Abstract
Previous studies examining the covariation among Attention Deficit Hyperactivity Disorder (ADHD), Oppositional Defiant Disorder (ODD) and Conduct Disorder (CD) have yielded inconsistent results. Some studies have concluded that the covariation among these symptoms is due to common genetic influences, whereas others have found a common environmental overlap. The present study investigated the genetic and environmental correlations among these three childhood disorders, based on a sample of 1,241 twins, age 9–10 years. A latent externalizing behavior factor was found to explain the covariance among ADHD, ODD and CD symptoms. Genetic influences explained more than half of the variance in this externalizing factor in both boys and girls. There were also unique genetic and environmental influences in each set of symptoms, suggesting some etiological independence of the three disorders. Our findings have implications for molecular genetic studies trying to identify susceptibility genes, as well as for possible prevention of externalizing disorders through identification of at-risk children early in life.
Keywords: ADHD, CD, ODD, covariation, genetic influences, twins
Attention Deficit Hyperactivity Disorder (ADHD), Oppositional Defiant Disorder (ODD) and Conduct Disorder (CD) are three of the most prevalent disruptive behavior disorders in children and adolescents. They affect approximately 1–15% of all school age children, and account for a large amount of childhood referrals to mental health clinics (Brown et al., 2001; Maughan et al., 2004). They often lead to stress and frustration in the affected children, their families, teachers and peers. Numerous studies have reported comorbidity among these disruptive behavior disorders in both epidemiological and clinical samples (Angold, Costello & Erkanli, 1999; Biederman, Newcorn & Sprich, 1991; Faraone et al., 1998b; Maughan et al., 2004), often making them difficult to isolate and understand individually. Such high levels of comorbidity among supposedly different domains of externalizing problems, such as inattention, hyperactivity, aggression and defiance make these various problem behaviors complex and particularly hard to treat. To date, the genetic and environmental etiology that underlies the comorbidity among these disruptive behavior disorders is unclear.
ADHD is characterized by pervasive and impairing symptoms of inattention, hyperactivity and impulsivity (American Psychiatric Association, 2004). The disorder is associated with academic underachievement, substance use and dependence, psychosocial problems and social maladjustment, including unemployment in adulthood (Biederman & Faraone, 2006; Kuperman et al., 2001).
Like ADHD, ODD is also a common psychiatric disorder in children (Maughan et al., 2004). The disorder typically occurs in early childhood and is characterized by a pattern of negativistic, disobedient, hostile and defiant behaviors. In contrast to ODD, CD is a more severe disorder; it often develops later than ODD, typically in early adolescence (American Psychiatric Association, 2004). In many cases, CD is found in individuals that have been diagnosed with ODD in early childhood (Biederman et al., 1996; Lahey et al., 2000), and it has therefore been suggested that ODD may be a developmental precursor to CD (Rowe et al., 2002). Regardless, the majority of empirical research supports a distinction between ODD and CD (Loeber et al., 2000). Both ODD and CD are of great concern due to their high prevalence rates and poor outcome. Children with ODD and CD are at higher risk for later substance use and dependence (White et al., 2001), and children with CD are more apt to develop other psychiatric disorders, including antisocial personality disorder during adulthood (Kim-Cohen et al., 2003; Robins, 1966).
There is evidence that children with these disruptive behavior disorders share a range of similar risk factors, including personality related factors, e.g., a tendency to be high in novelty seeking (Anckarsäter et al., 2006; Rettew et al., 2004), and family related factors, e.g., socio-demographic disadvantages (Fergusson & Horwood, 1998) and chaotic/disruptive family environments, such as those associated to the effects of parental alcoholism (Kuperman et al., 1999), marital conflict, and child abuse or neglect (Rutter, Giller & Hagell, 1998). Further, it is known from twin studies that genetic influences are important in the development of ADHD symptoms (Rhee et al., 1999; Rietveld et al., 2003; Thapar et al., 2000), whereas both genes and shared environmental influences (i.e., non-genetic influences that contribute to co-twin similarity) seem to be important in the development of ODD symptoms (Burt et al., 2001) and CD symptoms (Jacobson, Prescott & Kendler, 2002; Rose et al., 2004). In addition, family studies have shown that ADHD, ODD and CD are co-transmitted in families, and thus appear to share a common familial etiology (Faraone et al., 1998a). For instance, one study found that relatives of ADHD probands were at greater risk for developing ADHD and ODD than relatives of normal controls (Faraone et al., 1997). Although there are meaningful distinctions among ADHD, ODD and CD, especially in their correlates and outcomes (Schachar & Tannock, 1995), observation of their common vulnerabilities suggests that they share the same liability risk which could be either genetic, environmental or some combination of both in origin.
It is possible using a genetically informative design to investigate the nature of comorbidity, in which the covariation among symptoms of different disorders is explained by common (i.e., correlated) genetic risk factors, common environmental risk factors, or both (Rhee et al., 2007). If the same genetic factors influence these disorders, then a high genetic correlation would be found. Likewise, if the same environmental factors are important, a high environmental correlation would be found.
Several twin studies have been conducted to examine the covariation between ADHD and combined ODD/CD symptoms (Nadder et al., 2002; Silberg et al., 1996) and between ADHD and CD symptoms, excluding ODD symptoms (Thapar, Harrington & McGuffin, 2001). These studies reported that the covariation between ADHD and combined ODD/CD symptoms and between ADHD and CD symptoms is largely explained by common genetic risk factors. The few twin studies that have distinguished between ODD and CD symptoms and analyzed them independently have however, reached somewhat different conclusions. Burt et al. (2001; 2003; 2005) used a sample of 10–12 year old children and conducted face-to-face interviews with both twins and their mothers. In one study, maternal and child reports were combined into one symptom count for each disorder (i.e., a ’’best estimate’’ approach: each symptom was considered present if either the mother or child endorsed it). The associations between these combined symptom counts for ADHD, CD and ODD were found to depend mainly on common shared environmental risk factors (i.e., non-genetic influences that contribute to similarity within pairs of twins) (Burt et al., 2003; Burt et al., 2001). Combining information across reports in this way may not be ideal, however, given the potential rater effects which are known to be important in genetic models of childhood disorders (Bartels et al., 2003). It is thus difficult to compare the results from Burt et al. (2001) to other studies using separate raters. In another study from this same group, however, mother and child reports were separated into different symptom counts, i.e., ADHD, ODD and CD. Shared environmental influences were found to explain most of the variance common to maternal and child reports, but genetic influences were the most important effect explaining the co-occurrence of ADHD, CD and ODD unique to maternal and child reports (Burt et al., 2005). These results underscore the importance of examining different raters separately, since etiologies appear to be somewhat specific to each rater. Furthermore, Dick et al. (2005) conducted face-to-face interviews in a sample of 14 year old children and found that the relationships between these symptoms were largely due to common genetic risk factors (Dick et al., 2005). One shortcoming common to these studies, is the exclusion of opposite sex twins—either in the data collection or analyses—making it difficult to understand how etiologies may differ across males and females. By including opposite sex twins it is possible to investigate whether magnitude of genetic and environmental influences differs across sexes, and whether or not it is the same set of genes or shared environmental experiences that influences ADHD, ODD and CD symptoms in males and females. Given that boys generally show a higher prevalence of ADHD (Brown et al., 2001), ODD (Feehan et al., 1994) and CD (Maughan et al., 2004), it is interesting to examine both aspects of sex differences, which are whether the magnitude of genetic and environmental effects differs in males and females, and whether the genes that influence the liability to ADHD, ODD and CD symptoms are the same in the two sexes.
Furthermore, it has been suggested that different disruptive and problem behaviors within the psychopathological spectrum (e.g., substance use, antisocial behavior, ADHD, CD, impulsive and sensation seeking personality traits) can be united by an externalizing factor. This has consistently been found on a phenotypic level (please see (Krueger et al., 2007; Krueger et al., 2005). These problem behaviors may co-occur because they share a common genetic component. For instance, the genetic influences on a common externalizing factor describing CD, substance use, ADHD, and novelty seeking was found to account for more than 80% of the variation in an adolescent sample (Young et al., 2000). Similar findings were reported in a study linking antisocial personality disorder, CD, alcohol and drug dependence and unconstrained personality style (Krueger et al., 2002). Strong heritable influences on an externalizing factor of antisocial behavior, substance abuse and CD were also found in an adult sample (Kendler et al., 2003). There is at least one study that reported that the most important influence on a common externalizing factor linking ADHD, CD and ODD symptoms were a common shared environmental influence. However, that study combined maternal and child symptoms into one measure (Burt et al., 2003).
