Abstract
Functional magnetic resonance imaging and repetitive transcranial magnetic stimulation (rTMS) were used to explore the pathophysiology of auditory/verbal hallucinations (AVHs). Sixteen patients with schizophrenia-spectrum disorder were studied with continuous or near continuous AVHs. For patients with intermittent hallucinations (N = 8), blood oxygenation level-dependent (BOLD) activation maps comparing hallucination and nonhallucination periods were generated. For patients with continuous hallucinations (N = 8) correlations between BOLD signal time course in Wernicke’s area, and other regions were used to map functional coupling to the former. These maps were used to identify 3–6 cortical sites per patient that were probed with 1-Hz rTMS and sham stimulation. Delivering rTMS to left temporoparietal sites in Wernicke’s area and the adjacent supramarginal gyrus was accompanied by a greater rate of AVH improvement compared with sham stimulation and rTMS delivered to anterior temporal sites. For intermittent hallucinators, lower levels of hallucination-related activation in Broca’s area strongly predicted greater rate of response to left temporoparietal rTMS. For continuous hallucinators, reduced coupling between Wernicke’s and a right homologue of Broca’s area strongly predicted greater left temporoparietal rTMS rate of response. These findings suggest that dominant hemisphere temporoparietal areas are involved in expressing AVHs, with higher levels of coactivation and/or coupling involving inferior frontal regions reinforcing underlying pathophysiology.
Keywords: Broca’s area, hallucinations, language, schizophrenia, Wernicke’s area
Introduction
Auditory/verbal hallucinations (AVHs) of spoken speech occur in 60–80% of persons with schizophrenia (Sartorius et al. 1974; Andreasen and Flaum 1991). These hallucinations often produce high levels of distress, behavioral dyscontrol, and functional disability that are refractory to antipsychotic drugs. The pathophysiological basis of this syndrome remains uncertain, but if better understood, may lead to more effective treatments.
Neuroimaging studies have associated occurrences of AVHs with activation in diverse brain regions involved in speech generation, speech perception, and verbal memory (Suzuki et al. 1993; Woodruff et al. 1994; Silbersweig et al. 1995; Dierks et al. 1999; Lennox et al. 1999, 2000; Shergill et al. 2000; Copolov et al. 2003; Shergill et al. 2004; van de Van et al. 2005). Many of these studies have found prominent activation during AVHs in the right as well as left hemisphere (Woodruff et al. 1994; Lennox et al. 1999; Shergill et al. 2000) with considerable regional variation from one subject to the next (Lennox et al. 2000; Copolov et al. 2003). These findings have been difficult to interpret due to small sample sizes and uncertainty whether detected activation reflects brain processes directly involved in generating AVHs versus “downstream” consequences of these events such as secondary shifts in attention (van de Van et al. 2005), registration of hallucinations in verbal memory, or activation propagated to nonessential regions.
Our understanding of brain mechanisms producing AVHs may be advanced by research methods that can directly alter function of neurocircuitry components implicated in the genesis of these symptoms. One such tool is repetitive transcranial magnetic stimulation (rTMS), which delivers very brief, 1.5–2 Tesla magnetic pulses that pass relatively undistorted to small regions of the superficial cerebral cortex. Repetitive TMS delivered at a frequency of 1 Hz for 15–20 min duration can produce sustained reductions in excitability or reactivity of the cortical region directly stimulated (Chen et al. 1997; Boroojerdi et al. 2000), possibly via a mechanism analogous to long-term depression elicited by direct electrical stimulation of gray matter at the same frequency (for review see Hoffman and Cavus 2002). An early 15O positron emission tomography study found activation in temporoparietal regions associated with AVHs (Silbersweig et al. 1995). This finding prompted our group to undertake 2 clinical trials involving hallucinating patients using 1-Hz rTMS directed to the TP3 scalp site specified by 10/20 International Electroencephalography (EEG) system (Hoffman et al. 2000, 2003, 2005). The TP3 site overlies the junction of Brodmann area (BA) 39 and 40 in the inferior parietal lobule (Herwig et al. 2003), which neighbors temporoparietal areas highlighted in the study of Silbersweig et al. (1995). Our trials plus 2 others (Chibbaro et al. 2005; Poulet et al. 2005) demonstrated statistically greater symptomatic improvement in AVHs following rTMS delivered to the TP3 site compared with sham stimulation. However, later neuroimaging reports have not detected activation specifically at the BA 39/40 junction (corresponding to the TP3 site) during AVHs (see, for instance, Shergill et al. 2000; Copolov et al. 2003), and negative or equivocal results have been reported for 3 other rTMS trials of patients with AVHs targeting the TP3 site (McIntosh et al. 2004; Fitzgerald et al. 2005; Lee et al. 2005).
