Abstract
The observed age-related decline in neurogenesis may result from reduced proliferation or increased death rate of neuronal precursor cells (NPCs). We found that caspase-3, but not caspase-6, -7, or -9, was activated in NPCs in neurogenic regions of young, young-adult, middle-aged and aged rat brains. The number of capase-3-immunoreactive cells was highest in young and lowest in aged rats. Surprisingly, intraventricular administration of a caspase-3 inhibitor failed to restore the number of BrdU-positive cells in the aged dentate gyrus, suggesting that the age-related decline in neurogenesis may be attributable primarily to reduced proliferation. Additionally, we also found that NPCs in the subventricular zone of young-adult and aged rat brain were increased after focal cerebral ischemia, suggesting that the increase in neurogenesis induced by ischemia may result from an increase in the rate of NPC proliferation, but not from a decrease in NPC death. Thus, our results suggest that age-related and injury-induced changes in the rate of neurogenesis are controlled at the level of NPC proliferation. Furthermore, our results may imply that the mechanisms that maintain a stable population of NPCs in the normal adult and in the ischemic brain, which account for the observed age-dependent reduction or injury-induced increases in neurogenesis, impinge on the regulation of cell division at the NPC level.
Keywords: Neurogenesis, aging, cell death, ischemia, dentate gyrus, subventricular zone
1. Introduction
Neural precursor cells (NPCs), which reside in the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG) and in the subventricular zone (SVZ) lining the lateral ventricles, may provide an endogenous mechanism for brain repair and recovery from injury or disease [15]. Although such a role is largely conjectural, it has generated interest in the possibility that further stimulation of neurogenesis in the adult brain might yield benefit in neurological diseases or in cerebral dysfunction associated with aging. In support of this possibility, NPCs show increased proliferation in response to brain insults such as ischemia [18], migrate toward sites of brain damage [1], and can differentiate into functional neurons [25]. However, only a subset of newborn SVZ neurons migrate into the olfactory bulb (OB) and differentiate into mature neurons [9], whereas the majority undergo programmed cell death [13].
Programmed cell death is an important mechanism that determines the size and shape of the vertebrate nervous system [16], and genetic elimination of cell death leads to embryonic mortality or gross anatomical malformations [6]. Accordingly, programmed death of NPCs has been demonstrated in a variety of in vivo [21] and in vitro [24] systems, including the SVZ and DG of adult mouse brain [2]. Caspases are key mediators of programmed cell death. Although some caspases show activity as proenzymes, they generally exist in a dormant form that is activated by cleavage of their inhibitory prodomains, generating their active forms. Caspase-3 has been documented to play a role in NPC death during embryonic development [20]. NPCs from adult rat brain SVZ also undergo caspase-3-dependent cell death in vitro in response to staurosporine and 2,3-dimethoxy-1,4-naphthoquinone or 4-nonylphenol [10]. In addition, caspase inhibitors reduce constitutive [3] and seizure-induced [14] neurogenesis in the adult rat brain in vivo.
Although NPC proliferation in the DG and SVZ is reduced with aging [17,19,23], how programmed cell death of NPCs may be altered during normal brain aging is unknown. Therefore, we investigated the expression of caspase activation products in neuroproliferative zones of the young, young-adult, middle-aged and aged rat brain, as well as the effect of caspase inhibition on neurogenesis. In addition, although the total number of newly generated neurons is increased in the ischemic brain, it is possible that this process may be counterbalanced by the activation of programmed cell death. Therefore, we also compared the number of NPCs showing caspase-3 activation in the SVZ of young-adult and aged brain after focal ischemia.
2. Experimental and methods
2.1 Animals
Young (5-day-old), young-adult (3-month-old), middle-aged (11-month-old) and aged (24- month-old) male Fisher-344 rats were obtained from the National Institute of Aging, National Institutes of Health and housed in a temperature-controlled environment (21–23°C) with a 12 hr/12 hr light/dark cycle. All animal experiments were carried out in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals, and approved by the Institutional Animal Care and Use Committee (IACUC). Efforts were made to minimize animal suffering and to reduce the number of animals used.
2.2. Transient focal cerebral ischemia
Young-adult and aged male Fisher-344 rats were anesthetized with 5% isoflurane in a gas mixture of 30% oxygen and 70% nitrous oxide. Focal ischemia was performed as previously described [28]. Briefly, immediately thereafter, both common carotid arteries (CCAs) were occluded with microclips and rats underwent simultaneous occlusion of the middle cerebral artery (MCA) and both CCAs for 60 min. This model produces an infarct restricted to the cerebral cortex. Recirculation was established by removing the microclips and restoration of MCA blood flow was verified visually. Blood pressure, blood gases, and blood glucose were monitored via the left femoral artery. Rectal temperature and temporalis muscle temperature contralateral to MCA occlusion were monitored continuously and maintained at 37.0–37.5°C with heating pads.
