Abstract
Presenilin-1 is required for γ-secretase activity, which participates in Notch receptor processing, the pathogenesis of Alzheimer's disease and the modulation of Ca2+ signaling. We tested the hypothesis that γ-secretase proteolytic activity modulates store-operated Ca2+ entry (SOCE) in rat dorsal root ganglion neurons. Depletion of intracellular Ca2+ stores by blocking the endoplasmic reticulum Ca2+ pump with cyclopiazonic acid (CPA) evoked a transient increase in [Ca2+]i but no sustained Ca2+ influx. However, in cells expressing a dominant negative presenilin-1 mutant (PS1-D257A), γ-secretase activity was inhibited and treatment with CPA evoked sustained Ca2+ influx. Similarly, pharmacologic inhibition of γ-secretase with DAPT for 48 hrs enhanced SOCE. SKF96365, an inhibitor of store-operated channels, blocked SOCE in cells expressing PS1-D257A. Thus, γ-secretase proteolytic activity regulates a SOCE pathway in sensory neurons.
Keywords: γ-secretase, dorsal root ganglion neuron, capacitative Ca2+ entry, store-operated Ca2+ entry
The proteolytic enzyme γ-secretase participates in Notch receptor processing [10], the pathogenesis of Alzheimer's disease [17], and has recently been implicated in modulating Ca2+ signaling processes [26]. Dominant negative mutations in presenilin, which is required for γ-secretase activity, prevent the proteolytic processing of the Notch receptor resulting in developmental defects [14]. Mutations in the presenilins (PS1 and PS2) are the most common cause of early-onset familial Alzheimer's disease (FAD); they produce a gain-of-function that increases the level of amyloid-β42 [29]. Presenilins are integral membrane proteins found predominantly in the endoplasmic reticulum (ER) and are also present in mitochondria, golgi, endosomes, and the intracellular side of the plasma membrane [23]. Cells that express presenilin mutants also exhibit changes in store-operated Ca2+ entry (SOCE) [19, 11, 34].
SOCE results from opening plasma membrane Ca2+ channels following depletion of intracellular Ca2+ stores. Inhibition of ER Ca2+ pumps or activation of ER Ca2+ release channels produces a drop in ER Ca2+ sensed by stromal interacting molecule (STIM1), which redistributes in the ER to activate plasma membrane Ca2+ channels such as Orai1 and TRPC [12, 24]. SOCE was first described as a major route of Ca2+ influx in non-excitable cells and is now known to occur in excitable cells including neurons [3, 28, 31]. SOCE prolongs the elevation in [Ca2+]i that follows store mobilization and, depending on cell type, enhances secretion [9], activates transcription [25], alters synaptic transmission [2] and assists in refilling depleted Ca2+ stores [8].
Fibroblasts from FAD PS1 knock-in mice and neuronal cells expressing FAD PS1 mutants exhibit a marked impairment in SOCE and increased Ca2+ levels within the ER [19, 11, 34]. PS1 modulation of SOCE is independent of the expression of amyloid precursor protein [11] but, PS1-dependent effects on phosphoinositide-mediated Ca2+ signals are mediated through changes in γ-secretase activity [27]. Cortical neurons from PS1 knockout mice display a marked potentiation in SOCE [34]. Expression of a dominant negative PS1 mutant (PS1-D257A) produces a variety of effects including loss of γ-secretase activity, PS1 endoproteolysis, and impaired notch signaling [14, 33]. In neuronal cell lines expressing PS1-D257A or treated with a γ-secretase inhibitor, SOCE was potentiated and phosphoinositide-mediated elevations in [Ca2+]i were inhibited [34, 27].
Presenilin function has not been studied in sensory neurons and little is known about the regulation of SOCE in peripheral neurons. Because the mechanisms for regulating [Ca2+]i in peripheral neurons differ from those in central neurons, especially concerning the role of intracellular Ca2+ stores, we examined whether presenilin-sensitive SOCE is expressed in sensory neurons. We found that genetic and pharmacologic inhibition of γ-secretase significantly enhanced SOCE in dorsal root ganglion (DRG) neurons.
