VMAT-2 immunoreactivity and VMAT-2 ligand binding in this fraction. The first evidence of this phenomenon came from studies by Brown et al. (2001a) who demonstrated that a single administration of cocaine increases both DA uptake and dihydrotetrabenazine (DHTBZ) binding in the VMAT-2C subcellular fraction prepared from the striatum of treated rats: an effect inhibited by pretreatment with a D2, but not D1, antagonist (Brown et al., 2001b). A similar phenomenon was reported after bupropion treatment (Rau et al., 2005). Other reuptake inhibitors demonstrated to increase uptake via the VMAT-2C include amfonelic acid, GBR-12935, RTI-113, RTI-112.2, RTI-177 and RTI-514 (Brown et al., 2001a; Rau et al., 2005).
Riddle et al. (2002) later demonstrated that the cocaine-induced increases in VMAT-2C activity correspond temporally with increases and decreases in VMAT-2C and VMAT-2M immunoreactivity, respectively. Importantly, cocaine treatment is without effect on total synaptosomal VMAT-2 immunoreactivity (i.e., VMAT-2M + VMAT-2C), thus suggesting a redistribution of VMAT-2 protein within the nerve terminal.
A major focus of our studies assessing the impact of reuptake inhibitor administration on VMAT-2C has been methylphenidate (MPD). This stimulant was selected for study as it lacks neurotoxic potential (Zaczek et al., 1989; Yuan et al., 1997) and is used widely to treat disorders including attention deficit hyperactivity disorder. Studies reveal that like cocaine, MPD rapidly and reversibly increases DA uptake via, and ligand binding to, striatal VMAT-2C. In addition, MPD increases VMAT-2C immunoreactivity. Concurrently, MPD decreases VMAT-2M immunoreactivity. Importantly, MPD is without effect on total synaptosomal VMAT-2 immunoreactivity (i.e., VMAT-2M + VMAT-2C). Taken together, these data lead to the hypothesis that like cocaine, MPD causes a redistribution of VMAT-2M and associated vesicles from synaptosomal membranes to the cytoplasm (Sandoval et al., 2002; Volz et al., 2007).
Studies utilizing both tritiated DA uptake (Sandoval et al., 2002) and rotating disk electrode voltammetry (Volz et al., 2007) demonstrate that MPD increases the Vmax, with little or no change in Km, of DA uptake via the VMAT-2C. Further kinetic analysis indicates that MPD increases the density of kinetically active VMAT-2C, but does not affect either the catalytic rate constant or the rate constant for DA binding to the VMAT-2C (Volz et al., 2007). These results suggest that the kinetics of DA binding to the VMAT-2C and translocation of this substrate into the intravesicular space are not affected by MPD. Further, the increase in VMAT-2C and associated vesicle levels after MPD treatment is solely responsible for the increase in DA transport via the VMAT-2C.
Of particular interest are findings that despite decreasing VMAT-2M immunoreactivity, MPD treatment increases the Vmax of vesicular DA uptake of the VMAT-2M. This kinetic upregulation contributes to an increased total DA content in the VMAT-2M-containing subcellular fraction after MPD treatment. Further, MPD administration increases the magnitude and initial velocity of K+-stimulated DA release from striatal suspensions (Volz et al., 2007). This latter effect is prevented by pretreatment with the D2 antagonist, eticlopride. (Volz et al., in press).