2.3. Administration of caspase-3 inhibitor and Bromodeoxyuridine (BrdU)
The peptide methylketone caspase-3 inhibitor, N-Benzyloxycarbonyl-Asp(OMe)-Glu(OMe)-Val-Asp-(OMe)-fluoro-methylketone (Z-DEVD-FMK) (Calbiochem), was dissolved in DMSO and further diluted in artificial cerebrospinal fluid (aCSF) at the concentration of 20 mg/ml. Caspase-3 inhibitor or vehicle was infused at 1 μl/hr for 3 days with an osmotic minipump (Alzet 1003D; Alza Scientific Products) in the left ventricle (from the bregma: anteroposterior, −0.8 mm; lateral, 1.5 mm; depth, 3.5 mm). These treatments were assigned in a randomized and blinded manner. For BrdU counting after infusion of caspase inhibitor, BrdU (50 mg/kg in saline) was given by the intraperitoneal twice daily on the last day and rats were killed one day later. For the phenotype of the caspase-3 positive cells, BrdU was given twice daily for 3 days and rats were killed at 4th day. For BrdU counting after focal ischemia, BrdU (50 mg/kg in saline) was given by the intraperitoneal twice daily for 3 days from the day after focal ischemia and sham-operated groups, and rats were killed at 10th day. Brains (four per condition) were freshly isolated and fifty-micrometer coronal sections were cut with a cryostat and stored at −80°C. Some brains were also perfused with saline and 4% paraformaldehyde in PBS and embedded in paraffin.
2.4. Immunohistochemistry
To detect BrdU-labeled cells in tissue sections, paraffin sections were treated with 50% formamide, 280 mM NaCl, and 30 mM sodium citrate at 65°C for 2 hr, incubated in 2 M HCl at 37°C for 30 min, and rinsed in 0.1 M boric acid (pH 8.5) at room temperature for 10 min. Sections were then incubated in 1% H2O2 for 15 min, and in blocking solution (2% goat serum, 0.3% Triton X-100, and 0.1% bovine serum albumin in PBS) for 2 hr at room temperature, before being treated with mouse monoclonal anti-BrdU (Roche, Indianapolis, IN, USA; 2 μg/ml) overnight at 4 °C. To detect the expression of activated caspase cleavage products, immunohistochemistry was conducted on paraffin sections using a standard protocol with antigen retrieval, according to the manufacturer’s instructions (Vector Laboratories). The primary antibodies used were affinity-purified rabbit anti-cleaved caspase-3, -6, -7, and -9 (all from Cell Signaling; 1:200–500). Sections were washed with PBS, incubated with biotinylated goat anti-mouse or anti-rabbit IgG secondary antibody (Vector Laboratories; 1:200) for 2 hr at 25°C, washed, and placed in avidin–peroxidase conjugate solution (Vector Laboratories) for 1 hr. The horseradish peroxidase reaction was detected with 0.05% diaminobenzidine (DAB) and 0.03% H2O2. Processing was stopped with H2O and sections were dehydrated through graded alcohols, cleared in xylene, and coverslipped in permanent mounting medium (Vector Laboratories). Sections were examined with a Nikon E800 epifluorescence microscope. Controls included omitting the primary and secondary antibodies.
2.5. Double-label immunohistochemistry
Double-immunolabeling study was conducted as previous described [19]. Sections were incubated with blocking solution, then with primary antibodies at 4°C overnight, and with secondary antibodies in blocking solution at room temperature for 2 hr. The primary antibodies used are antibodies listed above and anti-doublecortin (DCX; Santa Cruz; 1:200). The secondary antibodies were rhodamine-conjugated rat-absorbed donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories Inc.; 1:200) and fluorescein isothiocyanate-conjugated pig anti-goat or goat anti-rabbit IgG (Jackson ImmunoResearch; 1:200). Sections were mounted with Vectashield (Vector) and fluorescence signals were detected with a Nikon PCM-2000 confocal laser-scanning microscopy. Simple PCI imaging software (Compix Inc.) was employed to confirm co-localization of BrdU and cell type-specific markers within individual cells.
2.6. TUNEL assay
The terminal transferase-mediated dUTP nick end labeling (TUNEL) method is widely used to visualize dead cells by detecting DNA double-strand breaks. TUNEL assay was performed by using the DeadEnd Fluorometric TUNEL System (Promega Inc.) according to the manufacture’s instruction. Briefly, the paraffin sections from young-adult, middle-aged and aged brain were incubated with Protein K solution (20μg/ml) for 10 min at room temperature. After two more washes, each slide was covered with equilibration buffer for 10 minutes. Then slides were incubated in a provided TUNEL assay mixture containing fluorescein-12-dUTP and recombinant Terminal Deoxynucleotidyl Transferase (TdT) within a moist air chamber at 37°C for 1 hr. After stopping the reaction, the brain slides were counterstained with DAPI (Vector Lab). The TUNEL-positive cells were identified as localized bright green cells in a blue background by fluorescence microscopy. Nonspecific labeling was investigated by omitting TdT during the initial step of the labeling procedure. Positive controls were obtained by using ischemic brain sections.
2.7. Cell counting
Both caspase-3-positive and BrdU-positive cells in the SVZ and the DG of rat brains were blindly counted in 5–7 DAB-stained coronal frozen sections (50-μm thickness) per animal, spaced 200 μm apart. Images of caspase-3-positive and BrdU-positive cells were acquired using a Nikon Eclipse-800 confocal microscope and a Nikon DXM 1200 digital camera. The number of immunoreactive cells in each 200× image was determined using Simple PCI software. TUNEL-positive cells in the SVZ and the DG of rat brains were blindly counted in 5–7 paraffin coronal sections (6-μm thickness) per animal using two-photon confocal microscopy. At least three animals were used per condition.
2.8. Statistical analysis
Quantitative results were expressed as the mean ± SEM. The statistical significance of differences between means was evaluated using one-way analysis of variance (ANOVA), and pair-wise comparison of means was accomplished by Scheffe’s post hoc test. Pearsons correlation test was applied to assess the association between BrdU-positive and caspase-3-positive cells. P<0.05 was regarded as statistically significant.