Rat DRG neurons were grown in primary culture as described previously [31] under a protocol approved by the University of Minnesota Institutional Animal Care and Use Committee. [Ca2+]i was recorded from single DRG neurons using indo-1 based photometry as previously described [32].
We used biolistic particle-mediated gene transfer [30] to express wild-type human PS1 (PS1-WT) and dominant negative PS1-D257A [33] in DRG neurons. Neurons were co-transfected with an enhanced green fluorescent protein (EGFP) expression vector in order to identify cells expressing the PS1 constructs. Cells transfected with EGFP expression vector alone served as controls (Sham). PS1-D257A inhibits γ-secretase activity [33] and as such decreases the production of the C-terminal fragment of beta-amyloid (AICD) and the C-terminal Notch fragment (NICD) [14]. Both AICD and NICD bind the transcriptional repressor CBF1 resulting in the activation of CBF1-repressed genes. Thus, to verify whether expression of D257A-PS1 inhibited γ-secretase activity in DRG neurons, we used a CBF1 luciferase reporter assay (4xwtCBF1Luc) [13]. 4xwtCBF1Luc was co-transfected with a plasmid containing Renilla luciferase under control of the CMV promoter (pRL-CMV), and the expression of firefly luciferase was normalized to constitutively expressed Renilla luciferase activity to correct for differences in transfection efficiency. γ-secretase cleavage products remove CBF1 repression, therefore a decrease in γ-secretase activity results in a reduction in normalized luciferase activity. As shown in Fig. 1A, cultures transfected with the PS1-D257A expression vector (n=3 coverslips) had 10 ± 8% of the γ-secretase activity of sham transfected cultures (n=3 coverslips). Luciferase expression in PS1-WT (n=3 coverslips) and sham-transfected neurons were not significantly different. These results demonstrate that PS1-D257A expression in rat DRG neurons inhibits endogenous γ-secretase activity.
Fig. 1.
PS1-D257A expression inhibits γ-secretase and prolongs SOCE in DRG neurons. (A) Expression of PS1-D257A inhibits CBF1-dependent transcription. Cultures were transfected with 4xwtCBF1Luc and pRL-CMV. Luciferase activity, expressed as the ratio of firefly to Renilla luminescence, was measured from populations of cells expressing EGFP alone (Sham) or EGFP and either PS1-D257A or PS1-WT. * p<0.05 PS1-D257A versus PS1-WT or sham. (B) Representative trace from a cell expressing PS1-D257A shows protocol for SOCE evoked by continuous exposure to 5 μM CPA. CPA was applied during the time indicated by the horizontal bar. (C) Bar graph depicts mean [Ca2+]i calculated for the variables listed. The letters correspond to the times indicated in B. *p<0.05, compared to Sham or PS1-WT. p=0.06, PS1-D257A compared to PS1-WT (D) Representative SOCE traces for Sham (n=28), PS1-D257A (n=34), and PS1-WT (n=14) expressing neurons are shown. (E) Time to recover to 75% of the peak SOCE response is shown for sham, PS1-D257A and PS1-WT expressing neurons. *p<0.05, compared to Sham or PS1-WT.
