Abstract
This report describes the synthesis of two cyclic RGD (Arg-Gly-Asp) conjugates, HYNIC-2PEG4-dimer (HYNIC = 6-hydrazinonicotinyl; 2PEG4-dimer = E[PEG4-c(RGDfK)]2; and PEG4 = 15-amino-4,7,10,13-tetraoxapentadecanoic acid) and HYNIC-3PEG4-dimer (3PEG4-dimer = PEG4-E[PEG4-c(RGDfK)]2), and evaluation of their 99mTc complexes [99mTc(HYNIC-2PEG4-dimer)(tricine)(TPPTS)] (99mTc-2PEG4-dimer: TPPTS = trisodium triphenylphosphine-3,3′,3″-trisulfonate) and [99mTc(HYNIC-3PEG4-dimer)(tricine)(TPPTS)] (99mTc-3PEG4-dimer) as novel radiotracers for imaging integrin αvβ3 expression in athymic nude mice bearing U87MG glioma and MDA-MB-435 breast cancer xenografts. The integrin αvβ3 binding affinities of RGD peptides were determined by competitive displacement of 125I-c(RGDyK) on U87MG glioma cells. It was found that the two PEG4 linkers between RGD motifs in HYNIC-2PEG4-dimer (IC50 = 2.8 ± 0.5 nM) and HYNIC-3PEG4-dimer (IC50 = 2.4 ± 0.7 nM) are responsible for their higher integrin αvβ3 binding affinity than that of HYNIC-PEG4-dimer (PEG4-dimer = PEG4-E[c(RGDfK)]2; IC50 = 7.5 ± 2.3 nM). Addition of extra PEG4 linker in HYNIC-3PEG4-dimer has little impact on integrin αvβ3 binding affinity. 99mTc-2PEG4-dimer and 99mTc-3PEG4-dimer were prepared in high yield with >95% radiochemical purity and the specific activity of > 10 Ci/μmol. Biodistribution studies clearly demonstrated that PEG4 linkers are particularly useful for improving the tumor uptake and clearance kinetics of 99mTc-2PEG4-dimer and 99mTc-3PEG4-dimer from non-cancerous organs. It was also found that there was a linear relationship between the tumor size and radiotracer tumor uptake expressed as %ID (percentage of the injected dose) in U87MG glioma and MDA-MB-435 breast tumor models. The blocking experiment showed that the tumor uptake of 99mTc-2PEG4-dimer is integrin αvβ3-mediated. In the metabolism study, 99mTc-2PEG4-dimer had high metabolic stability during its excretion from renal and hepatobiliary routes. 99mTc-3PEG4-dimer also remained intact during thee excretion from the renal route, but, had ~30% metabolism during the excretion from the hepatobiliary route. Planar imaging studies in U87MG glioma and MDA-MB-435 breast tumor models showed that the tumors of ~5 mm in diameter could be readily visualized with excellent contrast. Thus, 99mTc-3PEG4-dimer is a very promising radiotracer for the early detection of integrin αvβ3–positive tumors, and may have the potential for non-invasive monitoring of tumor growth or treatment efficacy.
Keywords: integrin αvβ3, 99mTc-labeled peptides, cyclic RGD dimers, tumor imaging
INTRODUCTION
Integrin αvβ3 plays a critical role in tumor angiogenesis and metastasis.1–8 The highly restricted expression of integrin αvβ3 during tumor growth, invasion and metastasis makes it an interesting molecular target for the development of integrin αvβ3-targeted therapeutic drugs and molecular imaging probes.9–18 Many high affinity cyclic RGD (Arg-Gly-Asp) peptides and non-peptide integrin αvβ3 antagonists have been identified and studied for their potential as therapeutic pharmaceuticals for the treatment of solid tumors.13–18 These anti-angiogenic agents were designed to block the formation of new blood vessels and destroy existing abnormal blood vessels by starving tumors and inhibiting their growth. They have also been shown to increase therapeutic efficacy in conjunction with other therapeutic agents or with external radiotherapy.19 Inhibition of integrin αvβ3 by cyclic RGD peptides has been shown to induce endothelial apoptosis,15 inhibit angiogenesis,17 and increase endothelial monolayer permeability.19, 20 The inhibition of integrin αvβ3 activity has been associated with the decreased tumor growth in breast cancer xenografts.19 Synergy of the cyclic RGD peptide EMD 121974 with radioimmunotherapy (RIT) increased the efficacy of therapy in a murine breast cancer model.19 However, there are several significant challenges for successful anti-angiogenic clinical trials: (1) selection of the right patients who will benefit the most, given that only the integrin αvβ3–positive patients are responsive to the specific anti-angiogenic treatment; (2) monitoring the therapeutic efficacy of the anti-angiogenic treatment; and (3) individualizing the optimal dose and schedule of anti-angiogenic treatment for specific patients. To achieve these goals, it is essential to develop an imaging agent that could be used for non-invasive visualization and quantification of the integrin αvβ3 expression level before and during the anti-angiogenic therapy.
Over the last decade, many radiolabeled cyclic RGD peptides have been evaluated as potential radiotracers for noninvasive imaging of integrin αvβ3-positive tumors by single photon emission computed tomography (SPECT) or positron emission tomography (PET).20–36 The integrin αvβ3-targeted radiotracers have recently been reviewed extensively.37–43 Among these radiotracers evaluated in different tumor-bearing animal models, [18F]Galacto-RGD (2-[18F]fluoropropanamide-c(RGDfK(SAA); SAA = 7-amino-L-glyero-L-galacto-2,6-anhydro-7-deoxyheptanamide) and [18F]-AH111585, the core peptide sequence of which was originally discovered from a phage display library (as ACDRGDCFCG),44 are currently under clinical investigation for noninvasive visualization of integrin αvβ3 expression.44–46 The PET imaging studies in cancer patients clearly show that the integrin αvβ3 expression level correlates well with radiotracer tumor uptake.44–46 Although they are able to target integrin αvβ3-positive tumors, the relatively low tumor uptake, suboptimal pharmacokinetics, high cost and lack of preparative modules for the 18F-labeled monomeric RGD peptides are significant challenges for their continued clinical applications.
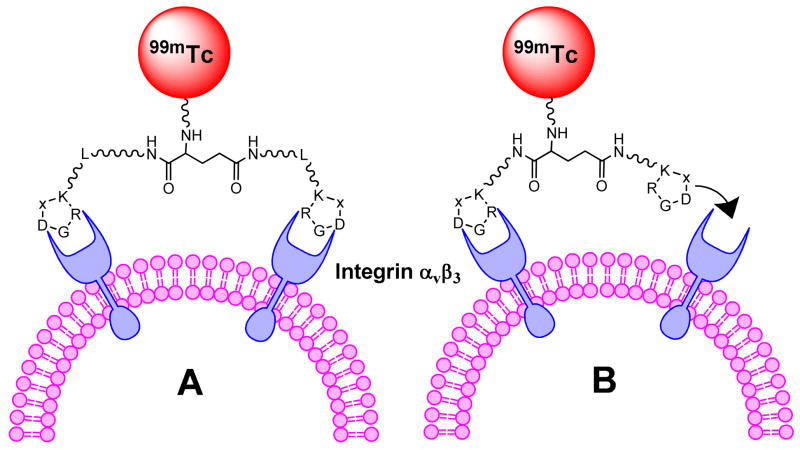
Since the natural mode of interactions between integrin αvβ3 and the RGD-containing proteins, such as fibronectin and vitronectin, may involve multiple binding sites, the idea to improve integrin αvβ3 affinity with multimeric cyclic RGD peptides may provide more effective integrin αvβ3 antagonists with better targeting capability and higher cellular uptake through the integrin-dependent endocytosis.47 Multivalent interactions are designed to make weak ligand-receptor interactions more biologically relevant.48 Over the last several years, we and others have been using multimeric cyclic RGD peptides, such as E[c(RGDxK)]2 and E{E[c(RGDxK)]2}2 (x = f (D-Phe) and y (D-Tyr)), to develop the integrin αvβ3-targeted radiotracers for imaging tumors by SPECT or PET.49–62 In general, there are two main factors (Figure 1: simultaneous integrin αvβ3 binding of two RGD motifs and the locally enriched RGD concentration) that contribute to the higher integrin αvβ3 binding affinity of E[c(RGDfK)]2 and E{E[c(RGDfK)]2}2 than that of their monomeric counterpart c(RGDfK). The key for “bivalency” is that the distance between two RGD motifs in multimeric cyclic RGD peptides must be long enough and flexible enough to achieve simultaneous integrin αvβ3 binding. If the distance between two cyclic RGD motifs is long enough, the multimeric cyclic RGD peptides would bind to the integrin αvβ3 in a “bidentate” fashion (Figure 1A). If the distance between two cyclic RGD motifs in multimeric cyclic RGD peptides is too short to achieve simultaneous integrin αvβ3 binding, the local RGD concentration would be significantly “enriched” in the vicinity of the neighboring integrin αvβ3 sites once the first RGD motif is bound to integrin αvβ3 (Figure 1B). In both cases, increasing the multiplicity would result in higher integrin αvβ3 binding affinity of multimeric cyclic RGD peptides and better tumor uptake with longer tumor retention time for their corresponding radiotracers. Although in vitro assays and ex-vivo biodistribution studies have demonstrated that the radiolabeled (99mTc, 18F, 64Cu and 111In) multimeric cyclic RGD peptides, such as E{E[c(RGDxK)]2}2 and E[c(RGDxK)]2 (x = f and y), have much better tumor targeting capability as evidenced by their higher integrin αvβ3 binding affinity and better radiotracer tumor uptake than their monomeric analogs,51–62 it remains unclear if the cyclic RGD motifs in E[c(RGDfK)]2 and E{E[c(RGDfK)]2}2 are able to achieve simultaneous integrin αvβ3 binding. In addition, the uptake of the radiolabeled (99mTc, 18F, 64Cu and 111In) multimeric cyclic RGD peptides in the kidneys, liver, lungs and spleen is also significantly increased as the peptide multiplicity increases.
Figure 1.
Schematic illustration of interactions between the cyclic RGD peptide dimers and integrin αvβ3. A: the distance between two RGD motifs is long enough, and the multimeric cyclic peptide is able to bind integrin αvβ3 in a “bidentate” fashion. B: the distance between two cyclic RGD motifs is not long enough for simultaneous integrin αvβ3 binding, but the RGD concentration is “locally enriched” in the vicinity of neighboring integrin αvβ3 once the first cyclic RGD motif is bounded. In both cases, the result would be higher integrin αvβ3 binding affinity and slower dissociation kinetic from integrin αvβ3 of multimeric cyclic RGD peptides.