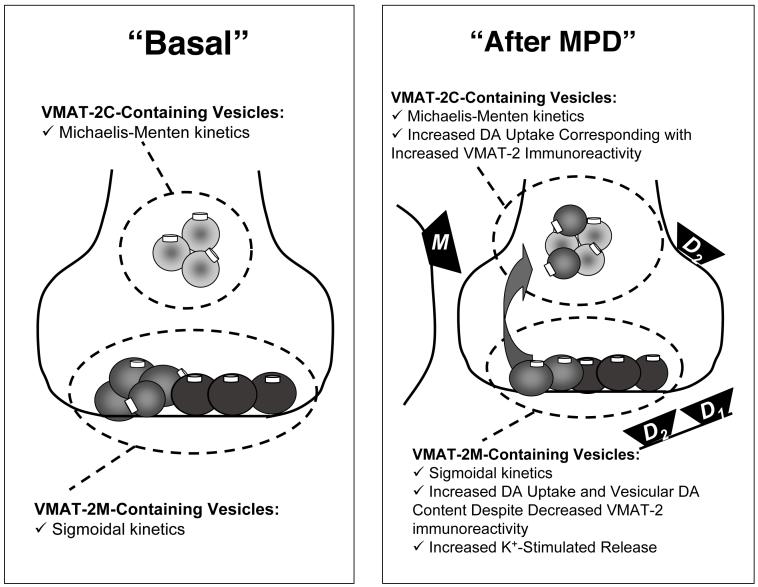
Findings that eticlopride inhibits MPD-induced DA release were unexpected since D2 agonist administration decreases K+-stimulated DA release (Starke et al., 1989; Pothos et al., 1998). Thus, while D2 receptors mediate the effects of MPD on vesicular DA transport, vesicle trafficking and vesicular DA content, other mechanisms must contribute to MPD-induced K+-stimulated release. Further investigation revealed that the muscarinic antagonist, scopolamine, inhibits the enhanced K+-stimulated release caused by MPD (Volz et al., in press). A summary of the acute effects of MPD on VMAT-2 localization and function is presented in Figure 1.
Figure 1.
Schematic representation illustrating the impact of MPD on VMAT-2C- and VMAT-2M-containing vesicles, and the receptors (e.g., D1, D2 and muscarinic (M)) that mediate these effects as described in text.
One potential functional consequence of the ability of MPD to increase DA sequestration via the VMAT-2C and VMAT-2M relates to findings that MPD post-treatment protects against the persistent dopaminergic deficits caused by METH (Sandoval et al., 2003). As noted above, it has been suggested that METH promotes aberrant cytoplasmic DA accumulation and the subsequent formation of DA-associated reactive oxygen species, thus leading to long-term damage. Accordingly, an MPD-induced sequestration of DA via the VMAT-2C and the higher capacity VMAT-2M may afford neuroprotection by preventing this degenerative process.
VI. Effects of DA receptor activation on VMAT-2C
Activation of D2 receptors contributes to the amphetamine analog-induced alteration in VMAT-2C function, as indicated by findings that the decreases in vesicular DA uptake caused by multiple MDMA or a single METH injection(s) are attenuated by pretreatment with a D2 antagonist (Brown et al., 2002; Hansen et al., 2002). However, the role of D2 receptors in affecting vesicular DA uptake is complex, as evidenced by findings noted above that D2 antagonist pretreatment also attenuates MPD- and cocaine-induced increases in VMAT-2C-mediated uptake and/or VMAT-2C distribution (Brown et al., 2001b; Sandoval et al., 2002). Moreover, D2 agonist administration increases VMAT-2C-mediated uptake, DHTBZ binding to the VMAT-2C, and VMAT-2C immunoreactivity (Brown et al., 2001b; Truong et al., 2003, 2004 a,b). Additional studies are required to sort out the significance of these findings.
VII. Effects of Development
Many thousands of adolescents and young adults have used high doses of illicit METH. This is of concern since, as noted above, METH treatment causes persistent dopaminergic deficits in adult animal models. Effects in adolescents have received less attention.
In rodent models, several studies have demonstrated that younger animals are more resistant than older animals to the monoaminergic damage caused by METH. There are age-dependent differences in the acute response of VMAT-2 to METH as well. In particular, repeated high-dose METH injections cause greater acute decreases in uptake via the VMAT-2C in postnatal day (PND) 90 (i.e., young adult) vs. PND 40 (i.e., adolescent) rats (Truong et al., 2005). Greater basal levels of VMAT-2C in PND 90 vs. PND 40 rats likely account for this difference in the magnitude of this METH effect. Findings of greater VMAT-2C levels in the adult animals were unexpected, as it was predicted that the older rats that were more susceptible to long-term METH-induced DA deficits would be so because these animals lack DA sequestration capacity. Instead, older rats appear to have more VMAT-2C-associated activity, perhaps to compensate for having greater striatal DA tissue content to manage (Truong et al., 2005).