The effect of PS1-D257A expression on SOCE in DRG neurons has not been studied. Figure 1B shows a representative SOCE response from a PS1-D257A-expressing cell evoked by continuous treatment with the sarcoplasmic-endoplasmic reticulum Ca2+ ATPase (SERCA) inhibitor, cyclopiazonic acid (CPA, 5μM) in the presence of extracellular Ca2+. CPA blocks Ca2+ uptake into the ER resulting in release of Ca2+ into the cytoplasm producing a transient rise in [Ca2+]i. The [Ca2+]i then recovers to a sustained plateau maintained by SOCE. Analysis of basal, peak and plateau [Ca2+]i from sham, PS1-WT and PS1-D257A transfected cells revealed that neither the resting [Ca2+]i nor the peak amplitude of the CPA-induced response was significantly different among the groups ((Fig. 1C). However, the plateau of the SOCE response, measured 2000 s after application of CPA, was elevated in PS1-D257A relative to either Sham or PS1-WT expressing neurons. The average plateau [Ca2+]i in PS1-D257A was 55 % higher than sham and 98 % greater than PS1-WT expressing neurons (Fig. 1C). The representative traces in Figure 1D show that the [Ca2+]i increase in PS1-D257A expressing cells was more sustained. The time to recover to 75% of the peak took 236±34 s in neurons expressing PS1-D257A compared to 150±27 s in PS1-WT and 180±23 s in sham expressing neurons (Fig. 1E). Because these experiments were run in the continuous presence of CPA, the prolonged [Ca2+]i increase observed in the PS1-D257A expressing neurons was independent of intracellular store-mediated Ca2+ uptake and release, suggesting the differences are likely due to prolonged activation of a Ca2+ influx pathway.
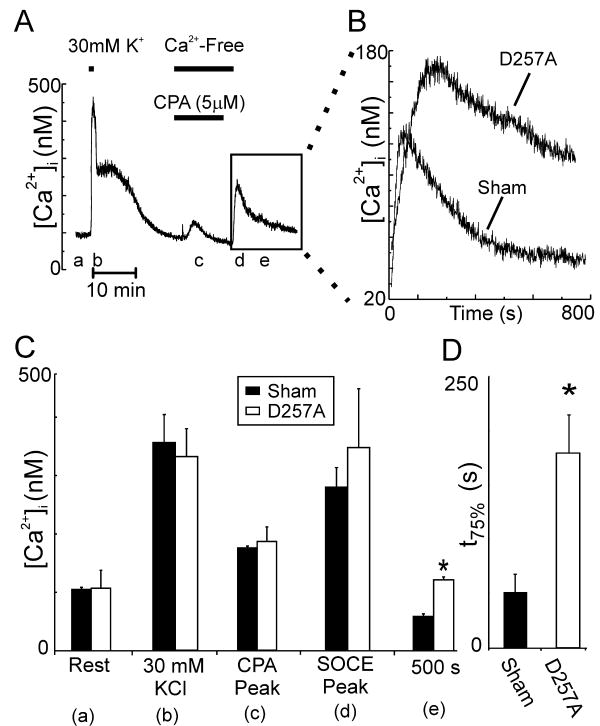
SOCE can also be studied by depleting the ER of Ca2+ and then initiating Ca2+ influx by return of Ca2+ to the medium. The effects of PS1-D257A expression on SOCE in DRG neurons were determined using a protocol slightly modified from one previously described [31]. Voltage-operated Ca2+ influx was evoked by depolarization with 30 mM K+ (Fig. 2A). PS1-D257A expression did not affect resting [Ca2+]i or the depolarization-induced [Ca2+]i increase (Fig. 2C). After allowing the cell to recover for 15 minutes, 5μM CPA was applied for 5 minutes in Ca2+-free medium (100 μM EGTA) to deplete intracellular Ca2+ stores. CPA was then removed in the absence of extracellular Ca2+. The return of extracellular Ca2+ (1.26 mM) produced an increase in [Ca2+]i mediated by SOCE. Expression of PS1-D257A did not affect the peak amplitude of the SOCE response (Fig. 2C). In Figure 2B the effects of PS1-D257A (n=6) expression on the shape of the SOCE response are compared to sham (n=6) transfected neurons. The duration of the SOCE-mediated [Ca2+]i increase was significantly prolonged in neurons expressing PS1-D257A. 500 s after returning Ca2+ to the extracellular medium, [Ca2+]i was 63±10 nM higher in the PS1-D257A expressing neurons compared to Sham transfected neurons (Fig. 2C). Furthermore, neurons expressing PS1-D257A took 207 ± 23 % longer to recover to 75 % of peak amplitude compared to sham cells (Fig. 2D). These data suggest that PS1-D257A expression prolongs SOCE in DRG neurons without affecting voltage-operated Ca2+ influx or Ca2+ clearance.