3. Results
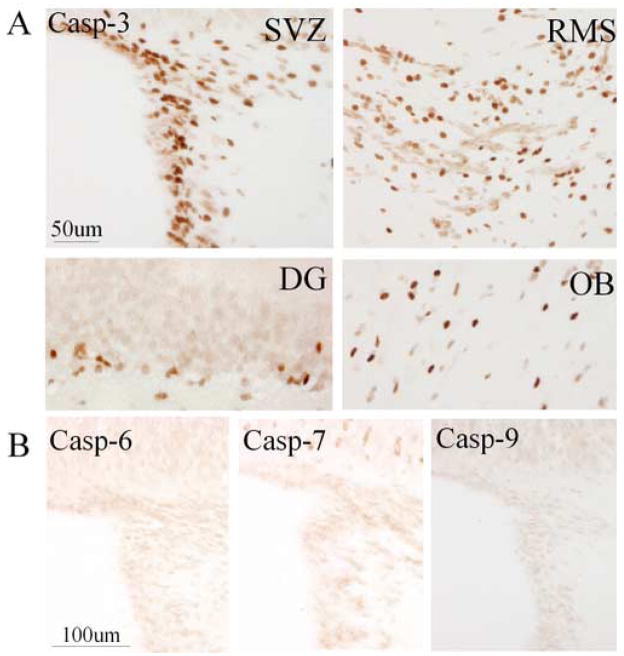
To explore the molecular mechanisms of NPC death in the DG and SVZ of young-adult brain, immunohistochemistry was performed on brain sections from young-adult (3-month-old) rats using antibodies against cleaved (activated) caspases. Cleaved caspase-3 was detected predominantly in cells located in the hippocampal SGZ, SVZ, rostral migratory stream (RMS) and OB (Fig. 1A), indicating that caspase-3-dependent programmed cell death in the adult brain was associated with neuroproliferative zones. In contrast, cleavage products of caspase-6, -7, and -9 were not observed in these regions (Fig. 1B). Similar to young-adult brain, activated caspase-3, but not other cleaved caspase family members mentioned above, was detected in the SGZ, SVZ, RMS and OB in the brain of 24-month-old rats.
Fig. 1.
Caspase activation in neuroproliferative regions of young-adult rat brain. (A) Cleaved caspase-3-positive cells (brown) were seen in the SVZ, RMS, DG SGZ, and OB of rat brain. (B) Cleavage products of capase-6, caspase-7, and caspase-9 were not detected in SVZ (or DG, not shown). Data shown are representative of at least four experiments per panel.
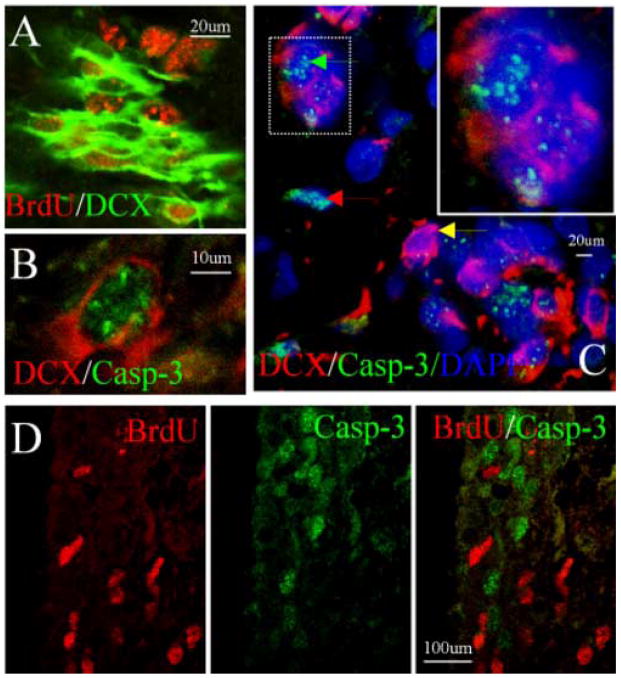
To determine the phenotype of cells exhibiting caspase-3 cleavage, double immunolabeling was conducted using antibodies against cleaved caspase-3 and against DCX, a NPC maker (14). DCX-positive cells incorporated BrdU (Fig. 2A), suggesting that these cells were proliferating NPCs. Cleaved caspase-3-positive cells in the DG (Fig. 2B) and SVZ (Fig. 2C) expressed DCX, confirming that these cells were NPCs. Some cleaved caspase-3-positive cells, however, did not express DCX, suggesting that these cells might be NPCs in the late stages of programmed cell death or cells of non-neuronal lineage. The majority of cleaved caspase-3-positive cells in hippocampal SGZ and SVZ did not incorporate BrdU (Fig. 2D); this suggests that BrdU labeling was a bona fide sign of cell division, and not a manifestation of attempted DNA repair or cell cycle entry in moribund cells.
Fig. 2.
Phenotypic features of cleaved caspase-3-positive cells in young-adult rat brain. (A) Co-localization of nuclear BrdU (red) and cytoplasmic DCX (green) in SVZ. (B) Co-localization of nuclear cleaved caspase-3 (green) and cytoplasmic DCX (red) in hippocampal SGZ. (C) Co-localization of nuclear cleaved caspase-3 (green) and cytoplasmic DCX (red) in some (green arrow) but not other (red and yellow arrows) cells in SVZ; DAPI (blue) was used to stain nuclei. High magnification view of a merged image (insert, top right) shows co-localization of caspase-3 and DCX. (D) Lack of nuclear co-localziation of BrdU (red) and cleaved caspase-3 (green) in SVZ of normal brain, indicating that most BrdU labeling is not a manifestation of cell death. Data shown are representative of at least three experiments per panel.