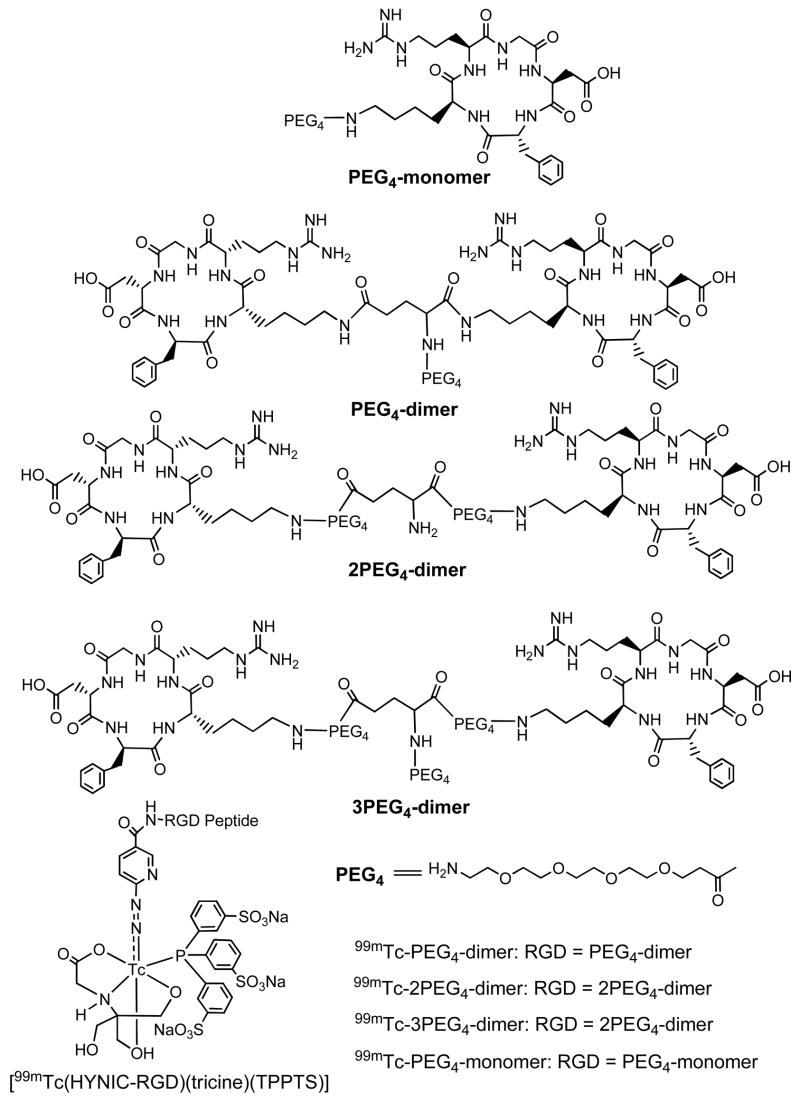
To solve these problems, we prepared a new cyclic RGD peptide dimer: E[PEG4-c(RGDfK)]2 (Figure 2: 2PEG4-dimer, PEG4 = 15-amino-4,7,10,13-tetraoxapentadecanoic acid). Two PEG4 linkers were used to increase the distance between two RGD motifs from 6 bonds in E[c(RGDfK)]2 to 38 bonds in 2PEG4-dimer excluding side arms of K-residues. We also prepared PEG4-E[PEG4-c(RGDfK)]2 (Figure 2: 3PEG4-dimer), in which an extra PEG4 was added to improve radiotracer excretion kinetics from non-cancerous organs, such as kidneys, liver and lungs. We are particularly interested in 99mTc due to its optimal nuclear characteristics (half-life, abundance and energy of γ-photons) and easy availability at low cost.11,63–65 For 99mTc-labeling, 6-hydrazinonicotinyl (HYNIC) was used as the bifunctional coupling agent whereas tricine and TPPTS (trisodium triphenylphosphine-3,3′,3″-trisulfonate) were used to stabilize the 99mTc-HYNIC core in 99mTc complexes: [99mTc(HYNIC-2PEG4-dimer)(tricine)(TPPTS)] (99mTc-2PEG4-dimer) and [99mTc(HYNIC-3PEG4-dimer)(tricine)(TPPTS)] (99mTc-3PEG4-dimer). An in vitro competition assay was used to determine the integrin αvβ3 binding affinity of HYNIC-2PEG4-dimer and HYNIC-3PEG4-dimer against 125I-c(RGDyK) bound to the integrin αvβ3-positive U87MG human glioma cells. Biodistribution characteristics of 99mTc-2PEG4-dimer and 99mTc-3PEG4-dimer were evaluated in athymic nude mice bearing U87MG glioma and MDA-MB-435 breast cancer xenografts. For comparison purposes, we also evaluated HYNIC-PEG4-c(RGDfK) (HYNIC-PEG4-monomer), HYNIC-PEG4-E[c(RGDfK)]2 (HYNIC-PEG4-dimer) and their 99mTc complexes, [99mTc(HYNIC-PEG4-monomer)(tricine)(TPPTS)] (99mTc-PEG4-monomer) and [99mTc(HYNIC-PEG4-dimer)(tricine)(TPPTS)] (99mTc-PEG4-dimer). The main objective of this study is to demonstrate that the use of PEG4 linkers will make it possible for the two cyclic RGD motifs in99mTc-2PEG4-dimer and 99mTc-3PEG4-dimer to achieve simultaneous integrin αvβ3 binding, thereby leading to higher integrin αvβ3 binding affinity for the cyclic RGD peptide dimer and better tumor uptake with longer tumor retention times for the corresponding 99mTc radiotracer. In addition, the presence of PEG4 linkers should improve the radiotracer excretion kinetics from kidneys, liver and lungs. Improving the radiotracer tumor uptake and its excretion kinetics from non-cancerous organs is critically important for the early detection of integrin αvβ3–positive tumors.
Figure 2.
HYNIC-conjugated cyclic RGD peptide dimers, HYNIC-PEG4-c(RGDfK) (PEG4-monomer), HYNIC-PEG4-E[c(RGDfK)]2 (PEG4-dimer), HYNIC-E[PEG4-c(RGDfK)]2 (2PEG4-dimer), HYNIC-PEG4-E[PEG4-c(RGDfK)]2 (3PEG4-dimer), and their ternary ligand 99mTc complexes [99mTc(HYNIC-RGD)(tricine)(TPPTS)] (RGD = PEG4-monomer, PEG4-dimer, 2PEG4-dimer and 3PEG4-dimer).
EXPERIMENTAL SECTION
Materials
Dicyclcohexylcarbodiimide (DCC), N-hydroxysuccinimide (NHS), succinic acid, trisodium triphenylphosphine-3,3′,3″-trisulfonate (TPPTS) and tricine were purchased from Sigma-Aldrich (St. Louis, MO). Cyclic RGD peptide dimers, 2PEG4-dimer and 3PEG4-dimer were custom-made by Peptide International, Inc. (Louisville, KY). PEG4-E[c(RGDfK)]2 (PEG4-dimer) was prepared according to the procedure described in our previous reports.52 Sodium succinimidyl 6-(2-(2-sulfonatobenzaldehyde)hydrazono)nicotinate (HYNIC-NHS) was prepared according to literature method.66 Na99mTcO4 was obtained from a commercial DuPont Pharma 99Mo/99mTc generator (North Billerica, MA). The ESI (electrospray ionization) mass spectral data were collected on a Finnigan LCQ classic mass spectrometer, School of Pharmacy, Purdue University.
HPLC Methods
HPLC Method 1 used a LabAlliance HPLC system (State College, PA) equipped with a UV/Vis detector (λ = 254 nm) and Zorbax C18 semi-prep column (9.4 mm × 250 mm, 100 Å pore size). The flow rate was 2.5 mL/min. The gradient mobile phase started with 90% solvent A (25 mM NH4OAc) and 10% solvent B (acetonitrile) to 85% solvent A and 15% solvent B at 5 min to 65% solvent A and 35% solvent B at 30 min, followed by an isocratic mobile phase with 50% solvent A and 50% solvent B at 32 – 36 min. The radio-HPLC method (Method 2) used the LabAlliance HPLC system equipped with a β-ram IN/US detector (IN/US System, Tampa, FL) and Zorbax C18 column (4.6 mm × 250 mm, 300 Å pore size; Agilent Technologies, Santa Clara, CA). The flow rate was 1 mL/min. The mobile phase was isocratic with 90% solvent A (25 mM NH4OAc, pH = 5.0) and 10% solvent B (acetonitrile) at 0 – 2 min, followed by a gradient mobile phase going from 10% B at 2 min to 15% B at 5 min and to 20% solvent B at 20 min.
HYNIC-E[PEG4-c(RGDfK)]2 (HYNIC-2PEG4-dimer)
HYNIC-NHS (4.6 mg, 11 μmol) and E[PEG4-c(RGDfK)]2 (5 mg, 2.8 μmol) were dissolved in 2.0 mL of a mixture containing DMF and water (50:50 = v:v). The pH in the mixture was adjusted to 8.5 – 9.0 using 0.1 N NaOH. The reaction mixture was then stirred overnight at room temperature. The product was isolated from the reaction mixture by HPLC purification (Method 1). The fraction at 19.6 min was collected. Lyophilization of the collected fractions afforded HYNIC-2PEG4-dimer as a white powder. The yield was 2.0 mg (~34%) with >95% HPLC purity. ESI-MS (positive mode): m/z = 1058.59 for [M + 2H]2+ (m/z = 1058.99 calcd. for [C94H140N24O30S]2+).
HYNIC-PEG4-E[PEG4-c(RGDfk)]2 (HYNIC-3PEG4-dimer)
HYNIC-NHS (2 mg, 4.8 μmol) and 3PEG4-dimer (2.4 mg, 1.2 μmol) were dissolved in a mixture (2 mL) of DMF and water (1:1 = v:v). The pH was adjusted to 8.5 – 9.0 with 0.1 N NaOH. The mixture was stirred overnight at room temperature. The product was isolated by HPLC purification (Method 1). Fractions at 20.6 min were collected. Lyophilization of the combined collections afforded the expected product HYNIC-3PEG4-dimer. The yield was 1 mg (~35%) with >95% HPLC purity. ESI-MS: m/z = 2364.33 for [M+H]+ (2364.1 calcd. For [C105H160N25O35S]+).
HYNIC-PEG4-c(RGDfK) (HYNIC-PEG4-Monomer)
HYNIC-PEG4-monomer was prepared using the same procedure using HYNIC-NHS (4.4 mg, 10.6 μmol) and PEG4-c(RGDfk) (3 mg, 3.53 μmol). Lyophilization of the combined collections ~19.5 min (Method 1) afforded the expected product HYNIC-PEG4-Monomer. The yield was 1.6 mg (~40%) with HPLC purity >95%. ESI-MS: m/z = 1154.17 for [M+H]+ (1154.49 calcd. For [C51H72N13O16S]+)
99mTc-Labeling
To a lyophilized vial containing 5 mg of TPPTS, 6.5 mg of tricine, 38.5 mg of disodium succinate hexahydrate, and 12.7 mg of succinic acid, 20 μg of the HYNIC-RGD conjugate (RGD = PEG4-monomer, 2PEG4-dimer or 3PEG4-dimer) was added 1.0 – 1.5 mL of Na99mTcO4 solution (10 – 50 mCi) in saline. The vial was heated at 100 °C for 20 – 25 min in a lead-shielded water bath. After heating, the vial was placed back into the lead pig, and allowed to stand at room temperature for ~10 min. A sample of the resulting solution was analyzed by the radio-HPLC. The radiochemical purity (RCP) was >95% for all new 99mTc radiotracers.
Dose Preparation for Animal Studies
For biodistribution studies, all new radiotracers were prepared and then purified by HPLC (Method 2). Volatiles in the HPLC mobile phase were removed under vacuum. Doses were prepared by dissolving the HPLC-purified radiotracer in saline to 20 – 30 μCi/mL. For imaging studies, doses were prepared by dissolving the radiotracer in saline to 5 – 10 mCi/mL. In the blocking experiment, E[c(RGDfK)]2 was dissolved in the solution containing the radiotracer to give a concentration of 1.75 mg/mL. The resulting solution was filtered with a 0.20 μ syringe-driven Millex-LG filter before being injected into animals. Each tumor-bearing mouse was injected with ~0.2 mL of the filtered dose solution.
Determination of Log P Values
The radiotracer was prepared and then purified by HPLC (Method 2). Volatiles in the HPLC mobile phase were removed under vacuum. The residue was dissolved in an equal volume (3 mL:3 mL) mixture of n-octanol and 25 mM phosphate buffer (pH = 7.4). After stirring vigorously for ~20 min, the mixture was centrifuged at a speed of 8,000 rpm for 5 min. Samples (in triplets) from both n-octanol and aqueous layers were obtained and counted in a Perkin Elmer Wizard – 1480 γ-counter (Shelton, CT). Partition coefficients were measured three different times. The log P values were reported as the average of three independent measurements plus the standard deviation.
In Vitro Whole-Cell Integrin αvβ3 Binding Assay
The in vitro integrin binding affinity and specificity of multimeric cyclic RGD peptides were determined via a competitive displacement assay using 125I-c(RGDyK) as the integrin-specific radioligand. Experiments were performed using the integrin αvβ3-positive U87MG human glioma cell line by slight modification of the literature method.39 For comparison purposes, we also evaluated c(RGDyK) using the same in vitro assay. Briefly, the U87MG glioma cells were grown in Gibco’s Dulbecco’s medium supplemented with 10% fetal bovine serum (FBS), 100 IU/mL penicillin and 100 μg/mL streptomycin (Invitrogen Co, Carlsbad, CA) at 37 °C in a humidified atmosphere containing 5% CO2. Multiscreen DV filter plates were seeded with ~105 cells in the binding buffer and incubated with 125I-c(RGDyK) in the presence of increasing concentrations of different cyclic RGD peptides or their HYNIC conjugates. After removing the unbound 125I-c(RGDyK), hydrophilic PVDF filters were collected and the radioactivity was determined using a gamma counter (Packard, Meriden, CT). The IC50 values were calculated by fitting the data by nonlinear regression using GraphPad Prism™ (GraphPad Software, Inc., San Diego, CA). All the in vitro experiments were carried out twice with triplicate samples. The IC50 values were calculated, and reported as an average of these samples plus the standard deviation. Comparison between two different cyclic RGD peptides was made using the two-way ANOVA test (GraphPad Prim 5.0, San Diego, CA). The level of significance was set at p < 0.05.
