Abstract
Rationale
Nicotine-induced cognitive enhancement may be a factor maintaining tobacco smoking, particularly in psychiatric populations suffering from cognitive deficits. Schizophrenia patients exhibit higher smoking rates compared with the general population, suggesting that attempts to self-medicate cognitive schizophrenia deficits may underlie these high smoking levels.
Objectives
The present study explored pro-cognitive effects of nicotine in a model of schizophrenia-like cognitive dysfunction to test this self-medication hypothesis.
Methods
We investigated whether chronic nicotine (3.16 mg/kg/day, base) would attenuate the performance disruption in the five-choice serial reaction time task (5-CSRTT, a task assessing various cognitive modalities, including attention) induced by repeated administration of phencyclidine (PCP), an N-methyl-d-aspartate receptor antagonist that induces cognitive deficits relevant to schizophrenia.
Results
Chronic nicotine administration shortened 5-CSRTT response latencies under baseline conditions. Nicotine-treated rats also made more correct responses and fewer omissions than vehicle-treated rats. Replicating previous studies, repeated PCP administration (2 mg/kg, 30 min before behavioral testing for 2 consecutive days followed 2 weeks later by 5 consecutive days of PCP administration) decreased accuracy and increased response latencies, premature responding, and timeout responding. Chronic nicotine did not attenuate these PCP-induced disruptions.
Conclusions
Chronic nicotine had pro-cognitive effects by itself, supporting the hypothesis that cognitive enhancement may contribute to tobacco smoking. At the doses of nicotine and PCP used, however, no support was found for the hypothesis that the beneficial effects of nicotine on cognitive deficits induced by repeated PCP administration, assessed in the 5-CSRTT, are larger than nicotine effects in the absence of PCP.
Keywords: cognition, schizophrenia, nicotine, phencyclidine, attention, processing speed, 5-CSRTT, rat
Introduction
Schizophrenia is a severe psychiatric disorder characterized by profound cognitive deficits (Elvevag and Goldberg 2000) that affect a wide range of cognitive modalities, including attention (Laurent et al. 1999), processing speed (Nelson et al. 1990), response inhibition/impulse control (Wykes et al. 2000), and cognitive flexibility (Morice 1990). Cognitive dysfunction in schizophrenia is highly correlated with functional impairment and long-term disability and is not well treated by currently available medications (Sharma and Antonova 2003).
Tobacco smoking rates in schizophrenia patients are strikingly high. While only 25-30% of the general population smoke tobacco, the incidence in schizophrenia patients is nearly 90% (Hughes et al. 1986), with most patients being heavy smokers (De Leon et al. 2002). Nicotine, one of the major psychoactive components of tobacco smoke, can improve aspects of cognition (Mangan 1983; Peeke and Peeke 1984), particularly attention (Rusted and Warburton 1992; Warburton et al. 1992; Parrott and Craig 1992; Bates et al. 1995). The desire for cognitive benefits derived from nicotine has been suggested to be a major motivational force driving tobacco smoking (Warburton 1990). Self-assessments from tobacco smokers support this hypothesis. In a survey in Wisconsin, 17% of light smokers, 21% of moderate smokers, and 27% of heavy smokers listed “helps me concentrate” as a reason for smoking tobacco (Meyer 2002). The high smoking rates among schizophrenia patients, therefore, may reflect an attempt to self-medicate the cognitive symptoms of the disorder. This observation also suggests that a dysfunction in nicotinic acetylcholine receptor signaling may be involved in the etiology of cognitive deficits in schizophrenia (Freedman et al. 1994).
Translational studies using experimental animals, such as rodents, allow for the investigation of the effects of nicotine on general cognitive function and schizophrenia-like cognitive deficits under tightly controlled conditions. In the present study, we used a rat model that employed phencyclidine (PCP), a dissociative anesthetic that acts as a noncompetitive antagonist at N-methyl-d-aspartate (NMDA) glutamate receptors, as the inducing condition. PCP intoxication mimics a variety of schizophrenia symptoms, including cognitive disruption, in both humans and animals (Luby et al. 1959; Pradhan 1984; Handelmann et al. 1987) and is widely used to induce a variety of behaviors and deficits relevant to schizophrenia (Javitt 1987; Steinpreis 1996; Jentsch and Roth 1999). Specifically, PCP exposure disrupts cognition in humans and animals, producing a wide range of deficits that include attentional impairment (Pearlson 1981; Pradhan 1984; Jin et al. 1997), decreased processing speed (Pearlson 1981; Pradhan 1984; Jin et al. 1997), decreased response inhibition (Sanger and Jackson 1989; Jin et al. 1997; Jentsch and Taylor 2001), and cognitive inflexibility (Jentsch and Taylor 2001; Rodefer et al. 2005). The profile of cognitive disruption induced by PCP administration, therefore, parallels deficits present in schizophrenia patients.
We measured cognitive performance and impairment using the 5-choice serial reaction time task (5-CSRTT) that assesses several cognitive modalities. The 5-CSRTT was originally developed as a test of attentional performance (Carli et al. 1983; Robbins 2002) and constitutes a well-established method to assess spatial sustained and divided attention. In addition, the 5-CSRTT includes measures of disinhibition of inappropriate responding and has become recognized as a test of impulsivity (Puumala et al. 1996; Evenden 1990). Furthermore, the task provides measures of processing speed and compulsivity or cognitive inflexibility (Robbins 2002). Nicotine improves 5-CSRTT performance, increasing attentional accuracy and processing speed (Stolerman et al. 2000; Hahn et al. 2002; Bizarro et al. 2004; Semenova et al. 2007). Repeated administration of PCP induces a profile of disruption in the 5-CSRTT that mirrors several of the cognitive deficits seen in schizophrenia, including impaired attention, increased impulsivity, cognitive inflexibility, and decreased processing speed (Amitai et al. 2007). These deficits are sensitive to attenuation by the atypical antipsychotic clozapine (Amitai et al. 2007) that has partial effectiveness in ameliorating cognitive dysfunction in schizophrenia (Meltzer and McGurk 1999; Bilder et al. 2002), indicating that the model of PCP-induced disruptions in the 5-CSRTT has predictive validity (Geyer and Markou 1995).
In the present study, we investigated the effects of chronic nicotine treatment on cognitive performance in the 5-CSRTT under both baseline conditions and during repeated PCP administration. We hypothesized that the cognition-enhancing effects of nicotine would be particularly pronounced in situations of impaired cognitive performance. Therefore, we predicted that chronic nicotine treatment would improve measures of 5-CSRTT performance, particularly attentional function, and that these beneficial effects on cognition would be larger when 5-CSRTT performance was degraded by repeated PCP administration than in the absence of PCP treatment.
Materials and methods
Animals
Twenty-four male Wistar rats (Charles River Laboratories, Wilmington, MA) were housed two per cage on a 12 h:12 h reversed light-dark cycle (lights off at 8:00 am). All behavioral testing was conducted during the animals’ dark cycle. Rats were allowed to reach a body weight of at least 300 g before being restricted to 20 g of food per day (in addition to the food pellets earned during testing) and initiation of behavioral training. Water was available ad libitum at all times except during testing. Animals were treated in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care and the National Research Council’s Guide for Care and Use of Laboratory Animals. All experiments were approved by the University of California, San Diego, Animal Care and Use Committee.
Drugs
Nicotine bitartrate was purchased from Sigma (St. Louis, MO), and d-phencyclidine hydrochloride (PCP) was obtained from the National Institute on Drug Abuse (Bethesda, MD). PCP and nicotine were dissolved in 0.9% saline solution. PCP was administered by subcutaneous (s.c.) injection in a volume of 2 ml/kg. Nicotine was delivered via subcutaneous osmotic minipumps.
Apparatus
Training and testing were conducted in operant testing chambers enclosed in sound-attenuating chambers (Med Associates, St. Albans, VT). Each testing chamber contained a curved rear wall with nine contiguous apertures. Metal inserts covered every alternate hole, leaving open holes 1, 3, 5, 7, and 9. A photocell beam located at the entrance of each aperture detected nosepoke responses, and a 3 W stimulus light was located at the rear of each aperture. Food pellets could be delivered automatically into a magazine located in the opposite wall via a food dispenser; a photocell beam detected head entries into the magazine. A computer running MedPC software (Med Associates, St. Albans, VT) controlled the apparatus.