Fig. 2.
PS1-D257A expression in DRG neurons prolongs SOCE. (A) Representative trace showing protocol used to study SOCE in rat DRG neurons. Treatments were applied at the times indicated by the horizontal bars. Cells were bathed in 1.26 mM Ca2+ except where indicated. The boxed region contains the SOCE response induced by returning Ca2+ to the extracellular solution. (B) Representative SOCE traces from Sham (n=6) and PS1-D257A (n=6) expressing cells are shown for the time indicated by the boxed region in A. (C) Bar graph summarizes the effects of PS1-D257A expression on [Ca2+]i for PS1-D257A and Sham transfected cells at the times listed. The letters in parentheses correspond to the times indicated in A. *p<0.05 versus Sham. (D) Bar graph shows time to recover to 75 % of the peak SOCE in Sham and PS1-D257A expressing neurons. *p<0.05, PS1-D257A versus sham.
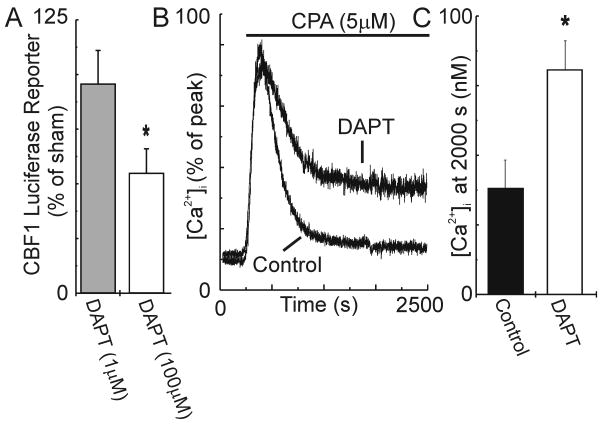
To address the question of whether PS1-D257A prolonged SOCE by virtue of its ability to inhibit proteolysis, we used an alternative pharmacological approach to inhibit γ-secretase activity. The cell-permeable, dipeptide compound DAPT blocks γ-secretase-mediated proteolysis [7]. We again used the CBF1 luciferase reporter assay to measure γ-secretase activity. As shown in Fig. 3A, 48 hr treatment of DRG neurons with 1 μM DAPT (n=7) did not significantly affect luciferase expression, whereas treatment with 100 μM DAPT (n=6) reduced transactivation of the CBF1-luciferase reporter by 45%. Treatment with 1μM DAPT did not significantly affect SOCE (n=7). However, 48 hr treatment with 100 μM DAPT significantly enhanced SOCE compared to vehicle-treated control cells (n=9; Fig. 3B,C). [Ca2+]i was 104 % higher in DAPT (100 μM) treated neurons 2000 s after evoking SOCE. Basal [Ca2+]i and the peak of the CPA-evoked response were not affected by DAPT. Treatment with DAPT 5 min prior to and during the recording did not affect the CPA-evoked response at 2000 s.
Fig. 3.
DAPT prolongs SOCE in DRG neurons. Neurons were treated in culture with the indicated concentration of DAPT or DMSO (control) for 48 hours. Cells were washed for 15 minutes before beginning the recording. Both wash and recording periods are in the absence of vehicle or drug. (A) DAPT inhibited transactivation of CBF1 luciferase. * p<0.05, 100 μM DAPT compared to sham. (B) Representative [Ca2+]i traces for control neurons (n=9) and neurons treated with 100 μM DAPT (n=17) were normalized to the peak [Ca2+]i and overlaid. (C) Bar graph summarizes the effects of 48 hr treatment with 100 μM DAPT on the [Ca2+]i measured after 2000 s in CPA (5 μM). *p<0.05, DAPT-treated (100 μM) compared to control.