Animal Model
Biodistribution and imaging studies were carried out using the athymic nude mice bearing U87MG human glioma and MDA-MB-435 human breast cancer xenografts in compliance with the NIH animal experiment guidelines (Principles of Laboratory Animal Care, NIH Publication No. 86-23, revised 1985). The animal protocol for these studies has been approved by the Purdue University Animal Care and Use Committee (PACUC). U87MG glioma and MDA-MB-435 breast cancer cells were grown at 37 °C in Minimal Essential Medium (Alpha Modification) containing 3.7 g of sodium bicarbonate/L, 10% fetal bovine serum v/v, in a humidified atmosphere of 5% carbon dioxide. Female athymic nu/nu mice were purchased from Harlan (Indianapolis, IN) at 4 – 5 weeks of age. Each mouse was implanted subcutaneously with 5 × 106 tumor cells into the left and right upper flanks (for the glioma model) or the left and right mammary fat pads (for the breast cancer model). Doing so allows us to assess the impact of tumor size on the radiotracer imaging quality in a single tumor-bearing mouse. Two to four weeks after inoculation, animals with tumors in the range of 0.05 – 1.7 g were used for biodistribution and imaging studies.
Biodistribution Protocol
Twelve tumor-bearing mice with the tumor size of 0.05 – 0.4 g were randomly divided into three groups. The 99mTc radiotracer (~2 μCi) in 0.1 mL saline was administered via tail vein. Four animals were sacrificed by sodium pentobarbital overdose (100 mg/kg) at 30, 60, and 120 min postinjection (p.i.). Blood samples were withdrawn from the heart. The tumor and normal organs (brain, eyes, spleen, lungs, liver, kidneys, muscle and intestine) were excised, washed with saline, dried with absorbent tissue, weighed, and counted on a Perkin Elmer Wizard – 1480 γ-counter (Shelton, CT). The organ uptake was calculated as a percentage of the injected dose per organ (%ID/organ) and a percentage of the injected dose per gram of organ tissue (%ID/g). For the blocking experiment, each animal was administered with ~2 μCi of 99mTc-2PEG4-dimer along with ~350 μg of E[c(RGDfK)]2 (~14 mg/kg). At 1 h p.i., four animals were sacrificed by sodium pentobarbital overdose (100 mg/kg) for organ biodistribution. The organ uptake (%ID/g) was compared to that obtained in the absence of excess E[c(RGDfK)]2 at the same time point.
Scintigraphic Imaging
Two tumor-bearing mice bearing U87MG glioma or MDA-MB-435 breast cancer xenografts were used for imaging studies with 99mTc-3PEG4-dimer. The tumor-bearing mice were anesthetized with intraperitoneal injection of Ketamine (40 – 100 mg/kg) and Xylazine (2 – 5 mg/kg). Each animal was administered with 0.5 – 1.0 mCi of 99mTc-3PEG4-dimer. Animals were placed prone on a single head mini γ-camera (Diagnostic Services Inc., NJ) equipped with a parallel-hole, low-energy, and high-resolution collimator. Static images were acquired at 30, 60, 120 and 240 min p.i. and stored digitally in a 128 × 128 matrix. The acquisition count limits were set at 500 K. For the blocking experiment with 99mTc-3PEG4-dimer in the U87MG glioma model, the same procedure was used except that E[c(RGDfK)]2 (~14 mg/kg or ~350 μg per mouse) was co-injected as the blocking agent. After imaging, the mice were euthanized by sodium pentobarbital overdose (100 – 200 mg/kg).
Metabolism
Two normal athymic nude mice were used for the metabolism study of each 99mTc radiotracer. Each mouse was administered with 100 – 120 μCi of the 99mTc radiotracer via tail vein. The urine samples were collected at 30 min and 120 min p.i. by manual void, and were mixed with equal volume of 20% acetonitrile aqueous solution. The mixture was centrifuged at 8,000 rpm. The supernatant was collected and filtered through a 0.20 μ Millex-LG syringe-driven filter unit. The filtrate was analyzed by radio-HPLC (Method 2). The feces samples were collected at 2 h p.i. and suspended in the 20% acetonitrile aqueous solution (2 mL). The resulting mixture was vortexed for 5 – 10 min. After centrifuging at 8,000 rpm for 5 min, the supernatant was collected and passed through a 0.20 μ Millex-LG syringe-driven filter unit to remove any precipitate or particles. The filtrate was analyzed by radio-HPLC (Method 2).
Data and Statistical Analysis
The biodistribution data and target-to-background (T/B) ratios are reported as an average plus the standard variation based on results from four tumor-bearing mice at each time point. Comparison between two different 99mTc radiotracers was made using the two-way ANOVA test (GraphPad Prim 5.0, San Diego, CA). The level of significance was set at p < 0.05.
RESULTS
HYNIC-RGD Conjugate Synthesis
Cyclic RGD peptide conjugates, HYNIC-PEG4-monomer, HYNIC-2PEG4-dimer and HYNIC-3PEG4-dimer were prepared by conjugation of PEG4-monomer, 2PEG4-dimer and 3PEG4-dimer, respectively, with excess HYNIC-NHS in DMF. All new HYNIC conjugates were purified by HPLC (Method 1) and characterized by ESI-MS. The ESI-MS data were completely consistent with their proposed formula. Their HPLC purity of HYNIC conjugates was >95% before being used for 99mTc-labeling and determination of their integrin αvβ3 binding affinity.
Integrin αvβ3 Binding Affinity
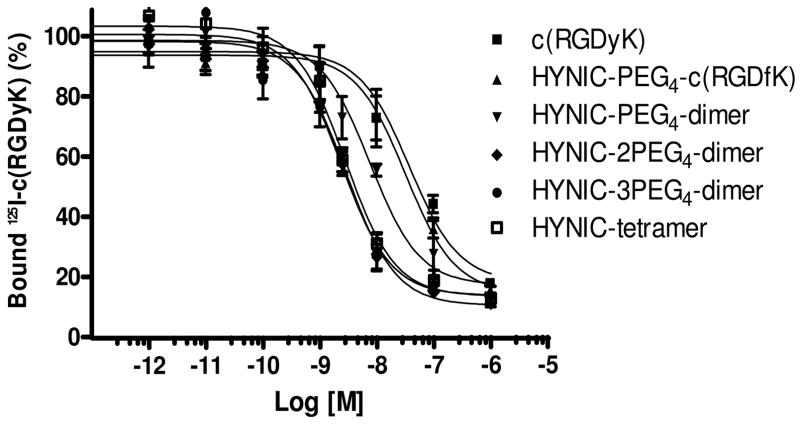
We determined the integrin αvβ3 binding affinity of HYNIC-PEG4-monomer, HYNIC-2PEG4-dimer and HYNIC-3PEG4-dimer by displacement of 125I-c(RGDyK) bound to U87MG human glioma cells. For comparison purposes, we also evaluated c(RGDyK), HYNIC-PEG4-dimer and HYNIC-tetramer in the same assay. IC50 values were calculated to be 37.3 ± 10.1, 31.7 ± 9.7, 7.5 ± 2.3, 2.9 ± 0.7, 2.4 ± 0.7 and 2.8 ± 0.5 nM for c(RGDyK), HYNIC-PEG4-monomer, HYNIC-PEG4-dimer, HYNIC-2PEG4-dimer, HYNIC-3PEG4-dimer and HYNIC-tetramer, respectively.
Radiochemistry
All three new radiotracers (99mTc-PEG4-monomer, 99mTc-2PEG4-dimer and 99mTc-3PEG4-dimer) were prepared in high yield (>95%) using the non-SnCl2 formulation.21,31,67 The specific activity was >5 mCi/μg (or 10 Ci/μmol of a ~2000 Dalton HYNIC-RGD conjugate) for both 99mTc-2PEG4-dimer and 99mTc-3PEG4-dimer. All three radiotracers were stable in the kit matrix for >6 h, which is consistent with the results from our previous studies.31,68–70 The HPLC retention times of 99mTc-PEG4-monomer, 99mTc-2PEG4-dimer and 99mTc-3PEG4-dimer are listed in Table 1. We also determined their partition coefficients (P values) in an equal volume mixture of n-octanol and 25 mM phosphate buffer (pH = 7.4). The log P values for 99mTc-PEG4-monomer, 99mTc-2PEG4-dimer and 99mTc-3PEG4-dimer were calculated to be −4.23 ± 0.21, −4.04 ± 0.15 and −3.96 ± 0.05, respectively. These values were slightly lower than that reported for 99mTc-PEG4-dimer (log P = −3.56 ± 0.16).60
Table 1.
HPLC retention time and log P values for 99mTc-labeled cyclic RGD peptides.
| Compound | RCP (%) | Retention Time (min) | Log P Value |
|---|---|---|---|
| 99mTc-PEG4-monomer | > 97 | 8.81 | −4.23 ± 0.21 |
| 99mTc-PEG4-dimer | > 92 | 12.61 | −3.56 ± 0.16 |
| 99mTc-2PEG4-dimer | > 97 | 15.69 | −4.04 ± 0.15 |
| 99mTc-3PEG4-dimer | > 95 | 22.91 | −3.96 ± 0.05 |
It is interesting to note that 99mTc-PEG4-monomer, 99mTc-2PEG4-dimer and 99mTc-3PEG4-dimer all showed a single radiometric peak in their radio-HPLC chromatograms using the chromatographic conditions described in this study. In ternary ligand complexes [99mTc(HYNIC-BM)(tricine)(TPPTS)] (BM = targeting biomolecule), the Tc chelate is chiral because the tricine coligand is “prochiral”. As a result, complexes [99mTc(HYNIC-BM)(tricine)(TPPTS)] are often formed as a 50%:50% mixture of two diastereomers if BM has one or more chiral centers in its backbone.65,68,70 Attempts to separate these two diastereomers in 99mTc-PEG4-monomer, 99mTc-2PEG4-dimer and 99mTc-3PEG4-dimer by using different reversed-phase HPLC columns, buffering agents, mobile phase gradients and flow rates were not successful. Apparently, the presence of PEG4 groups made separation of these two diastereomers much more difficult.
