Abstract
Rationale
Chronic stress perturbs modulatory brain neurotransmitter systems, including serotonin (5-HT), and is a risk factor for psychiatric disorders such as depression. Deficits in cognitive flexibility, reflecting prefrontal cortical dysfunction, are prominent in such disorders. Orbitofrontal cortex (OFC) has been implicated specifically in reversal learning, a form of cognitive flexibility modulated by 5-HT.
Objectives
Assess the effects of chronic intermittent cold (CIC) stress, a potent metabolic stressor, on performance of rats in an attentional set shifting test (AST). Assess a possible role for serotonin in CIC-induced deficits, and test the effects of acute serotonin reuptake blockade.
Methods
Male Sprague-Dawley rats were exposed to CIC stress (14 days × 6 hr/day at 4 °C) before testing on the AST. In subsequent experiments, brain 5-HT was depleted in naïve rats with para-chlorophenylalanine, or 5-HT release was increased acutely in CIC-stressed rats with citalopram (5 mg/kg, s.c.) given 30 min prior to the first reversal task. Microdialysis was used to assess CIC-induced changes in 5-HT release in OFC during testing.
Results
CIC-stressed rats exhibited a selective impairment on the first reversal task in the AST. 5-HT depletion induced a similarly selective deficit in reversal learning. The CIC-induced impairment in reversal learning was attenuated by acute 5-HT reuptake blockade. 5-HT release was reduced in OFC of CIC-stressed rats during behavioral testing.
Conclusions
The CIC stress-induced impairment of cognitive flexibility may involve dysregulation of 5-HT modulatory function in OFC. Such deficits may thus model relevant symptoms of neuropsychiatric disorders that respond positively to SSRI treatment.
Keywords: attention, chronic stress, cognition, depression, microdialysis, oribitofrontal cortex, PCPA, reversal learning, serotonin
Introduction
The prefrontal cortex (PFC) has been implicated in cognitive flexibility, i.e., the ability to attend to changes in the environment and adapt behavioral response strategies accordingly (Dalley et al. 2004; Ragozzino et al. 1999). Deficits in such processes, which can lead to perseverative cognitive and emotional biases, are thought to be important in the development and maintenance of depression, particularly as related to stress (Beck 1976; Beck et al. 1987). In addition to reduced motivation and an overall diminished cognitive capacity, depressed patients show abnormal responses to performance feedback, a narrowing of attentional focus to depression–relevant thoughts, and difficulty in shifting attentional set from one affective dimension to another (Murphy et al. 1999). This is consistent with the perseverative attention to themes of loss and worthlessness, and the persistent ruminations that are prevalent in this disorder (Murphy et al. 2003; Murphy et al. 1999). Neuropsychological studies of depressed patients have revealed impairments in executive functions related specifically to decreased frontal lobe activity, e.g., verbal fluency, cognitive set shifting, behavioral flexibility and perseveration (see Fossati et al. 1999). Imaging studies have shown associations between cognitive dysfunction and altered activity in prefrontal cortex in depression (Rogers et al. 2004; Sheline 2003). Successful treatment with cognitive behavioral therapy, or antidepressant drugs such as paroxetine, produced concomitant changes in prefrontal cortical function, also revealed in neuroimaging studies (Goldapple et al. 2004; Kennedy et al. 2001; Prasko et al. 2004).
The Wisconsin Card Sorting Test (WCST) is a neurocognitive test that reveals strategy-switching deficits in patients with frontal lobe damage or prefrontal cortical dysfunction (see (Tollefson 1996). To be able to study different aspects of cognitive flexibility, and to address the neural mechanisms underlying these complex cognitive processes, similar attentional set shifting tasks have been developed for non-human primates (Roberts et al. 1992) and for rodents (Birrell and Brown 2000). Studies using these tests have shown that distinct subregions of prefrontal cortex are involved in different components or forms of cognitive flexibility. For example, lesions or pharmacological manipulations of rat medial prefrontal cortex (mPFC) affected extradimensional (ED) set shifting specifically, leaving both intradimensional (ID) acquisition and reversal learning intact (Birrell and Brown 2000; Chudasama et al. 2001; Chudasama and Robbins 2003; Lapiz et al. 2007; Lapiz and Morilak 2006; McAlonan and Brown 2003; Ragozzino 2002; Ragozzino et al. 1999). In these tests, subjects must learn a series of reward contingencies, using cues that vary along multiple stimulus dimensions (e.g., odor and texture, shape and color, etc). ED set shifting involves the acquisition of a new contingency requiring a shift in attention (and responding) away from the stimulus dimension that had been established previously as the relevant or informative dimension (i.e., formation of a cognitive set), to a previously irrelevant stimulus dimension. Hence, the mPFC is involved in shifting attention between perceptual features of complex stimuli (Dias et al. 1996; 1997; Owen et al. 1991).
By contrast, the orbital frontal cortex (OFC) has been implicated specifically in reversal learning, another form of cognitive flexibility in which a previously positive cue becomes negative and a previously negative cue becomes positive, but within the same stimulus dimension. Lesions of the monkey OFC resulted in impairment of reversal learning, with normal acquisition and maintenance of attentional set (Dias et al. 1996). Similarly, selective impairments in reversal learning have been observed after lesions or temporary inactivation of the rat OFC (Bohn et al. 2003; Chudasama and Robbins 2003; Ferry et al. 2000; Kim and Ragozzino 2005; McAlonan and Brown 2003; Schoenbaum et al. 2002), again leaving new acquisition and ED set shifting capabilities intact (McAlonan and Brown 2003).
Different neurotransmitters may also modulate these cognitive processes differentially in subregions of prefrontal cortex. For instance, in a previous study, we have shown that increases in noradrenergic neurotransmission in mPFC selectively facilitated ED set shifting (Lapiz et al. 2007; Lapiz and Morilak 2006). By contrast, others have suggested that reversal learning may be modulated in OFC specifically by serotonergic neurotransmission (Clarke et al. 2004; Clarke et al. 2005; Clarke et al. 2007).
Chronic stress is a risk factor and antecedent both to depression and to dysregulation of brain monoaminergic neurotransmitter systems (Anisman and Zacharko 1986). We have shown previously that a chronic psychogenic stressor, chronic unpredictable stress, produced a deficit in ED set shifting, which was prevented by chronic treatment with desipramine, a selective norepinephrine reuptake blocker (Bondi et al. 2008). The effects of escitalopram, a serotonin reuptake blocker, were more complex, in that the detrimental effect of chronic unpredictable stress was attenuated, but escitalopram alone also induced a baseline deficit in ED set shifitng. The noradrenergic system is highly responsive to stress (e.g., see review in Morilak et al. 2005). However, depending on the nature of the stress, e.g., psychogenic or metabolic, different neural pathways and neurotransmitter systems may be invoked (e.g., see Herman et al. 2005; Sawchenko et al. 2000). For instance, recent reports have emphasized a potential role for changes serotonin (5-HT) activity in the prefrontal cortex specifically after uncontrollable versus controllable stress (Amat et al. 2005).
Chronic intermittent cold (CIC) stress is a potent metabolic stressor that has been shown to sensitize the noradrenergic response to acute stress (Ma and Morilak 2005; Mana and Grace 1997; Pardon et al. 2003). Thus, CIC stress may be a viable model for chronic stress as an antecedent to stress-related psychiatric disorders involving alterations in monoaminergic transmission. However, changes in cognitive function following CIC stress have not been investigated, nor has a possible effect of CIC stress on serotonergic function in frontal cortex been examined. Thus, as serotonin has also been implicated in such disorders, the purpose of the present study was to investigate the effects of CIC stress on cognitive performance of rats in the attentional set shifting test, and to explore a potential role for 5-HT in any detrimental effects observed on cognitive function in the rat prefrontal cortex. Portions of this work have been presented in abstract form (Lapiz-Bluhm et al. 2008; Morilak and Lapiz-Bluhm 2007).
Materials and Methods
Animals
A total of 101 adult male Sprague-Dawley rats, weighing 220–240 g on arrival, were used in these studies. They were initially group-housed (3 rats/cage) in 25 × 45 × 15 cm plastic cages, maintained on a 12/12-hr light/dark cycle (lights on at 0:700 hr), and given access to food and water ad libitum. After acclimatizing for a minimum of 7 days, rats were housed individually prior to any experimental manipulation. Experiments were conducted during the light portion of the cycle, between 09:00–17:00h. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio, and were consistent with NIH guidelines for the care and use of laboratory animals. All efforts were made to minimize animal pain, discomfort or suffering, and the number of rats used.