5-choice serial reaction time task procedure
Animals were allowed to habituate to the operant testing chambers for 20 min on 2 consecutive days. During these sessions, each open aperture and the food magazine were baited with 5-10 food pellets to encourage rats to explore them. Next, rats were trained to retrieve food rewards from the magazine in three daily 20 min sessions during which a food pellet was delivered noncontingently into the magazine every 20 s. Preliminary training sessions followed, in which all apertures were illuminated until the rat made a nosepoke response into any aperture that resulted in extinction of all apertures and delivery of a food pellet into the magazine. Head entries into the magazine initiated an intertrial interval (ITI) varying from 2-10 s, after which all apertures again were illuminated. Training on the 5-CSRTT task began once rats retrieved >70 rewards for two consecutive sessions. The training procedure for the 5-CSRTT was based on the protocol established by Robbins and colleagues (Carli et al. 1983). Briefly, trials were initiated by a head entry into the food magazine. An initial noncontingent food pellet was delivered into the magazine at the start of each session to facilitate initiation of the first trial. After a variable ITI ranging from 2-10 s, a light stimulus was presented in one of the response apertures. A nosepoke in this aperture within a limited hold period (correct response) resulted in the delivery of a food pellet into the magazine. Nosepokes in a wrong aperture (incorrect responses) or failures to respond within the limited hold period (omissions) were punished by a 5 s timeout, marked by extinction of the house light and no delivery of food reward. Nosepokes in any aperture made before presentation of the target stimulus (premature responses) likewise resulted in a timeout and no food reward. Nosepokes during the timeout period (timeout responses) reset the timeout period. Each session lasted 30 min or until 100 trials had been completed, whichever occurred first. The duration of the light stimulus and the limited hold period were initially set at 30 s and 60 s, respectively, and gradually decreased over the course of training to a 1 s stimulus duration and a 5 s limited hold. Rats were trained until they had achieved criterion performance (>70% accuracy and <30 total omissions) and stable baselines (<10% variation in accuracy over 5 consecutive days). On average, 40 sessions were required for rats to attain criterion performance. The following measures were recorded to assess task performance:
Accuracy: number of correct responses divided by the sum of correct and incorrect responses [# correct responses/(# correct responses + # incorrect responses) × 100]. Accuracy was only computed if correct + incorrect responses totaled 10 or more.
Percent correct responses: total number of correct responses divided by the total number of trials.
Percent omissions: total number of omissions divided by the total number of trials.
Premature responses: total number of responses performed during the ITI, before presentation of the light stimulus.
Timeout responses: total number of responses performed during a timeout period.
Latency to correct response: time from the onset of the light stimulus to the performance of a correct nosepoke response.
Latency to incorrect response: time from the onset of the light stimulus to the performance of a correct nosepoke response. This latency measure was assessed in all experiments but is not reported here because it exhibited essentially the same drug effects as latency to correct response.
Latency to reward retrieval: time from the performance of a correct response to the retrieval of the food reward from the magazine.
Total trials: total number of trials initiated during the session.
Osmotic minipump implantation and removal
Rats were anesthetized with a 1-3% isoflurane/oxygen vapor mixture, and an osmotic minipump (Alzet model 2ML1 14 day pump, Alza Corporation, Palo Alto, CA) containing either nicotine or saline (see experimental design below) was inserted subcutaneously on the back of the animal parallel to the spine, with the flow moderator directed posteriorly. The wound was stapled, and an antibacterial ointment was applied to the incision area. On day 14, an incision was made under anesthesia, the minipump was removed, the wound was closed with surgical staples, and an antibacterial ointment was applied.
Experimental design
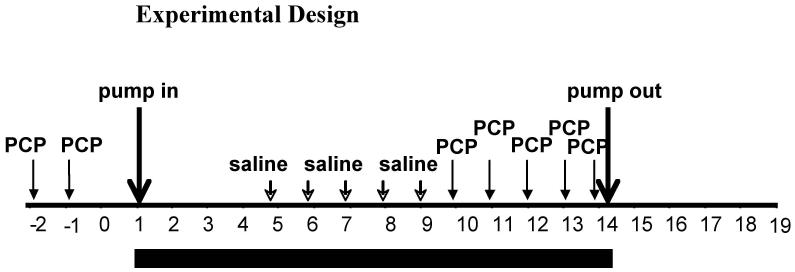
After establishment of stable performance in the 5-CSRTT (<10% variation in accuracy over 5 consecutive days for each individual rat), rats received two initial subcutaneous injections of 2 mg/kg PCP, separated by 24 h. The rats then were assigned to two groups that did not differ in accuracy, correct responses, incorrect responses, and premature responses both under baseline conditions and during this initial PCP exposure. This group-matching procedure was necessitated by the considerable variability in responsiveness to performance disruption with PCP, which cannot be predicted from an animal’s baseline performance. Balancing the groups based on both their performance in the absence of PCP and during PCP exposure ensured that differential responsiveness to PCP would not confound the results after nicotine treatment. One group (n = 12) was prepared with 14 day osmotic minipumps delivering 3.16 mg/kg/day nicotine base (9 mg/kg/day nicotine salt); the other group (n = 12) received pumps containing saline. After 3 initial days of nicotine/vehicle exposure, all rats received five consecutive daily subcutaneous saline injections 30 min before 5-CSRTT testing to habituate them to the injection procedure, followed by five consecutive daily subcutaneous injections with 2 mg/kg PCP 30 min before 5-CSRTT testing. Overall, this design allowed for the comparison of the effects of nicotine vs. vehicle (between-subjects factor) as well as of PCP vs. saline (within-subjects factor), with all factorial combinations (saline/saline, saline/PCP, nicotine/saline, nicotine/PCP) explored. The mixed within/between-subjects design was necessitated by the long training times required by the task and the large numbers of animals needed in each group to reduce the considerable variation in the behavioral effects of PCP commonly observed (Idris et al. 2005; Amitai et al. 2007). Previous studies in our laboratory demonstrated that the repeated injection procedure alone does not induce any changes in any of the task parameters (Amitai et al. 2007). Repeated injections of PCP were used because previous studies showed that a single injection of PCP produced a general, nonspecific response suppression that partially or completely occluded the observation of specific cognitive and other deficits relevant to schizophrenia (Amitai et al. 2007). Pumps were removed on the 14th day of nicotine/saline exposure, and rats were tested daily in the 5-CSRTT for 10 additional days to assess the return of performance to baseline levels (see Fig. 1 for a diagram of the experimental design). PCP was administered at a concentration of 2 ml/kg because irritation at the injection site had been observed with repeated PCP injections at 1 ml/kg, even when varying the injection site.
Figure 1.
Diagram of experimental design.
Data analyses
Data were analyzed using two-way mixed-design analyses of variance (ANOVA). To assess the effects of chronic nicotine by itself, average values from the 5 days preceding any drug treatment (“baseline days”) were compared with average values obtained during the 9 days of PCP-free pump treatment. Presence of Pump (before pump implantation/during pump exposure) was the within-subjects factor, and Pump Content (nicotine/vehicle) was the between-subjects factor. To assess the effects of chronic nicotine on performance disruption induced by repeated PCP administration, average values from the 5 days of saline injection during pump treatment were compared with average values from the 5 days of PCP administration during pump treatment. Drug Challenge (PCP/saline) was the within-subjects factor, and Pump Content (nicotine/saline) was the between-subjects factor. When statistically significant effects were found in the ANOVAs, post hoc comparisons among means were conducted using Bonferroni tests. The level of significance was set at 0.05. Data were analyzed using the GraphPad Prism statistical package (GraphPad, San Diego, CA).