SKF96365 inhibits SOCE [22]. Here, we determined the contribution of SKF96365-sensitive ion channels to the sustained elevation in [Ca2+]i induced by CPA. SKF96365 completely blocked SOCE in DRG neurons expressing PS1-D257A, as indicated by the rapid return of the CPA response to basal [Ca2+]i (Fig. 4A). Thus, 2000s after CPA application the [Ca2+]i in SKF96365-treated PS1-D257A-expressing cells (n=5) was significantly reduced relative to PS1-D257A-expressing cells in the absence of drug (n=5)(Fig. 4C). SKF96365 evoked complex effects in DAPT treated cells (Fig. 4B). After an initial rapid recovery toward basal levels, [Ca2+]i steadily rose for the remainder of the recording (n=13). The increase in [Ca2+]i evoked by SKF96365 did not require store-depletion as it was also observed in DAPT-treated cells in the absence of CPA (n=11).
Fig. 4.
SKF96365 inhibits SOCE in DRG neurons. (A-B) Representative traces show SOCE evoked by 5 μM CPA (applied at the times indicated by the horizontal bars) in PS1-D257A expressing cells (A) and in cells treated for 48 hrs with 100 μM DAPT (B) in the absence or presence of 10 μM SKF96365 (added 5 min prior to and during CPA treatment). (C) Bar graph summarizes the effects of 10 μM SKF96365 on the [Ca2+]i measured after 2000 s in CPA from untreated (control), PS1-D257A expressing and DAPT (48 hrs) treated cells (n≥5 for each group). *p<0.05, compared to vehicle-treated cells.
We have shown that inhibition of presenilin dependent proteolytic activity enhances SOCE in rat sensory neurons. These results are in good agreement with previous studies examining the role of presenilins in Ca2+ signaling. There is general consensus that FAD mutations in presenilin alter γ-secretase activity resulting in decreased SOCE and that inhibition of γ-secretase activity by dominant negative presenilins, such as the D257A mutant studied here, result in increased SOCE [6, 19, 34]. We did not observe a change in store capacity, as measured by the amplitude of the CPA-induced response, which differs from other reports [5]. Store refilling may depend on cell-specific factors such as other Ca2+ sources and SERCA activity.
Expression of PS1-D257A inhibited γ-secretase as indicated by reduced gene expression driven by the intracellular signaling domain. The marked changes in ion channel function following inhibition of γ-secretase raise some concerns about potential side effects should pharmacologic inhibitors be used to reduce β-amyloid production in Alzheimer's disease. Whether changes in γ-secretase activity are directly responsible for altered signaling in cells expressing mutant presenilins is controversial [11]. Changes in Ca2+ homeostasis resulting from dominant negative PS1 have been found to be both dependent and independent of changes in γ-secretase activity [1, 20]. Multiple factors might explain these differences including cell-type studied, protocols used to elicit responses, and the specific PS1 constructs or drugs used to inhibit y-secretase activity. PS1 mutations influence multiple cell signaling pathways including those regulated by phospholipase C [4], glycogen synthase kinase [15] and mitogen-activated protein kinase [16]. The multifunctional calcium binding protein, calsenilin, binds PS proteins and participates in Ca2+-dependent gene expression. Expression of mutant presenilins might also disrupt ER structure altering ER-SOCE coupling. However, experiments with DAPT indicate that inhibition of proteolytic activity produces the increase in SOCE. Interestingly, acute application of DAPT had no effect. Thus, the most parsimonious explanation for the up regulation of SOCE described here is a change in transcription resulting from γ-secretase inhibition.
Presumably the SOCE observed in cells expressing PS1-D257A is also present in non-transfected cells, although less prominent. Thus, PS1-D257A may be used to enhance SOCE to enable its study in sensory neurons. CPA-induced Ca2+ entry in cells expressing PS1-D257A was inhibited by SKF96365 consistent with the pharmacology of SOCE [22], although truly selective blockers for these channels are not available. In DAPT-treated cells, SKF96365 produced complicated effects on the CPA-induced [Ca2+]i response that included a slowly developing increase in [Ca2+]i. In some cells, SKF96365 activates a non-selective cation channel at high concentrations [22]. The appearance of this Ca2+ influx response in DAPT-treated, but not PS1-D257A-expressing cells, suggests that inhibition of γ-secretase is not sufficient to reveal this effect of SKF96365 in DRG neurons.