Rotating disk electrode voltammetry studies confirm findings of Truong et al. (2005) by demonstrating that the initial velocities of both VMAT-2C-mediated DA uptake and METH-induced DA efflux are less in PND 38 - 42 vs. PND 88 - 92 rats. The lower uptake is due to a lower Vmax with no change in the Km. Both the turnover number and rate constant for the association of DA with VMAT-2 are similar in these age groups (Volz et al., 2006b).
VIII. Conclusion
The preclinical studies described above have focused on the impact of stimulant administration on VMAT-2 function and distribution of associated vesicles within nerve terminals. These investigations have extended our understanding that changes in the localization and function of VMAT-2 and associated vesicles, especially those occurring in the first hours to days after drug treatment, represent processes beyond simply dopaminergic nerve terminal loss. Instead, these studies highlight the fact that the VMAT-2 and associated vesicles are highly regulatable, and may serve as important targets for pharmacological manipulations, with chemical or side effect implications.
In addition, it is important to note that the regulation of VMAT-2 is not limited to changes resulting from redistribution of the transporter and its associated DA-containing vesicles or to the kinetic differences described above for the VMAT-2C and VMAT-2M. For example, a distinctly different modulatory process involving targeting and trafficking of the VMAT-2 protein per se to particular subcellular structures (e.g., large dense-core vesicles) has been described in neuroendocrine PC12 cells (Li et al., 2005), and its elucidation may provide insight into mechanisms underlying regulation of vesicular trafficking in vivo. It is also worth mentioning that at least one agent that inhibits VMAT2 function, lobeline, attenuates both behavioral and neurochemical effects of amphetamine in rodents, thus further suggesting that VMAT2 may be an important therapeutic target (for review, see Zheng et al., 2006 and references therein). Future studies investigating the various mechanisms underlying alterations in VMAT-2 and its sequestration capacity are warranted as these will provide insight into both the physiolological regulation of dopaminergic systems, and the pathophysiology of a variety of DA-related disorders ranging from substance abuse to neurodegenerative diseases such as Parkinson’s disease.
IX. Acknowledgements
The authors thank the numerous graduate students, postdoctoral researchers and faculty who have contributed to many of the studies described in this manuscript. Support from the National Institutes of Health (DA000378, DA00869, DA04222, DA13367, DA11389 and DA019447) is also appreciated greatly.
IX. References
- Brown JM, Hanson GR, Fleckenstein AE. Methamphetamine rapidly decreases vesicular dopamine uptake. J. Neurochem. 2000;74:2221–2223. doi: 10.1046/j.1471-4159.2000.0742221.x. [DOI] [PubMed] [Google Scholar]
- Brown JM, Hanson GR, Fleckenstein AE. Regulation of the vesicular monoamine transporter-2: a novel mechanism for cocaine and other psychostimulants. J. Pharmacol. Exp. Ther. 2001a;296:762–767. [PubMed] [Google Scholar]
- Brown JM, Hanson GR, Fleckenstein AE. Cocaine-induced increases in vesicular dopamine uptake: role of dopamine receptors. J. Pharmacol. Exp. Ther. 2001b;298:1150–1153. [PubMed] [Google Scholar]
- Brown JM, Riddle EL, Sandoval V, Weston RK, Hanson JE, Crosby MJ, Ugarte YV, Gibb JW, Hanson GR, Fleckenstein AE. A single methamphetamine administration rapidly decreases vesicular dopamine uptake. J. Pharmacol. Exp. Ther. 2002;302:497–501. doi: 10.1124/jpet.302.2.497. [DOI] [PubMed] [Google Scholar]