Results
Effects of chronic nicotine on 5-CSRTT under baseline conditions
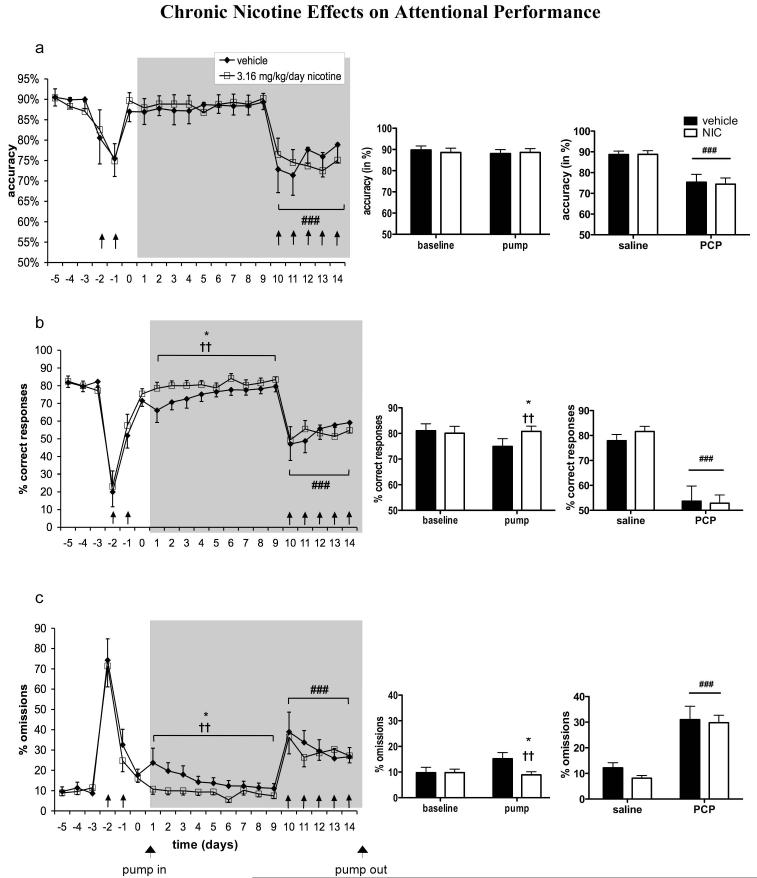
No effects of Presence of Pump and Pump Content on accuracy were found (Fig. 2a). A significant main effect of Presence of Pump on percent correct responses [F(1,22) = 5.98, p < 0.05] and a significant Presence of Pump × Pump Content interaction [F(1,22) = 9.40, p < 0.01] were seen, indicating that rats in the chronic nicotine group exhibited higher numbers of correct responses after pump implantation compared with rats in the vehicle group (Fig. 2b). A significant main effect of Presence of Pump [F(1,22) = 5.36, p < 0.05] and a significant Presence of Pump × Pump Content interaction [F(1,22) = 10.22, p < 0.01] also were observed for percent omissions, indicating that nicotine-treated rats had fewer omissions than vehicle-treated rats after pump implantation (Fig. 2c). To follow up on the possible effect of nicotine suggested by the Presence of Pump × Pump Content interaction, we performed an additional mixed-design ANOVA comparing percent omissions made by vehicle- vs. nicotine-treated rats on each individual day of PCP-free pump treatment (Time, i.e. pump treatment day, was the repeated-measures within-subjects factor, and Pump Content, i.e. nicotine vs. vehicle, was the between-subjects factor). This analysis revealed a significant main effect of Pump Content [F(1,176) = 5.33, p < 0.05], further indicating that nicotine decreased omissions. Post hoc testing revealed significantly fewer omissions in nicotine-treated compared with vehicle-treated rats on the first day of pump treatment (p < 0.05). A significant main effect of Presence of Pump on total number of trials completed indicated that total trials were reduced after pump implantation [F(1,22) = 8.97, p < 0.01], but no main effect of Pump Content and no Presence of Pump × Pump Content interaction were observed (data not shown). Average trials completed per group were 97.13 ± 1.73 during baseline and 93.05 ± 2.63 during pump exposure for vehicle-treated rats, and 97.32 ± 0.92 during baseline and 93.14 ± 2.75 during pump exposure for nicotine-treated rats.
Figure 2.
Effects of chronic nicotine administration on attentional performance in the 5-CSRTT under baseline conditions and during repeated PCP administration. Accuracy (a), percent correct responses (b), and percent omissions (c) are expressed as mean ± SEM. Bar graphs show binned averages from the 5 baseline days preceding any drug treatment and the 9 days of PCP-free pump treatment (left) and binned averages from the 5 days of saline injection during pump treatment and the 5 days of PCP administration during pump treatment (right). *p < 0.05, statistically significant differences compared with baseline performance. ††p < 0.01, statistically significant Presence of Pump × Pump Content interaction. ###p < 0.001, statistically significant difference compared with performance after saline injections. Gray field indicates the period of pump treatment. ↑ indicates a PCP injection.
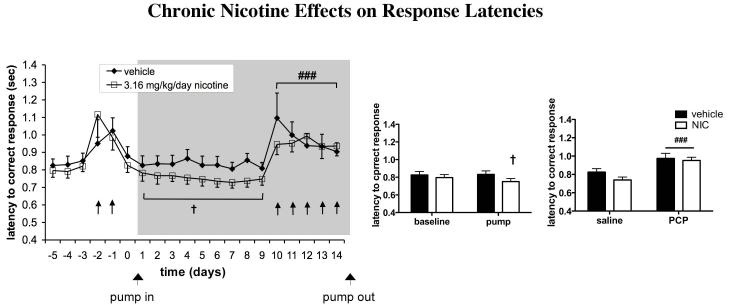
For latency to correct response, a significant Presence of Pump × Pump Content interaction was observed [F(1,22) = 7.30, p < 0.05], as well as a strong trend toward a main effect of Presence of Pump [F(1,22) = 4.08, p = 0.056], indicating a decrease in latency to correct response in nicotine-treated rats (Fig. 3). Latency to reward retrieval remained unaffected (data not shown).
Figure 3.
Effects of chronic nicotine administration on speed of processing in the 5-CSRTT under baseline conditions and during repeated PCP administration. Latencies to correct response are expressed as mean ± SEM. Bar graphs show binned averages from the 5 baseline days preceding any drug treatment and the 9 days of PCP-free pump treatment (left) and binned averages from the 5 days of saline injection during pump treatment and the 5 days of PCP administration during pump treatment (right). †p < 0.05, statistically significant Presence of Pump × Pump Content interaction. ###p < 0.001, statistically significant difference compared with performance after saline injections. Gray field indicates the period of pump treatment. ↑ indicates a PCP injection.
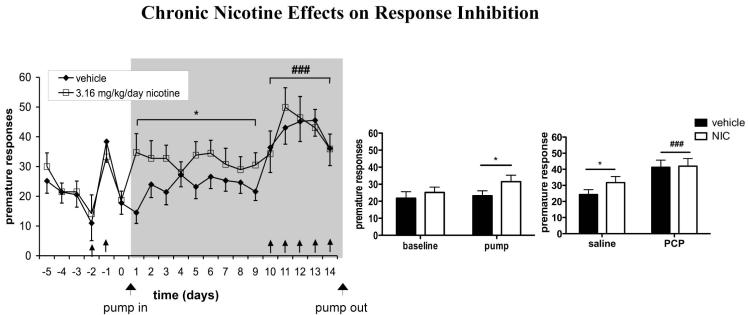
Presence of Pump also significantly increased premature responses [F(1,22) = 4.55, p < 0.05], but no significant main effect of Pump Content or Pump Exposure × Pump Content interaction were observed. However, post-hoc tests revealed that nicotine rats completed significantly more premature responses after pump implantation compared to baseline values (p < 0.05), whereas no significant difference was found in vehicle-treated rats (Fig. 4). No effects on timeout responses (data not shown) were found.
Figure 4.