Schönfeldt-Lecuona et al. (2004) tested the hypothesis that AVHs are instances of inner speech mislabeled as having a nonself origin as proposed by others (Frith and Done 1989; McGuire et al. 1993; McGuire, Silbersweig, Murray et al. 1996; McGuire, Silbersweig, Wright et al. 1996). Probing Broca’s area and primary auditory cortex at sites selected in light of an inner speech task eliciting functional magnetic resonance imaging (fMRI) activation in these regions did not yield consistent improvements in AVHs relative to sham stimulation.
In order to further clarify pathophysiological processes, the study described below used 1-Hz rTMS to probe multiple cortical sites in patients with AVHs determined using 2 alternative fMRI methods. For patients with intermittent AVHs with intervening silent periods, maps of blood oxygenation level-dependent (BOLD) activation comparing hallucination events signaled during scanning and nonhallucination periods were used for positioning the rTMS coil. For patients experiencing AVHs continuously, activation maps corresponding to hallucination events were not possible because there were no nonhallucination periods for comparison. For these patients, the stimulation coil was positioned based on maps of BOLD signal correlations used to detect functional coupling to Wernicke’s region. The rationale for the latter approach was based on: 1) speech percept-like qualities of AVHs (Junginger and Frame 1985; Nayani and David 1996), suggesting direct involvement of Wernicke’s region, 2) studies demonstrating that functional coupling between brain areas reflecting known network neuro-anatomy can be detected as BOLD signal correlations (Biswal et al. 1995; Lowe et al. 1998; Hampson et al. 2002), and 3) preliminary findings suggesting that BOLD signal correlations linking Wernicke’s area and Broca’s area in patients with AVHs were abnormally elevated when compared with normal subjects (Hampson M, Hoffman R, unpublished data). Our study objectives were to identify those cortical regions where rTMS produced significant improvements in AVHs and then assess the statistical relationship between clinical response in these regions and fMRI findings.
Methods
Subjects
Sixteen right-handed patients meeting Diagnostic and Statistical Manual - Version IV criteria for either schizophrenia or schizoaffective disorder based on the Structured Clinical Interview for Axis I disorders - Patient Edition Version 2.0 (First et al. 1995), and reporting continuous or near continuous AVHs were studied using the protocols described below. Eight patients experienced frequent, intermittent AVHs and 8 patients experienced continuous AVHs. Patients in the former group reported hallucination events on average at least once every 2 min with intervening nonhallucination (silent) periods. Patients in the latter group described continuous hallucinated speech during wakefulness with no intervening silent periods. Continuous hallucinators were studied to expand our sample size. Many of these patients had been referred to our research program due to nearly total resistance to pharmacological treatment. We had found in addition that continuous hallucinators did not respond to rTMS administered to the parietal TP3 EEG site in our previous trial (Hoffman et al. 2005). Positive results using fMRI-guided rTMS in this patient group would therefore suggest a methodological advance. Another potential advantage of studying continuous hallucinators is that the steady-state nature of their hallucinations in theory should be optimal for delineating functional coupling to Wernicke’s area via BOLD signal correlations, which was our method for positioning rTMS when BOLD signal activation maps of hallucination events could not be generated. The Table summarizes demographic and clinical characteristics of these 2 patient groups. Our study included 5 patients who had enrolled in a previous rTMS trial where stimulation was positioned at the TP3 site (Hoffman et al. 2000, 2005). Patients remained on their psychotropic medication at unchanged dosages for at least 4 weeks prior to initiation of the trial and for the duration of the trial itself.