- Chu PW, Seferian KS, Birdsall E, Truong JG, Riordan JA, Metcalf CS, Hanson GR, Fleckenstein AE. Differential regional effects of methamphetamine on dopamine transport. Eur. J. Pharmacol. doi: 10.1016/j.ejphar.2008.05.028. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubells JF, Rayport S, Rajendran G, Sulzer D. Methamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. J. Neurosci. 1994;14:2260–2271. doi: 10.1523/JNEUROSCI.14-04-02260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Gaffney M, Weihmuller FB, O’Dell SJ, Marshall JF. Striatal subregions are differentially vulnerable to the neurotoxic effects of methamphetamine. Brain Res. 1992;598:321–326. doi: 10.1016/0006-8993(92)90201-j. [DOI] [PubMed] [Google Scholar]
- Eyerman DJ, Yamamoto BK. Lobeline attenuates methamphetamine-induced changes in vesicular monoamine transporter 2 immunoreactivity and monoamine depletions in the striatum. J. Pharmacol. Exp. Ther. 2005;312:160–169. doi: 10.1124/jpet.104.072264. [DOI] [PubMed] [Google Scholar]
- Eyerman DJ, Yamamoto BK. A rapid oxidation and persistent decrease in the vesicular monoamine transporter 2 after methamphetamine. J. Neurochem. 2007;103:1219–1227. doi: 10.1111/j.1471-4159.2007.04837.x. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Wilkins DG, Gibb JW, Hanson GR. Interaction between hyperthermia and oxygen radical formation in the 5-hydroxytryptaminergic response to a single methamphetamine administration. J. Pharmacol. Exp. Ther. 1997;283:281–285. [PubMed] [Google Scholar]
- Frey KA, Koeppe RA, Kilbourn MR, Vander Borght TM, Albin RL, Gilman S, Kuhl DE. Presynaptic monoaminergic vesicles in Parkinson’s disease and normal aging. Ann. Neurol. 1996;40:873–884. doi: 10.1002/ana.410400609. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Gainetdinov RR, Wang YM, Valenzano KJ, Miller GW, Caron MG. Increased methamphetamine neurotoxicity in heterozygous vesicular monoamine transporter 2 knockout mice. J. Neurosci. 1999;19:2424–2431. doi: 10.1523/JNEUROSCI.19-07-02424.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb JW, Kogan FJ. Influence of dopamine synthesis on methamphetamine-induced changes in striatal and adrenal tyrosine hydroxylase activity. Naunyn Schmiedebergs Arch. Pharmacol. 1979;310:185–187. doi: 10.1007/BF00500283. [DOI] [PubMed] [Google Scholar]
- Giovanni A, Liang LP, Hastings TG, Zigmond MJ. Estimating hydroxyl radical content in rat brain using systemic and intraventricular salicylate: impact of methamphetamine. J. Neurochem. 1995;64:1819–1825. doi: 10.1046/j.1471-4159.1995.64041819.x. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Nihei MK, McGlothan JL, Howard AS. Methamphetamine-induced deficits of brain monoaminergic neuronal markers: distal axotomy or neuronal plasticity. Neurosci. 2003;122:499–513. doi: 10.1016/s0306-4522(03)00476-7. [DOI] [PubMed] [Google Scholar]
- Guillot TS, Richardson JR, Wang MZ, Li YJ, Taylor TN, Ciliax BJ, Zachrisson O, Mercer A, Miller GW. PACAP38 increases vesicular monoamine transporter 2 (VMAT2) expression and attenuates methamphetamine toxicity. Neuropeptides. 2008 Jun 2; doi: 10.1016/j.npep.2008.04.003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JP, Riddle EL, Sandoval V, Brown JM, Gibb JW, Hanson GR, Fleckenstein AE. Methylenedioxymethamphetamine decreases plasmalemmal and vesicular dopamine transport: mechanisms and implications for neurotoxicity. J. Pharmacol. Exp. Ther. 2002;300:1093–1100. doi: 10.1124/jpet.300.3.1093. [DOI] [PubMed] [Google Scholar]