In the present study, we investigated the genetic and environmental sources of ADHD, ODD and CD symptoms using data from a large sample of 9–10 year old same sex and opposite sex twins. ADHD, ODD and CD symptoms were rated by parents using face-to-face interviews. The discrepancy in the literature regarding the genetic and environmental influence on the covariation among ADHD, ODD and CD symptoms, and the lack of complete understanding of sex differences in their underlying etiology warrants further examination of these disorders and their common genetic and environmental influences in boys and girls. We therefore attempted to expand the existing literature by (1) testing for quantitative as well as qualitative sex differences in the genetic and environmental effects in ADHD, ODD and CD symptoms; (2) determining the extent to which the associations among these symptoms are due to common genetic factors, common environmental factors, or both; (3) testing whether a latent externalizing behavior problem factor explains the covariance among these symptoms; and (4) estimating the genetic and environmental influences for this underlying externalizing factor.
Method
Sample
The subjects were participants in the University of Southern California (USC) Twin Study of Risk Factors for Antisocial Behavior, which is an ongoing prospective longitudinal study of the interplay of genetic, environmental, social, and biological factors on the development of antisocial behavior from childhood to emerging adulthood. The study uses an extensive assessment protocol, including cognitive, behavioral, psychosocial and psychophysiological measures. The twins and their parents were recruited from the larger Los Angeles community and the sample is representative of the ethnic and socio-economic diversity of the greater Los Angeles area (Baker et al., 2007). The study includes 605 families, N=1,219 twins and triplets born between 1990 and 1995. Study participation required that the twins be proficient in English and 9 or 10 years old at 1st assessment (Baker et al., 2006). In addition, either English or Spanish proficiency was required for the twins’ primary caregiver. So far, two waves of data have been completed, and a third wave is currently being collected. The design and recruitment has been described in additional detail elsewhere (Baker et al., 2006; Baker et al., 2007).
Zygosity determination
Zygosity was based on DNA microsatellite analysis [> 7 concordant and zero discordant markers = monozygotic (MZ); one or more discordant markers = dizygotic (DZ)] for 87% of the same-sex twin pairs. For the remaining same-sex twin pairs, zygosity was established by questionnaire items about the twins’ physical similarity and the frequency with which people confuse them. The questionnaire was used only when DNA samples were insufficient for one or both twins. When both questionnaire and DNA results were available, there was a 90% agreement between the two (Baker et al., 2007).
The current study includes data from the first wave of assessment conducted in 2000–2004. During the first wave of data collection, the twins and their primary caregiver participated in a 6–8 hour laboratory assessment at USC. The assessment was divided into two sessions with a one hour lunch break. Caregiver participation was primarily the biological mothers (> 90 %) and the twins were 9–10 years old (mean age=9.6, SD=.60). Only twins where zygosity could be diagnosed and with data from parent ratings on ADHD, ODD or CD symptoms scores were included in the present study: N=1,208 (99% of the total sample N=1,219) children (n=1,181 twins and 9 sets of triplets n=27): 275 MZ male, 169 DZ male, 274 MZ female, 192 DZ female, and 298 opposite sex DZ pairs.
Measures
During the laboratory assessment, the caregivers were asked to report on their twins’ symptoms of ADHD, ODD and CD symptoms, and the twins were asked only to report on their own CD symptoms using the Diagnostic Interview Schedule for Children version IV (DISC-IV) (Shaffer et al., 2000). The DISC was not administered to teachers. As the DISC was administered to caregivers for all three disorder symptoms, we only used caregiver report in the present study. The DISC-IV is a highly structured interview that has been adapted from DSM-IV-TR (American Psychiatric Association, 2004) to assess psychiatric disorders and symptoms in children and adolescents age 6 to 17 years old. The DISC-IV has been designed to be administered by well-trained lay interviewers for epidemiological research. Responses of DISC interviews are mostly limited to yes and no, although some have an additional “sometimes” or “somewhat” response option or a close-ended frequency choice. Both symptom scores and diagnoses were provided through computerized scoring of the ADHD, ODD and CD modules. The DISC-IV distinguishes between symptoms present during the “past year” and symptoms that are “current” within the past 4 weeks. The present study only uses the past year ADHD, ODD and CD symptom scores for data analysis.
Attention Deficit Hyperactivity Disorder (ADHD) symptoms
As in the DSM-IV-TR, the DISC-IV divides ADHD into: (1) a predominantly inattentive subtype, including individuals with significant elevations of inattention in the absence of significant hyperactivity-impulsivity, (2) a predominantly hyperactive-impulsive subtype that includes children with significant elevations of hyperactivity-impulsivity but not inattention, and (3) a combined type that describes individuals with simultaneous elevations of inattention and hyperactivity-impulsivity. In our sample, 60 (4.99%) of the twins met a diagnosis for the inattentive subtype, 43 (3.57%) twins met a diagnosis for the hyperactive/impulsive subtype, 34 (2.82%) twins met a diagnosis for the combined type. In total, 137 (11.38 %) twins met the diagnoses for any type.
In the current study we used the total ADHD symptom score, summing both types of items (11 inattention items and 10 hyperactive/impulsive items). The caregivers’ reports indicated that 84% of the twins had at least one ADHD-symptom in the past year.
Oppositional Defiant Disorder (ODD) symptoms
The ODD symptom score was derived as the sum of 12 items from the Oppositional Defiant Disorder module of the DISC-IV (Shaffer et al., 2000). Of the twins in our sample, 120 (9.96%) met the diagnosis for ODD. Based on caregivers’ reports 90% of the twins had had at least one ODD-symptom in the past year.
Conduct Disorder (CD) symptoms
To measure CD symptoms, the sum of 25 items in the Conduct Disorder module from the DISC-IV was used (Shaffer et al., 2000). Of the twins in our sample, 24 (1.99%) of twins met the diagnosis for CD. The caregiver reports indicated that 48% of the twins had had at least one CD symptom in the past year.
A subset of twins from 30 families (exactly 30 boys and 30 girls) from the full sample was re-tested after approximately six months to evaluate test re-test reliability for ADHD, ODD and CD symptoms. The test re-test reliability was adequate for the measures: ADHD symptoms, r=.78, ODD symptoms r=.69, CD symptoms r=.81. The internal consistencies (Cronbach’s alpha) of the three sets of symptom counts were also sufficient in the full sample: ADHD symptoms α=.86, ODD symptoms α=.83, CD symptoms α=.70. Prior to analysis, the measures were independently transformed (log10(x+1)) to reduce the positive skew in their distributions.
All study participants were assured confidentiality. The laboratory procedures and all aspects of the study were reviewed by the University of Southern California Institutional Review Board and were compliant with Federal regulations at the time.
Statistical Analyses
Descriptive Statistics and Correlations
To get a first indication of the underlying sources of variance among ADHD, ODD and CD symptoms, an examination was made of within-person correlations (i.e., phenotypic correlations among ADHD, ODD and CD symptoms), intraclass twin correlations (i.e., Twin-1 and Twin-2 correlations within each measure) and cross-twin cross-trait correlations (e.g., ADHD symptom score in Twin-1 with ODD symptom score in Twin-2). For example, a DZ intraclass correlation approximately half the value of the MZ intraclass correlation would indicate the presence of genetic effects for a given set of symptoms, whereas a DZ intraclass correlation more than half the MZ intraclass correlation indicates the presence of both genetic and shared environmental effects (i.e., non-genetic influences that contribute to similarity within pairs of twins). The cross-twin cross-trait correlations give information about the genetic and environmental correlations between traits. Cross-twin cross-trait correlations are interpreted the same way as intraclass correlations—that is, greater values for MZ compared to DZ pairs suggests shared or correlated genetic influences for two particular sets of symptoms. This cursory examination of observed correlations gives a rough approximation of the genetic and environmental variation and covariation among the three sets of symptoms, although formal modeling is necessary to test the significance of the preliminary inferences made from these correlations.
Genetic analyses
Saturated models, which estimate the variances, covariances and means of ADHD, ODD and CD symptom scores were first fit in both univariate and multivariate analyses. As these summary statistics were obtained for each zygosity group, mean and variance differences between Twin-1 and Twin-2, between males and females, and between zygosity groups could be formally assessed through the comparison of various sub-models. For example, mean differences between Twin-1 and Twin-2 were ascertained by comparing a model that estimated separate means for Twin-1 and Twin-2 with one that constrains means to be the same across twins.
To estimate the relative contribution of genetic and environmental factors to individual differences in each of the symptom scores (ADHD, ODD and CD) univariate model fitting was carried out. By using both male and female same sex twins it is possible to test for quantitative sex differences; that is, to test whether the magnitudes of genetic and environmental effects differ in males and females for a specific trait or disorder or if they can be constrained to be equal. In addition, by also including opposite sex twins it is possible test for qualitative sex differences; that is, to test whether different genetic and shared environmental effects are important for males and females for a specific trait or disorder. If different genetic influences are important, then the opposite sex twins will be less genetically similar for the trait than DZ twins and their genetic correlation will be less than 0.5. This can be tested by allowing the genetic correlation for opposite sex twin pairs (rGmf) to be estimated in the model, rather than being fixed to 0.5. Alternatively, to evaluate whether qualitatively different environments are important in each sex, one can test whether the correlation between environments shared by male and female twins (rCmf) is less than 1.0 in opposite sex twin pairs (Neale & Cardon, 1992).