Scanning Protocols
For the first 7 patients (4 intermittent and 3 continuous hallucinators), magnetic resonance imaging employed a GE 1.5T Signa LX scanner. T1-weighted images of 14 contiguous 6-mm thick slices were acquired parallel to a line joining the anterior and posterior commissures (the AC-PC line). During these functional runs, intermittent hallucinators were asked to depress and then release a button that digitally marked the onset-offset of each hallucination event during image acquisition. For patients with continuous hallucinations, no behavioral task was assigned. Image acquisition runs lasted for 4 min, 6 s (164 gradient recalled, single-shot echo planar images for each slice; time repitition [TR] = 1500 ms, time echo [TE] = 60 ms, flip angle = 60° ,64 by 64 acquisition matrix, 3.125 × 3.125 × 6 mm resolution). The first 4 images of each slice in each run were discarded in order to allow magnetization to reach a steady state. Subjects completed 5–7 runs. In addition, functional data were collected that examined activation elicited when listening to external speech. These data were used to delineate a functionally defined Wernicke’s region in order to generate maps of BOLD signal correlations calculated relative to this region. For this purpose, identical image acquisition parameters were utilized except that 2 runs of 226 images were collected while subjects listened to recorded clips from a narrated story played at 45-s intervals over headphones with intervening 45-s silent periods.
For the last 9 patients, magnetic resonance imaging employed a Siemens 3T Trio scanner. Twenty-two 4-mm T1-weighted images were acquired parallel to the AC-PC line, with 0.8-mm skip between images, and functional imaging data was collected in the same slice locations. Functional runs were collected as described above, but imaging parameters were adjusted for the increased field strength (TR = 1500 ms, TE = 30 ms, flip angle = 80°, 64 × 64 acquisition matrix, 3.125 × 3.125 × 4.8 mm).
Image Analyses
All data were motion corrected using the Statistical Parametric Mapping algorithm. A spatial Gaussian filter was applied to the data with a width of 2 pixels at its half maximum and pixels with a median value over the time course that fell below 5% of the maximum time-course median pixel value were set to zero.
Intermittent hallucinators: mapping regional activation associated with hallucination periods
A hemodynamic lag of 3.5 s was assumed when classifying images in the hallucination versus nonhallucination condition. A statistical parametric map of t-scores was created by comparing signal intensity during hallucinations with signal intensity at rest for each brain pixel. Results from the different runs were averaged to produce a t-map for each subject.
Continuous hallucinators: mapping correlated activity relative to Wernicke’s region
A functionally delineated Wernicke’s region was ascertained by comparing activation while listening to external speech versus activation during intervening nonspeech periods. Neurally modulated hemodynamics in each listening or silent period were not assumed to be present until the third image collected during that period. A t-test was performed comparing each pixel’s signal level during speech presentation blocks with that during resting blocks. Results from runs with external speech were averaged to produce a t-map for each subject. The Wernicke’s reference region for correlation analyses was defined for each individual as the 30 most active pixels in the left posterior superior temporal gyrus (STG) corresponding to posterior BA 22. Correlation maps relative to the functionally defined Wernicke’s reference region were then generated for scan data collected during the functional runs acquired in the absence of external speech (Hampson et al. 2002). Data from each of those runs were first low-pass filtered with a cutoff frequency of 0.2 Hz. The partial correlation between the time course of each pixel in question and that of the Wernicke’s reference region within each of the runs was then calculated after removing the effects of the average time course of the slice in which the pixel was located. The correlations were averaged across runs and transformed to a Gaussian distribution via Fisher’s transformation. By fitting the distribution with a Gaussian curve (to full width at half maximum) and adjusting for mean and standard deviation, data were then transformed to a standard normal distribution (Lowe et al. 1998). This yielded a map representing the strength of correlations to Wernicke’s area in terms of standardized z values.
Region of Interest Analyses
Regions of interest (ROIs) selected for analyses of fMRI data were those implicated in language processing defined in terms of Talairach coordinates (Talairach and Tournoux 1988). There were 6 such regions: Broca’s area (BA 44/45), left primary auditory cortex (BA 41), left temporoparietal cortex (Wernicke’s area defined as left BA 22 posterior to y = −30 plus the left supramarginal region, BA 40), and the homologous right-sided region.
For intermittent hallucinators, percent signal change for hallucination periods relative to rest periods was averaged across all pixels in a given ROI to estimate activation in that ROI. For continuous hallucinators, z-maps of BOLD signal correlations relative to the functionally defined Wernicke’s region were transformed into Talairach space and then averaged across all pixels in the ROI to yield a quantitative estimate of correlation to Wernicke’s area for that subject in that ROI.