Chronic intermittent cold stress
The procedure for CIC stress exposure was as described previously (Ma and Morilak 2005), except that the duration of CIC treatment was increased to 14 days. Briefly, rats were individually-housed and randomly assigned to either control or CIC stress conditions. During the light phase of the light/dark cycle, rats in the CIC stress group were transported in their home cages, with food, water and bedding, into a cold room maintained at 4°C for a period of 6 h. After 6h, they were returned to the main housing room. This procedure was repeated every day for 14 consecutive days. Rats in the control condition were left undisturbed in the housing facility during this time. One week prior to behavioral testing (i.e., beginning on day 11 of CIC stress), all rats were maintained on a restricted diet of 14 g of food pellets per day, with water freely available. Following the 14-day period of CIC stress treatment, the rats were then taken through the 3-day attentional set shifting protocol, described below.
Attentional Set Shifting Test
The attentional set shifting test was conducted as described previously (Lapiz and Morilak 2006). The testing apparatus was a rectangular wooden arena (inner dimensions, length × width × height: 71 × 40 × 20 cm) painted white on all surfaces. A removable, white plexiglas divider separated one-third of the length of the arena from the rest, forming a start box. This also served as a holding area following each trial, allowing the experimenter to clean the arena and change pots. To begin each trial, a rat was placed in the start box, and given access to the rest of the arena by lifting this divider. A white plexiglas panel divided the opposite third of the arena into two sections. At testing, one digging bowl was placed in each section. This separation enabled the experimenter to quickly remove the rat following an incorrect response, thus preventing it from moving to the other bowl to retrieve the bait. The digging bowls consisted of small terracotta pots (internal rim diameter 7 cm; depth 6 cm). Each pot was defined by a pair of cues along two stimulus dimensions, the digging medium with which the pot was filled and an odor (see Table 1). To mark each pot with a distinct odor, two drops (10 μl/drop) of scented aromatic oil (Frontier Natural Brands, Boulder, CO, USA) was applied to the inner rim at least 5 days prior to use. Then, 3–5 μl of the same oil was reapplied a day before testing. A different pot was used for each combination of digging medium and odor, and only one odor was ever applied to a given pot. The bait, a 1/4 Honey Nut Cheerio (General Mills Cereals, Minneapolis, MN, USA), was buried 2 cm below the surface of the digging medium in the “positive” pot. At the beginning of all discrimination tasks, a very small quantity of powdered Cheerio was also sprinkled onto the digging medium in the unbaited pot to eliminate the possibility that the rat may locate the bait by smell rather than by learning the discrimination.
Table 1.
Behavioral protocol for the attentional set shifting test
| Discrimination Stage | Dimensions | Example Combinations | ||
|---|---|---|---|---|
| Relevant | Irrelevant | (+) | (−) | |
| Simple (SD) | Odor | Clove/Sawdust | Nutmeg/Sawdust | |
| Compound (CD) | Odor | Medium | Clove/Raffia | Nutmeg/Metallic Filler |
| Clove/Metallic Filler | Nutmeg/Raffia | |||
| Reversal 1 (R1) | Odor | Medium | Nutmeg/Raffia | Clove/Metallic Filler |
| Nutmeg/Metallic Filler | Clove/Raffia | |||
| Intradimensional Shift (ID) | Odor | Medium | Rosemary/Wood balls | Cinnamon/Plastic beads |
| Rosemary/Plastic beads | Cinnamon/Wood balls | |||
| Reversal 2 (R2) | Odor | Medium | Cinnamon/Plastic beads | Rosemary/Wood balls |
| Cinnamon/Wood balls | Rosemary/Plastic beads | |||
| Extradimensional shift (ED) | Medium | Odor | Velvet/Citronella | Crepe/Thyme |
| Velvet/Thyme | Crepe/Citronella | |||
| Reversal 3 (R3) | Medium | Odor | Crepe/Thyme | Velvet/Citronella |
| Crepe/Citronella | Velvet/Thyme | |||
Representative example of stimulus pairs, and the progression through stages of the attentional set shifting test protocol. In this example, odor was the initial discriminative stimulus dimension, shifting to digging medium in the ED stage. For each stage, the positive stimulus is in bold, and is paired randomly across trials with the two stimuli from the irrelevant dimension.
Digging was defined as a vigorous displacement of the medium to retrieve the reward buried within the pot. Simply investigating the rim of the pot or the surface of the digging medium with paws or snout without displacing material was not scored as a “dig”, and the trial continued until a “dig” response was executed. Thus, rats were able to access tactile, visual and olfactory characteristics of the pots to make their choices based on these stimulus dimensions.
The behavioral procedure entailed three days for each rat:
Day 1, Habituation
On the first day, the rats were trained to dig reliably in the pots to obtain a food reward. Two unscented pots, both baited, were placed in the home cage for a series of three exposures of 5 min each. With each exposure, the bait was covered with an increasing amount of sawdust. Once the rat was digging reliably, it was introduced into the test arena, and given 3 trials to retrieve reward from both of the sawdust-filled baited pots.
Day 2, Training
The following day, the rats were trained on a series of simple discriminations (SD), to a criterion of six consecutive correct trials. For these trials, the rats first had to learn to associate the food reward with an odor cue (lemon vs. rosewood, both pots filled with sawdust). After criterion was reached for the odor discrimination, the pots were changed, and they had to discriminate the digging media (felt strips vs. shredded paper, no odor). All rats were trained using the same pairs of stimuli and in the same order. The positive and negative cues for each rat were determined randomly and represented equally. These training stimuli were not used again in later testing trials.
Day 3, Testing
Following the training day, the rats were tested on a series of increasingly difficult discriminations (see Table 1). Testing continued at each stage until the rat reached a criterion of 6 consecutive correct trials, after which testing proceeded to the next stage.
The first stage was a simple discrimination (SD), involving only one stimulus dimension. Half the rats in any group were required at this stage to discriminate between 2 odors, only one of which was associated with reward, with both pots filled with sawdust. For the other half, this first discrimination involved digging media, with no odors applied to the pots (for the sake of clarity, the remainder of this description will only consider the example beginning with odor discrimination). The second stage was then a compound discrimination (CD), where the second, irrelevant stimulus was introduced. Only one odor was associated with reward, as in the SD stage, but two different digging media were now paired randomly with the odors. The third stage was a reversal of this discrimination (R1), in which the same odors and media were used, and odor was still the relevant dimension, but in these trials the negative odor from the previous stage was now positive (i.e., associated with the reward), and the positive odor from the previous stage was now negative (no reward). The fourth stage was an intradimensional (ID) shift, wherein odor was still the relevant dimension and the medium was still irrelevant, but all new stimuli were used (i.e., new odors and new media). The fifth stage was a reversal of this discrimination (R2), in which the previously negative odor was now positive, as in R1. The sixth stage required an extradimensional (ED) shift, in which all new stimuli were again introduced, but this time the relevant dimension was also changed, e.g., the digging medium became the relevant dimension and odor was now irrelevant. Finally, the seventh stage was another reversal (R3), where the previously negative medium was now positive. The assignment of each exemplar in a pair as being positive or negative in a given stage, and the left-right positioning of the pots in the arena on each trial, were randomized in advance. For half the rats, the discriminations began with odor as the relevant cue, as described above, and for half the discrimination began with medium as the salient cue. Table 1 outlines the progression through these stages, and provides examples of the cue combinations used, beginning in this case with an odor discrimination, shifting to medium in the ED shift stage. The dependent measure in this procedure was Trials to Criterion (TTC), the number of trials required to reach criterion of 6 consecutive correct responses at each test stage.
Experiment 1: Effects of 14-day CIC stress on attentional set shifting task performance
Twenty-four individually-housed rats were randomly assigned to 2 groups: control or 14-day CIC stress exposure. Seven days prior to testing, food was restricted to 14g/day, with water freely available. On days 15–17, rats were handled and underwent habituation and training as described above, with testing conducted 3 days after the last cold stress exposure (Day 17).