- Hogan KA, Staal RG, Sonsalla PK. Analysis of VMAT2 binding after methamphetamine or MPTP treatment: disparity between homogenates and vesicle preparations. J. Neurochem. 2000;74:2217–2220. doi: 10.1046/j.1471-4159.2000.0742217.x. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AJ, Morgan ME, Gibb JW. The long-term effects of multiple doses of methamphetamine on neostriatal tryptophan hydroxylase, tyrosine hydroxylase, choline acetyltransferase, and glutamate decarboxylase activities. Life Sci. 1979;25:1373–1378. doi: 10.1016/0024-3205(79)90414-4. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AJ, Gibb JW. Long-term effects of multiple doses of methamphetamine on tryptophan hydroxylase and tyrosine hydroxylase activity in rat brain. J. Pharmacol. Exp. Ther. 1980;214:257–262. [PubMed] [Google Scholar]
- Johanson CE, Frey KA, Lundahl LH, Keenan P, Lockhart N, Roll J, Galloway GP, Koeppe RA, Kilbourn MR, Robbins T, Schuster CR. Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology. 2006;185:327–328. doi: 10.1007/s00213-006-0330-6. [DOI] [PubMed] [Google Scholar]
- Kita T, Takahashi M, Kubo K, Wagner GC, Nakashima T. Hydroxyl radical formation following methamphetamine administration to rats. Pharmacol. Toxicol. 1999;85:133–137. doi: 10.1111/j.1600-0773.1999.tb00080.x. [DOI] [PubMed] [Google Scholar]
- Kogan FJ, Nichols WK, Gibb JW. Influence of methamphetamine on nigral and striatal tyrosine hydroxylase activities and on striatal dopamine levels. Eur. J. Pharmacol. 1976;36:363–371. doi: 10.1016/0014-2999(76)90090-x. [DOI] [PubMed] [Google Scholar]
- Larsen KE, Fon EA, Hastings TG, Edwards RH, Sulzer D. Methamphetamine-induced degeneration of dopaminergic neurons involves autophagy and upregulation of dopamine synthesis. J. Neurosci. 2002;22:8951–8960. doi: 10.1523/JNEUROSCI.22-20-08951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVoie MJ, Hastings TG. Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: evidence against a role for extracellular dopamine. J. Neurosci. 1999;19:1484–1491. doi: 10.1523/JNEUROSCI.19-04-01484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Waites CL, Staal RG, Dobryy Y, Park J, Sulzer DL, Edwards RH. Sorting of vesicular monoamine transporter 2 to the regulated secretory pathway confers the somatodendritic exocytosis of monoamines. Neuron. 2005;48:619–33. doi: 10.1016/j.neuron.2005.09.033. [DOI] [PubMed] [Google Scholar]
- Miller GW, Erickson JD, Perez JT, Penland SN, Mash DC, Rye DB, Levey AI. Immunochemical analysis of vesicular monoamine transporter (VMAT2) protein in Parkinson’s disease. Exp. Neurol. 1999;156:138–148. doi: 10.1006/exnr.1998.7008. [DOI] [PubMed] [Google Scholar]
- Morgan ME, Gibb JW. Short-term and long-term effects of methamphetamine on biogenic amine metabolism in extra-striatal dopaminergic nuclei. Neuropharmacology. 1980;10:989–995. doi: 10.1016/0028-3908(80)90010-6. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Predborski S, Davila V, Schmitz Y, Sulzer D. D2-like dopamine autoreceptor activation reduces quantal size in PC12 cells. J. Neurosci. 1998;18:5575–5585. doi: 10.1523/JNEUROSCI.18-15-05575.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau KS, Birdsall E, Hanson JE, Johnson-Davis KL, Carroll FI, Wilkins DG, Gibb JW, Hanson GR, Fleckenstein AE. Bupropion increases striatal vesicular monoamine transport. Neuropharmacology. 2005;49:820–830. doi: 10.1016/j.neuropharm.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Rau KS, Birdsall E, Volz TJ, Riordan JA, Baucum AJ, Adair BP, Bitter R, Gibb JW, Hanson GR, Fleckenstein AE. Methamphetamine administration reduces hippocampal vesicular monoamine transporter-2 uptake. J. Pharmacol. Exp. Ther. 2006;318:676–682. doi: 10.1124/jpet.105.099200. [DOI] [PubMed] [Google Scholar]