TMS Protocols
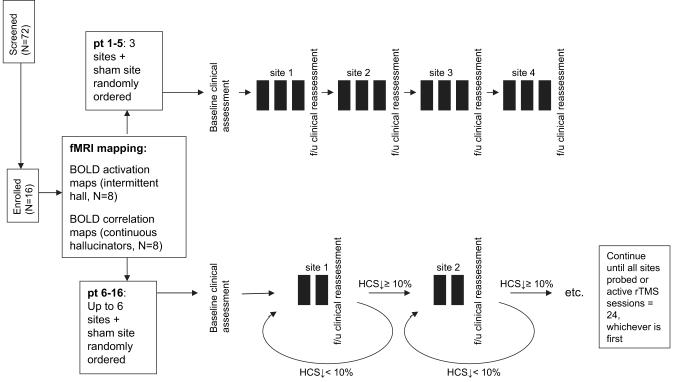
For the first 5 subjects in the study, the 3 most prominent cortical sites based on fMRI maps (either activation maps of hallucination events or Wernicke’s-referenced correlation maps) were selected as rTMS targets. Scalp locations overlying these cortical sites were then identified using a BrainLAB VectorVision frameless stereotactic system (BrainLAB AG, Munich, Germany). Each site plus a sham condition—where the stimulation coil was angled 45° off the scalp while positioned in a temporoparietal site—received 16 min of 1-Hz stimulation once per day for 3 successive days in a crossover design where order of sites was randomized. Except for weekends, different sites were probed sequentially on a once daily basis. Repetitive TMS was administered using a MAGSTIM SUPER system (MAGSTIM Ltd., Whitland, Wales) and a figure-8 coil. Stimulation was administered at 90% motor threshold defined as the minimum stimulation strength applied to primary motor cortex required to elicit visualized motor movements of fingers or thumb in 4/8 tries. Coil position was stabilized using a mechanical arm. Assessments of hallucination severity were conducted after each 3-day block of stimulations and reflected the 24-h period after the last stimulation administered for that block. Study participants, all clinical raters, and all personnel responsible for the clinical care of the patient remained masked to stimulation condition during this 12-day period. Patients received 3 more sessions of active rTMS to the site producing greatest clinical benefit following unmasking.
fMRI data for these first 5 subjects identified more than 3 cortical targets for rTMS per subject with minimal rTMS response observed in 2/5 patients. In order to increase the likelihood of locating regions that yielded rTMS response, the protocol was subsequently revised for the remaining patients so that up to 6 active sites (plus a sham stimulation site in a temporoparietal location) could be targeted. In order to accommodate a larger number of sites while limiting the total number of active stimulation sessions per subject to 24 for practical and safety reasons, continuation of stimulation at a given site was dependent on observed clinical response assessed after every second 16-min rTMS session. If active (or sham) rTMS to that site led to a reduction in hallucination severity ≥ 10% over that 2-day period, then 2 more daily sessions of active (or sham) rTMS were given to that site. If ≥ 10% reduction was not obtained, rTMS was shifted to the next site per a predetermined randomized order. After 2 more days of stimulation, hallucination severity was again assessed and this same decision process applied. This process was repeated until a total of 24 sessions of active rTMS were administered or all targeted sites were studied with active rTMS, whichever came first. Study participants, all clinical raters, and all personnel responsible for the clinical care of the patient remained masked to stimulation condition throughout this protocol.
Assessing Hallucination Severity
Many factors contribute to hallucination severity (e.g., loudness, frequency, and verbal content), and these factors appear to vary in significance from patient to patient. The primary outcome measure consequently was an individualized Hallucination Change Scale (HCS) anchored to each patient’s own descriptions of hallucinatory experience (Hoffman et al. 2003, 2005). Each patient generated a baseline narrative description of AVHs for the 24-h time period just prior to initiation of the trial, which was assigned a score of 10. The HCS was scored on subsequent days by requesting the patient to generate a new narrative description of AVHs for the 24-h period subsequent to the last stimulation session. Follow-up severity scores could exceed baseline, with a maximum score of 20 corresponding to AVHs twice as severe as baseline and a score of zero corresponding to no AVHs during the 24-h assessment window. Acceptable levels of interrater and test-retest reliability have been demonstrated for this scale (Hoffman et al. 2003, 2005).