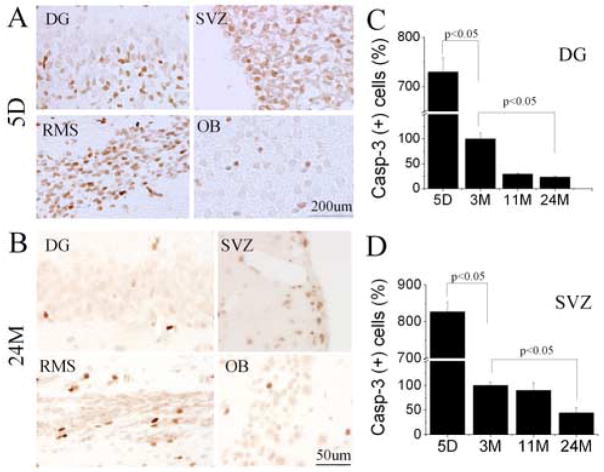
Our and other previous studies showed that proliferative cells are dramatically reduced in the SGZ and SVZ with increasing age, based on the total number of BrdU-positive cells present. To confirm these observations, we also performed double staining of BrdU and DCX in young-adult and aged rat brain. We found that the number of BrdU/DCX-positive cells was reduced in the SGZ of dentate gyrus and SVZ in aged as compared with young-adult rat brain (data not shown) (p<0.05), further confirming an age-dependent decline in neurogenesis. Neurogenesis in DG declines with aging due to diminished proliferation of NPCs [22]. Less is known about the mechanism accounting for reduced neurogenesis in the aged SVZ, or about how specific cell-death pathways may be affected. To investigate how caspase-3-dependent NPC death is influenced by aging, immunohistochemistry was performed on brain sections from rats of various ages. The number of cells showing caspase-3 cleavage in SGZ and SVZ decreased dramatically over the lifespan of these rats, as did the number of cells labeled by BrdU (Fig. 3A-D) and BrdU/DCX (data not shown). Although the largest age-related decline in caspase-3 cleavage that we observed occurred between 5 days and 3 months, and therefore corresponds to maturation rather than senescence, a further decline occurred between 3 months and 11 months in SGZ and between 11 months and 24 months in SVZ.
Fig. 3.
Age-dependent decline in NPC death in neuroproliferative zones of rat brain. Compared to 5-day-old (5D) rats (A), 24-month-old rats (B) show reduced numbers of cells with capase-3 cleavage product (brown) in DG, SVZ, RMS and OB. The number of cleaved caspase-3-immunopositive cells in DG SGZ (C) and SVZ (D) were also quantified for rats aged 5 days (5D), 3 months (3M), 11 months (11M) and 24 months (24M), which also showed an age-dependent reduction in this index of NPC death. Data shown are representative fields (A and B) or mean values ± SEM (C and D) from at least three experiments per panel.
If an increase in the susceptibility of NPCs to cell death contributes to decreased neurogenesis associated with senescence, then the number of cells that express cleaved caspase-3 might be expected to represent a larger percentage of the number of cells labeled by BrdU in aged than in non-aged adult rats. We and others have reported previously that between ~3 to ~24 months of age, the number of BrdU-labeled cells decreases by ~90% in DG and by ~50% in SVZ [19,23]. In the present study, the number of cleaved caspase-3-immunopositive cells decreased by ~75% in DG and by ~50% in SVZ (Fig. 3C). This observation is consistent with the similarity between age-dependent decreases in BrdU incorporation and cell death reported before in DG [17]. To further confirm these findings, TUNEL staining was performed on brain sections of rats at different ages. Surprisingly, few TUNEL-positive cells were found in the SGZ of dentate gyrus and SVZ either in young-adult or in aged brain (Fig. 4A and B). In contrast, the vast majority of TUNEL-positive cells were detected in the striatum, the infarction area adjacent to the SVZ after focal cerebral ischemia, suggesting that the method we used was sensitive enough to detect most of cells with DNA double-strand breaks (Fig. 4C). Nevertheless, the number of TUNEL-positive cells in the SGZ and SVZ in aged brain was still less than that in young-adult brain, further confirming that cell death in neurogenic regions might not contribute to the observed age-dependent decline in neurogenesis.
Fig. 4.