- Riddle EL, Topham MK, Haycock JW, Hanson GR, Fleckenstein AE. Differential trafficking of the vesicular monoamine transporter-2 by methamphetamine and cocaine. Eur. J. Pharmacol. 2002;449:71–74. doi: 10.1016/s0014-2999(02)01985-4. [DOI] [PubMed] [Google Scholar]
- Riddle EL, Hanson GR, Fleckenstein AE. Therapeutic doses of amphetamine and methylphenidate selectively redistribute the vesicular monoamine transporter-2. Eur. J. Pharmacol. 2007;571:25–28. doi: 10.1016/j.ejphar.2007.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli SO, Betz WJ. Synaptic vesicle pools. Nature Rev. 2005;6:49–61. doi: 10.1038/nrn1583. [DOI] [PubMed] [Google Scholar]
- Sandoval V, Riddle EL, Hanson GR, Fleckenstein AE. Methylphenidate alters vesicular monoamine transport and prevents methamphetamine-induced dopaminergic deficits. J. Pharmacol. Exp. Ther. 2003;304:1181–1187. doi: 10.1124/jpet.102.045005. [DOI] [PubMed] [Google Scholar]
- Sandoval V, Riddle EL, Hanson GR, Fleckenstein AE. Methylphenidate redistributes vesicular monoamine transporter-2: role of dopamine receptors. J. Neurosci. 2002;22:8705–8710. doi: 10.1523/JNEUROSCI.22-19-08705.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CJ, Ritter JK, Sonsalla PK, Hanson GR, Gibb JW. Role of dopamine in the neurotoxic effects of methamphetamine. J Pharmacol Exp Ther. 1985;233:539–544. [PubMed] [Google Scholar]
- Seiden LS, Fischman MW, Schuster CR. Long-term methamphetamine induced changes in brain catecholamines in tolerant rhesus monkeys. Drug Alcohol Depend. 1976;1:215–219. doi: 10.1016/0376-8716(76)90030-2. [DOI] [PubMed] [Google Scholar]
- Starke K, Gothert M, Kilbinger H. Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol. Rev. 69:864–989. doi: 10.1152/physrev.1989.69.3.864. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle revisited. Neuron. 2000;28:317–320. doi: 10.1016/s0896-6273(00)00109-4. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Tata DA, Raudensky J, Yamamoto BK. Augmentation of methamphetamine-induced toxicity in the rat striatum by unpredictable stress: contribution of enhanced hyperthermia. Eur. J. Neurosci. 2007;26:739–748. doi: 10.1111/j.1460-9568.2007.05688.x. [DOI] [PubMed] [Google Scholar]
- Teng L, Crooks PA, Sonsalla PK, Dwoskin LP. Lobeline and nicotine evoke [3H]overflow from rat striatal slices preloaded with [3H]dopamine: differential inhibition of synaptosomal and vesicular [3H]dopamine uptake. J Pharmacol Exp Ther. 1997;280:1432–1444. [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM. The newly synthesized pool of dopamine determines the severity of methamphetamine-induced neurotoxicity. J. Neurochem. 2008 doi: 10.1111/j.1471-4159.2007.05155.x. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong JG, Hanson GR, Fleckenstein AE. Apomorphine increases vesicular monoamine transporter-2 function: implications for neurodegeneration. Eur. J. Pharmacol. 2004a;492:143–147. doi: 10.1016/j.ejphar.2004.03.060. [DOI] [PubMed] [Google Scholar]
- Truong JG, Newman AH, Hanson GR, Fleckenstein AE. Dopamine D2 receptor activation increases vesicular dopamine uptake and redistributes vesicular monoamine transporter-2 protein. Eur. J. Pharmacol. 2004b;504:27–32. doi: 10.1016/j.ejphar.2004.09.049. [DOI] [PubMed] [Google Scholar]