Experiment 2: Effects of 5-HT depletion by PCPA on attentional set-shifting task performance
Twenty individually-housed rats were randomly assigned to 2 groups: vehicle or PCPA (4-chloro-DL-phenylalanine methyl ester hydrochloride; Sigma, St. Louis, MO, USA). PCPA was dissolved in 0.9% NaCl (40 mg/ml), adjusted to pH 6.0 by adding 0.1 M NaOH. Drug was prepared fresh daily, and administered at a dose of 200 mg/kg, i.p. (calculated as the free base) in a volume of 5 ml/kg on each of 4 consecutive days prior to the attentional set-shifting test. Rats were weighed daily to determine the drug dose and to monitor changes in weight gain, one of the reported side effects of PCPA (Dringenberg et al. 1995). The PCPA treatment regimen used in this study (4 × 200 mg/kg/day i.p.) was based on previously published studies, which showed significant 94% depletion of rat brain 5-HT and an absence of 5-HT immunolabeling in cortex and other forebrain regions (Kornum et al. 2006). Cryan et al. (2000) also reported a 90% reduction in 5-HT levels on Day 6 following a 3-day PCPA (150 mg/kg/day) treatment.
The extent of 5-HT depletion was assessed by measuring 5-HT content in frontal cortex. After behavioral testing, rats were decapitated, the frontal cortex dissected to 4 mm from the frontal pole, frozen in isopentane and stored at −80°C. Samples were homogenized in 0.1N perchloric acid, supernatant was obtained by centrifugation, and 5-HT was measured by HPLC (assay conducted by the Clinical Psychopharmacology Laboratory, UTHSCSA).
Experiment 3: Effects of acute citalopram administration on the performance of CIC-stressed rats on the attentional set-shifting task
Forty-three individually-housed rats were randomly assigned to 2 treatment groups, control or 14-day CIC stress exposure, which were each then further subdivided into 2 acute drug treatment conditions, citalopram or vehicle control. Seven days prior to testing, food was restricted to 14g/day, with water freely available. On days 15–17, rats were handled and underwent habituation and training as described above with testing conducted 3 days following the last cold stress exposure (Day 17). On the testing day, all rats were taken through the SD and CD stages, after which they were given an acute injection of saline vehicle (1 ml/kg, s.c.) or citalopram hydrobromide (5 mg/kg, calculated as the free base; H. Lundbeck A/S, Denmark). We have found previously using microdialysis that this dose elicits about a 5-fold increase in extracellular 5-HT levels in OFC acutely (unpublished data). Thirty min after drug administration, behavioral testing was resumed, beginning with the first reversal task (R1).
Experiment 4: Effects of CIC stress on 5-HT release in OFC during behavioral testing
Rats initially underwent surgery for microdialysis probe implantation. They were anesthetized with a cocktail of ketamine 43 mg/ml, acepromazine 1.4 mg/ml, xylazine 8.6 mg/ml, 1.0 ml/kg, i.m., with 25% supplementation as needed, and placed in a stereotaxic frame with the incisor bar set at −3.3 mm, adjusted to achieve a flat skull, indicated by equal dorsoventral (DV) coordinates for bregma and lambda. A microdialysis guide cannula (CMA/12; CMA Microdialysis, North Chelmsford, MA) was implanted, aimed at OFC with coordinates relative to bregma: AP +2.9 mm, ML ±2.3 mm, DV −4.2 mm, placing the tip 2 mm above the target (Paxinos and Watson 1998). Approximately half the rats in each group were implanted on the left side, and half on the right. The guide cannula was anchored to the skull with jeweler’s screws and dental acrylic, and an obdurator was inserted to maintain patency. After surgery, rats were given antibiotic (Penicillin G, 300, 000 IU/ml, 1.0 ml/kg, s.c.), hydrated with 1 ml saline (i.p.) returned to a fresh cage and housed singly.
Twenty-four operated rats were randomly assigned to 2 groups: control or 14-day CIC stress exposure. Seven days prior to AST testing, food was restricted to 14g/day, with water freely available. On days 15–17, rats were handled and underwent habituation and training as described above, with testing conducted 3 days after the last cold stress exposure (Day 17). On the testing day, a microdialysis probe (CMA/12), with 2 mm active membrane, 20 kDa MW cutoff, was inserted into the guide, extending 2 mm beyond the tip, centering the active membrane in OFC. The probe was perfused with ACSF (147 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, 0.85 mM MgCl2, pH 7.4) at 0.8 μl/min. Animals were allowed a 2 hr equilibration before sample collection was initiated. Collection time was 20 min, resulting in sample volume of 16 μl, collected into tubes containing 5 μl of 3 mM EDTA, pH 6.0. For all samples, a 9 min delay was incorporated into the sampling procedure to allow for the dead volume between the active membrane and the sample collection outlet. After collecting 4 baseline samples, behavioral testing began. Previous studies (Lapiz et al. 2007; Lapiz and Morilak 2006), have shown that some of the test stages require less time for criterion to be reached than the 20-min microdialysis sample interval. Thus, sampling over some of the behavioral tasks was combined: a single sample was collected during SD and CD testing (SD/CD), and also during ID and R2 testing (ID/R2). The time required to complete the other test stages (R1, ED and R3) was sufficient to allow collection of a single sample during each. The amount of 5-HT in a standard injection volume of 15.0 μl was measured by HPLC with electrochemical detection (BAS 502 system with a Unijet microbore column, 3μm ODS, 100×1 mm, Bioanalytical Systems Inc. West Lafayette, IN). The mobile phase contained 8% acetonitrile, 100 mM sodium acetate, 1.25 mM octylsulfonic acid, 0.5 mM EDTA, pH 5.0, at a flow rate of 90 μl/min. With these conditions, 5-HT had a retention time of ~4.6 min. The amount of 5-HT in each sample was quantified against a calibration curve run daily, with a detection limit of ~0.176 pg/sample (1.0 fmole). At the end of the experiment, rats were sacrificed by rapid decapitation, and the brains removed for histological localization of the probe track. Any case in which the probe was located outside of OFC was eliminated a priori.
Data analyses
In all experiments, investigators conducting the behavioral test were blind to the experimental treatment condition of the rats being tested. The dependent measure in all experiments was the number of trials to criterion on each test stage, expressed as mean ± SEM. For all experiments, the mean trials to criterion during the simple discrimination tasks conducted on the training day were first compared by analysis of variance (ANOVA), to ensure that training was equivalent between all control and treatment groups. For Experiment 1, data collected on the testing day were then analyzed by two-way ANOVA (Stress × Stage), with repeated measures over Stage. For Experiment 2, data were analyzed by two-way ANOVA (Drug × Stage) with repeated measures over Stage. In addition, differences in body weight change from the start of PCPA treatment to the testing day were analyzed by Student’s t-test. For Experiment 3, data were analyzed by three-way ANOVA (Stress × Drug × Stage), with repeated measures over Stage. For Experiment 4, the behavioral data were analyzed by two-way ANOVA, as in experiment 1. For the microdialysis data, the mean of the four baseline samples was first calculated for each animal, and these values were subjected to Student’s t-test to assess potential changes in baseline 5-HT levels induced by the CIC stress treatment. The effect of CIC stress on 5-HT levels collected during AST testing was then analyzed using two-way ANOVA (Stress × Stage) with repeated measures over Stage. Data from 6 rats (3 controls and 3 stressed) were included in the behavioral data in this experiment for purposes of replication, but were not included in the microdialysis data due to probe failure during the course of testing. In all experiments, where significant main effects or interactions were indicated, post hoc analyses were performed using the Newman-Keuls test to identify differences between treatment groups at specific test stages. For all analyses, significance was determined at p < 0.05.
Only rats that completed all stages of the AST were included in the analyses. A rat was eliminated if it stopped responding, or failed to complete all stages after 5.5 hr of testing (17:30 hr), as continuing to test beyond that time would have approached the transition to the dark phase of the light cycle. In Experiment 1, two control rats failed to finish the test. In Experiment 2, three PCPA-treated rats were excluded: two failed to complete the training trials conducted on day 2, and one failed to complete all stages on the test day. In Experiment 3, two citalopram-treated rats were excluded from the analysis because they failed to complete all tasks on testing day. In experiment 4, four rats were excluded because they failed to finish all stages on the testing day. Animals so excluded were not included in reporting the total number of rats used.