This basic univariate genetic model can be extended to the bivariate case in order to investigate the genetic and environmental overlap between ADHD and ODD symptoms, between ADHD and CD symptoms, and between ODD and CD symptoms (Enders & Bandalos, 2001). A Cholesky decomposition was used to estimate variance-covariance matrices for A, C and E effects in a correlated factors model (Davis et al., 2007) for each pairwise set of symptoms. This provides estimates of the genetic correlation (rg), the shared environmental correlation (rc), and the non-shared environmental correlation (re) between a pair of measures, in this case, between ADHD and ODD symptoms; or between ADHD and CD symptoms; or between ODD and CD symptoms. rg indicates the extent to which genetic effects on one measure overlap with genetic effects on another measure, while rc and re indicate overlap among shared and non-shared environmental factors for the different symptoms. These statistics vary from −1.0 and +1.0, and are independent of the magnitudes of genetic and environmental influence for each set of symptoms (Posthuma et al., 2003).
To investigate further the nature of the relationships among ADHD, ODD and CD symptoms, two types of multivariate models were also fit to the three sets of symptoms simultaneously: (1) an independent pathway model, and (2) a common pathway model. In an independent pathway model, genetic and environmental effects are of two types: common and specific. The model specifies common genetic (A); common shared environmental (C); and common non-shared environmental (E) factors that load on ADHD, ODD and CD symptoms, as well as genetic and environmental effects that are specific (plus error) to each measure. It should also be noted that an independent pathway model including three variables estimates the same number of parameters as a Cholesky model for three variables. In contrast, in a common pathway model, the common genetic (A), common shared environmental (C), and common non-shared environmental (E) factors are mediated through a shared latent factor (F) that represents the variance shared among the three externalizing behaviors. The common pathway model estimates fewer parameters than the independent pathway model and therefore, is more parsimonious. In addition to the genetic and environmental effects on the shared latent factor; As, Cs, and Es (which include measurement error) parameters that are specific to each measure are also estimated (McArdle & Goldsmith, 1990; Neale & Cardon, 1992). Fully saturated models, which estimate means and 6×6 matrices of covariances among the three externalizing symptom scores, were used as a baseline comparison.
All models were fit with the structural equation program Mx (Neale et al., 2003), using a maximum likelihood estimation procedure for raw data. This raw maximum likelihood approach yields a goodness of fit index calculated as two times the log-likelihood of the data given the model (-2LL). The difference between the fit index of a full model and that of a submodel, in which parameters are fixed to be zero or constrained to be equal, follows a χ2 distribution with the difference in the number of estimated parameters as the degrees of freedom. A non-significant difference indicates that the model with fewer parameters to be estimated fits the data better (Neale & Cardon, 1992). In addition to the likelihood-ratio χ2–test, Akaike’s information criterion (AIC = χ2 – 2 × degrees of freedom) was computed. The AIC represents the balance between model fit and the number of parameters (parsimony), with lower values of AIC indicating the most suitable model (Akaike, 1987). Finally, a third model-selection statistic was the Bayesian Information Criterion (BIC = -2LL + df ln N), where increasingly negative values correspond to increasingly better fitting models (Raftery, 1995).
Results
Descriptive statistics for ADHD, ODD, and CD symptoms at ages 9–10 years are presented in Table 1. No significant mean or variance differences were found between Twin-1 and Twin-2, nor were there any mean or variance differences across zygosity groups (MZ male, DZ male, MZ female, DZ female, opposite sex) for any of the measures (analyses available upon request). The lack of variance differences between MZ and DZ twins suggests that sibling imitation or contrast effects may not be important to these three groups of externalizing behavior symptoms at this age (Simonoff et al., 1998). Even so, bivariate and multivariate genetic models were run with and without opposite sex twins; that is, one set of analyses using five zygosity groups (MZ male, DZ male, MZ females, DZ females, and opposite sex twins) and one set of analyses using four zygosity groups (excluding opposite sex twins).
Table 1.
Means, standard deviations and number of participants (n) for ADHD, ODD and CD symptom scores, age 9–10 years, by sex and zygosity
| Males | Females | |||||
|---|---|---|---|---|---|---|
| MZ | DZ | DZ | MZ | DZ | DZ | |
| same sex | opposite sex | same sex | opposite sex | |||
| ADHD symptoms | 5.71 (4.54)
N=275 |
6.85 (5.64)
n=169 |
7.18 (6.08)
n=147 |
4.57 (4.70)
n=272 |
4.36 (4.47)
n=190 |
3.91 (4.21)
n=151 |
| ODD symptoms | 4.55(3.27)
N=275 |
5.01 (3.40)
n=169 |
5.01 (3.18)
n=147 |
4.22 (3.26)
n=272 |
4.65 (3.29)
n=191 |
4.23 (2.89)
n=151 |
| CD symptoms | 1.27 (1.77)
N=275 |
1.64 (2.13)
n=169 |
1.52 (2.31)
n=147 |
.89 (1.39)
n=274 |
.93 (1.71)
n=192 |
.85 (1.55)
n=151 |
Note. MZ = monozygotic; DZ = dizygotic; n = number of participants
No variance or covariance differences were found between boys and girls for any of the symptoms, apart for CD symptoms where some variance differences were found between DZ males and females (χ2= 6.86, df=2, p=.03) and between opposite sex males and females (χ2=4.34, df=1, p=.04). Significant mean differences were found between boys and girls for all three measures ADHD symptoms (χ2= 59.47, df=9, p<.001) ODD symptoms (χ2= 20.23, df=9, p=.02) CD symptoms (χ2= 36.56, df=9, p<.001). Therefore in all genetic models, mean values were estimated separately for boys and girls.
Moreover, the three measures were moderately correlated in this sample: ADHD symptoms and ODD symptoms: phenotypic correlation r=.40, df=1204, p<.001; ADHD symptoms and CD symptoms: r=.37, df=1204, p<.001; and ODD symptoms and CD symptoms: r=.45, df=1205, p<.001.
Twin Correlations
Intraclass and cross-twin cross-trait correlations are shown in Table 2. The MZ intraclass correlations were consistently greater than the corresponding DZ intraclass correlations, suggesting genetic influences for all three symptoms. Likewise, the cross-twin cross-trait correlations were also generally greater for MZ than DZ pairs, indicating that the genetic effects are important for the relationship between ADHD and ODD symptoms, between ADHD and CD symptoms, and between ODD and CD symptoms. The same-sex DZ intraclass correlations for ADHD symptoms were less than half the MZ intraclass correlations; this was most evident in females. This could indicate the influence of dominant genetic effects (D), consequently a univariate ADE model was compared to an AE model. However, an AE model provided a better fit (χ2=.72; df=2; p=.70). Moreover, the same-sex DZ intraclass correlations for ODD and CD symptoms were all greater than half the MZ intraclass correlations, indicating shared environmental influences. The lower DZ opposite sex intraclass correlation compared to the DZ same sex intraclass correlation for CD symptoms may be due to qualitative sex differences. Lastly, the MZ intraclass correlations were all less than one, which suggest influence of non-shared environment.
Table 2.
Intraclass and cross-twin cross-trait correlations between ADHD, ODD and CD symptoms, age 9–10 years, by sex and zygosity
| ADHD | ODD | CD | ADHD | ODD | CD | |
|---|---|---|---|---|---|---|
| MZ male | MZ female | |||||
| ADHD | .58* | .62* | ||||
| ODD | .29* | .59* | .32* | .62* | ||
| CD | .32* | .40* | .68* | .33* | .34* | .68* |
|
|
||||||
| DZ same sex male | DZ same sex female | |||||
|
|
||||||
| ADHD | .25* | .17 | ||||
| ODD | .15 | .37* | .13 | .48* | ||
| CD | .26* | .31* | .53* | .20* | .30* | .49* |
|
|
||||||
| DZ OS male (below diagonal)–female (above diagonal) | ||||||
|
|
||||||
| ADHD | .39* | .04 | .19* | |||
| ODD | .20* | .38* | .18* | |||
| CD | .12 | .25* | .33* | |||
Note. MZ = monozygotic, DZ = dizygotic; intraclass correlations on each diagonal in bold; cross-twin cross-trait correlations are below the diagonal;
= p < .05
Univariate Modeling Fitting Results
Table 3 displays univariate model-fitting results and parameter estimates for all five zygosity groups (both same-sex and opposite-sex twins included). Comparing Models I and II with Model III showed that qualitative sex differences were not significant for either ADHD (Δχ2=0.20; df=1; p=.65 for fixing rGmf=.5; Δχ2=0.00; df=1; p=1.0 for fixing rCmf=1.0) or ODD (Δχ2=0.00; df=1; p=1.00 for fixing rGmf; Δχ2=0.04; df=1; p=.53 for fixing rCmf =1.0). Moreover, comparing Models III and IV revealed that variance components could be constrained to be equal in males and females for both ADHD (Δχ2=0.18; df=3; p=.98) and ODD (Δχ2=1.07; df=3; p=.78). Genetic influences in ADHD symptoms accounted for 61% (p<.05) of the variance, while 39% (p<.05) was due to non-shared environmental influences. For ODD symptoms, 34% of the variance (p<.05) was due to genetic effects, 25% (p<.05) was due to shared environmental influences, and the non-shared environment accounted for the remaining 41% (p<.05).