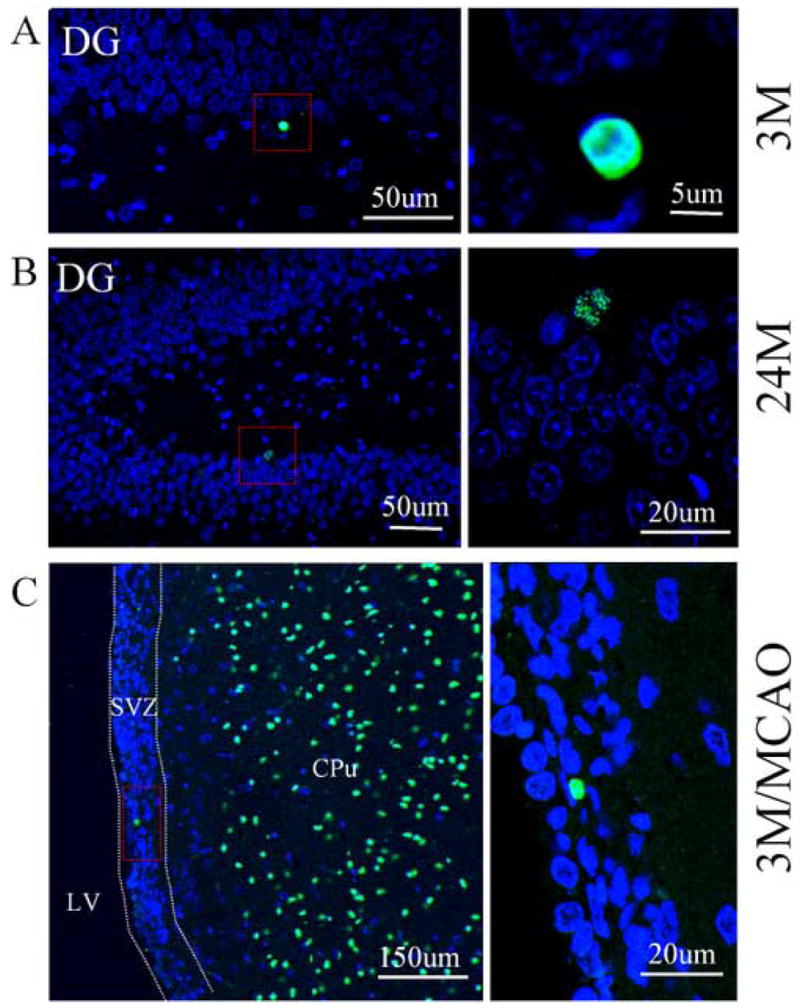
The numbers of TUNEL-positive cells in DG of young-adult and aged rat brain. Few TUNEL-positive cells (green) were found in the DG of young-adult (A) and aged rat brain (B). DAPI (blue) was used for counterstaining of nuclei. Paraffin sections from focal cerebral ischemic brain (3-month-old) were used as positive controls (C). A number of TUNEL-positive cells were observed in the infarction region (CPu), but only few TUNEL-positive cells were found in the SVZ. Left penal: low-magnification. Right penal: high-magnification. LV: lateral ventricle; CPu: caudate putamen. Images are representative of results from three experiments.
To determine the effect of inhibiting caspase-mediated NPC death in 24-month-old rats, the caspase inhibitor DEVD was infused in the left lateral ventricle following BrdU administration. The concentration and duration of infusion of DEVD were based on our previous study of its effectiveness in inhibiting caspase-3 activity in the hippocampus after global cerebral ischemia [11]. As shown in Fig. 5, caspase-3 positive cells were observed in DG of aged brain, but were dramatically reduced after administration of DEVD. However, the number of BrdU-positive cells in DG was not significantly increased by DEVD (p>0.05). Similar results were also found in young-adult rat (Fig. 5A and B), implying that the decline in neurogenesis in the aged SGZ and SVZ results primarily from reduced NPC proliferation rather than increased NPC death.
Fig. 5.
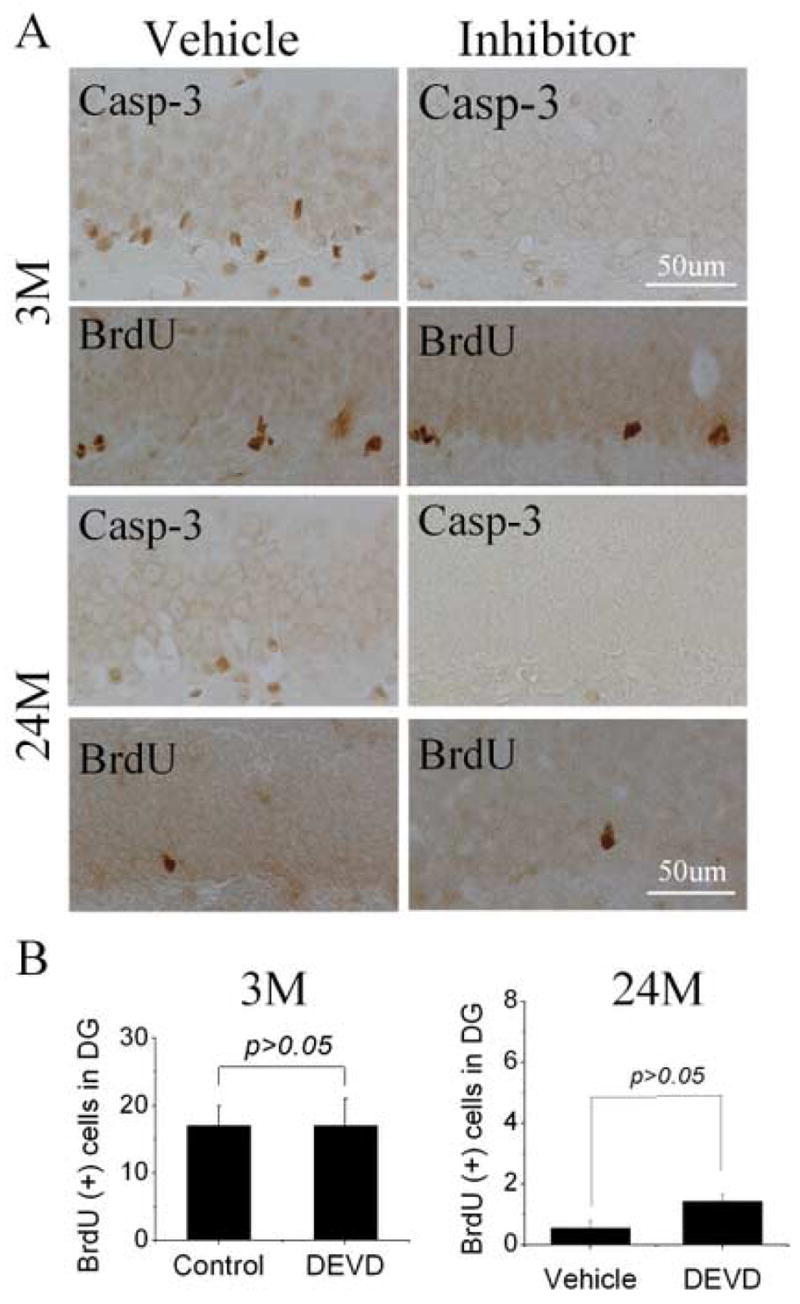
Effect of caspase inhibition on numbers of BrdU-positive cells in DG of young-adult and aged rat brain. (A) The caspase inhibitor DEVD (20 mg/ml) was infused at 1 μl/hr for 3 days into the left lateral ventricle of young-adult (3M) and aged (24M) rats, and BrdU (50 mg/kg) was given twice i.p. on the last day. Immunocytochemistry showed that DEVD reduced the number of cells showing caspase-3 cleavage, without affecting the number of cells that incorporated BrdU (P>0.05). (B) The number of BrdU-positive cells in the hippocampal SGZ after infusion of DEVD into the left lateral ventricle of young-adult (3M) and aged (24M) rat brain for 3 days and BrdU was given twice on the last day. Animals were killed one day later. Images (A) are representative of results from three experiments.