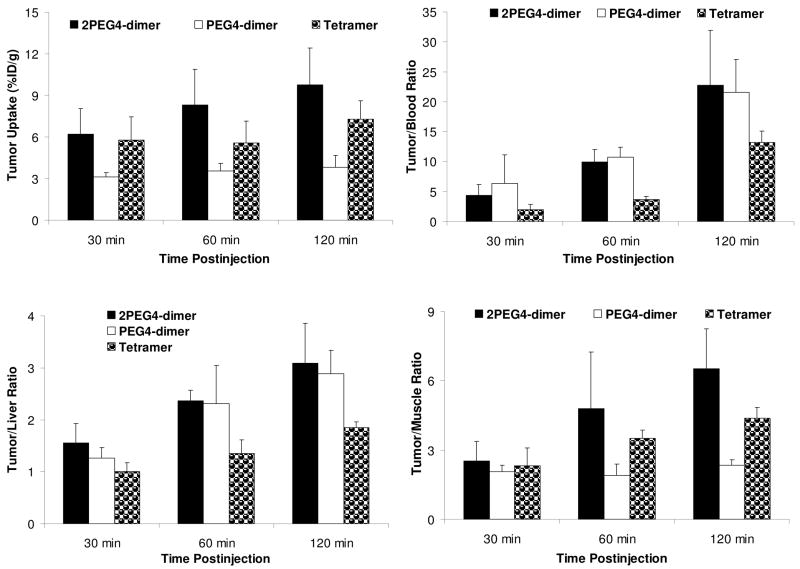
Biodistribution Characteristics in Breast Cancer Model
The athymic nude mice bearing MDA-MB-435 human breast cancer xenografts were used to study the biodistribution characteristics and excretion kinetics of 99mTc-2PEG4-dimer. For comparison purposes, we evaluated 99mTc-PEG4-dimer in the same animal model with very similar tumor size (0.1 – 0.5 g). Figure 4 compares the %ID/g tumor uptake and selected T/B ratios of 99mTc-PEG4-dimer, 99mTc-2PEG4-dimer and 99mTc-tetramer. Detailed biodistribution data for 99mTc-2PEG4-dimer and 99mTc-PEG4-dimer are listed in Tables SI and SII, respectively. The biodistribution data and T/B ratios of 99mTc-tetramer in the same tumor-bearing animal model were obtained from our previous report.53
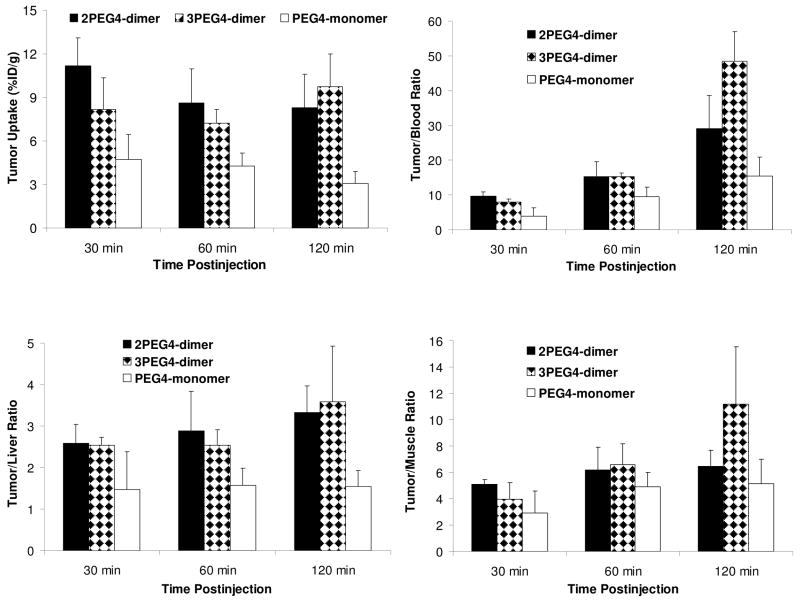
Figure 4.
Direct comparison of tumor uptake (%Id/g) and T/B ratios between 99mTc-2PEG4-dimer, 99mTc-PEG4-dimer and 99mTc-tetramer in athymic nude mice (n = 4) bearing MDA-MB-435 breast cancer xenografts.
The tumor uptake of 99mTc-2PEG4-dimer was 5.20 ± 1.74 %ID/g at 30 min p.i. and 9.77 ± 2.66 %ID/g at 120 min p.i., which was well comparable to that of 99mTc-tetramer (5.78 ± 0.67 %ID/g at 30 min p.i. and 7.30 ± 1.32 %ID/g at 120 min p.i.) within the experimental error. In contrast, the tumor uptake of 99mTc-PEG4-dimer was only 3.49 ± 0.62 %ID/g at 30 min p.i. and 3.82 ± 0.54 %ID/g at 120 min p.i. in the same model. 99mTc-2PEG4-dimer had a liver uptake of 2.97 ± 0.63 %ID/g at 30 min p.i. and 3.27 ± 1.09 %ID/g at 120 min p.i. The liver uptake of 99mTc-tetramer was 4.76 ± 0.72 %ID/g at 30 min p.i., and 4.09 ± 0.59 %ID/g at 120 min p.i.53 The kidney uptake of 99mTc-2PEG4-dimer (14.37 ± 4.09 %ID/g and 13.78 ± 5.36 % ID/g at 30 and 120 min p.i., respectively) was only half of that for 99mTc-tetramer (32.84 ± 4.27 %ID/g at 30 min p.i. and 25.93 ± 2.52 %ID/g at 120 min p.i.). The muscle uptake of 99mTc-2PEG4-dimer (1.76 ± 0.56 %ID/g at 30 min p.i. and 1.51 ± 0.35 %ID/g at 120 min p.i.) was slightly lower than that of 99mTc-tetramer (2.41 ± 0.54 %ID/g at 30 min p.i. and 2.09 ± 0.70 %ID/g at 120 min p.i.).53 The tumor/muscle ratio of 99mTc-2PEG4-dimer was 6.52 ± 1.72 at 120 min, which was much better (p < 0.01) than that of 99mTc-PEG4-dimer (2.91 ± 0.60 at 120 min).
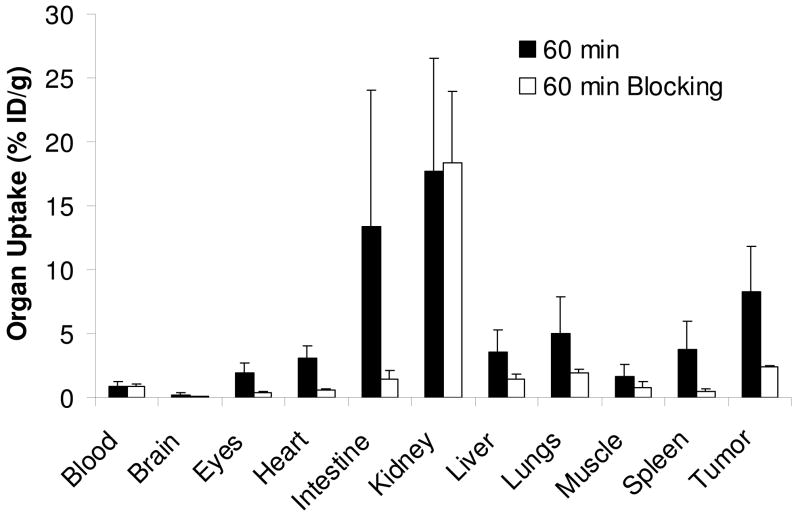
Blocking Experiment
In this experiment, 99mTc-2PEG4-dimer was used as radiotracer and E[c(RGDfK)]2 as the blocking agent (~14 mg/kg or ~350 μg per tumor-bearing mouse). Figure 5 compares the organ uptake of 99mTc-2PEG4-dimer at 60 min p.i. in the absence or presence of excess E[c(RGDfK)]2. Co-injection of excess E[c(RGDfK)]2 resulted in dramatic reduction in the tumor uptake of 99mTc-2PEG4-dimer (2.43 ± 0.07 %ID/g with E[c(RGDfK)]2 vs. 8.30 ± 3.57 %ID/g without E[c(RGDfK)]2). There was also a significant reduction of its uptake in the eyes, heart, intestine, lungs, liver and spleen (Figure 5), however, its uptake in the blood and kidneys in the presence of E[c(RGDfK)]2 was comparable to that obtained without E[c(RGDfK)]2.
Figure 5.
Comparison of organ uptake for 99mTc-2PEG4-dimer in the absence or presence of excess E[c(RGDfK)]2 at 60 min p.i.
Biodistribution Characteristics in Glioma Model
To further confirm our findings in the MDA-MB-435 breast cancer model, we also evaluated the tumor uptake and biodistribution properties of 99mTc-PEG4-monomer, 99mTc-2PEG4-dimer and 99mTc-3PEG4-dimer in athymic nude mice bearing U87MG glioma xenografts. The tumor size was very close (0.1 – 0.5 g) in these two tumor-bearing animal models. These studies were designed to: (1) demonstrate the superiority of 99mTc-2PEG4-dimer and 99mTc-3PEG4-dimer over 99mTc-PEG4-monomer; (2) assess the impact of an extra PEG4 group on radiotracer excretion kinetics in kidneys, liver and lungs; and (3) compare the tumor uptake of 99mTc-2PEG4-dimer in the xenografted glioma and breast tumors. Figure 6 compares their tumor uptake and selected T/B ratios. Detailed biodistribution data for 99mTc-2PEG4-dimer, 99mTc-3PEG4-dimer and 99mTc-PEG4-monomer are summarized in Tables SIII – SV.
Figure 6.
Comparison of the tumor uptake (%ID/g) and T/B ratios between 99mTc-PEG4-monomer, 99mTc-2PEG4-dimer and 99mTc-3PEG4-dimer in athymic nude mice (n = 4) bearing the U87MG glioma xenografts.
The normal organ uptake of 99mTc-2PEG4-dimer in the U87MG glioma-bearing mice was almost identical to that in mice bearing MDA-MB-435 breast cancer xenografts. Its tumor uptake was 11.17 ± 1.96 %ID/g and 8.31 ± 2.31 %ID/g at 30 and 120 min p.i., respectively. 99mTc-3PEG4-dimer also had a high tumor uptake (8.17 ± 0.68 %ID/g and 9.74 ± 3.22 %ID/g at 30 and 120 min p.i., respectively) with very fast blood clearance (1.02 ± 0.22 %ID/g at 30 min p.i. and 0.17 ± 0.02 %ID/g at 120 min p.i.). Its kidney uptake was 17.60 ± 0.73 %ID/g at 30 min p.i. and 9.89 ± 1.20 % ID/g at 120 min p.i. Its liver uptake was 3.14 ± 0.18 %ID/g and 2.92 ± 0.77 %ID/g at 30 and 120 min p.i., respectively. At 30 min p.i., its muscle uptake (1.45 ± 0.20 %ID/g) was lower than that of 99mTc-2PEG4-dimer (2.18 ± 0.37 %ID/g); but this difference diminished (1.00 ± 0.22 %ID/g for 99mTc-3PEG4-dimer and 1.27 ± 0.20 %ID/g for 99mTc-2PEG4-dimer) at 120 min p.i. The tumor/liver ratios of 99mTc-2PEG4-dimer and 99mTc-3PEG4-dimer were almost identical (Figure 6), but 99mTc-3PEG4-dimer had better (p < 0.01) tumor/blood and tumor/muscle ratios than those of 99mTc-2PEG4-dimer at 120 min p.i. The tumor uptake of 99mTc-PEG4-monomer was 4.74 ± 2.71 %ID/g and 3.08 ± 0.83 %ID/g at 30 and 120 min p.i., respectively, and was much lower than that of 99mTc-2PEG4-dimer and 99mTc-3PEG4-dimer (Figure 6). As a result, its tumor/blood (3.85 ± 2.47 at 30 min p.i. and 15.50 ± 5.47 at 120 min p.i.) and tumor/liver (1.47 ± 0.91 at 30 min p.i. and 1.55 ± 0.38 at 120 min p.i.) ratios were lower (p < 0.01) than those of 99mTc-2PEG4-dimer (tumor/blood = 9.63 ± 1.32 at 30 min p.i. and 29.12 ± 8.54 at 120 min p.i.; and tumor/liver = 2.59 ± 0.45 at 30 min p.i. and 3.33 ± 0.64 at 120 min p.i.) and 99mTc-3PEG4-dimer (tumor/blood = 6.72 ± 2.88 at 30 min p.i. and 48.84 ± 18.74 at 120 min p.i.; and tumor/liver = 2.13 ± 0.87 at 30 min p.i. and 3.49 ± 1.64 at 120 min p.i.). The kidney uptake of 99mTc-PEG4-monomer (10.78± 1.82 %ID/g at 30 min p.i. and 4.04 ± 0.69 % ID/g at 120 min p.i., respectively) was only half of that of 99mTc-2PEG4-dimer (20.81 ± 2.32 %ID/g at 30 min p.i. and 10.21 ± 1.37 %ID/g at 120 min p.i.) and 99mTc-3PEG4-dimer (17.60 ± 0.73 %ID/g at 30 min p.i. and 9.89 ± 1.20 %ID/g at 120 min p.i.). The muscle uptake of 99mTc-PEG4-monomer (1.65 ± 0.25 %ID/g at 30 min p.i. and 0.62 ± 0.15 %ID/g at 120 min p.i.) was favorably compared to that of 99mTc-3PEG4-dimer (1.45 ± 0.20 %ID/g at 30 min p.i. and 0.89 ± 0.10 %ID/g at 120 min p.i.) and within the experimental error.