A neuropsychological test battery was administered prior to and after the trial for each subject. Results of these assessments will be reported separately. The sequence of study procedures are summarized in Figure 1.
Figure 1.
Diagram of the clinical trial. Most common basis for excluding prospective subjects was insufficient hallucination frequency (N = 27). Black rectangle () represents a single, 16-min, 1-Hz rTMS (or sham) session. HCS, Hallucination change score. Open-label phase not reflected in figure.
Statistical Analyses
Change in hallucination severity for rTMS to a given site was quantified as percent change in HCS per stimulation day corrected for baseline HCS at initiation of stimulation for that site, defined as (HCSF – HCSB ) × 100/(HCSB × d), where HCSB = baseline HCS for the site in question, HCSF = final HCS for the site in question, and d = number of days of stimulation to that site. A recent clinical trial demonstrated a curvilinear response of HCS to rTMS as the number of stimulation sessions increased (Hoffman et al. 2005). These data suggested that as hallucination severity decreased during the course of the trial, subsequent HCS response rate decreased. Correction for site-specific baseline HCS was therefore incorporated into this outcome measure to avoid underestimating clinical effects of rTMS at sites probed later in the trial when partial improvements had already emerged. Because our outcome measure corrected for partial improvement at initiation of rTMS to a given site as well as the number of stimulation sessions delivered to the site, rate of AVH response to rTMS delivered across sites and subjects could be compared statistically.
Data limited to findings collected only during double-masked conditions are reported here. Data were grouped within broader functionally related regions in order to achieve sample sizes sufficient to compare response rates statistically. Along these lines, outcomes for rTMS delivered to right and left inferior frontal regions incorporating Broca’s region (BA 44 and 45) were pooled given that there were no trends suggesting lateralizing effects. For the same reason, results of stimulation delivered to primary auditory cortex (BA 41/42) and auditory association cortex (STG anterior to y = −30), collectively referred to as the anterior superior temporal region, were grouped together. Response data for the left temporoparietal cortex consisting of Wernicke’s area and the adjacent left supramarginal cortex (BA 40) were pooled given studies showing that these regions are functionally related and that both regions selectively activate in response to speech inputs (Kertesz et al. 1993; Benson et al. 2001; Matsumoto et al. 2004). Response data for rTMS delivered to the right-sided region homologous to Wernicke’s region and the right supramarginal cortex were grouped as the right temporoparietal region.
Response data for the left temporoparietal region departed significantly from normality and were analyzed nonparametrically. Effect size was estimated following partial renormalization via a square root transformation (X’ = SGN(X) × SQRT (ABS(X))) as follows:
where tp is the paired t-test, N = group size, and r = correlation between response rates in the 2 regions in question (Dunlap et al. 1996).
All statistical tests were 2-tailed using a P value of 0.05 as the cutoff for significance.
Results
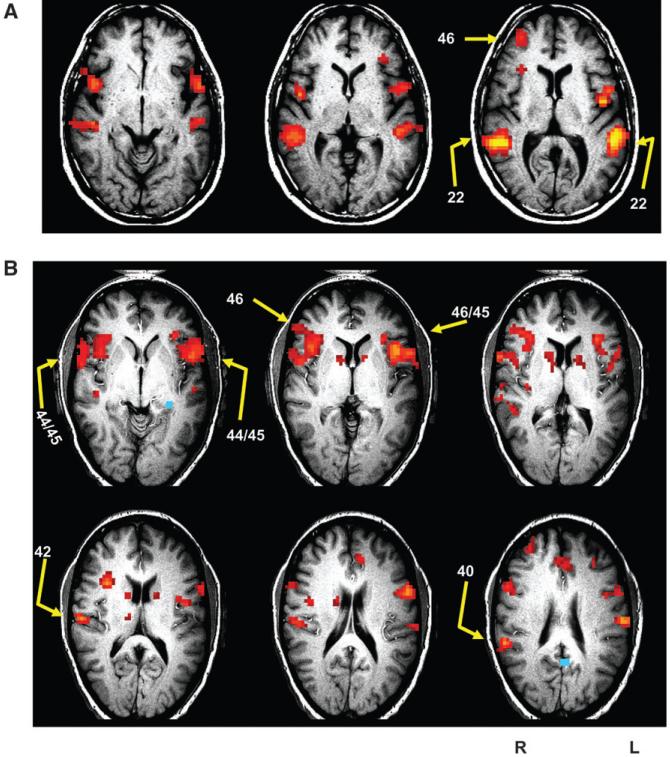
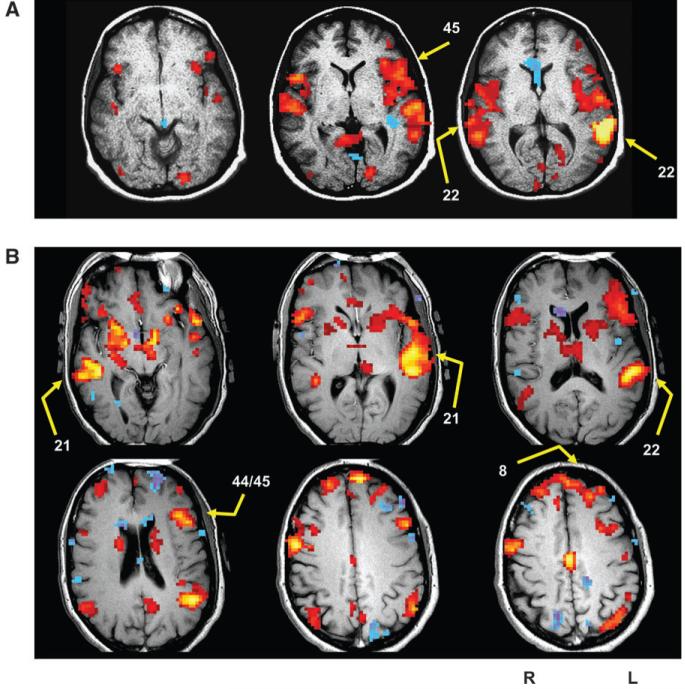
Figure 2 illustrates fMRI activation maps of hallucination events and sites targeted for rTMS for 2 patients with intermittent hallucinations. Figure 3 illustrates fMRI maps of correlations referenced to Wernicke’s area and corresponding rTMS sites for 2 continuous hallucinators.
Figure 2.
fMRI activation maps of hallucination events for 2 patients with intermittent hallucinations. Images are in the axial oblique orientation. Arrows illustrate the rTMS sites selected. Numbers next to arrows are Brodmann areas per Talairach and Tournoux (1988). Left side of the brain corresponds to the right side of images. (A) Representative subject in the 3-site version of the protocol. Activation associated with hallucination periods was focused bitemporally and in inferior frontal and prefrontal regions. Stimulation was limited to 3 sites and directed to posterior superior temporal cortex (BA 22), the right-sided homologous region, and a prefrontal region (BA 46). (B) Representative subject in the later version of the protocol where number of sites probed varied according to functional maps and site-specific clinical response. Activation in posterior temporal regions was largely absent with much more prominent involvement in frontal areas. Four sites were selected for rTMS to cover Broca’s area and right-sided homologous regions. The patient also received rTMS over primary auditory cortex and supramarginal cortex on the right side. Especially prominent temporalis muscle mass can be visualized on the right side over BA 44/45 as a thickening under the patient’s skin, which corresponds to the white rim in these images.
Figure 3.
Representative fMRI maps of correlations relative to Wernicke’s area for 2 patients with continuous hallucinations. Left side of the brain corresponds to the right side of images. (A) Subject in the 3-site version of the protocol. As expected, a prominent (auto) correlation emerged in Wernicke’s region itself, with correlations also expressed in anterior temporal and inferior frontal regions. The 3 sites selected for rTMS were posterior superior temporal regions on the left and right (BA 22) and a site in Broca’s area (BA 45). (B) Subject in the later version of the protocol where number of sites probed varied according to functional maps and site-specific clinical response. Besides the autocorrelation in Wernicke’s area, robust correlations were detected in left and right middle temporal gyrus (BA 21), which were each targeted for rTMS. In addition, a Broca’s region at the boundary of BA 44/45 was targeted along with an orbitofrontal area (BA 8).