Results
Experiment 1: Effects of CIC stress exposure on attentional set-shifting test performance
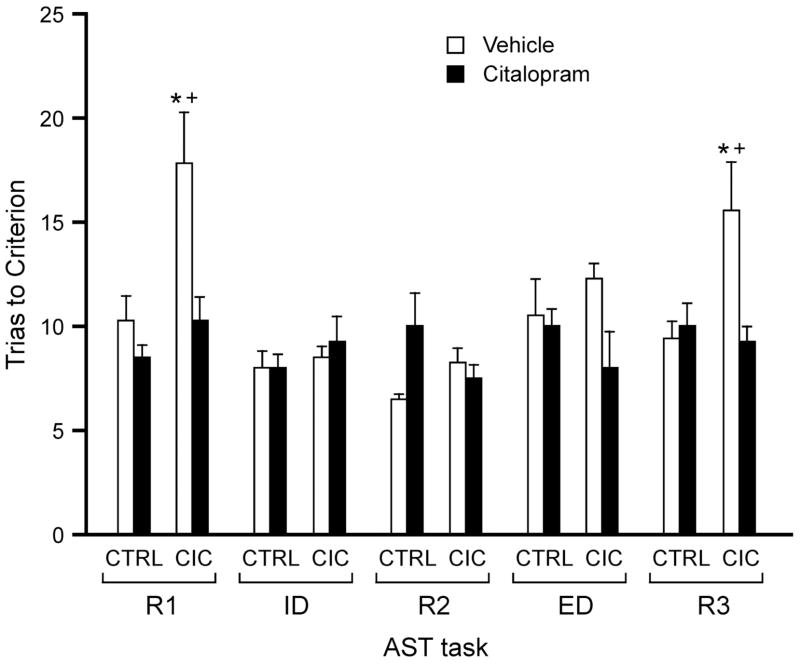
Both control and cold-stressed rats learned to dig in the baited bowls during habituation, and learned the simple discrimination during the training session comparably (F1,22 = 0.125, p = 0.73), indicating that the CIC stress treatment did not impair acquisition or the ability to perform on the test in general. Figure 1 shows the effect of 14-day CIC stress exposure on the TTC for each stage of the attentional set-shifting test. ANOVA revealed a significant main effect of Stage (F6,132 = 12.16, p <0.0001) and a Stress × Stage interaction (F6,132 = 3.85, p = 0.001). Post hoc analysis showed that cold-stressed rats required significantly more trials to reach criterion on the first reversal task (R1) compared to controls (p < 0.01). Trials to criterion on the R1 task for CIC-stressed rats were also significantly higher than on all other tasks for that group (p < 0.01).
Figure 1.
Effects of 14-day CIC stress exposure on the mean trials to criterion for each stage of the AST (n = 12 per group). CIC–stressed rats required significantly more trials to reach criterion on the R1 reversal task compared to non-stressed controls (*p < 0.01 compared to control rats on the same stage; +p < 0.05 compared to other tasks for the same group).
Experiment 2: Effects of PCPA-treatment on attentional set-shifting test performance
PCPA treatment resulted in a 96.6% depletion of 5-HT measured in frontal cortical extracts (14.18 ± 0.86 ng/ml for controls, compared to 0.48 ± 0.09 ng/ml for PCPA-treated rats). Analysis of body weight change from the start of food restriction to the testing day, which included the period of PCPA or vehicle treatment prior to testing, revealed a significantly greater loss of body weight in PCPA-treated rats (−21.2 ± 2.5 g for controls compared to −31.3 ± 3.9 g for PCPA-treated rats; t18 = 2.26, p < 0.05). Relative to starting body weight before food restriction, this represents a change of −6.1 ± 0.6% for vehicle-treated controls and −9.7 ± 1.1 % for PCPA-treated rats, consistent with an effective serotonin depletion by PCPA. Both groups learned to dig in the baited bowls during habituation, and both learned the simple discrimination comparably during the training session (F1,18 = 0.007, p = 0.93), indicating that PCPA treatment did not affect acquisition or performance capability in general.
Figure 2 shows the effect of PCPA treatment on the performance of rats on the attentional set-shifting test. ANOVA revealed significant main effects of Drug (F1,18 = 9.09, p < 0.01) and Stage (F6,108 = 14.40, p < 0.0001), and a Drug × Stage interaction (F6,108 = 6.44, p < 0.0001). Post hoc analysis showed that PCPA-treated rats required significantly more trials to reach criterion on the R1 task compared to vehicle-treated controls (p < 0.001). Trials to criterion on the R1 task were significantly higher than all other tasks for the PCPA-treated rats (p < 0.001).
Figure 2.
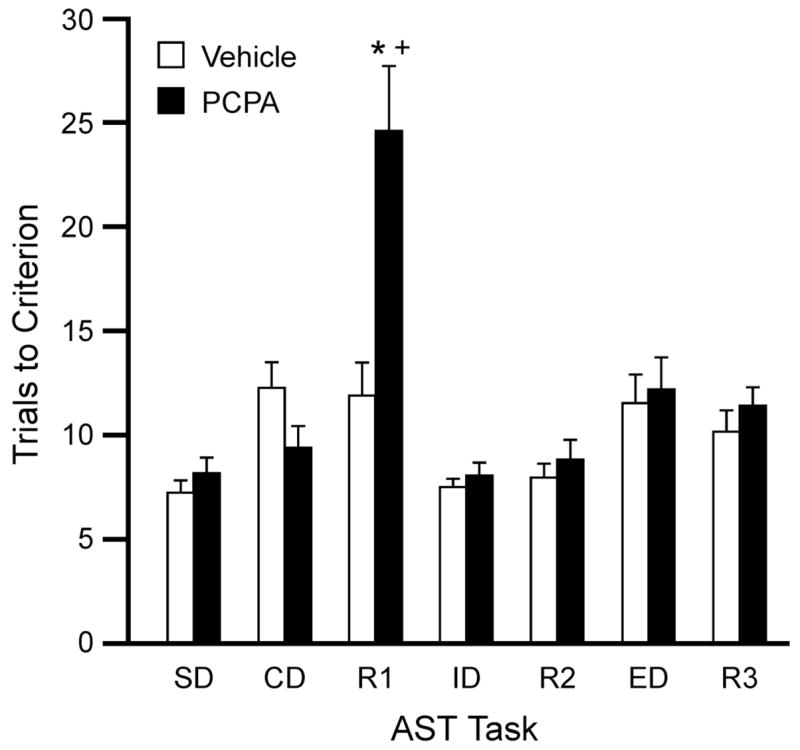
Effects of PCPA pretreatment (4 × 200 mg/kg/day) on performance of rats on the AST (n = 9–11 per group). PCPA-treated rats required significantly more trials to reach criterion on the R1 reversal task compared to vehicle-treated controls (*p < 0.001 compared to control rats on the same stage; +p < 0.001 compared to other tasks for the same group).
Experiment 3: Effects of acute systemic citalopram administration on the performance of CIC-stressed rats on the attentional set-shifting test
As in Experiment 1, both control and cold-stressed rats learned to dig in the baited bowls during habituation. Rats in both groups learned the simple discriminations during the training session comparably (F1,12 = 0.121, p = 0.73), confirming that the CIC stress treatment did not affect acquisition or performance in general.
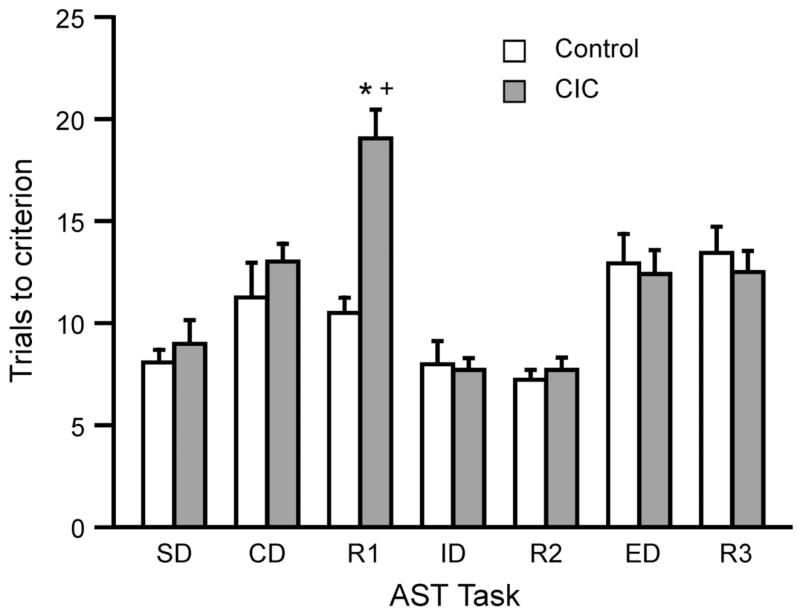
Figure 3 shows the effect of CIC stress and acute citalopram treatment on performance on the attentional set-shifting task beginning 30-min after citalopram injection (starting with the R1 task). ANOVA revealed significant main effects of Stress (F1,39= 9.417, p < 0.01), Drug (F1,39= 9.998, p < 0.01), and Stage (F4,156 = 6.438, p < 0.0001). There were significant interactions of Stress × Drug (F4,156 = 11.133, p < 0.01), and Drug × Task (F4,156 = 3.186, p < 0.05). The interaction of Stress × Stage approached significance (F4,156 = 2. 319, p = 0.059). Post hoc analysis showed that vehicle-treated CIC-stressed rats again required significantly more trials to criterion on the reversal learning tasks, specifically on R1 (p < 0.001) and also on R3 (p < 0.05) compared to vehicle–treated, non-CIC-stressed controls. Post hoc analysis further showed that acute citalopram treatment attenuated the effects of CIC stress on stimulus reversal learning, as citalopram-treated CIC-stressed rats required fewer trials to criterion on R1 (p < 0.001) and R3 (p < 0.05) compared to vehicle-treated CIC-stressed rats (Figure 3).