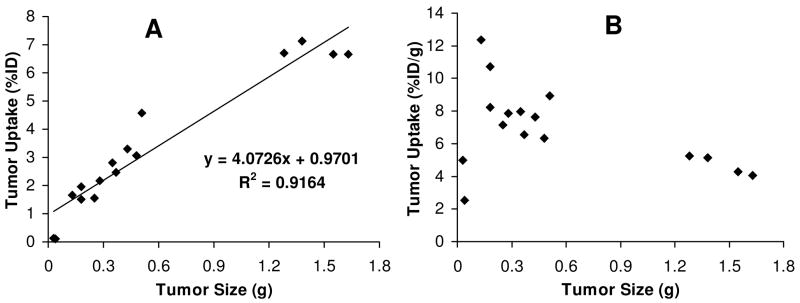
Effect of Tumor Size
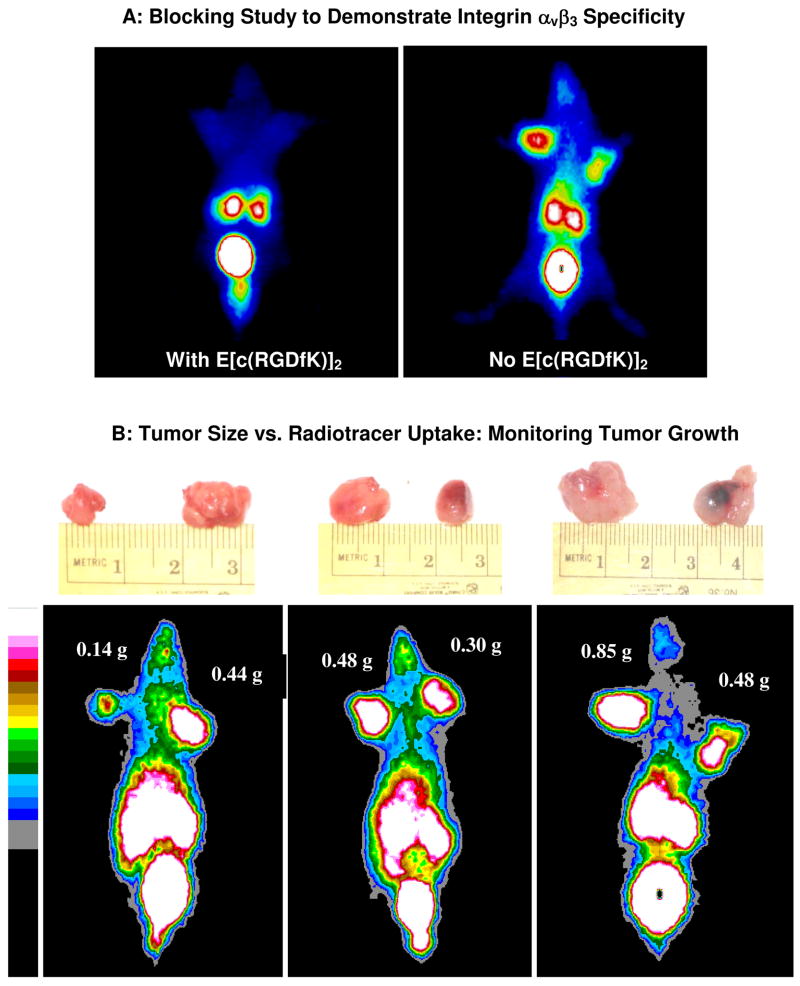
During biodistribution studies, we noticed that there is often a large variation in tumor size even if the same number of U87MG glioma cells were used for the same animal. In our previous report,62 we found that smaller tumors (< 0.5 g) had higher radiotracer uptake than large tumors regardless of the identity of radiotracers. To further clarify the relationship between tumor uptake and tumor size, we added four extra glioma-bearing mice (tumor size = 0.4 – 1.7 g) into the 120 min group of 99mTc-3PEG4-dimer. We found a linear relationship (Figure 5A) between the tumor size and %ID tumor uptake of 99mTc-3PEG4-dimer with R2 = 0.9164 for the eight tumor-bearing mice. As the tumor size increased, its %ID tumor uptake also increased in a linear fashion. If the tumor uptake is expressed as %/ID/g (Figure 5B), it appears that 99mTc-3PEG4-dimer had a narrow window to achieve the optimal tumor uptake. When the tumor size was in the range of 0.1 g – 0.5 g (100 – 500 mm3), its tumor uptake was 6.0 – 12.5 %ID/g. When the tumor size was too big (0.5 g – 1.6 g or 500 – 1700 mm3), the tumor uptake was in the range of 4.0 – 6.0 %ID/g. When the tumor size was too small (<0.05 g or <50 mm3), the tumor uptake of 99mTc-3PEG4-dimer was only 2.0 – 4.0 %ID/g.
Metabolic Properties
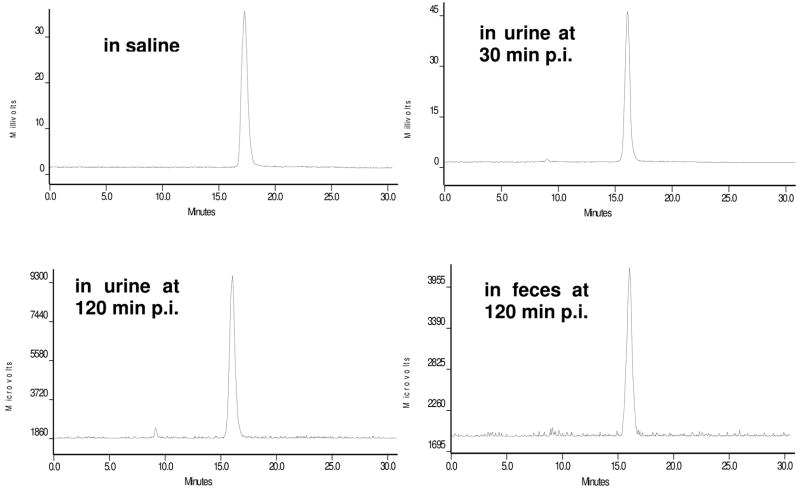
Normal athymic nude mice were used to examine the metabolic stability of 99mTc-PEG4-monomer, 99mTc-2PEG4-dimer and 99mTc-3PEG4-dimer. Figure 8 shows typical radio-HPLC chromatograms of 99mTc-2PEG4-dimer in saline (A), in urine at 30 min p.i. (B) and 120 min p.i. (C), and in feces at 120 min p.i. (D). There was very little metabolite detected in either urine or feces over the 2 h period. 99mTc-2PEG4-dimer remained intact during its excretion from both renal and hepatobiliary routes. A similar metabolic stability was observed for the 99mTc-PEG4-monomer (Figure SI). However, the 99mTc-3PEG4-dimer had ~30% metabolism during its excretion from the hepatobiliary route, whereas it remained intact during its excretion from the renal route (Figure SII).
Figure 8.
Radio-HPLC chromatograms of 99mTc-2PEG4-dimer in saline before injection, in urine at 30 min and 120 min p.i., and in feces at 120 min p.i. Two normal mice were used and each was administered with ~ 100 μCi of 99mTc-2PEG4-dimer.
Planar Imaging
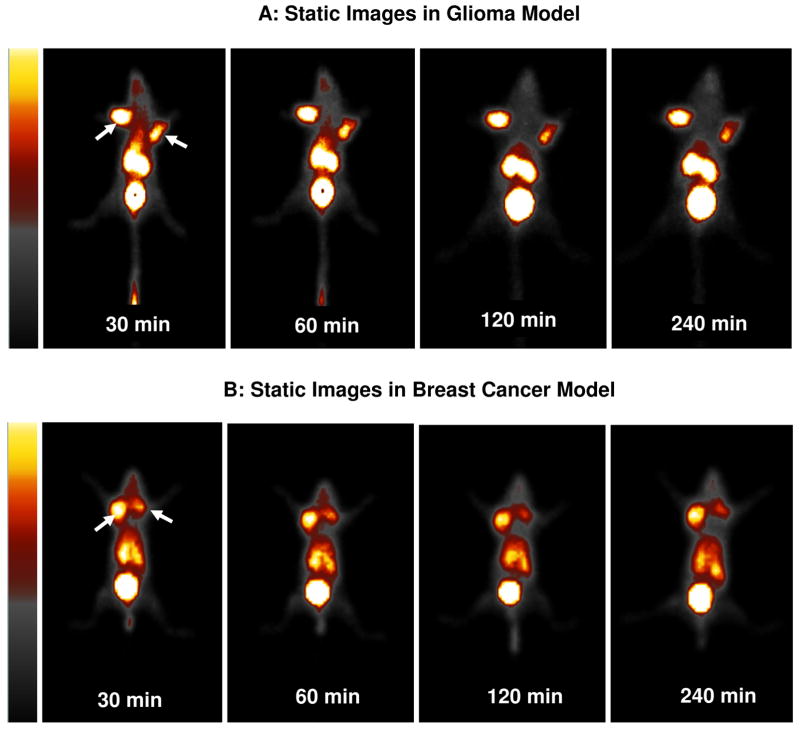
Figure 9 illustrates static images of the tumor-bearing mice administered with 99mTc-3PEG4-dimer at 30, 60, 120 and 240 min p.i. The glioma tumors were clearly visualized as early as 15 min p.i. with excellent contrast. No significant radioactivity accumulation was detected in the heart, liver and lungs. At >120 min p.i., the most visible organs are tumors, kidneys and bladder. The breast tumors with a similar size were also clearly visualized with high T/B contrast. The tumor uptake of 99mTc-3PEG4-dimer was significantly blocked (Figure 10A) by co-injection of excess E[c(RGDfK)]2, suggesting that its tumor localization was indeed integrin αvβ3–specific. Figure 10B compares the 60 min static images of the athymic nude mice bearing U87MG glioma xenografts (tumor size ranging from 0.14 g to 0.85 g or 5 mm – 10 mm in diameter) administered with 99mTc-3PEG4-dimer. It was found that the tumors of ~5 mm in diameter could be readily visualized as soon as 99mTc-3PEG4-dimer was injected. Larger tumors (>350 mm3 or >7 mm in diameter) had much better visualization than the smaller ones (<100 mm3 or <5 mm in diameter) even though the %ID/g tumor uptake in larger tumors (400 – 1000 mm3) was lower than those smaller ones (100 – 300 mm3).
Figure 9.
Representative static images of the tumor-bearing mice (top: U87MG glioma xenografts; bottom: MDA-MB-435 breast cancer xenografts) injected with 500 – 800 μCi of 99mTc-3PEG4-dimer and at 30, 60, 120 and 240 min p.i. Arrows indicate the presence of tumors.
Figure 10.
Panel A: The 60 min static images of 99mTc-3PEG4-dimer in the absence/presence of E[c(RGDfK)]2 at 60 min p.i. to demonstrate its integrin αvβ3 specificity. Co-injection of excess E[c(RGDfK)]2 resulted in significant reduction in the uptake of 99mTc-3PEG4-dimer in the tumor and organs in the abdominal area. Panel B: The 60 min static images of 99mTc-3PEG4-dimer in the athymic nude mice with different glioma tumor sizes (0.14 g – 0.85 g or 140 – 850 mm3).
DISCUSSION
Many multimeric cyclic RGD peptides have been successfully used to develop the integrin αvβ3-targeted radiotracers.31–36,49–62 For example, Liu et al have been using cyclic RGD dimers, such as E[c(RGDfK)]2, to develop diagnostic and therapeutic radiotracers.31,33–35 Chen and coworkers have been using E[c(RGDyK)]2 and E{E[c(RGDyK)]2}2 to prepare the integrin αvβ3-targeted 64Cu PET radiotracers.51,56,71 Kessler et al reported the HEG (hexaethylene glycolic acid) and poly(lysine)-tethered cyclic RGDfE dimers and tetramers with integrin αvβ3 binding affinity better than their monomeric analogs.49,50 HEG or poly(lysine) linkers were used to increase the distance between the adjacent cyclic RGDfE motifs. In this study, we determined the integrin αvβ3 binding affinities of HYNIC-2PEG4-dimer and HYNIC-3PEG4-dimer by competitive displacement of 125I-c(RGDyK) bound to U87MG glioma cells (Figure 3). We found that the integrin αvβ3 binding affinity of HYNIC conjugates follows the order of HYNIC-2PEG4-dimer ~ HYNIC-3PEG4-dimer ~ HYNIC-tetramer > HYNIC-PEG4-dimer > HYNIC-PEG4-monomer ~ c(RGDyK). The addition of PEG4 linkers between two cyclic RGD motifs in HYNIC-2PEG4-dimer and HYNIC-3PEG4-dimer significantly increased their integrin αvβ3 binding affinity. However, the addition of an extra PEG4 linker between HYNIC and 2PEG4-dimer had little impact on the integrin αvβ3 binding affinity. It is very important to note that the IC50 value depends largely on the radioligand (125I-c(RGDyK) vs. 125I-echistatin) and tumor cell lines (U87MG vs. MDA-MB-435) used in the competitive displacement assay. Caution should be taken when comparing the IC50 values of cyclic RGD peptides with those reported in the literature.