Effects of chronic nicotine administration on inhibition of inappropriate responding in the 5-CSRTT under baseline conditions and during repeated PCP administration. Premature responses are expressed as mean ± SEM. Bar graphs show binned averages from the 5 baseline days preceding any drug treatment and the 9 days of PCP-free pump treatment (left) and binned averages from the 5 days of saline injection during pump treatment and the 5 days of PCP administration during pump treatment (right). *p < 0.05, statistically significant differences compared with baseline performance. ###p < 0.001, statistically significant difference compared with performance after saline injections. Gray field indicates the period of pump treatment. ↑ indicates a PCP injection.
Effects of chronic nicotine on PCP-induced disruptions in the 5-CSRTT
Repeated PCP administration significantly decreased accuracy [F(1,22) = 40.39, p < 0.0001; Fig. 2a] and percent correct responses [F(1,22) = 61.89, p < 0.0001; Fig. 2b] and increased percent omissions [F(1,22) = 45.04, p < 0.0001; Fig. 2c], latency to correct response [F(1,22) = 35.08, p < 0.0001; Figure 3], premature responses [F(1,18) = 15.18, p < 0.001; Fig. 4], and timeout responses [F(1,22) = 44.83, p < 0.0001; data not shown]. Latency to reward retrieval was unaffected (data not shown). Total trials were decreased by repeated PCP administration [F(1,22) = 105.00, p < 0.0001]. No main effect of nicotine on total trials was detected, but a significant PCP × nicotine interaction [F(1,22) = 5.61, p < 0.05] reflected a slightly lower number of trials completed by nicotine- vs. vehicle-treated rats during repeated PCP exposure (data not shown). None of the other measures showed a main effect of nicotine or a PCP × nicotine interaction. Average trials completed per group were 95.13 ± 2.18 during saline administration and 74.7 ± 4.67 during PCP administration for vehicle-treated rats, and 95 ± 1.82 during saline administration and 62.28 ± 3.76 during PCP administration for nicotine-treated rats. The minimum number of trials completed by any rat during any testing session was 19.
Discussion
Rats treated with chronic nicotine completed more correct responses and made fewer omissions in the 5-CSRTT than saline-treated rats in the absence of PCP (Fig. 2b, c), suggesting enhanced attention. Moreover, they displayed shorter latencies to correct response (Fig. 3), indicating increased processing speed. This performance-enhancing effect of chronic nicotine under baseline conditions in the absence of PCP is consistent with previous data indicating that nicotine enhances cognitive performance. Studies in humans found enhanced attentional performance after cigarette smoking or other nicotine administration (Wesnes and Warburton 1983, 1984a, b; Rusted and Warburton 1992; Warburton et al. 1992; Parrott and Craig 1992; Bates et al. 1995). However, the use of research subjects who were abstinent smokers raises the possibility that the improvements observed were attributable to relief of nicotine withdrawal-induced cognitive deficits (Hughes 1991; Heishman et al. 1994; Heishman 1998). Studies investigating the effects of nicotine in nonsmokers were mixed, with some studies finding improved cognitive performance (Wesnes and Warburton 1984b; Foulds et al. 1996; Levin et al. 1998b), while others reported no effect or even impaired cognitive performance (Dunne et al. 1986; Heishman et al. 1993; Heishman and Henningfield 2000).
In animal studies, a single administration of nicotine produced similarly mixed results, with some studies showing improved cognitive performance (Evenden et al. 1993; Levin 1994; Levin et al. 1997; Mirza and Stolerman 1998; Stolerman et al. 2000; Hahn and Stolerman 2002; Bizarro et al. 2004; Rezvani et al. 2005), while others reported no improvements or cognitive impairment (Mundy and Iwamoto 1988a, b; Dunnett and Martel 1990). Chronic nicotine, however, which may better mimic the constant nicotine exposure experienced by long-term tobacco smokers, improved attention in the 5-CSRTT (Hahn and Stolerman 2002; Semenova et al. 2007) and enhanced working memory and acquisition of a working memory task (Levin et al. 1990, 1992, 1993, 1996a) more reliably than acute nicotine administration. Thus, our finding that chronic nicotine improved attention and processing speed further corroborates the potential of nicotine to improve cognitive performance, especially when administered chronically. This observation lends support to the hypothesis that cigarette smoking is at least partially driven by the desire for nicotine-induced cognitive benefits.
However, saline-treated rats appeared to experience small deficits in 5-CSRTT performance after minipump implantation, with decreases in correct responses and increases in omissions (Fig. 2b, c). While the performance of nicotine-treated rats was better than performance of saline-treated rats after pump implantation, it did not appear to exceed pre-pump implantation baseline levels. Therefore, the possibility cannot be excluded that nicotine may have simply prevented detrimental consequences of pump implantation, possibly by preventing a disruptive effect of mild post-surgical discomfort through its analgesic actions. In the case of latency to correct response, however, no such performance decrement below baseline levels was seen after minipump surgery (Fig. 3), confirming that the latencies of nicotine-treated animals were indeed faster than baseline levels and that nicotine enhanced processing speed. Chronic nicotine did not affect latency to reward retrieval, indicating that the shortening of correct response latencies was not simply attributable to a general increase in speed of locomotion.
Consistent with previous findings (Stolerman et al. 2000; Hahn et al. 2002; Bizarro et al. 2004; Semenova et al. 2007), nicotine also increased premature responding in the 5-CSRTT (Fig. 4), suggesting increased impulsivity. The pattern of results seen in our study underscores the fact that, even though accuracy and premature responses often are negatively correlated in the 5-CSRTT (Puumala et al. 1996), the two measures are dissociable. Nicotine, therefore, may improve certain aspects of cognitive performance (processing speed, attention) while negatively affecting another cognitive modality (response inhibition/impulsivity).
Nicotine administration also has been reported to improve cognitive performance in schizophrenia patients (Adler et al. 1993; Levin et al. 1996b; Depatie et al. 2002; Smith et al. 2002, 2006; Sacco et al. 2005), although only some studies find nicotine-induced improvements in nonsmoking schizophrenia patients (Harris et al. 2004), while others do not (Myers et al. 2004). We therefore explored the effects of chronic nicotine treatment on schizophrenia-like cognitive disruptions induced by repeated PCP administration. Replicating previous findings (Amitai et al. 2007), repeated PCP administration resulted in decreased accuracy and correct responding (Fig. 2a, b), increased incorrect responding and omissions (Fig. 2c), increased latencies to correct response (Fig. 3), and increased premature responding (Fig. 4) and timeout responding in the 5-CSRTT. PCP intoxication thus created a schizophrenia-like profile of cognitive disruption comprising impaired attention, increased impulsivity, cognitive inflexibility, and decreased speed of processing. Chronic nicotine treatment did not affect any of the performance deficits induced by repeated PCP administration (Fig. 2-4).
These findings did not confirm our prediction that the pro-cognitive effects of nicotine would be more pronounced under circumstances in which cognitive performance was degraded by antagonist actions at the NMDA receptor. This lack of effect was not likely due to the use of an insufficient nicotine dose. The dose chosen here (3.16 mg/kg/day nicotine base) generates stable plasma nicotine levels (44 ng/ml) comparable to those achieved by heavy smokers consuming ∼30 cigarettes per day (Benowitz 1988) and produces dependence in rats within as little as 7 days (Malin et al. 1992). This dose, therefore, is physiologically relevant, although the possibility remains that higher nicotine doses may be effective in reversing PCP-induced attentional deficits.
The PCP dose (2 mg/kg) and regimen (see Methods and Fig. 1) chosen for this study were based on extensive previous observations indicating that lower PCP doses (≤1 mg/kg) had little effect on 5-CSRTT performance. Higher PCP doses (≥3 mg/kg) led to profound nonspecific response suppression and overall behavioral disruption, including ataxia, while at even higher doses, paralysis of the hind limbs was observed. Finally, repeated administration of 2 mg/kg PCP induced selective cognitive deficits in the 5-CSRTT (Amitai et al. 2007). Although a number of studies used higher PCP doses than those used here to induce cognitive impairment (Jentsch et al. 1997; Jentsch and Taylor 2001; Rodefer et al. 2005, 2007), these studies examined cognitive performance in the drug-free state after a significant washout period from the last PCP administration. In contrast, we investigated cognitive deficits during acute PCP re-challenges after repeated PCP exposures, based on the observation that the schizophrenia-like state evoked by PCP in humans is present during PCP intoxication, not during PCP withdrawal or during prolonged post-PCP abstinence (Pradhan 1984).