Figure 3.
Effect of acute citalopram treatment (5mg/kg, s.c.), given 30 min prior to testing on the R1 task, on the reversal deficit induced by 14-day CIC stress exposure. Only data from the R1 stage on, i.e., after drug injection, are shown. As in experiment 1, vehicle-treated CIC-stressed rats required significantly more trials to reach criterion on the R1 reversal stage compared to vehicle-treated non-stressed control rats, and also compared to CIC-stressed rats given an acute injection of citalopram. Note that in this experiment, CIC stress also induced a deficit in the third reversal task (R3), that was similarly reversed by citalopram. Citalopram-treated CIC-stressed rats did not differ from non-stressed vehicle-treated control rats on any test stage. (*p < 0.05 vehicle-treated rats, CIC-stressed compared to non-stressed controls; +p < 0.05 CIC-stressed rats, vehicle-treated compared to citalopram-treated; n = 10–11 per group).
Experiment 4: Effects of CIC stress exposure on attentional set-shifting test performance and extracellular 5-HT levels measured in OFC during testing
Rats surgically implanted with microdialysis cannulae and subsequently exposed to either control or cold-stressed conditions learned to dig in the baited bowls during habituation. Both control and cold-stressed rats had comparable performance during the simple discrimination tasks on training day (F1,22 = 1.88, p = 0.60), indicating that neither surgery nor CIC stress impaired acquisition or the ability to perform on the test in general. Figure 4a shows the behavioral effects of CIC stress on rats tested while collecting microdialysis samples from OFC. As in experiment 1, ANOVA revealed significant main effects of Stress (F1,22 = 5.534, p < 0.05) and Stage (F6,132 = 21.366, p < 0.001), and a significant Stress × Stage interaction (F6.132 = 7.275, p < 0.001). Post hoc analysis showed a significant increase in the number of trials required to reach criterion on the R1 task in CIC-stressed rats compared to controls, replicating the results from Experiment 1. The replication of behavioral results from Experiment 1 also indicated that microdialyis conducted during testing did not interfere with performance on the AST, nor did it alter the ability to detect the cognitive effects of CIC stress. In addition, in this experiment, trials to criterion were also higher on the CD stage for CIC-stressed rats.
Figure 4.
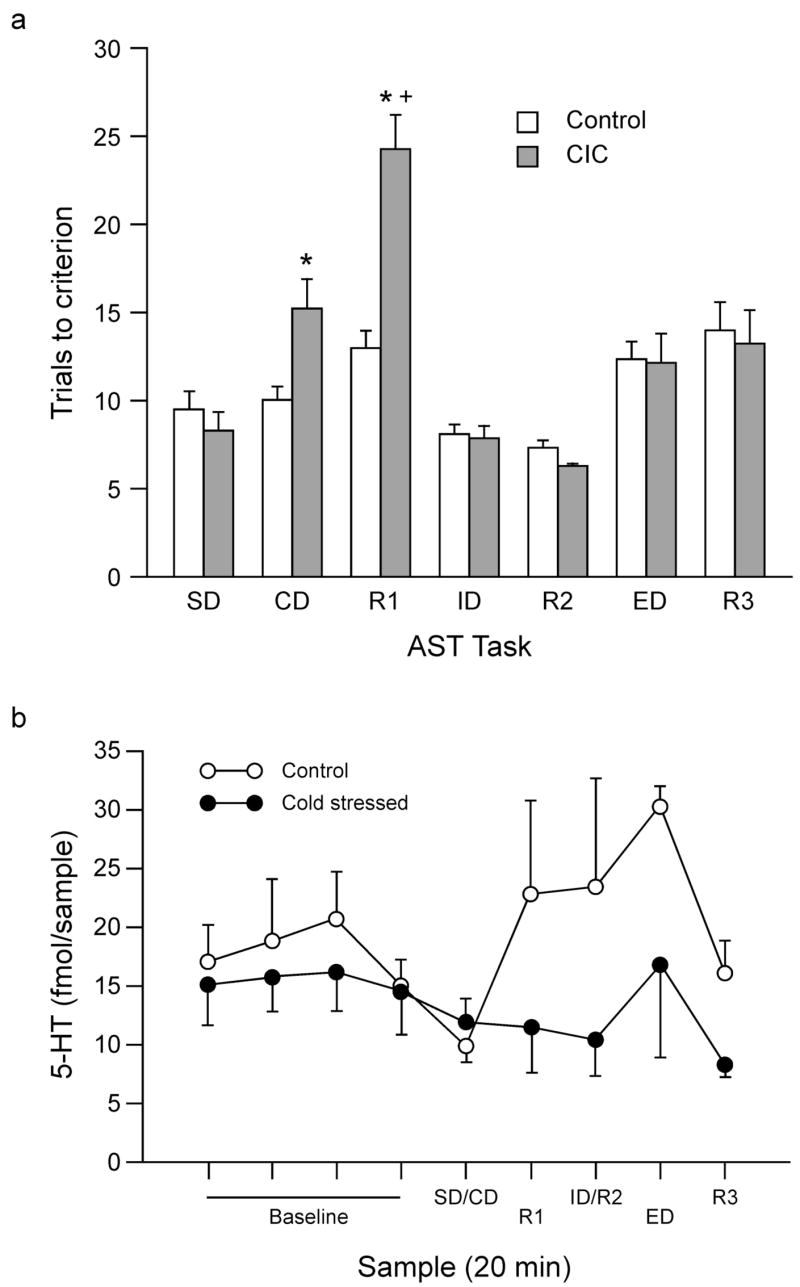
Effects of 14-day CIC stress exposure on reversal learning and on extracellular 5-HT levels measured in OFC during behavioral testing on the AST. a) In replication of experiment 1, CIC-stressed rats required more trials to reach criterion during R1 compared to controls (n= 11–13 per group; *p < 0.05 compared to control rats on the same stage; +p < 0.05 compared to other tasks for the same group). b) Extracellular 5-HT levels, collected in OFC by microdialysis during behavioral testing, were reduced in CIC-stressed rats compared to control rats (n = 9 per group; p < 0.05 main effect of CIC stress compared to control).
Figure 4b shows the concentration of 5-HT in microdialysate samples collected in the OFC during baseline and during behavioral testing on the AST. Mean baseline levels of 5-HT were comparable in the two groups (17.91 ± 2.92 fmoles/sample for controls, and 15.41 ± 3.0 fmoles/sample for CIC-stressed rats; t16 = 0.595, p = 0.56). Analysis of 5-HT levels in dialysate samples collected in OFC during behavioral testing (including the single mean baseline value) revealed a significant main effect of Stress (F1, 16 = 5.389, p < 0.05), an effect of Stage that only approached significance (F5, 80 = 2.097, p = 0.074), and no Stress × Stage interaction (F5,80 = 1.046, p = 0.396). This indicates that 5-HT release in OFC was reduced in CIC-stressed rats during behavioral testing, but this effect was similar across test stages (see Figure 4b).
Discussion
Reversal learning has been studied as a measure of cognitive flexibility in humans (Fellows and Farah 2003; Murphy et al. 2002; Rogers et al. 2000; Rolls et al. 1994), nonhuman primates (Clarke et al. 2004; Clarke et al. 2005; Clarke et al. 2007; Dias et al. 1996; Lee et al. 2007), and rats (Birrell and Brown 2000; Boulougouris et al. 2007a; Boulougouris et al. 2007b; Idris et al. 2005; McAlonan and Brown 2003; van der Meulen et al. 2007). Effective reversal learning involves a number of specific and distinct operations, including: 1) detection of the shift in contingency (i.e., “error detection”); 2) inhibition of the prepotent, previously learned positive response; 3) overcoming learned avoidance of the previously negative stimulus; and 4) acquisition of the new association. The results of the present study showed that chronic exposure to a metabolic stressor, intermittent cold stress, impaired some aspect of reversal learning in the attentional set shifting test, without interfering with the acquisition of new contingencies, as the simple discriminations, compound discriminations, and intra-dimensional set-shifting tasks were all generally unaffected in these studies. Moreover, not all forms of cognitive flexibility were altered by CIC stress, as extra-dimensional set-shifting remained intact as well. The relatively selective effect of CIC stress was mimicked by 5-HT depletion, using the tryptophan hydroxylase inhibitor, PCPA. Conversely, the CIC stress-induced impairment in reversal learning was attenuated by acute administration of the selective serotonin reuptake inhibitor, citalopram. These pharmacological results, along with the decrease in extracellular 5-HT levels observed in microdialysate samples collected in OFC during testing, indicate a possible dysregulation of serotonergic modulatory function in the OFC of rats exposed to CIC stress.