Figure 3.
In vitro inhibition curves of 125I-c(RGDyK) bound to U87MG glioma cells by c(RGDyK), HYNIC-PEG4-monomer, HYNIC-PEG4-dimer, HYNIC-2PEG4-dimer, HYNIC-3PEG4-dimer and HYNIC-tetramer. Their IC50 values were calculated to be 37 ± 5 nM, 32 ± 2 nM, 7.5 ± 0.5 nM, 2.9 ± 0.3 nM, 2.4 ± 0.3 nM and 2.8 ± 0.4 nM, respectively.
The distance between the two RGD motifs in 2PEG4-dimer is 38 bonds, whereas the longest distance between RGD motifs in E[c(RGDfK)]2 is only 6 bonds (excluding side arms of K-residues). The lower integrin αvβ3 binding affinity of HYNIC-PEG4-dimer (IC50 = 7.5 ± 2.3 nM) than that of HYNIC-2PEG4-dimer (IC50 = 2.9 ± 0.7 nM) strongly suggests that the distance between two cyclic RGD motifs in E[c(RGDfK)]2 is too short for simultaneous integrin αvβ3 binding. Thus, the higher integrin αvβ3 binding affinity of HYNIC-PEG4-dimer (IC50 = 7.5 ± 2.3 nM) than that of HYNIC-PEG4-monomer (IC50 = 31.7 ± 9.7 nM) is caused by the “enhanced local RGD concentration” (Figure 1B). This might also explain the higher tumor uptake for the radiolabeled (18F, 99mTc, 111In and 64Cu) cyclic RGD dimers (E[c(RGDfK)]2 and E[c(RGDyK)]2) as compared to their monomeric analogs.51,52,54–62,72
The longest distance between adjacent cyclic RGD motifs in the tetramer E[E[c(RGDfK)]2]2 is 16 bonds (excluding side arms of K-residues). HYNIC-tetramer has an integrin αvβ3 binding affinity (IC50 = 2.8 ± 0.5 nM) almost identical to those of HYNIC-2PEG4-dimer (IC50 = 2.9 ± 0.7 nM) and HYNIC-3PEG4-dimer (IC50 = 2.4 ± 0.7 nM). However, it is not clear if its higher binding affinity than that of HYNIC-PEG4-dimer (IC50 = 7.5 ± 2.3 nM) is caused by the simultaneous integrin αvβ3 binding (Figure 1A) of two adjacent cyclic RGD motifs or is simply due to the presence of four cyclic RGD motifs in E[E[c(RGDfK)]2]2. It must be noted that the ability of a multimeric RGD peptide to achieve simultaneous integrin αvβ3 binding depends largely on integrin αvβ3 density. If the density is high, the distance between two neighboring integrin αvβ3 sites will be short enough so that it is much easier for the multimeric RGD peptide to achieve simultaneous integrin αvβ3 binding. If the integrin αvβ3 density is low, the distance between two neighboring integrin αvβ3 sites will be long, and it might be more difficult for the same multimeric RGD peptide to achieve simultaneous integrin αvβ3 binding.
The integrin αvβ3 binding affinity difference between HYNIC-2PEG4-dimer and HYNIC-PEG4-dimer is also reflected by the tumor uptake of their corresponding ternary ligand 99mTc complexes, 99mTc-2PEG4-dimer and 99mTc-PEG4-dimer. The fact that 99mTc-2PEG4-dimer has twice the tumor uptake as 99mTc-PEG4-dimer (Figure 4) in the same animal model with similar tumor size (100 – 500 m3) provides direct in vivo evidence to support our conclusion that the two cyclic RGD motifs in 2PEG4-dimer are indeed able to achieve simultaneous integrin αvβ3 binding while PEG4-dimer is “monodentate” due to the short distance between its two cyclic RGD motifs. If they were bound to integrin αvβ3 in the same fashion, 99mTc-2PEG4-dimer and 99mTc-PEG4-dimer would have had similar tumor uptake in the same tumor-bearing animal model. Therefore, 2PEG4-dimer is a better targeting biomolecule than PEG4-dimer for future development of integrin αvβ3-targeted radiotracers. Since 99mTc-tetramer has the tumor uptake very close to that of 99mTc-2PEG4-dimer (Figure 4) in the same tumor-bearing animal model, one might suggest that the higher tumor uptake of 99mTc-tetramer as compared to that of 99mTc-PEG4-dimer is caused by the simultaneous integrin αvβ3 binding of two adjacent cyclic RGDfK motifs in E{E[c(RGDfK)]2}2. Alternatively, it is also reasonable to believe that the higher tumor uptake of 99mTc-tetramer than that of 99mTc-PEG4-dimer is due to the presence of four cyclic RGD motifs in E[E[c(RGDfK)]2]2.
Both 99mTc-2PEG4-dimer and 99mTc-3PEG4-dimer have advantages over 99mTc-tetramer with respect to their accumulation in non-cancerous organs. For example, the uptake of 99mTc-2PEG4-dimer and 99mTc-3PEG4-dimer in kidneys and liver was much lower (p < 0.01) than that of 99mTc-tetramer (Figure 4). As a result, their tumor/liver and tumor/kidney ratios are significantly better than those for 99mTc-tetramer. The higher kidney uptake of 99mTc-tetramer is probably caused by the presence of more R-residues in E{E[c(RGDfK)]2}2. In addition, the cost for the synthesis of E{E[c(RGDfK)]2}2 is much higher than both 2PEG4-dimer and 3PEG4-dimer. On the basis of these facts, we strongly believe that 2PEG4-dimer and 3PEG4-dimer are better targeting biomolecules than E{E[c(RGDfK)]2}2 for the future development of integrin αvβ3-targeted radiotracers.
The tumor uptake of 99mTc-2PEG4-dimer is partially blocked (Figure 5) by co-injection of excess E[c(RGDfK)]2, indicating that the tumor uptake of 99mTc-2PEG4-dimer is integrin αvβ3-mediated. The uptake blockage in the eyes, heart, intestine, lungs, liver and spleen suggests that the accumulation of 99mTc-2PEG4-dimer in these organs is also integrin αvβ3-mediated. This conclusion is supported by the immunohistopathological studies, which showed a strong positive staining of endothelial cells of small glomeruli vessels in the kidneys and weak staining in branches of the hepatic portal vein.71,72
The glioma uptake of 99mTc-2PEG4-dimer (11.17 ± 1.96 %ID/g) is higher (p < 0.01) than that in breast tumor (5.20 ± 1.74 %ID/g) at 30 min p.i., which is consistent with the fact that U87MG glioma cells have a higher integrin αvβ3 expression than MDA-MB-435 breast tumor cells.69,70 However, this difference disappears at 120 min p.i. (8.31 ± 2.31 %ID/g in glioma and 9.77 ± 2.66 %ID/g in breast tumor). The glioma uptake of 99mTc-3PEG4-dimer is comparable to that of 99mTc-2PEG4-dimer. The extra PEG4 linker in 3PEG4-dimer does not significantly change the tumor uptake, which is consistent with the similar integrin αvβ3 binding affinity for HYNIC-2PEG4-dimer (IC50 = 2.9 ± 0.7 nM) and HYNIC-3PEG4-dimer (IC50 = 2.4 ± 0.7 nM). The glioma uptake of 99mTc-3PEG4-dimer is almost 3× better than that for 99mTc-PEG4-monomer over the 2 h study period (Figure 6), further demonstrating the superiority of 3PEG4-dimer over PEG4-monomer as the integrin αvβ3-targeting biomolecules.
Non-invasive imaging of molecular markers, such as integrin αvβ3, is highly desirable for patient selection before anti-angiogenic treatment as it can provide more effective monitoring of therapeutic efficacy in the integrin αvβ3-positive cancer patients.73 The %ID tumor uptake reflects the integrin αvβ3 expression level on tumor cells and tumor neovasculature while the %ID/g tumor uptake reflects the integrin αvβ3 density. When tumors are <0.05 g (~50 mm3), there is little integrin αvβ3 expression. As a result, the tumor uptake (%ID and %ID/g) is low. When tumors are in the rapid growing stage (0.1 – 0.5 g or 100 – 500 mm3), the microvessel and integrin αvβ3 density is higher. The radiotracer %ID/g tumor uptake is higher (Figure 5B) even though its %ID tumor uptake is low (Figure 5A). As tumors grow, the total number of integrin αvβ3 sites on tumor cells and tumor neovasculature becomes larger. As a result, the %ID tumor uptake increases (Figure 5A). However, the microvessel density decreases due to the maturation of blood vessels, and the integrin αvβ3 density also decreases due to larger interstitial space and higher collagen concentrations.74 In addition, parts of the tumor may become necrotic, leading to the lower integrin αvβ3 density and the low radiotracer %ID/g uptake in larger tumors (Figure 5B).
Since tumors must have sufficient radioactivity counts to be detectable by planar or SPECT imaging, the total %ID tumor uptake is critically important for early tumor detection and noninvasive monitoring of the tumor growth or shrinkage during anti-angiogenic treatment in cancer patients. In this study, we found that tumors of ~5 mm in diameter are readily visualized with excellent contrast as early as 30 min p.i. At 60 min p.i., the only organs visible are tumors, kidneys and bladder of the tumor-bearing mouse (Figure 10B). Larger tumors have much better visualization than the smaller ones even though the %ID/g tumor uptake in larger tumors might be lower than that in smaller ones (Figure 8). The tumor detection limit is about 5 mm in diameter with 99mTc-3PEG4-dimer as the radiotracer. These data clearly show that 99mTc-3PEG4-dimer is very useful for early detection of the integrin αvβ3–positive tumors and has potential applications for noninvasive monitoring of the tumor growth or shrinkage during anti-angiogenic treatment.
Extensive metabolic degradation was observed for the 99mTc-labeled cyclic RGD monomer,75 dimer,58,60 tetramer,53,54 and the 64Cu-labeled cyclic RGD tetramer in the kidneys and urine samples.56 In this study, we found that 99mTc-2PEG4-dimer has very high metabolic stability during its excretion from both renal and hepatobiliary routes (Figure 6). Similar metabolic stability is also observed for 99mTc-PEG4-monomer (Figure SI). In contrast, 99mTc-3PEG4-dimer has ~30% metabolism during its excretion from the hepatobiliary route (Figure SII). The metabolic stability difference between 99mTc-2PEG4-dimer and 99mTc-3PEG4-dimer is due to the extra PEG4 in 3PEG4-dimer. It remains unclear how the extra PEG4 linker influences metabolic stability of the 99mTc-labeled cyclic RGD peptide dimers.
CONCLUSION
In this study, we evaluated two new radiotracers, 99mTc-2PEG4-dimer and 99mTc-3PEG4-dimer, for non-invasive imaging of tumor integrin αvβ3 expression in athymic nude mice bearing U87MG glioma and MDA-MB-435 breast cancer xenografts. We found that (1) the tumor-targeting capability could be significantly enhanced by addition of two PEG4 linkers between two cyclic RGD motifs, which allow them to achieve simultaneous integrin αvβ3 binding; (2) PEG4 linkers are particularly useful for improving the clearance kinetics of 99mTc-2PEG4-dimer and 99mTc-3PEG4-dimer from non-cancerous organs, such as the kidneys, liver and lungs; and (3) the %ID tumor uptake is correlated well with the tumor size in a linear fashion. 99mTc-3PEG4-dimer has the best profile with respect to tumor uptake, T/B ratios and pharmacokinetics among the 99mTc-labeled cyclic RGD peptide dimers and tetramers evaluated in the xenografted U87MG glioma and MDA-MB-435 breast cancer animal models. In addition, 99mTc-3PEG4-dimer can be readily prepared in high yield and radiochemical purity (RCP >95%) with very high specific activity (>5 mCi/μg or 10 Ci/μmol) from a single-vial kit formulation. 99mTc-3PEG4-dimer has significant advantages over the 18F-labeled cyclic RGD peptides with respect to cost, availability and easiness of routine clinical preparation. All these factors make 99mTc-3PEG4-dimer a very attractive SPECT radiotracer for the early detection of integrin αvβ3–positive tumors and for noninvasive monitoring of tumor growth or treatment efficacy in clinical settings.