The severe cognitive disruptions induced by PCP at this dose may have been too profound to be counteracted by nicotine, whose pro-cognitive effects tend to be mild. Interestingly, in a previous study, chronic clozapine effected a partial, but significant, attenuation of disrupted attention and impulsivity induced in the 5-CSRTT using a PCP dose and administration regimen identical to that used in the present study (Amitai et al. 2007). This finding demonstrates that at the dose used in the present study, PCP induces cognitive deficits that can be attenuated by pharmacological manipulations. Clozapine has a complex biochemical profile, interacting with a variety of receptors, including serotonin, dopamine, norepinephrine, and acetylcholine (Schmidt et al. 2001). Several of these receptor actions have been proposed to play a role in cognitive enhancement and amelioration of NMDA receptor antagonist-induced deficits, including antagonism of 5-HT2A (Schmidt et al. 1995; Meltzer 1999), 5-HT6 (Hatcher et al. 2005; Rodefer et al. 2007), and 5-HT7 (Pouzet et al. 2002; Semenova et al. 2008) serotonin receptors. Moreover, in addition to nicotinic receptors, muscarinic acetylcholine receptors are involved in cognitive function (Levin 1988; Brandeis et al. 1995). Perhaps actions at several receptors and neurotransmitter systems are required for reversal of the profound and complex changes induced by repeated PCP administration. Nevertheless, the possibility remains that at a slightly different dose, PCP may induce 5-CSRTT deficits that are more sensitive to attenuation by nicotine treatment.
During PCP exposure, the 5-CSRTT performance of nicotine-treated rats did not differ significantly from that of vehicle-treated rats (Fig. 2-4), suggesting that the performance enhancement induced by nicotine was blocked by the NMDA receptor antagonist. Nicotine-induced increases in glutamate release (Gioanni et al. 1999) may mediate cognitive-enhancing effects of nicotine. Noncompetitive antagonism of NMDA receptors by PCP may abolish the pro-cognitive increases in glutamate transmission induced by nicotine, as shown previously (Quarta et al. 2007). Nevertheless, nicotine-induced increases in glutamate transmission through non-NMDA receptors could potentially overcome the effects of NMDA blockade on downstream systems. Furthermore, chronic nicotine administration increases expression of high-affinity α4β2 nicotinic receptors (Marks et al. 1985; Schwartz and Kellar 1985) involved in cognitive function (Levin 2002). NMDA receptor antagonist administration attenuates nicotine-induced increases in α4β2 nicotinic receptor expression (Levin et al. 2005; Rezvani et al. 2008b). If nicotine effects on cognition depend on enhanced glutamate transmission and/or increased α4β2 nicotinic receptor expression that are countered by NMDA receptor antagonism, it is unlikely that nicotine could succeed in ameliorating PCP-induced cognitive disruptions.
Nevertheless, nicotine reduced the attentional disruption induced by a different noncompetitive NMDA receptor antagonist, dizocilpine, in a two-lever visual signal detection task (Rezvani and Levin 2003; Rezvani et al. 2008a). Improvement of dizocilpine-induced deficits in working and reference memory with nicotine also has been found (Levin et al. 1998a). However, the attenuation of dizocilpine-induced deficits by nicotine was seen only on one measure of attentional performance, percent correct rejections, while on another measure, percent hits, nicotine was ineffective and at some doses even exacerbated dizocilpine-induced disruptions at shorter stimulus durations (Rezvani and Levin 2003). In another study, nicotine attenuated the deficit in percent hits induced by dizocilpine, but to a lesser extent than percent correct rejections (Rezvani et al. 2008a). This pattern of results suggests that reversal of cognitive deficits produced by NMDA receptor antagonists can be difficult to demonstrate consistently and may be highly sensitive to doses of both the NMDA receptor antagonist and the putative “therapeutic” compounds, as well as task parameters.
Furthermore, PCP and dizocilpine exhibit subtle differences in their pharmacology that may account for the differences in results with the two drugs. Dizocilpine is more potent in its affinity for the NMDA receptor, and its behavioral actions show a slower onset and longer duration than PCP (Winger et al. 2002). PCP also has more affinity for the σ opioid receptor (Quirion et al. 1981) and greater effectiveness as a dopamine reuptake inhibitor than dizocilpine (Johnson and Jones 1990). However, nicotine has been found to ameliorate a PCP-induced acquisition deficit in monkeys (Thompson and Winsauer 1986). Furthermore, PCP-induced disruption of preattentional sensory gating in mice is attenuated by nicotine (Spielewoy and Markou 2004; Andreasen et al. 2006). Deficits in these cognitive modalities may be more sensitive to nicotine effects than attentional disruptions. This would appear surprising, however, because the pro-cognitive effects of nicotine under baseline conditions seem to preferentially improve attention (Rusted and Warburton 1992).
The studies discussed above show attenuation of NMDA receptor antagonist-induced deficits with acute administration of nicotine, and not with chronic nicotine treatment as used in the present study. However, the lack of reversal of PCP effects with nicotine in the present study is unlikely to be due to tolerance, because no tolerance to the pro-cognitive effects of nicotine is observed when the effects of nicotine are assessed under baseline conditions. In fact, as mentioned above, chronic nicotine may produce more reliable pro-cognitive effects under baseline conditions than acute nicotine, and in some cases, enhanced pro-cognitive effects of nicotine are seen with chronic administration (Semenova et al. 2007).
In summary, the present study found that at the doses investigated here, chronic nicotine did not exert beneficial effects on cognitive performance disrupted by the psychotomimetic NMDA receptor antagonist PCP, as assessed by the 5-CSRTT. However, we found evidence that chronic nicotine by itself may improve attentional function and processing speed, providing further evidence for the hypothesis that a desire for cognitive benefits may be among the factors driving the initiation and maintenance of tobacco smoking.
Acknowledgements
Supported by National Institute of Mental Health grant MH062527 and Tobacco-Related Disease Research Program (TRDRP) grant 15RT-0022 from the State of California to AM and TRDRP Individual Pre-doctoral Fellowship 15DT-0048 from the State of California to NA. The authors would like to thank Dr. Svetlana Semenova for intellectual input to this work, Mrs. Jessica Benedict and Ms. Chelsea Onifer for technical assistance, Mr. Pete Sharp for excellent assistance with electronics and computer software, and Mr. Mike Arends for outstanding editorial assistance.