Table 3.
Univariate model fit indices and parameter estimates (and 95% confidence intervals) for ADHD, ODD and CD symptom, age 9–10 years
| Overall fit | Parameter estimates (95 % CI) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| -2LL | Df | BIC | AIC | χ2 | df | p | A | C | E | A | C | E | |
| Saturated | 1026.08 | 1188 | −3297.53 | ADHD-symptoms | |||||||||
| I: Boys ≠ Girls | |||||||||||||
| rGmf free | 1034.28 | 1196 | −3319.09 | −7.93 | 8.07 | 8 | .43 | ||||||
| II: Boys ≠ Girls | |||||||||||||
| rCmf free | 1034.35 | 1196 | −3319.05 | 7.73 | 8.27 | 8 | .41 | ||||||
| III: Boys ≠ Girls | |||||||||||||
| rGmf, rCmf fixed | 1034.35 | 1197 | −3322.26 | −9.73 | 8.27 | 9 | .51 | ||||||
| IV: Boys = Girls | |||||||||||||
| 1034.53 | 1200 | −3331.79 | −15.55 | 8.45 | 12 | .75 | .61 (.46–.68) | .00 (.00–.13) | .39 (.32–.46) | Same as males | |||
| Saturated | 433.072 | 1189 | −3597.24 | ODD-symptoms | |||||||||
| I: Boys ≠ Girls | |||||||||||||
| rGmf free | 437.10 | 1197 | −3620.89 | −11.97 | 4.03 | 8 | .85 | ||||||
| II: Boys ≠ Girls | |||||||||||||
| rCmf free | 437.06 | 1197 | −3620.90 | −12.01 | 3.99 | 8 | .86 | ||||||
| III: Boys ≠ Girls | |||||||||||||
| rGmf, rCmf fixed | 437.10 | 1198 | −3624.09 | −13.97 | 4.03 | 9 | .91 | ||||||
| IV: Boys = Girls | |||||||||||||
| 438.17 | 1201 | −3633.18 | −18.90 | 5.10 | 12 | .95 | .34 (.13–.56) | .25 (.06–.43) | .41 (.34–.48) | Same as males | |||
| Saturated | 84.18 | 1192 | −3783.26 | CD-symptoms | |||||||||
| I: Boys ≠ Girls | |||||||||||||
| rGmf free | 94.56 | 1200 | −3803.74 | −5.62 | 10.38 | 8 | .24 | .39 (.12–.70) | .32 (.03–.56) | .28 (.22–.37) | .44 (.17–.74) | .26 (.00–.50) | .30 (.23–.39) |
| II: Boys ≠ Girls | |||||||||||||
| rCmf free | 99.19 | 1200 | −3801.42 | −0.99 | 15.01 | 8 | .06 | ||||||
| III: Boys ≠ Girls | |||||||||||||
| rGmf, rCmf fixed | 97.64 | 1201 | −3805.41 | −4.54 | 13.46 | 9 | .14 | ||||||
| IV: Boys = Girls | |||||||||||||
| 114.32 | 1204 | −3806.69 | 6.14 | 30.14 | 12 | .003 | |||||||
| IVa: Boys = Girls | |||||||||||||
| rGmf free | 107.23 | 1203 | −3807.03 | 1.05 | 23.05 | 11 | .017 | ||||||
Note. Model I: estimates different variance components and different genetic effects in the sexes; Model II: estimates different variance components and different shared environmental effects in the sexes; Model III: estimates different variance components in the sexes; Model IV: estimates same variance components in both sexes; Model IVa (fit only for CD symptoms) estimates different genetic effects but with equal magnitude of variance components in both sexes. Models I thru IVa are compared to the saturated covariance model in each case. A = variance due to additive genetic influences; C = variance due to shared environmental influences; E = variance due to non-shared environmental influences.
For CD symptoms, there were no qualitative sex differences in environmental influences, as indicated by the lack of significant drop in fit when fixing the shared environmental correlation for opposite sex twins (rCmf) to be 1.0 (Model III) compared to it being freely estimated (Model II) (Δχ2=1.56; df=1; p=.21). The best fitting model according to AIC indicated qualitative sex differences in genetic influences, as well as quantitative sex differences. The genetic correlation for opposite sex twins rGmf was estimated to be .00, with a 95% CI that included both boundary values of 0 and .5. Nonetheless, fixing the genetic correlation between males and females to be 0.50 (Model III) produced only a marginally significant drop in fit compared to Model I where the male-female genetic correlation was freely estimated (Δχ2=3.08; df=1; p=.08), and the lowest BIC value was obtained for Model III with quantitative but no qualitative sex differences in either genetic or environmental influences. This discrepancy in fit values for various constraints on sex effects suggested that one additional model be considered for CD symptoms, namely one in which qualitative sex differences in genetic effects are allowed but the magnitude of variance components constrained to be equal for boys and girls (Model IVa in Table 2). This model did not fit well, and was significantly worse than Model I (Δχ2=12.67, df=3, p<.05), further suggesting the possibility of some quantitative sex differences for CD. Given the ambiguity of results concerning the qualitative sex differences in CD symptoms, the full model estimates are presented in Table 2. As shown, the ACE estimates are actually remarkably similar for boys and girls, with heritability only slightly larger in girls (44%; p<.05) than in boys (39%; p<.05), and the shared environment being only slightly larger in boys (32%; p<.05) than in girls (26%). It should however, be kept in mind, that CD symptoms were rare at this age, and CD generally peaks at a later age, therefore these observed sex differences—both quantitative and qualitative—should be treated with caution. Larger samples including opposite sex twins are required to resolve these sex differences most clearly.
Bivariate Model Fitting Results
Table 4 displays genetic, shared environmental and non-shared environmental correlations between ADHD and ODD symptoms; ADHD and CD symptoms; and ODD and CD symptoms. In order to evaluate the extent to which opposite-sex twin correlations may be driving these estimates (especially in light of the somewhat lower DZ male-female correlation for CD symptoms), results are presented with and without the DZ male-female twins. The first three columns in Table 4 contain results including opposite sex twins and the last three rows contain results excluding opposite sex twins. For analyses including opposite-sex twins, the model that constrained genetic and environmental parameter estimates to be equal in males and females fit best for ADHD and ODD symptoms (χ2=5.03; df=9; p=.83) . Likewise, a model with sex equality for ACE effects fit best for ADHD and CD symptoms (χ2=15.39; df=9; p=.08) and for ODD and CD symptoms (χ2=15.05; df=9; p=.09). The observed sex differences in CD symptoms were not seen the bivariate analyses, which is probably due in part to their effects being very slight combined with the variance-covariance structure being similar across sexes. The same pattern of non-significant sex differences was found when excluding opposite sex DZ twins (model-fitting indices are available upon request). As seen in Table 3, the pattern of results is highly similar for analyses with and without the male-female twins, suggesting these are not unduly affecting these parameter estimates.
Table 4.