Previous studies have shown that exposure to acute cold stress impaired performance on a delayed match-to-sample task, attributed to effects on short-term or working memory (Ahlers et al. 1991; Shurtleff et al. 1993; Thomas et al. 1989; Thomas et al. 1991). Rats immersed in cold water (17°C) were also reported to be more behaviorally depressed than controls (Rauch and Lieberman 1980). Analogous patterns of cognitive impairment and adverse mood changes have been reported in humans. Acute cold exposure during cognitive testing degraded performance, including vigilance, reaction time, reasoning skills and short-term memory (e.g., see Coleshaw et al. 1983; Mahoney et al. 2007; Patil et al. 1995; Shurtleff et al. 1993). In addition to decrements in performance on a 4-choice reaction task and impairment in delayed match-to-sample tasks, cold-stressed individuals also reported higher levels of tension, confusion and depression on the subscale of the Profile of Mood States questionnaire, and had significantly higher “total mood disturbance” scores (Mahoney et al. 2007). Taken together, these results indicate that cold stress can impair cognitive performance and evoke adverse changes in both behavior and mood.
In agreement with previous studies which have established a role of the 5-HT system in reversal learning (Clarke et al. 2004; Clarke et al. 2005; Clarke et al. 2007), impairment in the first reversal task was also seen in the present study following depletion of central serotonin by PCPA, comparable to that induced by chronic cold stress, suggesting a possible involvement of the serotonergic system in the CIC stress-induced reversal deficit. Further evidence for involvement of 5-HT in the stress-induced impairment of reversal learning was seen in Experiment 3, in which acute treatment with the 5-HT reuptake inhibitor citalopram attenuated the detrimental effect of CIC stress on the reversal task. Finally, a possible serotonergic involvement was further supported by the reduced extracellular 5-HT levels detected in OFC during behavioral testing in rats exposed to CIC stress.
The cognitive deficit seen following both CIC stress and 5-HT depletion was specific to the reversal task. In previous studies, 5-HT depletion similarly impaired the ability to switch responding between stimuli on reversal trials in a visual discrimination task (Clarke et al. 2004; Clarke et al. 2007), but left cognitive set-shifting ability intact (Clarke et al. 2005). Further, PCPA treatment did not affect spatial navigation in a water maze or passive avoidance learning (Riekkinen et al. 1992), arguing against a general decline in cognitive capability. Although reduced body weight gain was observed in the present study, PCPA treatment also did not appear to affect general sensory-motor capabilities, nor motivation to learn the task and obtain the food reward, as the PCPA-treated rats trained and habituated, acquired the simple discriminations, and performed comparably to controls on all test stages with the exception of the first reversal.
In the present study, only the first reversal (R1) was consistently affected in all experiments. The second reversal (R2) was unaffected by any treatment, and the third reversal (R3) was not consistently affected, although cold stress induced a deficit in R3 as well as R1 in experiment 3, and both deficits were attenuated by citalopram treatment. Similarly inconsistent effects on the later reversal tasks have been reported previously, including studies employing serotonergic pharmacological manipulations (e.g., Bondi et al. 2008; Boulougouris et al. 2007b; Hatcher et al. 2005; Tait and Brown 2008). Although we hesitate to speculate extensively on a possible reason for this in the absence of experimental evidence, it seems that R1 is most vulnerable to manipulation, and this may be due to differences in the difficulty involved in reversing the contingencies established in the stages immediately preceding each reversal task. R2 is very similar in nature to R1; it follows a new acquisition within the same stimulus dimension as R1, and the animal has just had the prior experience of R1. The resulting ease with which rats typically master R2 may thus reflect “learning-to-learn”, and may also make R2 more resistant to disruption. By contrast, R3 is preceded by the ED set-shift stage, in which rats must abandon the cognitive set that had been reinforced repeatedly in all stages up to that point. Thus, the new contingency acquired in the ED stage may not be as “strong” as those in the earlier acquisitions, and less “flexibility” may be required to achieve the subsequent reversal in R3, i.e., less perseveration must be overcome, again making R3 less consistently prone to disruption.
The effect of 5-HT depletion was unlike that reported after catecholamine depletion, in which cognitive set shifting was disrupted, but with no effect on reversal learning (Crofts et al. 2001; Roberts et al. 1994). We and others have found that catecholamine neurotransmitter systems in the brain can also be affected by cold stress. Acute cold stress has been shown to increase norepinephrine release (Yeghiayan et al. 2001; Young et al. 1986), and chronic (2–4 weeks) cold stress exposure has been shown to increase noradrenergic receptor sensitivity, enhance the noradrenergic response to acute stress, and sensitize the acute excitability of noradrenergic neurons in locus coeruleus (Gresch et al. 1994; Jedema and Grace 2003; Ma and Morilak 2005; Mana and Grace 1997; Pardon et al. 2003). In addition, a variety of stressors, including cold stress, also increase dopamine utilization, leading to a reduction in central levels of both catecholamines (e.g., Dunn and File 1983; Finlay and Zigmond 1997; Reinstein et al. 1984). Impairments in cognitive function, specifically in working memory, have been demonstrated following alterations of central norepinephrine and dopamine function (Arnsten and Li 2005; Brozoski et al. 1979), and chronic reductions in monoaminergic neurotransmitter levels have been proposed as a potential mechanism by which chronic stress may predispose to depression (Anisman and Zacharko 1986).
The present results suggest that chronic metabolic stressors, such as CIC stress, may also alter central serotonergic modulation of reversal learning. This is in distinct contrast to the cognitive effects reported previously after a psychogenic stress treatment, chronic unpredictable stress, which induced a consistent deficit in extradimensional set-shifting capability and a less consistent effect on reversal learning (Bondi et al. 2008). The cognitive deficit following chronic unpredictable stress was prevented by concurrent chronic treatment with the selective norepinephrine reuptake inhibitor, desipramine, as well as the 5-HT reuptake inhibitor, escitalopram (Bondi et al. 2008), although escitalopram alone also decreased extradimensional set-shifting performance. Improvement in ED set-shifting has also been observed following elevation of noradrenergic neurotransmission, either by chronic desipramine treatment (Lapiz et al. 2007), or by systemic administration of atipamezole, an α2-autoreceptor antagonist (Lapiz and Morilak 2006). Moreover, the atipamezole-induced enhancement was blocked specifically by local microinjection of a post-synaptic α1–adrenergic receptor antagonist directly into medial prefrontal cortex (mPFC), indicating that extradimensional set-shifting is modulated specifically by noradrenergic transmission in mPFC (Lapiz and Morilak 2006).
By contrast, reversal learning may depend specifically on function of the orbitofrontal cortex, as suggested in humans (Hornak et al. 2004; Rolls et al. 1994), non-human primates (Clarke et al. 2004; Dias et al. 1996), and more recently in rats (McAlonan and Brown 2003). Thus, it appears that different types of chronic stress may have different effects on cognitive flexibility, dependent upon distinct subregions of prefrontal cortex, and potentially involving dysregulation of different monoaminergic systems. Whereas certain types of stressors, (e.g, chronic unpredictable stress) might produce effects that model changes in noradrenergic function, and deficits (e.g., extradimensional set-shifting) related to functional changes in the medial prefrontal cortex (see Bondi et al. 2008), other stressors (e.g., chronic intermittent cold stress) may produce dysregulation of serotonergic function, and cognitive deficits (e.g., reversal learning) attributable more to altered function of orbitofrontal cortex. It would be of interest to determine if the full range of behavioral and physiological changes produced by chronic unpredictable stress might be preferentially responsive to treatment with norepinephrine reuptake blockers, and if the behavioral and physiological consequences of chronic metabolic stress exposure might be preferentially responsive to 5-HT reuptake blockers. If such presumed regional and neurochemical specificity is indeed supported, one important implication would be that a more careful and precise evaluation of the cognitive deficit exhibited by a given depressed patient might better inform an optimally effective treatment strategy for that patient.