Supplementary Material
Detailed biodistribution data and T/B ratios for 99mTc-labeled cyclic RGD peptide dimers are listed in Tables SI – SV. Figure SI illustrates representative radio-HPLC chromatograms of 99mTc-PEG4-monomer in saline before injection (A), in urine at 30 min p.i. (B), in urine at 120 min p.i. (C), and in feces at 120 min p.i. (D). Figure SII shows typical radio-HPLC chromatograms of 99mTc-3PEG4-dimer in saline before injection (A), in urine at 30 min p.i. (B), in urine at 120 min p.i. (C), and in feces at 120 min p.i. (D). This information is available free of charge via Internet at http://pubs.acs.org.
Figure 7.
The relationship between tumor size and tumor uptake of 99mTc-3PEG4-dimer at 120 min p.i. in the athymic nude mice (n = 16) bearing the U87MG glioma xenografts.
Acknowledgments
The authors would like to thank Dr. Sulma I. Mohammed, the Director of Purdue Cancer Center Drug Discovery Shared Resource, Purdue University, for her assistance with the tumor-bearing animal model. This work is supported, in part, by Purdue University and research grants: R01 CA115883 A2 (S.L.) from the National Cancer Institute (NCI), R21 EB003419-02 (S.L.) from the National Institute of Biomedical Imaging and Bioengineering (NIBIB), R21 HL083961-01 (S.L.) from the National Heart, Lung, and Blood Institute (NHLBI), DE-FG02-08ER64684 (S.L.) from the Department of Energy, and R01 CA119053 (X.C.) from NCI.
References
- 1.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 2.Mousa SA. Mechanism of angiogenesis in vascular disorders: potential therapeutic targets. Drugs of the Future. 1998;23:51–60. [Google Scholar]
- 3.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 4.Meitar D, Crawford SE, Rademaker AW, Cohn SL. Tumor angiogenesis correlates with metastatic disease, N-myc-amplification, and poor outcome in human neuroblastoma. J Clinical Oncol. 1996;14:405–414. doi: 10.1200/JCO.1996.14.2.405. [DOI] [PubMed] [Google Scholar]
- 5.Gasparini G, Brooks PC, Biganzoli E, Vermeulen PB, Bonoldi E, Dirix LY, Ranieri G, Miceli R, Cheresh DA. Vascular integrin αvβ3: a new prognostic indicator in breast cancer. Clinical Can Res. 1998;4:2625–2634. [PubMed] [Google Scholar]
- 6.Falcioni R, Cimino L, Gentileschi MP, D’Agnano I, Zupi G, Kennel SJ, Sacchi A. Expression of beta 1, beta 3, beta 4, and beta 5 integrins by human lung carcinoma cells of different histotypes. Exp Cell Res. 1994;210:113–122. doi: 10.1006/excr.1994.1017. [DOI] [PubMed] [Google Scholar]
- 7.Sengupta S, Chattopadhyay N, Mitra A, Ray S, Dasgupta S, Chatterjee A. Role of αvβ3 integrin receptors in breast tumor. J Exp Clin Cancer Res. 2001;20:585–590. [PubMed] [Google Scholar]
- 8.Zitzmann S, Ehemann V, Schwab M. Arginine-Glycine-Aspartic acid (RGD)-peptide binds to both tumor and tumor endothelial cells in vivo. Cancer Res. 2002;62:5139–5143. [PubMed] [Google Scholar]
- 9.Weber WA, Haubner R, Vabuliene E, Kuhnast B, Webster HJ, Schwaiger M. Tumor angiogenesis targeting using imaging agents. Q J Nucl Med. 2001;45:179–182. [PubMed] [Google Scholar]
- 10.Costouros NG, Diehn FE, Libutti SK. Molecular imaging of tumor angiogenesis. J Cell Biol Suppl. 2002;39:72–78. doi: 10.1002/jcb.10426. [DOI] [PubMed] [Google Scholar]
- 11.Liu S, Edwards DS. Fundamentals of receptor-based diagnostic metalloradiopharmaceuticals. Topics in Current Chem. 2002;222:259–278. [Google Scholar]
- 12.Van de Wiele C, Oltenfreiter R, De Winter O, Signore A, Slegers G, Dieckx RA. Tumor angiogenesis pathways: related clinical issues and implications for nuclear medicine imaging. Eur J Nucl Med Mol Imag. 2002;29:699–709. doi: 10.1007/s00259-002-0783-8. [DOI] [PubMed] [Google Scholar]
- 13.van Hinsbergh VWM, Collen A, Koolwijk P. Angiogenesis and antiangiogenesis: perspectives for the treatment of solid tumors. Ann Oncol. 1999;4:S60–S63. [PubMed] [Google Scholar]
- 14.Brower V. Tumor angiogenesis-new drug on the block. Nature Biol. 1999;17:963–968. doi: 10.1038/13654. [DOI] [PubMed] [Google Scholar]
- 15.Brooks PC, Montgomery AMP, Rosenfeld M, Reisenfeld R, Hu T, Klier G, Cheresh DA. Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 16.Giannis A, Rübsam F. Integrin antagonists and other low molecular weight compounds as inhibitors of angiogenesis: new drugs in cancer therapy. Angew Chem Int Ed Engl. 1997;36:588–590. [Google Scholar]
- 17.Haubner R, Finsinger D, Kessler H. Stereoisomeric peptide libraries and peptidomimetics for designing selective inhibitors of the αvβ3 integrin for a new cancer therapy. Angew Chem Int Ed Engl. 1997;36:1374–1389. [Google Scholar]
- 18.Gottschalk KE, Kessler H. The structure of integrins and integrin-ligand complexes: Implications for drug design and signal transduction. Angew Chem Int Ed Engl. 2002;41:1374–1389. doi: 10.1002/1521-3773(20021018)41:20<3767::AID-ANIE3767>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 19.Burke PA, DeNardo SJ. Antiangiogenic agents and their promising potential for combined therapy. Cancer Res. 2001;48:7022–7032. doi: 10.1016/s1040-8428(01)00115-9. [DOI] [PubMed] [Google Scholar]
- 20.Qiao R, Yan W, Lum H, Malik AB. Arg-Gly-Asp peptide increases endothelial hydraulic conductivity: comparison with thrombin response. Am J Physiol. 1995;268:C110–C117. doi: 10.1152/ajpcell.1995.269.1.C110. [DOI] [PubMed] [Google Scholar]
- 21.Huabner R, Wester HJ, Senekowitsch-Schmidtke R, Diefenbach B, Kessler H, Stöcklin G, Schwaiger M. RGD-peptides for tumor targeting: biological evaluation of radioiodinated analogs and introduction of a novel glycosylated peptide with improved biokinetics. J Labelled Compounds & Radiopharmaceuticals. 1997;40:383–385. [Google Scholar]
- 22.Sivolapenko GB, Skarlos D, Pectasides D, Stathopoulou E, Milonakis A, Sirmalis G, Stuttle A, Courtenay-Luck NS, Konstantinides K, Epenetos AA. Imaging of metastatic melanoma utilizing a technetium-99m labeled RGD-containing synthetic peptide. Eur J Nucl Med. 1998;25:1383–1389. doi: 10.1007/s002590050312. [DOI] [PubMed] [Google Scholar]
- 23.Haubner R, Wester HJ, Reuning U, Senekowisch-Schmidtke R, Diefenbach B, Kessler H, Stöcklin G, Schaiger M. Radiolabeled αvβ3 integrin antagonists: a new class of tracers for tumor targeting. J Nucl Med. 1999;40:1061–1071. [PubMed] [Google Scholar]
- 24.Haubner R, Wester HJ, Weber WA, Mang C, Ziegler SI, Goodman SL, Senekowisch-Schmidtke R, Kessler H, Schwaiger M. Noninvasive imaging of αvβ3 integrin expression using 18F-labeled RGD-containing glycopeptide and positron emission tomography. Cancer Res. 2001;61:1781–1785. [PubMed] [Google Scholar]
- 25.Haubner R, Wester HJ, Burkhart F, Senekowisch-Schmidtke R, Weber W, Goodman SL, Kessler H, Schwaiger M. Glycosylated RGD-containing peptides: tracer for tumor targeting and angiogenesis imaging with improved biokinetics. J Nucl Med. 2001;42:326–336. [PubMed] [Google Scholar]
- 26.Poethko T, Schottelius M, Thumshirn G, Hersel U, Herz M, Henriksen G, Kessler H, Schaiger M, Wester HJ. Two-step methodology for high yield routine radiohalogenation of peptides: 18F-labeled RGD and octreotide analogs. J Nucl Med. 2004;45:892–902. [PubMed] [Google Scholar]
- 27.Chen X, Park R, Tohme M, Shahinian AH, Bading JR, Conti PS. MicroPET and autoradiographic imaging of breast cancer αv-integrin expression using 18F- and 64Cu-labeled RGD peptide. Bioconj Chem. 2004;15:41–49. doi: 10.1021/bc0300403. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Shahinian A, Park R, Bozorgzadeh MH, Bading JR, Conti PS. 18F-labeled cyclic RGD peptide for PET imaging of tumor angiogenesis. J Nucl Med. 2003;44(5 Suppl):47–48. [Google Scholar]
- 29.Chen X, Park R, Shahinian AH, Tohme M, Khankaldyyan V, Bozorgzadeh MH, Bading JR, Moats R, Laug WE, Conti PS. 18F-labeled RGD peptide: initial evaluation for imaging brain tumor angiogenesis. Nucl Med Biol. 2004;31:179–189. doi: 10.1016/j.nucmedbio.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Chen X, Park R, Shahinian AH, Bading JR, Conti PS. Pharmacokinetics and tumor retention of 125I-labeled RGD peptide are improved by PEGylation. Nucl Med Biol. 2004;31:11–19. doi: 10.1016/j.nucmedbio.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Liu S, Edwards DS, Ziegler MC, Harris AR, Hemingway SJ, Barrett JA. 99mTc-Labeling of a hydrazinonicotinamide-conjugated vitronectin receptor antagonist useful for imaging tumors. Bioconj Chem. 2001;12:624–629. doi: 10.1021/bc010012p. [DOI] [PubMed] [Google Scholar]
- 32.Dijkgraaf I, Rijinders AY, Soede A, Dechesne AC, van Esse GW, Brouwer AJ, Corstens FHM, Boerman OC, Rijkers DTS, Liskamp RMJ. Synthesis of DOTA-conjugated multivalent cyclic-RGD peptide dendrimers via 1,3-dipolar cycloaddition and their biological evaluation: implications for tumor-targeting and tumor imaging purposes. Org Biomol Chem. 2007;5:935–944. doi: 10.1039/b615940k. [DOI] [PubMed] [Google Scholar]
- 33.Liu S, Cheung E, Rajopadhye M, Ziegler MC, Edwards DS. 90Y- and 177Lu-labeling of a DOTA-conjugated vitronectin receptor antagonist for tumor therapy. Bioconj Chem. 2001;12:559–568. doi: 10.1021/bc000146n. [DOI] [PubMed] [Google Scholar]
- 34.Janssen M, Oyen WJG, Massuger LFAG, Frielink C, Dijkgraaf I, Edwards DS, Rajopadyhe M, Corsten FHM, Boerman OC. Comparison of a monomeric and dimeric radiolabeled RGD-peptide for tumor targeting. Cancer Biother & Radiopharm. 2002;17:641–646. doi: 10.1089/108497802320970244. [DOI] [PubMed] [Google Scholar]
- 35.Janssen M, Oyen WJG, Dijkgraaf I, Massuger LFAG, Frielink C, Edwards DS, Rajopadyhe M, Boonstra H, Corsten FH, Boerman OC. Tumor targeting with radiolabeled integrin αvβ3 binding peptides in a nude mouse model. Cancer Res. 2002;62:6146–6151. [PubMed] [Google Scholar]
- 36.Line BR, Mitra A, Nan A, Ghandehari H. Targeting tumor angiogenesis: comparison of peptide and polymer-peptide conjugates. J Nucl Med. 2005;46:1552–1560. [PubMed] [Google Scholar]
- 37.Liu S, Robinson SP, Edwards DS. Integrin αvβ3 directed radiopharmaceuticals for tumor imaging. Drugs of the Future. 2003;28:551–564. [Google Scholar]