Correlations from bivariate models between ADHD and ODD symptoms, between ADHD and CD symptoms, and between ODD and CD symptom, age 9–10 years
| Analyses including opposite sex twins | Analyses excluding opposite sex twins | |||||
|---|---|---|---|---|---|---|
| Correlations | ||||||
| rg | rc | re | rg | rc | re | |
| ADHD – ODD | .49 (.17 – .81) | 1.00 (−1.00 – 1.00) | .30 (.19 – .40) | .45 (.08 – .79) | 1.00 (−1.00 – 1.00) | .30 (.19 – .40) |
| ADHD – CD | .43 (.23 – .64) | 1.00 (−1.00 – 1.00) | .10 (−.02 – .21) | .39 (.08 – .67) | 1.00 (.98 – 1.00) | .09 (−.03 – .21) |
| ODD – CD | .51 (.21 – .83) | .81 (.12 – 1.00) | .21 (.11 – .33) | .43 (−.03 – .85) | .77 (.25 – 1.00) | .22 (.10 – .33) |
Note. rg = genetic correlation; rc = shared environmental correlation; re = non-shared environmental correlation
The genetic correlation (rg) of .49 in the full sample analyses, including opposite sex pairs, suggests a significant overlap between genetic influences in ADHD and ODD symptoms. Although the shared environmental correlation (rc) was estimated at its upper boundary value, it was non-significant. Non-shared environmental influences also show a significant overlap across ADHD and ODD symptoms (re=.30, p<.05). It should be noted that the non-shared environmental correlation (re) could also reflect correlated measurement error between the two measures. Likewise, the genetic correlation between ADHD and CD symptoms was rg=.43 (p<.05), whereas both the shared environmental correlation and the non-shared environmental correlation were non significant. The genetic correlation between ODD and CD symptoms was rg=.51, consistent with the hypothesis of a significant overlap of genetic influences between these two disorders. The shared environmental correlation was rc=.81 (p<.05) and the non-shared environmental correlation was re=.21 (p<.05). The results from the analyses without opposite sex twins were similar to the results from the analyses including them, with the exception that rc is highly significant between ADHD and CD when opposite sex twins are excluded. This is possibly due to the presence of some shared environmental influences in ADHD and the fact that the shared environmental influences in CD were significant. We note that in all cases, the confidence interval for rg did not include 1.0, indicating that in spite of the significant genetic correlations among these disorders there is some genetic specificity for each set of symptoms as well.
Multivariate Model Fitting Results
To investigate whether a latent externalizing behavior factor explains the covariance among these three symptoms, an independent pathway and a common pathway model were tested using the full sample, including opposite sex pairs (see Table 5). There was no significant loss in fit in the independent pathway compared to the saturated model (Δχ2=109.48; Δdf=93; p=.12). The common pathway model, which tests whether ADHD, ODD and CD symptoms share one underlying latent factor, did not worsen the fit compared to the independent pathway model (Δχ2=14.59; Δdf=8; p=.07). The common pathway model also had a smaller AIC, indicating that it is a more parsimonious model. In addition, the common pathway model had a smaller BIC, indicating a better fit.
Table 5.
Multivariate model fit indices for ADHD, CD and ODD symptoms, age 9–10 years
| Overall Fit | Model comparisons | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Models# | -2LL | DF | AIC | BIC | χ2 | df | p | Δχ2 | Δdf | p |
| 1 Saturated | 921.59 | 3482 | -- | -- | -- | -- | -- | -- | -- | -- |
| 2 Independent pathway | 1031.07 | 3575 | −76.52 | −10957.29 | 109.48 | 93 | 0.12 | -- | -- | -- |
| 3 Common pathway | 1045.66 | 3583 | −77.93 | −10975.67 | 124.07 | 101 | 0.06 | 14.59 | 8 | 0.07 |
| 3a Equate factor loadings in males and females | 1049.11 | 3586 | −80.48 | −10983.58 | 127.52 | 104 | 0.06 | 3.45 | 3 | 0.33 |
| 3b Equate factor loadings and common ACE in males and females | 1051.89 | 3589 | −83.70 | −10991.81 | 130.30 | 107 | 0.06 | 6.22 | 6 | 0.40 |
| 3c Equate factor loadings, common ACE and unique ACE in males and females | 1064.58 | 3598 | −89.01 | −11014.35 | 142.99 | 116 | .045 | 18.91 | 15 | 0.22 |
Note. -2LL = −2(log-likelihood) AIC = Akaike’s Information Criterion; BIC = Bayesian Information Criterion; df = degrees of freedom; Δχ2 = difference in log-likelihoods between nested models, Model 2 is compared to Model 1, Model 3 is compared to 2, Models 3a thru 3c are compared to Model 3.
The genetic and environmental factor structure was similar for boys and girls. In fact, constraining the factor loadings (i.e., the paths from the latent factor to ADHD, ODD and CD symptoms, see Figure 1) to be equal for boys and girls resulted in a non-significant decrease in fit (Δχ2=3.45; Δdf=3; p=.33). Next, we constrained common A, common C and common E (i.e., the latent A, C, and E that explain the latent externalizing factor, see Figure 1) to be equal in boys and girls, and doing this did not reduced the fit of the model (Δχ2=6.22; Δdf=6 p=.40). Also, the measurement specific effects could be constrained to be equal in boys and girls (Δχ2=18.91; Δdf=15, p=.22).
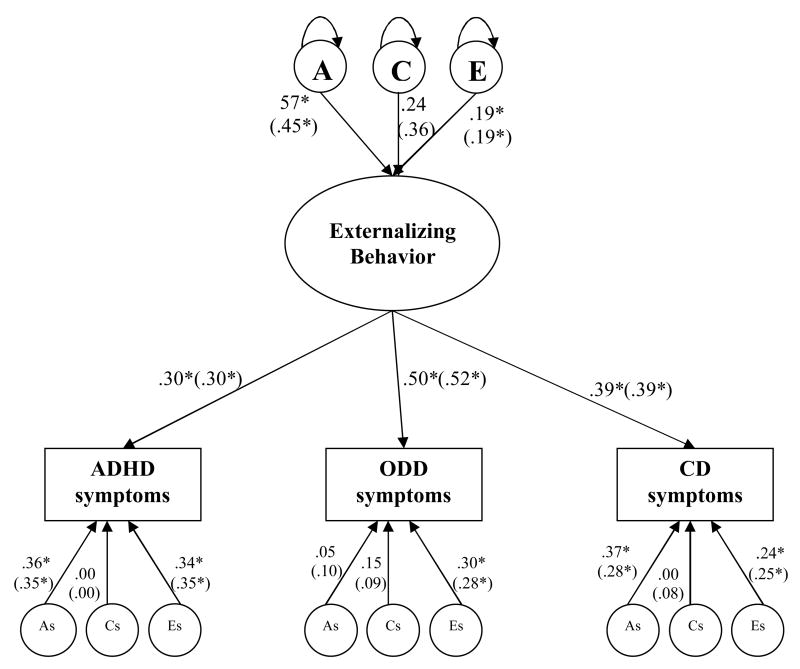
Figure 1.
Squared standardised parameter estimates from a common pathway model for ADHD, ODD and CD. Latent (unobserved) factors are depicted in circle where A refers to additive genetic factor; C refers to shared environmental factor, and E refers to non-shared environmental factor. Oval denotes the latent underlying factor (i.e., Externalizing Behavior). Observed variables are in rectangular, in this case: ADHD, ODD and CD symptoms. As (additive genetic): represents residual variance specific to each measure, likewise for Cs (shared environment), and Es (non-shared environment). Significant estimates are marked with an asterisk. For simplicity, only one twin in a pair is depicted. Results from analyses excluding opposite sex twins are presented within parentheses.
Figure 1 displays squared standardized parameter estimates from the common pathway model. The variation in the latent factor labelled externalizing behavior was explained 57% (p<.05) by the genetic factor (A), 24% (non-significant) by the shared environmental factor (C), and 19% (p<.05) by the non-shared environmental factor (E). The measurement specific genetic effects (AS) accounted for 36% (p<.05) of variance in ADHD symptoms, 5% (non-significant) in ODD symptoms, and 37% (p<.05) in CD symptoms. The measurement-specific shared environmental effect explained 15% (non-significant) of variance in ODD symptoms, but none of the variance in either ADHD or CD symptoms. The measurement-specific non-shared environmental effects, including measurement error, were significant in all three symptoms and explained variance from 24 to 34%. The results from the analyses without opposite sex twins are presented within the parentheses, and these were very similar to the results from the analyses including the opposite sex twins. Model-fitting indices excluding opposite sex twins are available upon request.
Discussion
Parental ratings of ADHD, ODD and CD symptoms in a large sample of 9–10 old twins were used to investigate the covariation among these three areas of child behavior problems. Most importantly, our findings demonstrated that a latent externalizing behavior factor explains the covariance among ADHD, ODD and CD symptoms. Genetic influences (57%) explained more than half of the variance in this externalizing factor, shared environmental influences were non-significant, and 19% was accounted for by the non-shared environment. There were also some unique genetic and environmental influences in each symptom type suggesting some independence and etiological distinction among these symptoms.
In our sample, ADHD symptoms were highly heritable, with 61% of the phenotypic variance being explained by genetic factors. These results support the findings of previous research with heritability being approximately in the range of 55–80% for ADHD (Rhee et al., 1999; Rietveld et al., 2003; Thapar et al., 2000). In keeping with previous research, non-shared environmental effects explained the remaining variance, whereas shared environmental influences seemed to be of negligible importance for ADHD symptoms (Rietveld et al., 2003). In contrast to our findings for ADHD symptoms, we found that the variation in ODD symptoms was not only due to genetic and non-shared environmental influences, but also due to shared environmental influences. That is, heritability and shared environmental influences explained approximately one third and one of quarter each of the total variance in ODD symptoms, respectively. This finding that is consistent with earlier studies on ODD symptoms (Burt et al., 2001). The current study also provides further support for the general finding of the importance of genetic, as well as shared environmental influences for the development of CD symptoms (Jacobson et al., 2002; Rose et al., 2004).