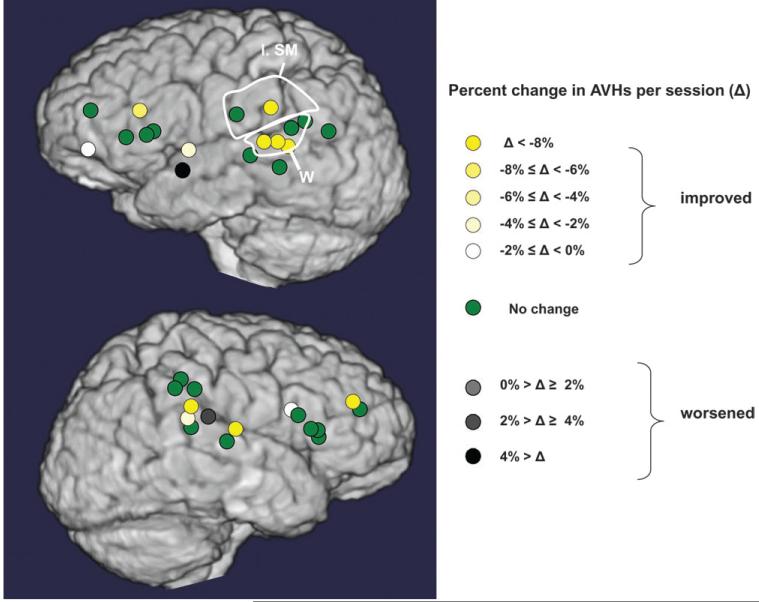
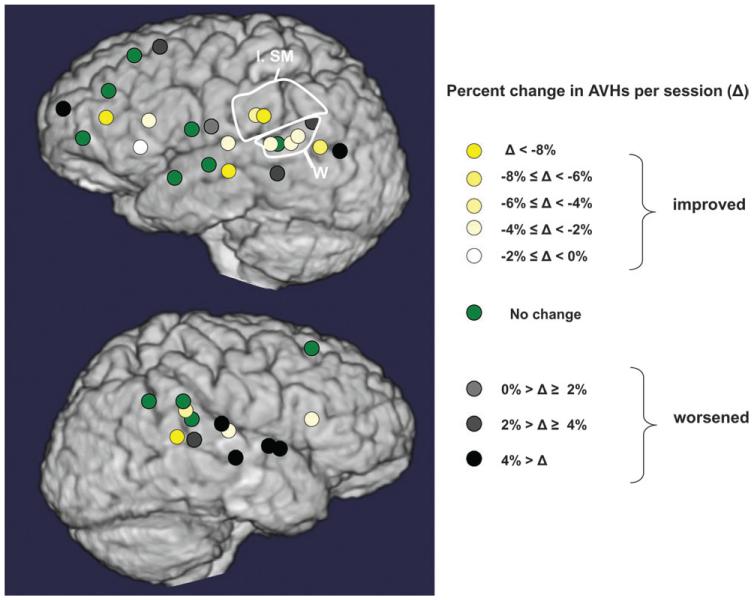
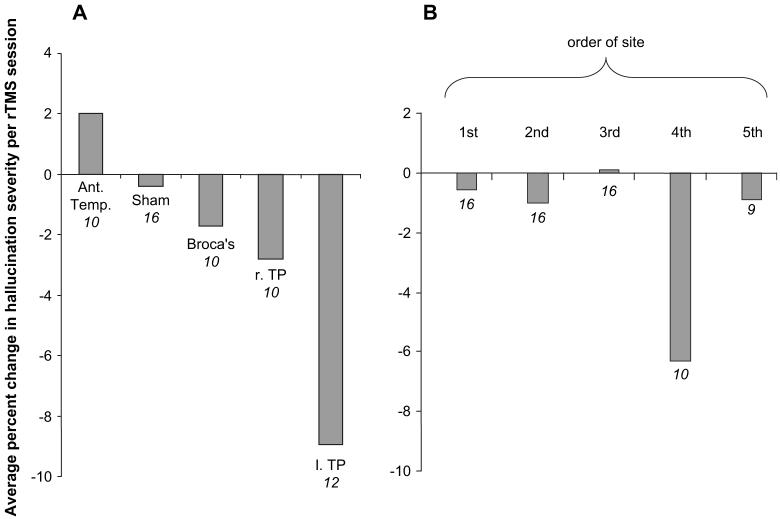
Figures 4 and 5 illustrate changes in hallucination scores for cortical sites probed with active rTMS for subjects with intermittent hallucinations and continuous hallucinations, respectively. Quantitative comparisons of clinical outcomes for rTMS delivered to ROIs are summarized in Figure 6A. Only rTMS directed to the left temporoparietal region produced a greater rate of clinical improvement relative to sham stimulation (Wilcoxon signed ranks test, z = 2.60, P = 0.009, estimated effect size = 1.01). Greater rate of improvement was also observed for rTMS delivered to this region compared with rTMS delivered to anterior temporal regions (Wilcoxon signed ranks test, z = 2.20, P = 0.028, estimated effect size = 1.27).