The modulation of SOCE by presenilin-dependent proteolytic activity most likely results from altered expression of the store operated channel or its regulator. We speculate that release of the intracellular signaling domain following proteolytic cleavage of a substrate such as Notch results in transcription of genes encoding channel or regulatory proteins such as STIM1, Orai and TRPC. Expression of a dominant-negative PS1 mutant in HEK cells enhanced agonist-induced Ca2+ influx through TRPC6 channels suggesting that PS might control an intermediate protein, either via transcription or direct binding, which then regulates channel activity [21]. TRPC channels participate in SOCE [24] and several TRPC channel isoforms are expressed in DRG neurons [18].
The role for SOCE and PS1 in sensory physiology is unclear at present although the dominant negative PS1 mutant should prove useful for studying these signaling pathways. The altered channel function described here suggests that SOCE and presenilins might cooperate to produce long-term changes in neuronal excitability.
Acknowledgments
Grants from the NSF (IOS0814549) and NIH (DA007304, DA011806) supported this work. We thank Dr. D. Selkoe for generously providing us with presenilin expression vectors and we thank Dr. S. Hayward for kindly providing us with the CBF1 luciferase reporter plasmid.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akbari Y, Hitt BD, Murphy MP, Dagher NN, Tseng BP, Green KN, Golde TE, LaFerla FM. Presenilin regulates capacitative calcium entry dependently and independently of gamma-secretase activity. Biochem Biophys Res Commun. 2004;322:1145–1152. doi: 10.1016/j.bbrc.2004.07.136. [DOI] [PubMed] [Google Scholar]
- 2.Baba A, Yasui T, Fujisawa S, Yamada RX, Yamada MK, Nishiyama N, Matsuki N, Ikegaya Y. Activity-Evoked Capacitative Ca2+ Entry: Implications in Synaptic Plasticity. J Neurosci. 2003;23:7737–7741. doi: 10.1523/JNEUROSCI.23-21-07737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouron A. Activation of a capacitative Ca2+ entry pathway by store depletion in cultured hippocampal neurones. FEBS Lett. 2000;470:269–272. doi: 10.1016/s0014-5793(00)01340-5. [DOI] [PubMed] [Google Scholar]
- 4.Cedazo-Minguez A, Popescu BO, Ankarcrona M, Nishimura T, Cowburn RF. The presenilin 1 Delta E9 mutation gives enhanced basal phospholipase C activity and a resultant increase in intracellular calcium concentrations. J Biol Chem. 2002;277:36646–36655. doi: 10.1074/jbc.M112117200. [DOI] [PubMed] [Google Scholar]
- 5.Chan SL, Culmsee C, Haughey N, Klapper W, Mattson MP. Presenilin-1 mutations sensitize neurons to DNA damage-induced death by a mechanism involving perturbed calcium homeostasis and activation of calpains and caspase-12. Neurobiol Dis. 2002;11:2–19. doi: 10.1006/nbdi.2002.0542. [DOI] [PubMed] [Google Scholar]
- 6.Cheng I, Yoo AS, Tanzi RE, Kim TW. Familial Alzheimer's disease-linked mutant presenilins attenuate capacitative calcium entry, Alzheimer's Disease : Advances in Etiology. Pathogenesis and Therapeutics. 2001:515–519. [Google Scholar]
- 7.Dovey HF, John V, Anderson JP, Chen LZ, de Saint Andrieu P, Fang LY, Freedman SB, Folmer B, Goldbach E, Holsztynska EJ, Hu KL, Johnson-Wood KL, Kennedy SL, Kholodenko D, Knops JE, Latimer LH, Lee M, Liao Z, Lieberburg IM, Motter RN, Mutter LC, Nietz J, Quinn KP, Sacchi KL, Seubert PA, Shopp GM, Thorsett ED, Tung JS, Wu J, Yang S, Yin CT, Schenk DB, May PC, Altstiel LD, Bender MH, Boggs LN, Britton TC, Clemens JC, Czilli DL, Dieckman-McGinty DK, Droste JJ, Fuson KS, Gitter BD, Hyslop PA, Johnstone EM, Li WY, Little SP, Mabry TE, Miller FD, Audia JE. Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J Neurochem. 2001;76:173–181. doi: 10.1046/j.1471-4159.2001.00012.x. [DOI] [PubMed] [Google Scholar]