Our data indicated no sex differences in the genetic and environmental effects in ADHD. Previous studies on ADHD have also suggested the absence of quantitative, as well as qualitative sex differences in larger samples of parent, teacher and self-reported data (Derks et al., 2008; Haberstick et al., 2008), although there are some exceptions (Larsson, Larsson & Lichtenstein, 2004; Rhee et al., 1999). Nor did we find any significant sex differences in ODD symptoms, which is in line with previous studies on ODD (Derks et al., 2007; Dick et al., 2005). However, we found some suggestion of qualitative sex differences in the genetic influences, as well as slight but significant quantitative sex differences in the univariate analyses for CD symptoms. Genetic influences were slightly larger in girls, and the shared environment somewhat larger in boys. There is also some possibility of different genetic influences being important in boys and girls, given the somewhat lower correlation for CD symptoms in DZ male-female twin pairs. Earlier research on conduct problems have yielded conflicting results, with some studies reporting significant sex differences (Jacobson et al., 2002; Rose et al., 2004), and others finding no sex differences (Dick et al., 2005; Subbarao et al., 2008). These aforementioned studies all used self-report measures in larger samples. The inconsistency in the literature regarding sex differences in the genetic and environmental effects on externalizing problem behaviors suggests that this warrants further investigation in studies including large samples of both same-sex, and perhaps especially opposite sex twins.
Genetic influences comprised a substantial amount of the variance in ADHD, ODD and CD symptoms and the covariance among them also seemed largely to stem from a common genetic factor. In fact, the covariation among these symptoms could be explained by a latent externalizing behavior factor, and 57% of the total variance in this externalizing behavior factor was explained by a common genetic risk factor. This suggests that a common genetic influence operates to bring about the co-occurrence of ADHD, ODD and CD symptoms. Further, the genetic correlation between ADHD and ODD symptoms was largely due to the influence of genes that increase the risk for both disorders. The same pattern was found for the relationship between ADHD and CD symptoms, and for ODD and CD symptoms. These findings replicate previous research investigating the covariation among these externalizing symptoms (Dick et al., 2005; Nadder et al., 2002; Silberg et al., 1996; Thapar et al., 2001). However, our findings differ from other published studies, which found that a common shared environmental factor linking ADHD, ODD and CD symptoms was the most important influence (Burt et al., 2003; Burt et al., 2001). A possible explanation for the differences in our findings compared to Burt et al. is that they combined data from parent and child reports into one measure, whereas in the present study we only used parental ratings. When using one rater (either self-report (Dick et al., 2005; Young et al., 2000) or caregiver report (Nadder et al., 2002; Silberg et al., 1996; Thapar et al., 2001)) a common genetic factor seems to account for the variance among these symptoms. However, when data is combined from different raters, a common shared environmental factor seems to account for their covariation (Burt et al., 2003; Burt et al., 2001).
The extent to which the same genes contribute to the co-occurrence of these disorders has implications for molecular genetic studies attempting to identify specific genes involved in these disorders. A recent study has in fact found evidence of linkage to a region of chromosome 7, which appears to contain genes conferring risk to the externalizing spectrum (including alcohol, drug dependence, CD, antisocial personality disorder, novelty and sensation seeking) (Dick et al., 2008). Together these findings have implications for how these disorders are grouped and classified. Specifically, if these different disorders are brought about by the same underlying genetic liability, then it is possible that certain environmental experiences determine which disorder is expressed in an individual (Kendler et al., 1987).
However, not all genetic influences were in common among ADHD, ODD and CD symptoms. This was indicated by the genetic correlations between these symptoms being significantly less than one. A non-overlapping genetic variance suggests that these disruptive behavior disorders are somewhat independent in their underlying biological substrates. Further, not all genetic influences were accounted for in the externalizing behavior factor, that is, there were some unique genetic influences in these symptoms, especially in ADHD and CD symptoms. This indicates that there are genetic effects that uniquely impact these symptoms, over and above the genetic influence from the externalizing behavior factor. In other words, there are also some etiological distinctions among these externalizing disorders. Nonetheless, our findings indicate that a common genetic vulnerability puts the individual at risk for developing more than one disorder. It is possible that a common set of genes not only influence these disruptive behavior disorders (Dick et al., 2005; Nadder et al., 2002; Silberg et al., 1996), but also other externalizing problem behaviors e.g., substance use, and certain personality traits such as novelty seeking, reflecting a common genetic vulnerability for externalizing psychopathology (Kendler et al., 2003; Krueger et al., 2002; Young et al., 2000).
Apart from finding a strong genetic overlap among these symptoms, we also found that shared environmental influences were of modest importance for their covariation. A common shared environmental influence in the externalizing factor indicates that common vulnerabilities within the shared environment increase the risk for the co-occurrence of these symptoms. This seems especially true for the co-occurrence of ODD and CD symptoms because the shared environmental correlation between them was significant. Common shared environmental risk factors for these symptoms may for example include family related factors (e.g., poor child rearing practices, maltreatment) (Caspi et al., 2002; Rutter et al., 1998) and contextual factors (e.g., neighborhood disadvantage and poverty) (Sampson, Raudenbush & Earls, 1997). Further research is needed to understand the important components in shared environmental risk factors in relationship to the co-occurrence of these symptoms. Finally, the current study found that non-shared environmental influences contributed to the covariation among these symptoms. It should be noted that the model used in the present study partitions measurement error away from variance in the latent externalizing behavior factor. When doing this, the non-shared environment for latent externalizing behavior factor was found to account for 19%, thus accounting for a significant amount of variance independent of measurement error. Common non-shared environmental risk factors include any experience that is unique to the individual and not shared by his/her co-twin, e.g., head trauma, belonging to different peer groups. We also found significant measurement-specific non-shared environmental effects, which includes measurement error.
Strengths and Limitations of the Present Study
There are several strengths in our study. The twin sample is large and the sample is representative of the ethnic and socio-economic diversity of the greater Los Angeles area (Baker et al., 2006). We used the DISC-IV (Shaffer et al., 2000), a well-standardized instrument to assess ADHD, ODD and CD symptoms. We used face-to-face interviews with the parents and our interview staff was well-trained in the procedures of the DISC. In addition, to fully explore potential sex differences, same sex twins as well as opposite sex twins were included. However, we also have to consider the limitations of this study and how these might have influenced our findings.
First, ADHD, ODD and CD symptoms were assessed using structured interviews with parents in a population-based twin sample; hence results should not be extrapolated to clinical settings. It should however be noted that population-based samples, as opposed to clinically referred samples, do not introduce referral and selection biases (Angold et al., 1999). Furthermore, it should also be mentioned that the children included in this study are in their pre-adolescent years, and CD problem behaviors generally peaks in mid-adolescence (Moffitt, 1993). It remains to be seen how genetic and environmental influences in all three sets of symptoms will change over time. Given the longitudinal nature of the present study, it will be possible to explore these developmental changes in future waves of assessment.
Second, our study is comprised of a cross-sectional sample of 9–10 year old twins, and therefore does not consider any developmental changes in the different disorders, or whether one disorder precedes the other disorder. Future longitudinal data will be required to investigate temporal changes in these childhood disorders. In addition, ADHD, ODD and CD symptoms were based on data from only one informant, which is a further limitation to our study.
A third limitation relates to rater bias. Rater bias occurs when parents either stress the similarities or differences in their children. If there is rater bias, variance differences between MZ and DZ twins are expected. An effect of parents stressing their twins’ similarities is confounded with shared environmental influences, and an effect of parents contrasting their twins’ is confounded with dominant effects (Rietveld et al., 2003; Vierikko et al., 2003). This suggests that a very low DZ correlation, which may indicate dominant effects, in combination with larger variance among DZ twins would be explained by rater bias. That is, in which parents tend to contrast their twins (Simonoff et al., 1998). However, the patterns of intraclass correlations and variances suggest that the effects of rater bias are of limited importance for ADHD symptoms in our data. We found shared environmental influences for ODD and CD symptoms. Even though no variance differences were found, it is possible that part of the shared environment is due to rater bias. On the other hand, because we conducted interviews for each twin separately, this may have reduced the tendency for parents to give similar ratings for both co-twins. A high shared environmental estimate is possibly due to rater bias which might be greater in studies using questionnaires to obtain parental ratings. In addition, we ran all our analyses including and excluding opposite sex twins, and when doing this we found similar results.