References
- Adler LE, Hoffer LD, Wiser A, Freedman R. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry. 1993;150:1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- Amitai N, Semenova S, Markou A. Cognitive-disruptive effects of the psychotomimetic phencyclidine and attenuation by atypical antipsychotic medications in rats. Psychopharmacology (Berl) 2007;193:521–537. doi: 10.1007/s00213-007-0808-x. [DOI] [PubMed] [Google Scholar]
- Andreasen JT, Andersen KK, Nielsen EO, Mathiasen L, Mirza NR. Nicotine and clozapine selectively reverse a PCP-induced deficit of PPI in BALB/cByJ but not NMRI mice: comparison with risperidone. Behav Brain Res. 2006;167:118–127. doi: 10.1016/j.bbr.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Bates T, Mangan G, Stough C, Corballis P. Smoking, processing speed and attention in a choice reaction time task. Psychopharmacology (Berl) 1995;120:209–212. doi: 10.1007/BF02246195. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Pharmacological aspects of cigarette smoking and nicotine addiction. N Engl J Med. 1988;319:1318–1330. doi: 10.1056/NEJM198811173192005. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Goldman RS, Volavka J, Czobor P, Hoptman M, Sheitman B, Lindenmayer JP, Citrome L, McEvoy J, Kunz M, Chakos M, Cooper TB, Horowitz TL, Lieberman JA. Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol in patients with chronic schizophrenia or schizoaffective disorder. Am J Psychiatry. 2002;159:1018–1028. doi: 10.1176/appi.ajp.159.6.1018. [DOI] [PubMed] [Google Scholar]
- Bizarro L, Patel S, Murtagh C, Stolerman IP. Differential effects of psychomotor stimulants on attentional performance in rats: nicotine, amphetamine, caffeine and methylphenidate. Behav Pharmacol. 2004;15:195–206. [PubMed] [Google Scholar]
- Brandeis R, Sapir M, Hafif N, Abraham S, Oz N, Stein E, Fisher A. AF150(S): a new functionally selective M1 agonist improves cognitive performance in rats. Pharmacol Biochem Behav. 1995;51:667–674. doi: 10.1016/0091-3057(94)00435-l. [DOI] [PubMed] [Google Scholar]
- Carli M, Robbins TW, Evenden JL, Everitt BJ. Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res. 1983;9:361–380. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- De Leon J, Tracy J, McCann E, McGrory A, Diaz FJ. Schizophrenia and tobacco smoking: A replication study in another US psychiatric hospital. Schizophr Res. 2002;56:55–65. doi: 10.1016/s0920-9964(01)00192-x. [DOI] [PubMed] [Google Scholar]
- Depatie L, O’Driscoll GA, Holahan AL, Atkinson V, Thavundayil JX, Kin NN, Lal S. Nicotine and behavioral markers of risk for schizophrenia: a double-blind placebo-controlled, cross-over study. Neuropsychopharmacology. 2002;27:1056–1070. doi: 10.1016/S0893-133X(02)00372-X. [DOI] [PubMed] [Google Scholar]
- Dunne MP, MacDonald D, Hartley LR. The effects of nicotine upon memory and problem solving performance. Physiol Behav. 1986;37:849–854. [PubMed] [Google Scholar]
- Dunnett SB, Martel FL. Proactive interference effects on short-term memory in rats: I. Basic parameters and drug effects. Behav Neurosci. 1990;104:655–665. doi: 10.1037//0735-7044.104.5.655. [DOI] [PubMed] [Google Scholar]
- Elvevag B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14:1–21. [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1990;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Turpin M, Oliver L, Jennings C. Caffeine and nicotine improve visual tracking by rats: a comparison with amphetamine, cocaine and apomorphine. Psychopharmacology (Berl) 1993;110:169–176. doi: 10.1007/BF02246968. [DOI] [PubMed] [Google Scholar]
- Foulds J, Stapleton J, Swettenham J, Bell N, McSorley K, Russell MA. Cognitive performance effects of subcutaneous nicotine in smokers and never-smokers. Psychopharmacology (Berl) 1996;127:31–38. doi: 10.1007/BF02805972. [DOI] [PubMed] [Google Scholar]
- Freedman R, Adler LE, Bickford P, Byerley W, Coon H, Cullum CM, Griffith JM, Harris JG, Leonard S, Miller C, Myles-Worsley M, Nagamoto HT, Rose G, Waldo M. Schizophrenia and nicotinic receptors. Harv Rev Psychiatry. 1994;2:179–192. doi: 10.3109/10673229409017136. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Markou A. Animal models of psychiatric disorders. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the fourth generation of progress. Raven Press; New York: 1995. pp. 787–798. [Google Scholar]
- Gioanni Y, Rougeot C, Clarke PB, Lepousé C, Thierry AM, Vidal C. Nicotinic receptors in the rat prefrontal cortex: increase in glutamate release and facilitation of mediodorsal thalamo-cortical transmission. Eur J Neurosci. 1999;11:18–30. doi: 10.1046/j.1460-9568.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- Hahn B, Shoaib M, Stolerman IP. Nicotine-induced enhancement of attention in the five-choice serial reaction time task: the influence of task demands. Psychopharmacology (Berl) 2002;162:129–137. doi: 10.1007/s00213-002-1005-6. [DOI] [PubMed] [Google Scholar]
- Hahn B, Stolerman IP. Nicotine-induced attentional enhancement in rats: effects of chronic exposure to nicotine. Neuropsychopharmacology. 2002;27:712–722. doi: 10.1016/S0893-133X(02)00348-2. [DOI] [PubMed] [Google Scholar]
- Handelmann GE, Contreras PC, O’Donohue TL. Selective memory impairment by phencyclidine in rats. Eur J Pharmacol. 1987;140:69–73. doi: 10.1016/0014-2999(87)90635-2. [DOI] [PubMed] [Google Scholar]
- Harris JG, Kongs S, Allensworth D, Martin L, Tregellas J, Sullivan B, Zerbe G, Freedman R. Effects of nicotine on cognitive deficits in schizophrenia. Neuropsychopharmacology. 2004;29:1378–1385. doi: 10.1038/sj.npp.1300450. [DOI] [PubMed] [Google Scholar]
- Hatcher PD, Brown VJ, Tait DS, Bate S, Overend P, Hagan JJ, Jones DN. 5-HT6 receptor antagonists improve performance in an attentional set shifting task in rats. Psychopharmacology (Berl) 2005;181:253–259. doi: 10.1007/s00213-005-2261-z. [DOI] [PubMed] [Google Scholar]
- Heishman SJ. What aspects of human performance are truly enhanced by nicotine? Addiction. 1998;93:317–320. doi: 10.1080/09652149835864. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Henningfield JE. Tolerance to repeated nicotine administration on performance, subjective, and physiological responses in nonsmokers. Psychopharmacology (Berl) 2000;152:321–333. doi: 10.1007/s002130000541. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Snyder FR, Henningfield JE. Performance, subjective, and physiological effects of nicotine in non-smokers. Drug Alcohol Depend. 1993;34:11–18. doi: 10.1016/0376-8716(93)90041-n. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Taylor RC, Henningfield JE. Nicotine and smoking: a review of effects on human performance. Exp Clin Psychopharmacol. 1994;2:345–395. [Google Scholar]
- Hughes JR. Distinguishing withdrawal relief and direct effects of smoking. Psychopharmacology (Berl) 1991;104:409–410. doi: 10.1007/BF02246044. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA. Prevalence of smoking among psychiatric outpatients. Am J Psychiatry. 1986;143:993–997. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- Idris NF, Repeto P, Neill JC, Large CH. Investigation of the effects of lamotrigine and clozapine in improving reversal-learning impairments induced by acute phencyclidine and d-amphetamine in the rat. Psychopharmacology (Berl) 2005;179:336–348. doi: 10.1007/s00213-004-2058-5. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Negative schizophrenic symptomatology and the PCP (phencyclidine) model of schizophrenia. Hillside J Clin Psychiatry. 1987;9:12–35. [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impaired inhibition of conditioned responses produced by subchronic administration of phencyclidine to rats. Neuropsychopharmacology. 2001;24:66–74. doi: 10.1016/S0893-133X(00)00174-3. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Tran A, Le D, Youngren KD, Roth RH. Subchronic phencyclidine adminstration reduces mesoprefrontal dopamine utilization and impairs cortical-dependent cognition in the rat. Neuropsychopharmacology. 1997;17:92–99. doi: 10.1016/S0893-133X(97)00034-1. [DOI] [PubMed] [Google Scholar]