Next, we asked whether increased cell proliferation following ischemia might result from decreased cell death in SVZ. We calculated the number of BrdU-positive cells in SVZ at 10 days after ischemia in young-adult and aged rats compare with control groups respectively. Late post-ischemia was selected as time for counting BrdU- and caspase-3-positive cells, to ensure detection of both rapid and slowly dividing cells and also of dead cells arising from secondary injury after focal ischemia. Consistent with previous findings, the number of BrdU-labeled cells in the SVZ was increased in both young-adult and in aged postischemic brain (Fig. 6A). The number of cleaved caspase 3-positive cells were significantly higher both in young-adult (3M) and aged (24M) ischemic rat SVZ (P < 0.001 and P < 0.001 respectively as a result of Tukey’s post-hoc test applied to a significant difference between experimental groups by one-way ANOVA, P < 0.0001). The ischemia-induced increase in caspase-3 positive cells in young and aged brains, however, was not significantly different (P > 0.05). If the observed ischemia-induced increases in numbers of replicating NPCs both in young and aged animals was due to a decrease in cell death, we would expect to observe that numbers of BrdU-positive cells and cleaved caspase-3 positive cells would be negatively correlated. Numbers of BrdU-positive cells and numbers of cleaved caspase 3-positive cells were strongly and positively correlated (Fig. 6C). This demonstrates that the observed increases in cleaved caspase-3-positive cells is directly proportional to the increase in numbers of replicating NPCs, ruling out a role of decreased cell death in the observed ischemia-induced increases in numbers of BrdU-positive cells. Therefore, our results suggest that NPC death might not contribute to cerebral ischemia-induced neurogenesis.
Fig. 6.
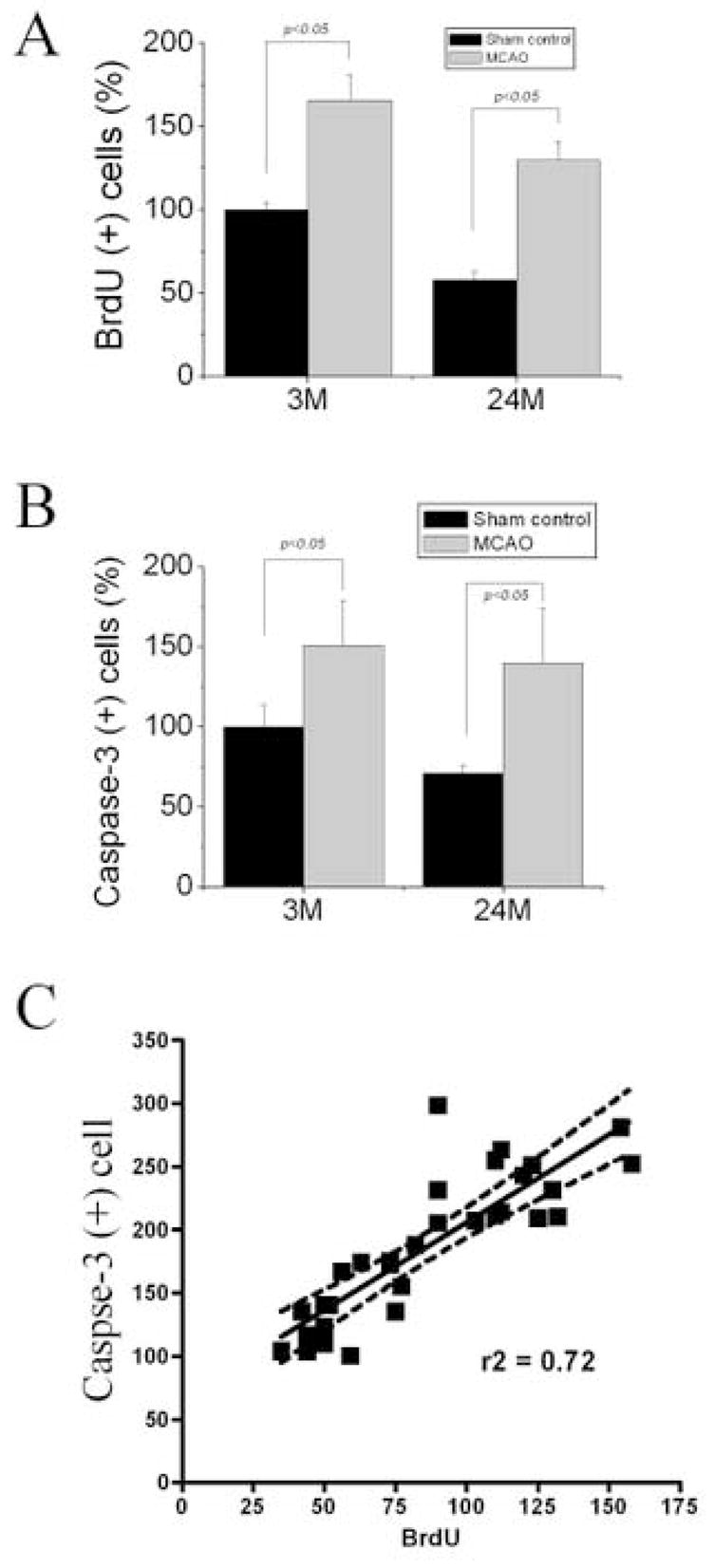
NPC proliferation and death in the SVZ after focal ischemia. The percentage of BrdU-immunoreactive cell (A) & caspase-3-positive cells (B) in the SVZ of sham-operated and ischemic brains in young-adult and aged rats is shown. The number of cleaved caspase 3-positive cells was significantly higher both in young (3M) and aged (24M) ischemic rat SVZ (P < 0.001 and P < 0.001 respectively). The ischemia-induced increase in caspase-3-positive cells in young-adult and aged brains, however, was not significantly different (P > 0.05). Pearson’s correlation analyses showed a positive correlation between numbers of BrdU-positive cells and numbers of cleaved caspase-3-positive cells in the SGZ in all experimental conditions examined (P < 0.0001, r2 = 0.7225) (C). Data shown are mean values ± SEM from at least three experiments per panel.