- 38.Haubner R, Wester HJ. Radiolabeled tracers for imaging of tumor angiogenesis and evaluation of antiangiogenic therapies. Curr Pharm Design. 2004;10:1439–1455. doi: 10.2174/1381612043384745. [DOI] [PubMed] [Google Scholar]
- 39.D’Andrea LD, Del Gatto A, Pedone C, Benedetti E. Peptide-based molecules in angiogenesis. Chem Biol Drug Desgin. 2006;67:115–126. doi: 10.1111/j.1747-0285.2006.00356.x. [DOI] [PubMed] [Google Scholar]
- 40.Meyer A, Auremheimer J, Modlinger A, Kessler H. Targeting RGD recognizing integrins: drug development biomaterial research tumor, imaging and targeting. Curr Pharm Design. 2006;12:2723–2747. doi: 10.2174/138161206777947740. [DOI] [PubMed] [Google Scholar]
- 41.Chen X. Multimodality imaging of tumor integrin αvβ3 expression. Mini-Rev Med Chem. 2006;6:227–234. doi: 10.2174/138955706775475975. [DOI] [PubMed] [Google Scholar]
- 42.Cai W, Chen X. Multimodality imaging of tumor angiogenesis. J Nucl Med. 2008;49:113S–128S. doi: 10.2967/jnumed.107.045922. [DOI] [PubMed] [Google Scholar]
- 43.Liu S. Radiolabeled multimeric cyclic RGD peptides as integrin αvβ3-targeted radiotracers for tumor imaging. Mol Pharm. 2006;3:472–487. doi: 10.1021/mp060049x. [DOI] [PubMed] [Google Scholar]
- 44.Kenny LM, Coombes RC, Oulie I, Contractor KB, Miller M, Spinks TJ, McParland B, Cohen PS, Hui A, Palmieri C, Osman S, Glaser M, Turton D, Al-Nahhas A, Anoagye EO. Phase I trial of the positron-emitting Arg-Gly-Asp (RGD) peptide radioligand 18F-AH111585 in breast cancer patients. J Nucl Med. 2008;49:879–886. doi: 10.2967/jnumed.107.049452. [DOI] [PubMed] [Google Scholar]
- 45.Beer AJ, Haubner R, Goebel M, Luderschmidt S, Spilker ME, Wester HJ, Weber WA, Schwaiger M. Biodistribution and pharmacokineticss of the αvβ3-selective tracer 18F-Galacto-RGD in cancer patients. J Nucl Med. 2005;46:1333–1341. [PubMed] [Google Scholar]
- 46.Haubner R, Weber WA, Beer AJ, Vabulience E, Reim D, Sarbia M, Becker KF, Goebel M, Hein R, Wester HJ, Kessler H, Schwaiger M. Noninvasive visualization of the activated αvβ3 integrin in cancer patients by positron emission tomography and [18F]Galacto-RGD. PLOS Medicine. 2005;2:e70, 244–252. doi: 10.1371/journal.pmed.0020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boturyn D, Coll JL, Garanger E, Favrot MC, Dumy P. Template assembled cyclopeptides as multimeric system for integrin targeting and endocytosis. J Am Chem Soc. 2004;126:5730–5739. doi: 10.1021/ja049926n. [DOI] [PubMed] [Google Scholar]
- 48.Mammen M, Choi SK, Whitesides GM. Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Angew Chem Int Ed. 1998;37:2755–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 49.Poethko T, Thumshirn G, Hersel U, Rau F, Haubner R, Schwaiger M. Improved tumor uptake, tumor retention and tumor/background ratios of pegylated RGD multimers. J Nucl Med. 2003;44:46P. [Google Scholar]
- 50.Thumshirn G, Hersel U, Goodman SL, Kessler H. Multimeric cyclic RGD peptides as potential tools for tumor targeting: solid-phase peptide synthesis and chemoselective oxime ligation. Chem Eur J. 2003;9:2717–2725. doi: 10.1002/chem.200204304. [DOI] [PubMed] [Google Scholar]
- 51.Chen X, Liu S, Hou Y, Tohme M, Park R, Bading JR, Conti PS. MicroPET imaging of breast cancer integrin αvβ3 expression with 64Cu-labeled dimeric RGD-containing cyclic peptides. Mol Imag Biol. 2004:350–359. doi: 10.1016/j.mibio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 52.Chen X, Tohme M, Park R, Hou Y, Bading JR, Conti PS. MicroPET imaging of breast cancer αv-integrin expression with 18F-labeled dimeric RGD peptide. Mol Imaging. 2005;3:96–104. doi: 10.1162/15353500200404109. [DOI] [PubMed] [Google Scholar]
- 53.Liu S, Hsieh WY, Jiang Y, Kim YS, Sreerama SG, Chen X, Jia B, Wang F. Evaluation of a 99mTc-labeled cyclic RGD tetramer for noninvasive imaging integrin αvβ3-positive breast cancer. Bioconj Chem. 2007;18:438–446. doi: 10.1021/bc0603081. [DOI] [PubMed] [Google Scholar]
- 54.Liu S, Kim YS, Hsieh WY, Sreerama SG. Coligand effects on solution stability, biodistribution and metabolism of 99mTc-labeled cyclic RGDfK tetramer. Nucl Med Biol. 2008;35:111–121. doi: 10.1016/j.nucmedbio.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jia B, Liu Z, Liu ZF, Yu ZL, Zhi Yang Z, Zhao HY, He ZJ, Liu S, Wang F. Linker effects on biological properties of 111In-labeled DTPA conjugates of a cyclic RGDfK dimer. Bioconj Chem. 2008;19:201–210. doi: 10.1021/bc7002988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu Y, Zhang X, Xiong Z, Cheng Z, Fisher DR, Liu S, Gambhir SS, Chen X. MicroPET imaging of glioma integrin αvβ3 expression using 64Cu-labeled tetrameric RGD peptide. J Nucl Med. 2005;46:1707–1718. [PubMed] [Google Scholar]
- 57.Dijkgraaf I, Kruijtzer JAW, Liu S, Soede A, Oyen WJG, Corstens FHM, Liskamp RMJ, Boerman OC. Improved targeting of the αvβ3 integrin by multimerization of RGD peptides. Eur J Nucl Med Mol Imaging. 2007;34:267–273. doi: 10.1007/s00259-006-0180-9. [DOI] [PubMed] [Google Scholar]
- 58.Liu S, Hsieh WY, Kim YS, Mohammed SI. Effect of coligands on biodistribution characteristics of ternary ligand 99mTc complexes of a HYNIC-conjugated cyclic RGDfK dimer. Bioconj Chem. 2005;16:1580–1588. doi: 10.1021/bc0501653. [DOI] [PubMed] [Google Scholar]
- 59.Jia B, Shi J, Yang Z, Xu B, Liu Z, Zhao H, Liu S, Wang F. 99mTc-labeled cyclic RGDfK dimer: initial evaluation for SPECT imaging of glioma integrin αvβ3 expression. Bioconj Chem. 2006;17:1069–1076. doi: 10.1021/bc060055b. [DOI] [PubMed] [Google Scholar]
- 60.Liu S, He ZJ, Hsieh WY, Kim YS, Jiang Y. Impact of PKM linkers on biodistribution characteristics of the 99mTc-labeled cyclic RGDfK dimer. Bioconj Chem. 2006;17:1499–1507. doi: 10.1021/bc060235l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dijkgraaf I, Liu S, Kruijtzer JAW, Soede AC, Oyen WJG, Liskamp RMJ, Corstens FHM, Boerman OC. Effect of linker variation on the in vitro and in vivo characteristics of an 111In-labeled RGD Peptide. Nucl Med Biol. 2007;34:29–35. doi: 10.1016/j.nucmedbio.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 62.Wang JJ, Kim YS, Liu S. 99mTc-labeling of HYNIC-conjugated cyclic RGDfK dimer and tetramer using EDDA as coligand. Bioconj Chem. 2008;19:634–642. doi: 10.1021/bc7004208. [DOI] [PubMed] [Google Scholar]
- 63.Liu S, Edwards DS, Barrett JA. 99mTc-labeling of highly potent small peptides. Bioconj Chem. 1997;8:621–636. doi: 10.1021/bc970058b. [DOI] [PubMed] [Google Scholar]
- 64.Liu S, Edwards DS. 99mTc-labeled small peptides as diagnostic radiopharmaceuticals. Chem Rev. 1999;99:2235–2268. doi: 10.1021/cr980436l. [DOI] [PubMed] [Google Scholar]
- 65.Liu S. 6-Hydrazinonicotinamide derivatives as bifunctional coupling agents for 99mTc-labeling of small biomolecules. Topics in Current Chem. 2005;252:117–153. [Google Scholar]
- 66.Harris TD, Sworin M, Williams N, Rajopadhye M, Damphousse PR, Glowacka D, Poirier MJ, Yu K. Synthesis of stable hydrazones of a hydrazinonicotinyl-modified peptide for the preparation of 99mTc-labeled radiopharmaceuticals. Bioconj Chem. 1999;10:808–814. doi: 10.1021/bc9900237. [DOI] [PubMed] [Google Scholar]
- 67.Liu S, Edwards DS, Harris AR, Ziegler MC, Poirier MJ, Ewels BA, DiLuzio WR, Hui P. Towards developing a non-SnCl2 formulation for RP444: a new radiopharmaceutical for thrombus imaging. J Pharm Sci. 2001;90:114–123. doi: 10.1002/1520-6017(200102)90:2<114::aid-jps2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 68.Edwards DS, Liu S, Barrett JA, Harris AR, Looby RJ, Ziegler MC, Heminway SJ, Carroll TR. A new and versatile ternary ligand system for technetium radiopharmaceuticals: water soluble phosphines and tricine as coligands in labeling a hydrazino nicotinamide-modified cyclic glycoprotein IIb/IIIa receptor antagonist with 99mTc. Bioconj Chem. 1997;8:146–154. doi: 10.1021/bc970002h. [DOI] [PubMed] [Google Scholar]
- 69.Liu S, Edwards DS, Harris AR. A novel ternary ligand system for 99mTc-labeling of hydrazinonicotinamide-modified biologically active molecules using imine-N containing heterocycles as coligands. Bioconj Chem. 1998;9:583–595. doi: 10.1021/bc9800116. [DOI] [PubMed] [Google Scholar]
- 70.Liu S, Edwards DS, Harris AR, Heminway SJ, Barrett JA. Technetium complexes of a hydrazinonicotinamide-conjugated cyclic peptide and 2-hydrazinopyridine: Synthesis and characterization. Inorg Chem. 1999;38:1326–1335. doi: 10.1021/ic980973o. [DOI] [PubMed] [Google Scholar]
- 71.Wu Z, Li Z, Chen K, Cai W, He L, Chin FT, Li F, Chen X. Micro-PET of tumor integrin αvβ3 expression using 18F-labeled PEGylated tetrameric RGD peptide (18F-FPRGD4) J Nucl Med. 2007;48:1536–1544. doi: 10.2967/jnumed.107.040816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang X, Xiong Z, Wu Y, Cai W, Tseng JR, Gambhir SS, Chen X. Quantitative PET imaging of tumor integrin αvβ3 expression with 18F-FPRGD2. J Nucl Med. 2006;47:113–121. [PMC free article] [PubMed] [Google Scholar]
- 73.Cai W, Rao J, Gambhir SS, Chen X. How molecular imaging is speeding up antiangiogenic drug development. Mol Cancer Ther. 2006;5:2624–2633. doi: 10.1158/1535-7163.MCT-06-0395. [DOI] [PubMed] [Google Scholar]
- 74.Jain RK. Transport of molecules in the tumor interstitium: a review. Cancer Res. 1987;47:3039–3051. [PubMed] [Google Scholar]
- 75.Haubner R, Bruchertseifer F, Bock M, Schwaiger M, Wester HJ. Synthesis and biological evaluation of 99mTc-labeled cyclic RGD peptide for imaging integrin αvβ3 expression. Nuklearmedizin. 2004;43:26–32. doi: 10.1267/nukl04010026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed biodistribution data and T/B ratios for 99mTc-labeled cyclic RGD peptide dimers are listed in Tables SI – SV. Figure SI illustrates representative radio-HPLC chromatograms of 99mTc-PEG4-monomer in saline before injection (A), in urine at 30 min p.i. (B), in urine at 120 min p.i. (C), and in feces at 120 min p.i. (D). Figure SII shows typical radio-HPLC chromatograms of 99mTc-3PEG4-dimer in saline before injection (A), in urine at 30 min p.i. (B), in urine at 120 min p.i. (C), and in feces at 120 min p.i. (D). This information is available free of charge via Internet at http://pubs.acs.org.