- Truong JG, Rau KS, Hanson GR, Fleckenstein AE. Pramipexole increases vesicular dopamine uptake: implications for treatment of Parkinson’s neurodegeneration. Eur. J. Pharmacol. 2003;474:223–226. doi: 10.1016/s0014-2999(03)02080-6. [DOI] [PubMed] [Google Scholar]
- Truong JG, Wilkins DG, Baudys J, Crouch DJ, Johnson-Davis KL, Gibb JW, Hanson GR, Fleckenstein AE. Age-dependent methamphetamine-induced alterations in vesicular monoamine transporter-2 function: Implications for neurotoxicity. J. Pharmacol. Exp. Ther. 2005;314:1087–1092. doi: 10.1124/jpet.105.085951. [DOI] [PubMed] [Google Scholar]
- Vergo S, Johansen JL, Leist M, Lotharius J. Vesicular monoamine transporter 2 regulates the sensitivity of rat dopaminergic neurons to disturbed cytosolic dopamine levels. Brain Res. 2007;1185:18–32. doi: 10.1016/j.brainres.2007.09.028. [DOI] [PubMed] [Google Scholar]
- Volz TJ, Farnsworth SJ, King JL, Riddle EL, Hanson GR, Fleckenstein AE. Methylphenidate administration alters vesicular monoamine transporter-2 function in cytoplasmic and membrane-associated vesicles. J. Pharmacol. Exp. Ther. 2007;323:738–745. doi: 10.1124/jpet.107.126888. [DOI] [PubMed] [Google Scholar]
- Volz TJ, Farnsworth SJ, Rowley SD, Hanson GR, Fleckenstein AE. Methylphenidate-induced increases in vesicular dopamine sequestration and dopamine release in the striatum: The role of muscarinic and dopamine D2 receptors. J. Pharmacol., Exp. Ther. doi: 10.1124/jpet.108.139386. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz TJ, Hanson GR, Fleckenstein AE. Measurement of kinetically resolved vesicular dopamine uptake and efflux using rotating disk electrode voltammetry. J. Neurosci. Methods. 2006a;155:109–115. doi: 10.1016/j.jneumeth.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Volz TJ, Hanson GR, Fleckenstein AE. Kinetic analysis of age-dependent changes in vesicular monoamine transporter-2 function. Synapse. 2006b;60:474–477. doi: 10.1002/syn.20321. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J. Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Res. 1980;181:151–160. doi: 10.1016/0006-8993(80)91265-2. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Lucot JB, Schuster CR, Seiden LS. Alpha-methyltyrosine attenuates and reserpine increases methamphetamine-induced neuronal changes. Brain Res. 1983;270:285–288. doi: 10.1016/0006-8993(83)90602-9. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat. Med. 1996a;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Levey AI, Rajput A, Ang L, Guttman M, Shannak K, Niznik HB, Hornykiewicz O, Pifl C, Kish SJ. Differential changes in neurochemical markers of striatal dopamine nerve terminals in idiopathic Parkinson’s disease. Neurology. 1996b;47:718–726. doi: 10.1212/wnl.47.3.718. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Zhu W. The effects of methamphetamine on the production of free radicals and oxidative stress. J Pharmacol Exp Ther. 1998;287:107–114. [PubMed] [Google Scholar]
- Yuan J, McCann U, Ricaurte G. Methylphenidate and brain dopamine neurotoxicity. Brain Res. 1997;767:172–175. doi: 10.1016/s0006-8993(97)00771-3. [DOI] [PubMed] [Google Scholar]
- Zaczek R, Battaglia G, Contrera JF, Culp S, De Souza EB. Methylphenidate and pemoline do not cause depletion of rat brain monoamine markers similar to that observed with methamphetamine. Toxicol Appl Pharmacol. 1989;100:227–233. doi: 10.1016/0041-008x(89)90309-8. [DOI] [PubMed] [Google Scholar]
- Zheng G, Dwoskin LP, Crooks PA. Vesicular monoamine transporter 2: role as a novel target for drug development. AAPS J. 2006;8:E682–92. doi: 10.1208/aapsj080478. [DOI] [PMC free article] [PubMed] [Google Scholar]