In conclusion, it is important to understand the causes of covariation among ADHD, ODD and CD symptoms, as the individuals exhibiting only one problem behavior are likely to have different prognoses, treatment responses, and risk factors compared with individuals who have multiple disruptive behavior disorders. The current study provides evidence that a highly heritable externalizing behavior factor explains the covariance among ADHD, ODD and CD symptoms. Our findings have implications for molecular genetic studies trying to identify susceptibility genes for these disorders. Furthermore, shared and non-shared environmental influences were also of importance in the externalizing behavior factor. This suggests that aspects of the environment, on both a family and a community level, may be important to consider in prevention and intervention strategies targeting the co-occurrence of these symptoms.
Acknowledgments
This study was funded by NIMH (R01 MH58354). Catherine Tuvblad was supported by post-doctoral stipends from the Swedish Council for Working Life and Social Research (Project 2006-1501) and the Sweden-America Foundation. Adrian Raine was supported by NIMH (Independent Scientist Award K02 MH01114-08). We thank the Southern California Twin Project staff for their assistance in collecting data, and the twins and their families for their participation.
References
- Akaike AC. Factor analysis and AIC. Psychometrika. 1987;52:317–32. [Google Scholar]
- American Psychiatric Association. American Psychiatric Association DSM-IV-TR Diagnostic and Statistical Manual of Mental disoders. Washington, DC: 2004. [Google Scholar]
- Anckarsäter H, Stahlberg O, Larson T, Hakansson C, Jutblad SB, Niklasson L, Nydén A, Wentz E, Westergren S, Cloninger CR, Gillberg C, Rastam M. The impact of ADHD and autism spectrum disorders on temperament, character, and personality development. American Journal of Psychiatry. 2006;163:1239–44. doi: 10.1176/ajp.2006.163.7.1239. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello JE, Erkanli A. Comorbidity. Journal of Child Psychology and Psychiatry. 1999;40:57–87. [PubMed] [Google Scholar]
- Baker LA, Barton M, Lozano DI, Raine A, Fowler JH. The Southern California Twin Register at the University of Southern California: II. Twin Research and Human Genetics. 2006;9:933–40. doi: 10.1375/183242706779462912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LA, Jacobson KC, Raine A, Lozano DI, Bezdjian S. Genetic and environmental bases of childhood antisocial behavior: A multi-informant twin study. Journal of Abnormal Psychology. 2007;116:219–35. doi: 10.1037/0021-843X.116.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels M, Hudziak JJ, van den Oord EJCG, van Beijsterveldt CEM, Rietveld MJH, Boomsma D. Co-occurrence of aggressive behavior and rule-breaking behavior at age 12: multi-rater analyses. Behavior Genetics. 2003;33:607–21. doi: 10.1023/a:1025787019702. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV. The effects of attention-deficit/hyperactivity disorder on employment and household income. Medscape General Medicine. 2006;18:3–12. [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Milberger S, Jetton JG, Chen L, Mick E, Greene RW, Russell RL. Is childhood oppositional defiant disorder a precursor to adolescent conduct disorder? Findings from a four-year follow-up study of children with ADHD. Journal of the American Academy Child and Adolescent Psychiatry. 1996;35:1193–204. doi: 10.1097/00004583-199609000-00017. [DOI] [PubMed] [Google Scholar]
- Biederman J, Newcorn J, Sprich S. Comorbidity of attention deficit hyperactivity disorder with conduct, depressive, anxiety, and other disorders. American Journal Psychiatry. 1991;148:564–77. doi: 10.1176/ajp.148.5.564. [DOI] [PubMed] [Google Scholar]
- Brown RT, Freeman WS, Perrin JM, Stein MT, Amler RW, Feldman HM, Pierce K, Wolraich ML. Prevalence and assessment of attention-deficit/hyperactivity disorder in primary care settings. Pediatrics. 2001;107:E43. doi: 10.1542/peds.107.3.e43. [DOI] [PubMed] [Google Scholar]
- Burt AS, Krueger R, McGue M, Iacono WG. Parent-child conflict and the comorbidity among childhood externalizing disorders. Archives of General Psychiatry. 2003;60:505–13. doi: 10.1001/archpsyc.60.5.505. [DOI] [PubMed] [Google Scholar]
- Burt AS, Krueger RF, McGue M, Iacono WG. Sources of covariation among attention-deficit/hyperactivity disorder, oppositional defiant disorder, and conduct disorder: The importance of shared environment. Journal of Abnormal Psychology. 2001;110:516–25. doi: 10.1037/0021-843X.110.4.516. [DOI] [PubMed] [Google Scholar]
- Burt SA, McGue M, Krueger RF, Iacono WG. Sources of covariation among the child-externalizing disorders: informant effects and the shared environment. Psychological Medicine. 2005;35:1133–44. doi: 10.1017/S0033291705004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–4. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Davis OSP, Kovas Y, Harlaar N, Busfield P, McMillan A, Frances J, Petrill SA, Dale PS, Plomin R. Generalist genes and the internet generation: etiology of learning abilities by web testing at age 10. Genes, Brain and Behavior. 2007:1–25. doi: 10.1111/j.1601-183X.2007.00370.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derks EM, Dolan CV, Hudziak JJ, Neale MC, Boomsma DI. Assessment and etiology of attention deficit hyperactivity disorder and oppositional defiant disorder in boys and girls. Behavior Genetics. 2007;37:559–66. doi: 10.1007/s10519-007-9153-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derks EM, Hudziak JJ, Dolan CV, van Beijsterveldt TC, Verhulst FC, Boomsma DI. Genetic and environmental influences on the relation between attention problems and attention deficit hyperactivity disorder. Behavior Genetics. 2008;38:11–23. doi: 10.1007/s10519-007-9178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Aliev F, Wang JC, Grucza RA, Schuckit M, Kuperman S, Kramer J, Hinrichs A, Bertelsen S, Budde JP, Hesselbrock V, Porjesz B, Edenberg HJ, Bierut LJ, Goate A. Using Dimensional Models of Externalizing Psychopathology to Aid in Gene Identification. Archives of General Psychiatry. 2008;65:310–8. doi: 10.1001/archpsyc.65.3.310. [DOI] [PubMed] [Google Scholar]
- Dick DM, Viken RJ, Kaprio J, Pulkkinen L, Rose RJ. Understanding the covariaton among childhood externalizing symptoms: genetic and environmental influences on conduct disorder, attention deficit hyperactivity disorder, and oppositional defiant disorder symptoms. Journal of Abnormal Child Psychology. 2005;33:219–29. doi: 10.1007/s10802-005-1829-8. [DOI] [PubMed] [Google Scholar]
- Enders CK, Bandalos DL. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling. 2001;8:430–57. [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Jetton JG, Tsuang M. Attention deficit hyperactivity disorder and conduct disorder: longitudinal evidence for a familial subtype. Psychological Medicine. 1997;27:291–300. doi: 10.1017/s0033291796004515. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Mennin D, Russell R, Tsuang MT. Familial subtypes of attention deficit hyperactivity disorder: a 4-year follow-up study of children from antisocial-ADHD families. Journal of Child Psychology and Psychiatry. 1998a;39:1045–53. [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Weber W, Russell R. Psychiatric, neuropsychological, and psychosocial features of DSM-IV subtypes of attention-deficit/hyperactivity disorder: results from a clinically referred sample. Journal of American Academy of Child and Adolescent Psychiatry. 1998b;37:185–93. doi: 10.1097/00004583-199802000-00011. [DOI] [PubMed] [Google Scholar]
- Feehan M, McGee R, Raja SN, Williams SM. DSM-III-R disorders in New Zealand 18-year-olds. Aust N Z J Psychiatry. 1994;28:87–99. doi: 10.3109/00048679409075849. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ. Early conduct problems and later life opportunities. Journal of Child Psychology and Psychiatry. 1998;39:1097–108. [PubMed] [Google Scholar]
- Haberstick BC, Timberlake D, Hopfer CJ, Lessem JM, Ehringer MA, Hewitt JK. Genetic and environmental contributions to retrospectively reported DSM-IV childhood attention deficit hyperactivity disorder. Psychological Medicine. 2008;38:1057–66. doi: 10.1017/S0033291707001584. [DOI] [PubMed] [Google Scholar]
- Jacobson KC, Prescott CA, Kendler K. Sex differences in the genetic and environmental influences on the development of antisocial behavior. Developmental Psychology. 2002;14:395–416. doi: 10.1017/s0954579402002110. [DOI] [PubMed] [Google Scholar]
- Kendler K, Heath AC, Martin NG, Eaves L. Symptoms of anxiety and symptoms of depression. Archives of General Psychiatry. 1987;44:451–7. doi: 10.1001/archpsyc.1987.01800170073010. [DOI] [PubMed] [Google Scholar]