Channon (1996) emphasized the importance of executive dysfunction related to prefrontal cortical dysregulation, specifically perseveration, or the failure to alter behavior in response to environmental feedback, in the diagnosis and treatment of depression. Indeed, cognitive-behavioral approaches (Beck 1976; 2005) postulate that depression is associated with a ruminative perseveration within a set of negative perceptions and beliefs about the self, the world and the future. Accordingly, the cognitive-behavioral therapeutic approach is intended to help the patient challenge such biases, and establish a more flexible, balanced, realistic and adaptive cognitive schema based on hypothesis-testing and the use of available evidence to modify thoughts and behaviors. It would be interesting and informative to determine if a more precise evaluation of the specific cognitive impairments exhibited by depressed patients (e.g., reversal- or cognitive set-shifting deficit) might predict a differential response to different antidepressant drug classes, e.g., serotonin-specific, norepinephrine-specific, or dual reuptake inhibitors.
Acknowledgments
We thank Ms. Julianne Doyen and Ms. Teresa F. Burke for expert technical assistance, and also thank Dr. Dania Rossi for her input and assistance with the microdialysis experiments. The authors have no conflicts of interest to report, nor any involvement to disclose, financial or otherwise, that may bias the conduct, interpretation or presentation of this work. All experiments were conducted in compliance with current law in the country in which they were performed.
Funding: Support was provided by a Young Investigator Award from NARSAD: The Mental Health Association (to MDSLB), and by research grants from the National Institutes of Health (MH53851 and MH72672 to DAM, and MH52369 to JGH).
References
- Ahlers ST, Thomas JR, Berkey DL. Hippocampal and body temperature changes in rats during delayed matching-to-sample performance in a cold environment. Physiol Behav. 1991;50:1013–1018. doi: 10.1016/0031-9384(91)90430-v. [DOI] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nature Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Anisman H, Zacharko RM. Behavioral and neurochemical consequences associated with stressors. Ann N Y Acad Sci. 1986;467:205–225. doi: 10.1111/j.1749-6632.1986.tb14630.x. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Li B-M. Neurobiology of executive functions: Catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Beck AT. Cognitive therapy and the emotional disorders. Int. Univ. Press, Int. Univ. Press; 1976. [Google Scholar]
- Beck AT. The current state of cognitive therapy: A 40-year retrospective. Arch Gen Psychiatry. 2005;62:953–959. doi: 10.1001/archpsyc.62.9.953. [DOI] [PubMed] [Google Scholar]
- Beck AT, Brown G, Steer RA, Eidelson JI, Riskind JH. Differentiating anxiety and depression: A test of the cognitive content-specificity hypothesis. J Abnormal Psychol. 1987;96:179–183. doi: 10.1037//0021-843x.96.3.179. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn I, Giertler C, Hauber W. Orbital prefrontal cortex and guidance of instrumental behaviour in rats under reversal conditions. Beh Brain Res. 2003;143:49–56. doi: 10.1016/s0166-4328(03)00008-1. [DOI] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33:320–331. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Beh Brain Res. 2007a;179:219–228. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Glennon JC, Robbins TW. Dissociable effects of selective 5-HT2A and 5-HT2C receptor antagonists on serial spatial reversal learning in rats. Neuropsychopharmacology. 2007b doi: 10.1038/sj.npp.1301584. ePub: in press. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Channon S. Executive dysfunction in depression: The Wisconsin Card Sorting Test. J Affective Disord. 1996;39:107–114. doi: 10.1016/0165-0327(96)00027-4. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Bussey TJ, Muir JL. Effects of selective thalamic and prelimbic cortex lesions on two types of visual discrimination and reversal learning. Eur J Neurosci. 2001;14:1009–1020. doi: 10.1046/j.0953-816x.2001.01607.x. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Crofts HS, Dalley JW, Robbins TW, Roberts AC. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. J Neurosci. 2005;25:532–538. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cerebral Cortex. 2007;17:18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- Coleshaw SRK, RNM VS, Wolff AH, Davis HM, Keatinge WR. Impaired memory registration and speed of reasoning caused by low body temperature. J Appl Physiol. 1983;55:27–31. doi: 10.1152/jappl.1983.55.1.27. [DOI] [PubMed] [Google Scholar]
- Crofts HS, Dalley JW, Collins P, Van Denderen JC, Everitt BJ, Robbins TW, Roberts AC. Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cerebral Cortex. 2001;11:1015–1026. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Harkin A, Naughton M, Kelly JP, Leonard BE. Characterization of D-fenfluramine-induced hypothermia: Evidence for multiple sites of action. Eur J Pharmacol. 2000;390:275–285. doi: 10.1016/s0014-2999(00)00012-1. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal cognitive and executive functions in rodents: Neural and neurochemical substrates. Neurosci Biobehav Revs. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: Effects of excitotoxic lesions of the prefrontal cortex of the marmoset. Behav Neurosci. 1996;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: Restriction to novel situations and independence from “on-line” processing. J Neurosci. 1997;17:9285–9297. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringenberg HC, Hargreaves EL, Baker GB, Cooley RK, Vanderwolf CH. P-chlorophenylalanine-induced serotonin depletion: Reduction in exploratory locomotion but no obvious sensory motor deficits. Beh Brain Res. 1995;68:229–237. doi: 10.1016/0166-4328(94)00174-e. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, File SE. Cold restraint alters dopamine metabolism in frontal cortex, nucleus accumbens and neostriatum. Physiol Behav. 1983;31:511–513. doi: 10.1016/0031-9384(83)90074-4. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: Evidence from reversal learning paradigm. Brain. 2003;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Ferry AT, Lu XC, Price JL. Effects of excitotoxic lesions in the ventral striatopallidal--thalamocortical pathway on odor reversal learning: inability to extinguish an incorrect response. Exp Brain Res. 2000;131:320–335. doi: 10.1007/s002219900240. [DOI] [PubMed] [Google Scholar]
- Finlay JM, Zigmond MJ. The effects of stress on central dopaminergic neurons: Possible clinical implications. Neurochem Res. 1997;22:1387–1394. doi: 10.1023/a:1022075324164. [DOI] [PubMed] [Google Scholar]
- Fossati P, Amar G, Raoux N, Ergis AM, Allilaire JF. Executive functioning and verbal memory in young patients with unipolar depression and schizophrenia. Psychiatry Res. 1999;89:171–187. doi: 10.1016/s0165-1781(99)00110-9. [DOI] [PubMed] [Google Scholar]
- Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, Mayberg H. Modulation of cortico-limbic pathways in major depression: Treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry. 2004;61:34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- Gresch PJ, Sved AF, Zigmond MJ, Finlay JM. Stress-induced sensitization of dopamine and norepinephrine efflux in medial prefrontal cortex of the rat. J Neurochem. 1994;63:575–583. doi: 10.1046/j.1471-4159.1994.63020575.x. [DOI] [PubMed] [Google Scholar]
- Hatcher PD, Brown VJ, Tait DS, Bate S, Overend P, Hagan JJ, Jones DNC. 5-HT6 receptor antagonists improve performance in an attentional set shifting task in rats. Psychopharmacology. 2005;181:253–259. doi: 10.1007/s00213-005-2261-z. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: Hypothalamo-pituitary-adrenocortical axis. Prog Neuro-Psychopharmacol Biol Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hornak J, O’Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, Polkey CE. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. J Cognitive Neurosci. 2004;16:463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- Idris NF, Repeto P, Neill JC, Large CH. Investigation of the effects of lamotrigmine and clozapine in improving reversal-learning impairments induced by acute phencyclidine and D-amphetamine in the rat. Psychopharmacology (Berl) 2005;179:336–348. doi: 10.1007/s00213-004-2058-5. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Grace AA. Chronic exposure to cold stress alters electrophysiological properties of locus coeruleus neurons recorded in vitro. Neuropsychopharmacology. 2003;28:63–72. doi: 10.1038/sj.npp.1300020. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Evans KR, Kruger S, Mayberg HS, Meyer JH, McCann S, Arifuzzman AI, Houle S, Vaccarino FJ. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- Kim J, Ragozzino ME. The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiol Learn Mem. 2005;83:125–133. doi: 10.1016/j.nlm.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornum BR, Licht CL, Weikop P, Knudsen GM, Aznar S. Central serotonergic depletion affects rat brain areas differently: A qualitative and quantitative comparison between different treatment schemes. Neurosci Lett. 2006;392:129–132. doi: 10.1016/j.neulet.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Lapiz MDS, Bondi CO, Morilak DA. Chronic treatment with desipramine improves cognitive performance of rats in an attentional set shifting test. Neuropsychopharmacology. 2007;32:1000–1010. doi: 10.1038/sj.npp.1301235. [DOI] [PubMed] [Google Scholar]