- 8.Fanger CM, Hoth M, Crabtree GR, Lewis RS. Characterization of t cell mutants with defects in capacitative calcium entry - genetic evidence for the physiological roles of crac channels. J Cell Biol. 1995;131:655–667. doi: 10.1083/jcb.131.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fomina AF, Nowycky MC. A current activated on depletion of intracellular Ca2+ stores can regulate exocytosis in adrenal chromaffin cells. J Neurosci. 1999;19:3711–3722. doi: 10.1523/JNEUROSCI.19-10-03711.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fortini ME. Gamma-secretase-mediated proteolysis in cell-surface-receptor signalling. Nat Rev Mol Cell Biol. 2002;3:673–684. doi: 10.1038/nrm910. [DOI] [PubMed] [Google Scholar]
- 11.Herms J, Schneider I, Dewachter I, Caluwaerts N, Kretzschmar H, Van Leuven F. Capacitive calcium entry is directly attenuated by mutant presenilin-1, independent of the expression of the amyloid precursor protein. J Biol Chem. 2003;278:2484–2489. doi: 10.1074/jbc.M206769200. [DOI] [PubMed] [Google Scholar]
- 12.Hewavitharana T, Deng X, Soboloff J, Gill DL. Role of STIM and Orai proteins in the store-operated calcium signaling pathway. Cell Calcium. 2007;42:173–182. doi: 10.1016/j.ceca.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh JJ, Henkel T, Salmon P, Robey E, Peterson MG, Hayward SD. Truncated mammalian Notch1 activates CBF1/RBPJk-repressed genes by a mechanism resembling that of Epstein-Barr virus EBNA2. Mol Cell Biol. 1996;16:952–959. doi: 10.1128/mcb.16.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jack C, Berezovska O, Wolfe MS, Hyman BT. Effect of PS1 deficiency and an APP gamma-secretase inhibitor on Notch1 signaling in primary mammalian neurons. Brain Res Mol Brain Res. 2001;87:166–174. doi: 10.1016/s0169-328x(01)00010-9. [DOI] [PubMed] [Google Scholar]
- 15.Kang DE, Soriano S, Frosch MP, Collins T, Naruse S, Sisodia SS, Leibowitz G, Levine F, Koo EH. Presenilin 1 facilitates the constitutive turnover of beta-catenin: differential activity of Alzheimer's disease-linked PS1 mutants in the beta-catenin-signaling pathway. J Neurosci. 1999;19:4229–4237. doi: 10.1523/JNEUROSCI.19-11-04229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim MY, Park JH, Choi EJ, Park HS. Presenilin acts as a positive regulator of basal level activity of ERK through the Raf-MEK1 signaling pathway. Biochem Biophys Res Commun. 2005;332:609–613. doi: 10.1016/j.bbrc.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Kimberly WT, Wolfe MS. Identity and function of gamma-secretase. J Neurosci Res. 2003;74:353–360. doi: 10.1002/jnr.10736. [DOI] [PubMed] [Google Scholar]
- 18.Kress M, Karasek J, Ferrer-Montiel A, Scherbakov N, Haberberger R. TRPC channels and diacylglycerol dependent calcium signaling in rat sensory neurons. Histochem Cell Biol. 2008;130:655–667. doi: 10.1007/s00418-008-0477-9. [DOI] [PubMed] [Google Scholar]