- Kendler K, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–37. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Moffitt TE, Harrington H, Milne BJ, Poulton R. Prior juvenile diagnoses in adults with mental disorder: developmental follow-back of a prospective-longitudinal cohort. Archives of General Psychiatry. 2003;60:709–17. doi: 10.1001/archpsyc.60.7.709. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologoc connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–24. [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, and personality: an integrative quantitative model of the adult externalizing spectrum. Journal of Abnormal Psychology. 2007;116:645–66. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Iacono WG. Externalizing Psychopathology in Adulthood: A Dimensional-Spectrum Conceptualization and Its Implications for DSM–V. Journal of Abnormal Psychology. 2005;114:537–50. doi: 10.1037/0021-843X.114.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperman S, Schlosser SS, Kramer JR, Bucholz K, Hesselbrock V, Reich T, Reich W. Developmental sequence from disruptive behavior diagnosis to adolescent alcohol dependence. American Journal of Psychiatry. 2001;158:2022–6. doi: 10.1176/appi.ajp.158.12.2022. [DOI] [PubMed] [Google Scholar]
- Kuperman S, Schlosser SS, Lidral J, Reich W. Relationship of child psychopathology to parental alcoholism and antisocial personality disorder. Journal of the American Academy Child and Adolescent Psychiatry. 1999;38:686–92. doi: 10.1097/00004583-199906000-00015. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Schwab-Stoen M, Goodman SH, Waldman ID, Canino G, Rathouz PJ, Miller TL, Dennis KD, Bird H, Jensen PS. Age and gender differences in oppositional behaviour and conduct problems: a cross-sectional household study of middle childhood and adolescence. Journal of Abnormal Psychology. 2000;109:488–503. [PubMed] [Google Scholar]
- Larsson JO, Larsson H, Lichtenstein P. Genetic and environmental contributions to stability and change of ADHD symptoms between 8 and 13 years of age: a longitudinal twin study. Journal of the American Academy Child and Adolescent Psychiatry. 2004;43:1267–75. doi: 10.1097/01.chi.0000135622.05219.bf. [DOI] [PubMed] [Google Scholar]
- Loeber R, Burke JD, Lahey BB, Winters A, Zera M. Oppositional defiant and conduct disorder: a review of the past 10 years, part I. Journal of the American Academy Child and Adolescent Psychiatry. 2000;39:1468–84. doi: 10.1097/00004583-200012000-00007. [DOI] [PubMed] [Google Scholar]
- Maughan B, Rowe R, Messer J, Goodman R, Meltzer H. Conduct Disorder and Oppositional Defiant Disorder in a national sample: developmental epidemiology. Journal of Child Psychology and Psychiatry. 2004;45:609–21. doi: 10.1111/j.1469-7610.2004.00250.x. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Goldsmith HH. Alternative common factor models for multivariate biometric analyses. Behavior Genetics. 1990;20:569–608. doi: 10.1007/BF01065873. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: a developmental taxonomy. Psychological Review. 1993;100:674–701. [PubMed] [Google Scholar]
- Nadder TS, Rutter M, Silberg J, Maes H, Eaves L. Genetic effects on the variation and covariation of attention deficit-hyperactivity disorder (ADHD) and oppositional-defiant disorder/conduct disorder (ODD/CD) symptomatologies across informant and occasion of measurement. Psychological Medicine. 2002;32:39–53. doi: 10.1017/s0033291701004792. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes H. Mx: Statistical modeling. Richmond, VA: Department of Psychiatry, Medical College of Virginia; 2003. [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Dordrecht: The Netherlands: Kluwer Academic Publications; 1992. [Google Scholar]
- Posthuma D, Beem AL, de Geus EJC, van Baal CM, von Hjelmborg JB, Iachine I, Boomsma DI. Theory and practice in quantitative genetics. Twin Research and Human Genetics. 2003;6:361–76. doi: 10.1375/136905203770326367. [DOI] [PubMed] [Google Scholar]
- Raftery AE. Bayesian model selection in social research. Sociological Methodology. 1995;25:111–63. [Google Scholar]
- Rettew DC, Copeland W, Stanger C, Hudziak JJ. Associations between temperament and DSM-IV externalizing disorders in children and adolescents. Journal of Developmental and Behavioral Pediatrics. 2004;25:383–91. doi: 10.1097/00004703-200412000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, Waldman ID, Hay DA, Levy F. Sex differences in genetic and environmental influences on DSM-III-R attention-deficit/hyperactivity disorder. Journal of Abnormal Psychology. 1999;108:24–41. doi: 10.1037/0021-843X.108.1.24. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Willcutt EG, Hartman CA, Pennington BF, DeFries JC. Test of alternative hypotheses explaining the comorbidity between attention-deficit/hyperactivity disorder and conduct disorder. Journal of Abnormal Child Psychology. 2007 doi: 10.1007/s10802-007-9157-9. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Rietveld MJ, Hudziak JJ, Bartels M, van Beijsterveldt CE, Boomsma DI. Heritability of attention problems in children: I. cross-sectional results from a study of twins, age 3–12 years. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2003;117:102–13. doi: 10.1002/ajmg.b.10024. [DOI] [PubMed] [Google Scholar]
- Robins LN. Deviant children grown up. Baltimore, MD: Wiliams & Wilkins; 1966. [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Kaprio J. Genetic and environmental effects on conduct disorder and alcohol dependence symptoms and their covariation at age 14. Alcoholism: Clinical and Experimental Research. 2004;28:1541–8. doi: 10.1097/01.alc.0000141822.36776.55. [DOI] [PubMed] [Google Scholar]
- Rowe R, Maughan B, Pickles A, Costello EJ, Angold A. The relationship between DSM-IV oppositional defiant disorder and conduct disorder: findings from the Great Smoky Mountains Study. Journal of Child Psychology and Psychiatry. 2002;43:365–73. doi: 10.1111/1469-7610.00027. [DOI] [PubMed] [Google Scholar]
- Rutter M, Giller H, Hagell A. Antisocial Behavior by Young People. Cambridge, UK: Cambridge University Press; 1998. [Google Scholar]
- Sampson RJ, Raudenbush SW, Earls F. Neighbourhoods and violent crime: A multilevel study of collective efficacy. Science. 1997;277:918–24. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- Schachar R, Tannock R. Test of four hypotheses for the comorbidity of attention-deficit hyperactivity disorder and conduct disorder. Journal of the American Academy Child and Adolescent Psychiatry. 1995;34:639–48. doi: 10.1097/00004583-199505000-00016. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas C, Comer J. Diagnostic Interview Schedule for Children - DISC IV. NY: Columbia University; 2000. [Google Scholar]
- Silberg J, Rutter M, Meyer J, Maes H, Hewitt JK, Simonoff E, Pickles A, Loeber M, Eaves L. Genetic and environmental influences on the covariation between hyperactivity and conduct disturbance in juvenile twins. Journal of Child Psychology and Psychiatry. 1996;37:803–16. doi: 10.1111/j.1469-7610.1996.tb01476.x. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Hervas A, Silberg JL, Rutter M, Eaves L. Genetic influences on childhood hyperactivity: contrast effects imply parental rating bias, not sibling interaction. Psychological Medicine. 1998;28:825–37. doi: 10.1017/s0033291798006886. [DOI] [PubMed] [Google Scholar]
- Subbarao A, Rhee SH, Young SE, Ehringer MA, Corley RP, Hewitt JK. Common genetic and environmental influences on major depressive disorder and conduct disorder. Journal of Abnormal Child Psychology. 2008;36:433–44. doi: 10.1007/s10802-007-9189-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, Harrington H, Ross K, McGuffin P. Does the definition of ADHD affect heritabiity? Journal of the American Academy Child and Adolescent Psychiatry. 2000;39:1528–1536. doi: 10.1097/00004583-200012000-00015. [DOI] [PubMed] [Google Scholar]
- Thapar A, Harrington R, McGuffin P. Examining the comorbidity of ADHD-related behaviours and conduct problems using a twin study design. British Journal of Psychiatry. 2001;179:224–9. doi: 10.1192/bjp.179.3.224. [DOI] [PubMed] [Google Scholar]
- Vierikko E, Pulkkinen L, Kaprio J, Viken RJ, Rose RJ. Sex differences in genetic and environmental effects on aggression. Aggressive Behavior. 2003;29:55–68. [Google Scholar]
- White HR, Xie M, Thompson W, Loeber R, Stouthamer-Loeber M. Psychopathology as a predictor of adolescent drug use trajectories. Psychology of Addictive Behaviors. 2001;15:210–8. [PubMed] [Google Scholar]
- Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. American Journal of Medical Genetics (Neuropsychiatric Genetics) 2000;96:684–95. [PubMed] [Google Scholar]