- Jin J, Yamamoto T, Watanabe S. The involvement of σ receptors in the choice reaction performance deficits induced by phencyclidine. Eur J Pharmacol. 1997;319:147–152. doi: 10.1016/s0014-2999(96)00858-8. [DOI] [PubMed] [Google Scholar]
- Johnson KM, Jones SM. Neuropharmacology of phencyclidine: basic mechanisms and therapeutic potential. Annu Rev Pharmacol Toxicol. 1990;30:707–750. doi: 10.1146/annurev.pa.30.040190.003423. [DOI] [PubMed] [Google Scholar]
- Laurent A, Saoud M, Bougerol T, d’Amato T, Anchisi AM, Biloa-Tang M, Dalery J, Rochet T. Attentional deficits in patients with schizophrenia and in their non-psychotic first-degree relatives. Psychiatry Res. 1999;89:147–159. doi: 10.1016/s0165-1781(99)00109-2. [DOI] [PubMed] [Google Scholar]
- Levin ED. Psychopharmacological effects in the radial-arm maze. Neurosci Biobehav Rev. 1988;12:169–175. doi: 10.1016/s0149-7634(88)80008-3. [DOI] [PubMed] [Google Scholar]
- Levin ED. Nicotine effects and working memory performance. Rec Adv Tobacco Sci. 1994;20:49–66. [Google Scholar]
- Levin ED. Nicotinic receptor subtypes and cognitive function. J Neurobiol. 2002;53:633–640. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bettegowda C, Weaver T, Christopher NC. Nicotine-dizocilpine interactions and working and reference memory performance of rats in the radial-arm maze. Pharmacol Biochem Behav. 1998a;61:335–340. doi: 10.1016/s0091-3057(98)00109-9. [DOI] [PubMed] [Google Scholar]
- Levin ED, Briggs SJ, Christopher NC, Rose JE. Persistence of chronic nicotine-induced cognitive facilitation. Behav Neural Biol. 1992;58:152–158. doi: 10.1016/0163-1047(92)90399-o. [DOI] [PubMed] [Google Scholar]
- Levin ED, Briggs SJ, Christopher NC, Rose JE. Chronic nicotinic stimulation and blockade effects on working memory. Behav Pharmacol. 1993;4:179–182. [PubMed] [Google Scholar]
- Levin ED, Conners CK, Silva D, Hinton SC, Meck WH, March J, Rose JE. Transdermal nicotine effects on attention. Psychopharmacology (Berl) 1998b;140:135–141. doi: 10.1007/s002130050750. [DOI] [PubMed] [Google Scholar]
- Levin ED, Kaplan S, Boardman A. Acute nicotine interactions with nicotinic and muscarinic antagonists: working and reference memory effects in the 16-arm radial maze. Behav Pharmacol. 1997;8:236–242. [PubMed] [Google Scholar]
- Levin ED, Kim P, Meray R. Chronic nicotine working and reference memory effects in the 16-arm radial maze: interactions with D1 agonist and antagonist drugs. Psychopharmacology (Berl) 1996a;127:25–30. doi: 10.1007/BF02805971. [DOI] [PubMed] [Google Scholar]
- Levin ED, Lee C, Rose JE, Reyes A, Ellison G, Jarvik M, Gritz E. Chronic nicotine and withdrawal effects on radial-arm maze performance in rats. Behav Neural Biol. 1990;53:269–276. doi: 10.1016/0163-1047(90)90509-5. [DOI] [PubMed] [Google Scholar]
- Levin ED, Wilson W, Rose JE, McEvoy J. Nicotine-haloperidol interactions and cognitive performance in schizophrenics. Neuropsychopharmacology. 1996b;15:429–436. doi: 10.1016/S0893-133X(96)00018-8. [DOI] [PubMed] [Google Scholar]
- Levin ED, Tizabi Y, Rezvani AH, Caldwell DP, Petro A, Getachew B. Chronic nicotine and dizocilpine effects on regionally specific nicotinic and NMDA glutamate receptor binding. Brain Res. 2005;1041:132–142. doi: 10.1016/j.brainres.2005.01.104. [DOI] [PubMed] [Google Scholar]
- Luby ED, Cohen BD, Rosenbaum G, Gottlieb JS, Kelley R. Study of a new schizophrenomimetic drug, sernyl. AMA Arch Neurol Psychiatry. 1959;81:363–369. doi: 10.1001/archneurpsyc.1959.02340150095011. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Newlin-Maultsby P, Roberts LK, Lanier JG, Carter VA, Cunningham JS, Wilson OB. Rodent model of nicotine abstinence syndrome. Pharmacol Biochem Behav. 1992;43:779–784. doi: 10.1016/0091-3057(92)90408-8. [DOI] [PubMed] [Google Scholar]
- Mangan GL. The effects of cigarette smoking on verbal learning and retention. J Gen Psychol. 1983;108:203–210. doi: 10.1080/00221309.1983.9711494. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Collins AC. Time course study of the effects of chronic nicotine infusion on drug response and brain receptors. J Pharmacol Exp Ther. 1985;235:619–628. [PubMed] [Google Scholar]
- Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21(2 Suppl):106S–115S. doi: 10.1016/S0893-133X(99)00046-9. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, McGurk SR. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull. 1999;25:233–255. doi: 10.1093/oxfordjournals.schbul.a033376. [DOI] [PubMed] [Google Scholar]
- Meyer G. Why people smoke: action paper number 1. In: Ahrens D, editor. Insights: smoking in Wisconsin. Center for Tobacco Research and Intervention, University of Wisconsin Medical School; Madison WI: 2002. [Google Scholar]
- Mirza NR, Stolerman IP. Nicotine enhances sustained attention in the rat under specific task conditions. Psychopharmacology (Berl) 1998;138:266–274. doi: 10.1007/s002130050671. [DOI] [PubMed] [Google Scholar]
- Morice R. Cognitive inflexibility and pre-frontal dysfunction in schizophrenia and mania. Br J Psychiatry. 1990;157:50–54. doi: 10.1192/bjp.157.1.50. [DOI] [PubMed] [Google Scholar]
- Mundy WR, Iwamoto ET. Actions of nicotine on the acquisition of an autoshaped lever-touch response in rats. Psychopharmacology (Berl) 1988a;94:267–274. doi: 10.1007/BF00176858. [DOI] [PubMed] [Google Scholar]
- Mundy WR, Iwamoto ET. Nicotine impairs acquisition of radial maze performance in rats. Pharmacol Biochem Behav. 1988b;30:119–122. doi: 10.1016/0091-3057(88)90433-9. [DOI] [PubMed] [Google Scholar]
- Myers CS, Robles O, Kakoyannis AN, Sherr JD, Avila MT, Blaxton TA, Thaker GK. Nicotine improves delayed recognition in schizophrenic patients. Psychopharmacology (Berl) 2004;174:334–340. doi: 10.1007/s00213-003-1764-8. [DOI] [PubMed] [Google Scholar]
- Nelson HE, Pantelis C, Carruthers K, Speller J, Baxendale S, Barnes TRE. Cognitive functioning and symptomatology in chronic schizophrenia. Psychol Med. 1990;20:357–365. doi: 10.1017/s0033291700017670. [DOI] [PubMed] [Google Scholar]
- Parrott AC, Craig D. Cigarette smoking and nicotine gum (0, 2 and 4 mg): effects upon four visual attention tasks. Neuropsychobiology. 1992;25:34–43. doi: 10.1159/000118807. [DOI] [PubMed] [Google Scholar]
- Pearlson GD. Psychiatric and medical syndromes associated with phencyclidine (PCP) abuse. Johns Hopkins Med J. 1981;148:25–33. [PubMed] [Google Scholar]
- Peeke SC, Peeke HV. Attention, memory, and cigarette smoking. Psychopharmacology (Berl) 1984;84:205–216. doi: 10.1007/BF00427447. [DOI] [PubMed] [Google Scholar]
- Pouzet B, Didriksen M, Arnt J. Effects of the 5-HT7 receptor antagonist SB-258741 in animal models for schizophrenia. Pharmacol Biochem Behav. 2002;71:655–665. doi: 10.1016/s0091-3057(01)00744-4. [DOI] [PubMed] [Google Scholar]
- Pradhan SN. Phencyclidine (PCP): some human studies. Neurosci Biobehav Rev. 1984;8:493–501. doi: 10.1016/0149-7634(84)90006-x. [DOI] [PubMed] [Google Scholar]
- Puumala T, Ruotsalainen S, Jakala P, Koivisto E, Riekkinen P, Jr, Sirvio J. Behavioral and pharmacological studies on the validation of a new animal model for attention deficit hyperactivity disorder. Neurobiol Learn Mem. 1996;66:198–211. doi: 10.1006/nlme.1996.0060. [DOI] [PubMed] [Google Scholar]