- 19.Leissring MA, Akbari Y, Fanger CM, Cahalan MD, Mattson MP, LaFerla FM. Capacitative calcium entry deficits and elevated luminal calcium content in mutant presenilin-1 knockin mice. J Cell Biol. 2000;149:793–797. doi: 10.1083/jcb.149.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leissring MA, Murphy MP, Mead TR, Akbari Y, Sugarman MC, Jannatipour M, Anliker B, Muller U, Saftig P, De Strooper B, Wolfe MS, Golde TE, LaFerla FM. A physiologic signaling role for the gamma -secretase-derived intracellular fragment of APP. Proc Natl Acad Sci U S A. 2002;99:4697–4702. doi: 10.1073/pnas.072033799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lessard CB, Lussier MP, Cayouette S, Bourque G, Boulay G. The overexpression of presenilin2 and Alzheimer's-disease-linked presenilin2 variants influences TRPC6-enhanced Ca2+ entry into HEK293 cells. Cellular Signalling. 2005;17:437–445. doi: 10.1016/j.cellsig.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Leung YM, Kwan CY. Current perspectives in the pharmacological studies of store-operated Ca2+ entry blockers. Japanese journal of pharmacology. 1999;81:253–258. doi: 10.1254/jjp.81.253. [DOI] [PubMed] [Google Scholar]
- 23.Levitan D, Greenwald I. Effects of SEL-12 presenilin on LIN-12 localization and function in Caenorhabditis elegans. Development. 1998;125:3599–3606. doi: 10.1242/dev.125.18.3599. [DOI] [PubMed] [Google Scholar]
- 24.Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, Birnbaumer L. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc Natl Acad Sci U S A. 2008;105:2895–2900. doi: 10.1073/pnas.0712288105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loh C, Carew JA, Kim J, Hogan PG, Rao A. T-cell receptor stimulation elicits an early phase of activation and a later phase of deactivation of the transcription factor NFAT1. Mol Cell Biol. 1996;16:3945–3954. doi: 10.1128/mcb.16.7.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattson MP, Chan SL. Neuronal and glial calcium signaling in Alzheimer's disease. Cell Calcium. 2003;34:385–397. doi: 10.1016/s0143-4160(03)00128-3. [DOI] [PubMed] [Google Scholar]
- 27.Popescu BO, Cedazo-Minguez A, Benedikz E, Nishimura T, Winblad B, Ankarcrona M, Cowburn RF. gamma -Secretase activity of presenilin 1 regulates acetylcholine muscarinic receptor mediated signal transduction. J Biol Chem. 2003 doi: 10.1074/jbc.M306041200. [DOI] [PubMed] [Google Scholar]
- 28.Putney JW. Capacitative calcium entry in the nervous system. Cell Calcium. 2003;34:339–344. doi: 10.1016/s0143-4160(03)00143-x. [DOI] [PubMed] [Google Scholar]
- 29.Selkoe DJ. Translating cell biology into therapeutic advances in Alzheimer's disease. Nature. 1999;399:A23–31. doi: 10.1038/399a023. [DOI] [PubMed] [Google Scholar]
- 30.Usachev YM, Khammanivong A, Campbell C, Thayer SA. Particle-mediated gene transfer to rat neurons in primary culture. Pflugers Arch. 2000;439:730–738. doi: 10.1007/s004249900240. [DOI] [PubMed] [Google Scholar]
- 31.Usachev YM, Thayer SA. Ca2+ influx in resting rat sensory neurones that regulates and is regulated by ryanodine-sensitive Ca2+ stores. J Physiol. 1999;519:115–130. doi: 10.1111/j.1469-7793.1999.0115o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Werth JL, Usachev YM, Thayer SA. Modulation of calcium efflux from cultured rat dorsal root ganglion neurons. J Neurosci. 1996;16:1008–1015. doi: 10.1523/JNEUROSCI.16-03-01008.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- 34.Yoo AS, Cheng I, Chung S, Grenfell TZ, Lee H, Pack-Chung E, Handler M, Shen J, Xia W, Tesco G, Saunders AJ, Ding K, Frosch MP, Tanzi RE, Kim TW. Presenilin-mediated modulation of capacitative calcium entry. Neuron. 2000;27:561–572. doi: 10.1016/s0896-6273(00)00066-0. [DOI] [PubMed] [Google Scholar]