- Lapiz MDS, Morilak DA. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience. 2006;137:1039–1049. doi: 10.1016/j.neuroscience.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Lapiz-Bluhm MD, Soto-Pina A, Morilak DA. Serotonergic involvement in the effects of chronic cold stress on reversal learning in the rats. FASEB J. 2008;22:906.4. [Google Scholar]
- Lee B, Groman S, London ED, Jentsch JD. Dopamine D2/D3 receptors play a specific role in the reversal of a learned visual discrimination in monkeys. Neuropsychopharmacology. 2007;32:2125–2134. doi: 10.1038/sj.npp.1301337. [DOI] [PubMed] [Google Scholar]
- Ma S, Morilak DA. Chronic intermittent cold stress sensitizes the HPA response to a novel acute stress by enhancing noradrenergic influence in the rat paraventricular nucleus. J Neuroendocrinol. 2005;17:761–769. doi: 10.1111/j.1365-2826.2005.01372.x. [DOI] [PubMed] [Google Scholar]
- Mahoney CR, Castellani J, Kramer FM, Young A, Lieberman HR. Tyrosine supplementation mitigates working memory decrements during cold exposure. Physiol Behav. 2007;92:575–582. doi: 10.1016/j.physbeh.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Mana MJ, Grace AA. Chronic cold stress alters the basal and evoked electrophysiological activity of rat locus coeruleus neurons. Neuroscience. 1997;81:1055–1064. doi: 10.1016/s0306-4522(97)00225-x. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatr. 2005;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Lapiz-Bluhm MD. Chronic intermittent cold stress induces impairment in reversal learning in the rat on an attentional set-shifting task. Soc Neurosci Abstr. 2007;33 Online: Program number 643.14. [Google Scholar]
- Murphy FC, Michael A, Robbins TW, Sahakian BJ. Neuropsychological impairments in patients with major depressive disorder: The effects of feedback on task performance. Psychol Med. 2003;33:455–467. doi: 10.1017/s0033291702007018. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, Paykel ES. Emotional bias and inhibitory control processes in mania and depression. Psychol Med. 1999;29:1307–1321. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Smith KA, Cowen PJ, Robbins TW, Sahakian BJ. The effects of tryptophan depletion on cognitive and affective processing in healthy volunteers. Psychopharmacology (Berl) 2002;163:42–53. doi: 10.1007/s00213-002-1128-9. [DOI] [PubMed] [Google Scholar]
- Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalohippocampectomy. Neuropsychologia. 1991;29:993–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
- Pardon M-C, Ma S, Morilak DA. Chronic cold stress sensitizes brain noradrenergic reactivity and noradrenergic facilitation of the HPA stress response in Wistar Kyoto rats. Brain Res. 2003;971:55–65. doi: 10.1016/s0006-8993(03)02355-2. [DOI] [PubMed] [Google Scholar]
- Patil PG, Apfelbaum JL, Zacny JP. Effects of a cold-water stressor on psychomotor and cognitive functioning in humans. Physiol Behav. 1995;58:1281–1286. doi: 10.1016/0031-9384(95)02071-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Prasko J, Hornacek J, Zalesky R, Kopecek M, Novak T, Paskova B, Skrdlantova L, Belohlavek O, Hoschl C. The change of regional brain metabolism (18FDG PET) in panic disorder during the treatment with cognitive behavioral therapy or antidepressants. Neuro Endocrinol Lett. 2004;25:340–348. [PubMed] [Google Scholar]
- Ragozzino ME. The effects of dopamine D(1) receptor blockade in the prelimbic-infralimbic areas on behavioral flexibility. Learning & Memory. 2002;9:18–28. doi: 10.1101/lm.45802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch TM, Lieberman HR. Tyrosine pretreatment reverses hypothermia-induced behavioral depression. Brain Res Bull. 1980;24:147–150. doi: 10.1016/0361-9230(90)90299-f. [DOI] [PubMed] [Google Scholar]
- Reinstein DK, Lehnert H, Scott NA, Wurtman RJ. Tyrosine prevents behavioral and neurochemical correlates of an acute stress in rats. Life Sci. 1984;34:2225–2231. doi: 10.1016/0024-3205(84)90209-1. [DOI] [PubMed] [Google Scholar]
- Riekkinen P, Jr, Riekkinen M, Sirviö J, Riekkinen P. Effects of concurrent nicotinic antagonist and PCPA treatments on spatial and passive avoidance learning. Brain Res. 1992;575:247–250. doi: 10.1016/0006-8993(92)90086-o. [DOI] [PubMed] [Google Scholar]
- Roberts AC, De Salvia MA, Wilkinson LS, Collins P, Muir JL, Everitt BJ, Robbins TW. 6-Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analog of the Wisconsin Card Sort test: Possible interactions with subcortical dopamine. J Neurosci. 1994;14:2531–2544. doi: 10.1523/JNEUROSCI.14-05-02531.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AC, Robbins TW, Everitt BJ, Muir JL. A specific form of cognitive rigidity following excitotoxic lesions of the basal forebrain in marmosets. Neuroscience. 1992;47:251–264. doi: 10.1016/0306-4522(92)90241-s. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Kasai K, Koji M, Fukuda R, Iwanami A, Nakagome K, Fukuda M, Kato N. Executive and prefrontal dysfunction in unipolar depression: A review of neuropsychological and imaging evidence. Neurosci Res. 2004;50:1–11. doi: 10.1016/j.neures.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Andrews TC, Grasby PM, Brooks DJ, Robbins TW. Contrasting cortical and subcortical activations produced by attentional set shifting and reversal learning in humans. J Cognitive Neurosci. 2000;12:142–162. doi: 10.1162/089892900561931. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchenko PE, Li H-Y, Ericsson A. Circuits and mechanisms governing hypothalamic responses to stress: A tale of two paradigms. Prog Brain Res. 2000;122:61–78. doi: 10.1016/s0079-6123(08)62131-7. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent SL, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;7:885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry. 2003;54:338–352. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- Shurtleff D, Thomas JR, Ahlers ST, Schrot J. Tyrosine ameliorates a cold-induced delayed matching-to-sample performance decrement in rats. Psychopharmacology (Berl) 1993;112:228–232. doi: 10.1007/BF02244915. [DOI] [PubMed] [Google Scholar]
- Tait DS, Brown VJ. Lesions of the basal forebrain impair reversal learning but not shifting of attentional set in rats. Beh Brain Res. 2008;187:100–108. doi: 10.1016/j.bbr.2007.08.035. [DOI] [PubMed] [Google Scholar]
- Thomas JR, Ahlers ST, House JF, Schrot J. Repeated exposure to moderate cold impairs matching-to-sample performance. Aviat Space Environ Med. 1989;60:1063–1067. [PubMed] [Google Scholar]
- Thomas JR, Ahlers ST, Shurtleff D. Thermal stress modulates temporal patterns of responding on a multiple DRL-FR schedule. Physiol Behav. 1991;50:437–442. doi: 10.1016/0031-9384(91)90091-2. [DOI] [PubMed] [Google Scholar]
- Tollefson GD. Cognitive function in schizophrenic patients. J Clin Psychiatry. 1996;57:31–39. [PubMed] [Google Scholar]
- van der Meulen JAJ, Joosten RNJMA, de Bruin JPC, Feenstra MGP. Dopamine and noradrenaline efflux in the medial prefrontal cortex during serial reversals and extinction of instrumental goal-directed behavior. Cerebral Cortex. 2007;17:1444–1453. doi: 10.1093/cercor/bhl057. [DOI] [PubMed] [Google Scholar]
- Yeghiayan SK, Luo S, Shukitt-Hale B, Lieberman HR. Tyrosine improves behavioral and neurochemical deficits caused by cold exposure. Physiol Behav. 2001;72:311–316. doi: 10.1016/s0031-9384(00)00398-x. [DOI] [PubMed] [Google Scholar]
- Young AJ, Muza SR, Sawka MN, Gonzalez RR, Pandolf KB. Human thermoregulatory responses to cold air are altered by repeated cold water immersion. J Appl Physiol. 1986;60:1542–1548. doi: 10.1152/jappl.1986.60.5.1542. [DOI] [PubMed] [Google Scholar]