- Quarta D, Naylor CG, Morris HV, Patel S, Genn RF, Stolerman IP. Different effects of ionotropic and metabotropic glutamate receptor antagonists on attention and the attentional properties of nicotine. Neuropharmacology. 2007;53:421–430. doi: 10.1016/j.neuropharm.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Quirion R, Hammer RP, Jr, Herkenham M, Pert CB. Phencyclidine (angel dust)/σ “opiate” receptor: visualization by tritium-sensitive film. Proc Natl Acad Sci U S A. 1981;78:5881–5885. doi: 10.1073/pnas.78.9.5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Caldwell DP, Levin ED. Nicotinic-serotonergic drug interactions and attentional performance in rats. Psychopharmacology (Berl) 2005;179:521–528. doi: 10.1007/s00213-004-2060-y. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Kholdebarin E, Dawson E, Levin ED. Nicotine and clozapine effects on attentional performance impaired by the NMDA antagonist dizocilpine in female rats. Int J Neuropsychopharmacol. 2008a;11:63–70. doi: 10.1017/S1461145706007528. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Tizabi Y, Getachew B, Hauser SR, Caldwell DP, Hunter C, Levin ED. Chronic nicotine and dizocilpine effects on nicotinic and NMDA glutamatergic receptor regulation: Interactions with clozapine actions and attentional performance in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2008b;32:1030–1040. doi: 10.1016/j.pnpbp.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Nicotinic-glutamatergic interactions and attentional performance on an operant visual signal detection task in female rats. Eur J Pharmacol. 2003;465:83–90. doi: 10.1016/s0014-2999(03)01439-0. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Rodefer JS, Murphy ER, Baxter MG. PDE10A inhibition reverses subchronic PCP-induced decifits in attentional set-shifting in rats. Eur J Neurosci. 2005;21:1070–1076. doi: 10.1111/j.1460-9568.2005.03937.x. [DOI] [PubMed] [Google Scholar]
- Rodefer JS, Nguyen TN, Karlsson JJ, Arnt J. Reversal of subchronic PCP-induced deficits in attentional set shifting in rats by sertindole and a 5-HT6 receptor antagonist: comparison among antipsychotics. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301654. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Rusted JM, Warburton DM. Facilitation of memory by post-trial administration of nicotine: evidence for an attentional explanation. Psychopharmacology (Berl) 1992;108:452–455. doi: 10.1007/BF02247420. [DOI] [PubMed] [Google Scholar]
- Sacco KA, Termine A, Seyal A, Dudas MM, Vessicchio JC, Krishnan-Sarin S, Jatlow PI, Wexler BE, George TP. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: involvement of nicotinic receptor mechanisms. Arch Gen Psychiatry. 2005;62:649–659. doi: 10.1001/archpsyc.62.6.649. [DOI] [PubMed] [Google Scholar]
- Sanger DJ, Jackson A. Effects of phencyclidine and other N-methyl-D-aspartate antagonists on the schedule-controlled behavior of rats. J Pharmacol Exp Ther. 1989;248:1215–1221. [PubMed] [Google Scholar]
- Schmidt AW, Lebel LA, Howard HR, Jr, Zorn SH. Ziprasidone: a novel antipsychotic agent with a unique human receptor binding profile. Eur J Pharmacol. 2001;425:197–201. doi: 10.1016/s0014-2999(01)01188-8. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Sorensen SM, Kehne JH, Carr AA, Palfreyman MG. The role of 5-HT2A receptors in antipsychotic activity. Life Sci. 1995;56:2209–2222. doi: 10.1016/0024-3205(95)00210-w. [DOI] [PubMed] [Google Scholar]
- Schwartz RD, Kellar KJ. In vivo regulation of [3H]acetylcholine recognition sites in brain by nicotinic cholinergic drugs. J Neurochem. 1985;45:427–433. doi: 10.1111/j.1471-4159.1985.tb04005.x. [DOI] [PubMed] [Google Scholar]
- Semenova S, Stolerman IP, Markou A. Chronic nicotine administration improves attention while nicotine withdrawal induces performance deficits in the 5-choice serial reaction time task in rats. Pharmacol Biochem Behav. 2007;87:360–368. doi: 10.1016/j.pbb.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenova S, Geyer MA, Sutcliffe JG, Markou A, Hedlund PB. Inactivation of the 5-HT7 receptor partially blocks phencyclidine-induced disruption of prepulse inhibition. Biol Psychiatry. 2008;63:98–105. doi: 10.1016/j.biopsych.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma T, Antonova L. Cognitive function in schizophrenia: deficits, functional consequences, and future treatment. Psychiatr Clin North Am. 2003;26:25–40. doi: 10.1016/s0193-953x(02)00084-9. [DOI] [PubMed] [Google Scholar]
- Smith RC, Singh A, Infante M, Khandat A, Kloos A. Effects of cigarette smoking and nicotine nasal spray on psychiatric symptoms and cognition in schizophrenia. Neuropsychopharmacology. 2002;27:479–497. doi: 10.1016/S0893-133X(02)00324-X. [DOI] [PubMed] [Google Scholar]
- Smith RC, Warner-Cohen J, Matute M, Butler E, Kelly E, Vaidhyanathaswamy S, Khan A. Effects of nicotine nasal spray on cognitive function in schizophrenia. Neuropsychopharmacology. 2006;31:637–643. doi: 10.1038/sj.npp.1300881. [DOI] [PubMed] [Google Scholar]
- Spielewoy C, Markou A. Strain-specificity in nicotine attenuation of phencyclidine-induced disruption of prepulse inhibition in mice: relevance to smoking in schizophrenia patients. Behav Genet. 2004;34:343–354. doi: 10.1023/B:BEGE.0000017878.75206.fd. [DOI] [PubMed] [Google Scholar]
- Steinpreis RE. The behavioral and neurochemical effects of phenyclidine in humans and animals: some implications for modeling psychosis. Behav Brain Res. 1996;74:45–55. doi: 10.1016/0166-4328(95)00162-x. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Mirza NR, Hahn B, Shoaib M. Nicotine in an animal model of attention. Eur J Pharmacol. 2000;393:147–154. doi: 10.1016/s0014-2999(99)00886-9. [DOI] [PubMed] [Google Scholar]
- Thompson DM, Winsauer PJ. Nicotine can attenuate the disruptive effects of phencyclidine on repeated acquisition in monkeys. Pharmacol Biochem Behav. 1986;25:185–190. doi: 10.1016/0091-3057(86)90251-0. [DOI] [PubMed] [Google Scholar]
- Warburton DM. Psychopharmacological aspects of nicotine. In: Wonnacott S, Russell MAH, Stolerman IP, editors. Nicotine psychopharmacology: molecular, cellular and behavioural aspects. Oxford Science Publications; Oxford: 1990. pp. 77–112. [Google Scholar]
- Warburton DM, Rusted JM, Fowler J. A comparison of the attentional and consolidation hypotheses for the facilitation of memory by nicotine. Psychopharmacology (Berl) 1992;108:443–447. doi: 10.1007/BF02247418. [DOI] [PubMed] [Google Scholar]
- Wesnes K, Warburton DM. Effects of smoking on rapid information processing performance. Neuropsychobiology. 1983;9:223–229. doi: 10.1159/000117969. [DOI] [PubMed] [Google Scholar]
- Wesnes K, Warburton DM. The effects of cigarettes of varying yield on rapid information processing performance. Psychopharmacology (Berl) 1984a;82:338–342. doi: 10.1007/BF00427682. [DOI] [PubMed] [Google Scholar]
- Wesnes K, Warburton DM. Effects of scopolamine and nicotine on human rapid information processing performance. Psychopharmacology (Berl) 1984b;82:147–150. doi: 10.1007/BF00427761. [DOI] [PubMed] [Google Scholar]
- Winger G, Hursh SR, Casey KL, Woods JH. Relative reinforcing strength of three N-methyl-d-aspartate antagonists with different onsets of action. J Pharmacol Exp Ther. 2002;301:690–697. doi: 10.1124/jpet.301.2.690. [DOI] [PubMed] [Google Scholar]
- Wykes T, Reeder C, Corner J. The prevalence and stability of an executive processing deficit, response inhibition, in people with chronic schizophrenia. Schizophr Res. 2000;46:241–253. doi: 10.1016/s0920-9964(99)00233-9. [DOI] [PubMed] [Google Scholar]