4. Discussion
Although greater than 9,000 new cells are produced per day in the adult rodent hippocampus [7], 60–80% of them degenerate within one month [13]. We and others have reported a gradual decline of neurogenesis with normal aging in rats and mice [8,19,23], which could be due to either decreased NPC neuroproliferation or increased NPC death.
Here we report that activated caspase-3 is associated with the naturally occurring death of NPCs in SGZ and SVZ throughout life, and that it declines roughly in parallel with the age-related decrease in proliferating NPCs detected by BrdU labeling. The active forms of all other caspases tested do not appear to be associated with this form of physiological cell death. Surprisingly, inhibiting caspase activity failed to increase the number of BrdU-labeled cells in DG of the aged rat brain, consistent with the view that the age-related decline in neurogenesis is attributable primarily to reduced proliferation of NPCs. Caspase family members are key elements of programmed cell death in the developing nervous system [26], where this form of cell death affects both NPCs and postmitotic neurons. Activation of caspase-3 is required for embryonic and perinatal apoptosis of NPCs [21], which is inhibited by Bcl-2 [12]. In addition, in vitro studies show that caspase-3 is activated in NPCs undergoing neurotoxin-induced death in the adult brain [10]. Our results show that caspase-3 activation may also be important for programmed death of NPCs in the adult SGZ and SVZ in vivo. Consistent with this idea, programmed cell death in the adult hippocampus is dramatically reduced in Bax-knockout mice [27] and after caspase inhibition in rats. Caspase-8 and -9 are upstream initiators of caspase-3-mediated death in what is considered ‘classical’ apoptosis [26]. In addition, we find that number of TUNEL-positive cells in the DG and SVZ of aged rat brain is less than that of caspase-3-positive cells. Therefore, our results suggest that NPC death in the adult brain may be affected by different forms of cell death programs involving the activation of caspase-3. This suggestion is supported by the observation that death of mature neurons in vivo does not strictly conform to apoptotic pathways as described in other cell types [5]. Thus, although therapeutic enhancement of neurogenesis may prove to have a role in treatment of age-related cognitive impairment or neurodegenerative diseases, our results suggest that efforts directed at inhibiting caspase-dependent cell death are unlikely to be helpful in this regard. Instead, measures designed to enhance neuronal proliferation, differentiation or migration appear to have greater promise.
In addition, we also found that ischemia increased both neurogenesis and caspase-3 activation in the SVZ. Previous studies showed that activation of caspase-3 was induced in the hippocampal SGZ of the gerbil after forebrain ischemia. These caspase-positive cells expressed an early neuronal marker [4], suggesting that caspase-mediated programmed cell death could mediate the loss of neurogenic cells in the SGZ. Functional repair is probably reduced in animals by inducing the programmed cell death of NPC and other immature neurons after focal ischemia. Though neurogenesis was enhanced after ischemic injury, the surviving NPCs were not sufficient for recovery after injury, since the majority of NPCs die during migration [1]. Thus, amplification of self-renewal mechanisms by stimulation of cell division at the level of NPCs, combined with reducing the number of NPC death and other newly generated neurons during migration might in the future come to be of therapeutic value for patients affected by ischemic brain injuries.
Acknowledgments
This work was supported by National Institute of Health (NIH) grant AG21980.
Footnotes
Disclosure Statement
There are no any actual or potential conflicts of interest including any financial, personal or other relationships with other people or organizations within three years of beginning the work submitted that could inappropriately influence (bias) our work. All animal experiments were carried out in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and approved by the IACUC. Efforts were made to minimize animal suffering and to reduce the number of animals used.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8(9):963–70. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 2.Biebl M, Cooper CM, Winkler J, Kuhn HG. Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neurosci Lett. 2000;291(1):17–20. doi: 10.1016/s0304-3940(00)01368-9. [DOI] [PubMed] [Google Scholar]
- 3.Biebl M, Winner B, Winkler J. Caspase inhibition decreases cell death in regions of adult neurogenesis. Neuroreport. 2005;16(11):1147–50. doi: 10.1097/00001756-200508010-00003. [DOI] [PubMed] [Google Scholar]
- 4.Bingham B, Liu D, Wood A, Cho S. Ischemia-stimulated neurogenesis is regulated by proliferation, migration, differentiation and caspase activation of hippocampal precursor cells. Brain Res. 2005;1058(1–2):167–77. doi: 10.1016/j.brainres.2005.07.075. [DOI] [PubMed] [Google Scholar]
- 5.Bredesen DE, Rao RV, Mehlen P. Cell death in the nervous system. Nature. 2006;443(7113):796–802. doi: 10.1038/nature05293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buss RR, Oppenheim RW. Role of programmed cell death in normal neuronal development and function. Anat Sci Int. 2004;79(4):191–7. doi: 10.1111/j.1447-073x.2004.00088.x. [DOI] [PubMed] [Google Scholar]
- 7.Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435(4):406–17. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 8.Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2(10):894–7. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- 9.Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56(2):337–44. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- 10.Ceccatelli S, Tamm C, Sleeper E, Orrenius S. Neural stem cells and cell death. Toxicol Lett. 2004;149(1–3):59–66. doi: 10.1016/j.toxlet.2003.12.060. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Nagayama T, Jin K, Stetler RA, Zhu RL, Graham SH, Simon RP. Induction of caspase-3-like protease may mediate delayed neuronal death in the hippocampus after transient cerebral ischemia. J Neurosci. 1998;18(13):4914–28. doi: 10.1523/JNEUROSCI.18-13-04914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng A, Chan SL, Milhavet O, Wang S, Mattson MP. p38 MAP Kinase Mediates Nitric Oxide-induced Apoptosis of Neural Progenitor Cells. J Biol Chem. 2001;276(46):43320–7. doi: 10.1074/jbc.M107698200. [DOI] [PubMed] [Google Scholar]
- 13.Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460(4):563–72. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- 14.Ekdahl CT, Mohapel P, Elmer E, Lindvall O. Caspase inhibitors increase short-term survival of progenitor-cell progeny in the adult rat dentate gyrus following status epilepticus. Eur J Neurosci. 2001;14(6):937–45. doi: 10.1046/j.0953-816x.2001.01713.x. [DOI] [PubMed] [Google Scholar]
- 15.Goldman S. Stem and progenitor cell-based therapy of the human central nervous system. Nat Biotechnol. 2005;23(7):862–71. doi: 10.1038/nbt1119. [DOI] [PubMed] [Google Scholar]
- 16.Haydar TF, Kuan C-Y, Flavell RA, Rakic P. The Role of Cell Death in Regulating the Size and Shape of the Mammalian Forebrain. Cereb Cortex. 1999;9(6):621–6. doi: 10.1093/cercor/9.6.621. [DOI] [PubMed] [Google Scholar]
- 17.Heine VM, Maslam S, Joels M, Lucassen PJ. Prominent decline of newborn cell proliferation, differentiation, and apoptosis in the aging dentate gyrus, in absence of an age-related hypothalamus-pituitary-adrenal axis activation. Neurobiol Aging. 2004;25(3):361–75. doi: 10.1016/S0197-4580(03)00090-3. [DOI] [PubMed] [Google Scholar]
- 18.Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A. 2001;98(8):4710–5. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin K, Sun Y, Xie L, Batteur S, Mao XO, Smelick C, Logvinova A, Greenberg DA. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell. 2003;2(3):175–83. doi: 10.1046/j.1474-9728.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- 20.Kudo C, Kori M, Matsuzaki K, Yamai K, Nakajima A, Shibuya A, Niwa H, Kamisaki Y, Wada K. Diclofenac inhibits proliferation and differentiation of neural stem cells. Biochem Pharmacol. 2003;66(2):289–95. doi: 10.1016/s0006-2952(03)00235-1. [DOI] [PubMed] [Google Scholar]
- 21.Levison SW, Rothstein RP, Brazel CY, Young GM, Albrecht PJ. Selective apoptosis within the rat subependymal zone: a plausible mechanism for determining which lineages develop from neural stem cells. Dev Neurosci. 2000;22(1–2):106–15. doi: 10.1159/000017432. [DOI] [PubMed] [Google Scholar]
- 22.Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- 23.Luo J, Daniels SB, Lennington JB, Notti RQ, Conover JC. The aging neurogenic subventricular zone. Aging Cell. 2006;5(2):139–52. doi: 10.1111/j.1474-9726.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- 24.Michelin S, del Rosario Perez M, Dubner D, Gisone P. Increased activity and involvement of caspase-3 in radiation-induced apoptosis in neural cells precursors from developing rat brain. Neurotoxicology. 2004;25(3):387–98. doi: 10.1016/j.neuro.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury. Cell. 2002;110(4):429–41. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 26.Sommer L, Rao M. Neural stem cells and regulation of cell number. Prog Neurobiol. 2002;66(1):1–18. doi: 10.1016/s0301-0082(01)00022-3. [DOI] [PubMed] [Google Scholar]
- 27.Sun W, Winseck A, Vinsant S, Park O-h, Kim H, Oppenheim RW. Programmed Cell Death of Adult-Generated Hippocampal Neurons Is Mediated by the Proapoptotic Gene Bax. J Neurosci. 2004;24(49):11205–13. doi: 10.1523/JNEUROSCI.1436-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Won SJ, Xie L, Kim SH, Tang H, Wang Y, Mao X, Banwait S, Jin K. Influence of age on the response to fibroblast growth factor-2 treatment in a rat model of stroke. Brain Res. 2006;1123(1):237–44. doi: 10.1016/j.brainres.2006.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]