Figure 4.
Cortical maps of rates of improvement/worsening of AVHs associated with rTMS delivered to alternative sites for all patients with intermittent hallucinations. Left supramarginal (l. sm.) region and Wernicke’s (W) region are circled.
Figure 5.
Cortical maps of rates of improvement/worsening of AVHs associated with rTMS delivered to alternative sites for all patients with continuous hallucinations. Left supramarginal (l. sm.) region and Wernicke’s (W) region are circled.
Figure 6.
(A) Data generated under double-masked conditions averaged across all subjects receiving stimulation at designated site. Ant. Temp., anterior superior temporal regions including BA 41/42 and anterior BA 22 bilaterally; Broca’s, BA 44/45 bilaterally; l. TP, left temporoparietal region defined as Wernicke’s area (BA 22 posterior to y = −30) plus left supramarginal cortex; r. TP, homologous right temporoparietal region. If 2 rTMS sites fell within the same region for a given subject, rTMS response rates for the 2 sites were averaged. Response data for other frontal and middle temporal sites not shown because number of subjects receiving rTMS in these areas were too small to permit comparative statistics. (B) Response rates graphed according to sequential order of site that was probed. Because only 3 subjects received stimulation in 6 sites, these results were not included. Negative values on the y axis reflect reductions in hallucination severity, and numbers below bars correspond to numbers of subjects receiving rTMS to that region or order in sequence.
It is possible that rTMS response for sites probed late in the sequence for a given subject expressed carryover effects arising from stimulating prior sites. If left temporoparietal sites clustered late in the order of sites, this may have contributed to improved response rates in this region. This possibility was assessed by comparing rate of response relative to site order probed with rTMS (Fig. 6B). For the 10 patients probed in at least 4 sites, the fourth site probed was associated with greater improvement than the first site (Wilcoxon signed rank test, z = 2.02, P = 0.043). The number of subjects receiving rTMS directed to left temporoparietal sites at different steps in the sequence was as follows: first in sequence (5), second in sequence (3), third in sequence (4), and fourth in sequence (3). These data indicate that sequential order of left temporoparietal rTMS did not appear to contribute to elevated response rate observed for this region.
ROI data for fMRI maps were assessed as possible predictors of rate of improvement while receiving left temporoparietal rTMS. For intermittent hallucinators alone (N = 6), negative correlations with rate of improvement for rTMS delivered to this region were significant for hallucination-related activation in Broca’s region (Spearman rank rho = –0.93, P = 0.008, Fig. 7) and the right-sided homologous region (Spearman rank rho = −.87, P = 0.024) but not for activation in the left temporoparietal region itself (Spearman rank rho = −0.70, P = 0.12). The correlation with Broca’s hallucination-related activation remained significant after Bonferroni correction (activation variables utilized in this analysis corresponded to left and right Broca’s, anterior temporal, and temporoparietal ROIs yielding a total of 6 variables) with adjusted P value = 0.008 × 6 = 0.048. Confounding effects of 2 factors that may have influenced detected level of activation as well as rTMS response were considered. Number of scanning runs, which could influence robustness of activation maps, may have been influenced by symptom severity (patients with greater symptoms may tolerate extended scanning with more difficulty), which, in turn, could have altered rTMS response. Similarly, frequency of hallucination events during scanning is an index of hallucination severity that could have influenced robustness of activation maps as well as rTMS response. Correlations with rate of improvement for left temporoparietal rTMS for these 2 variables did not, however, approach statistical significance (for number of scan runs per subject, Spearman rank rho = −0.26, P = 0.62; for frequency of AVHs during scanning, Spearman rank rho = .32, P = 0.54).
Figure 7.
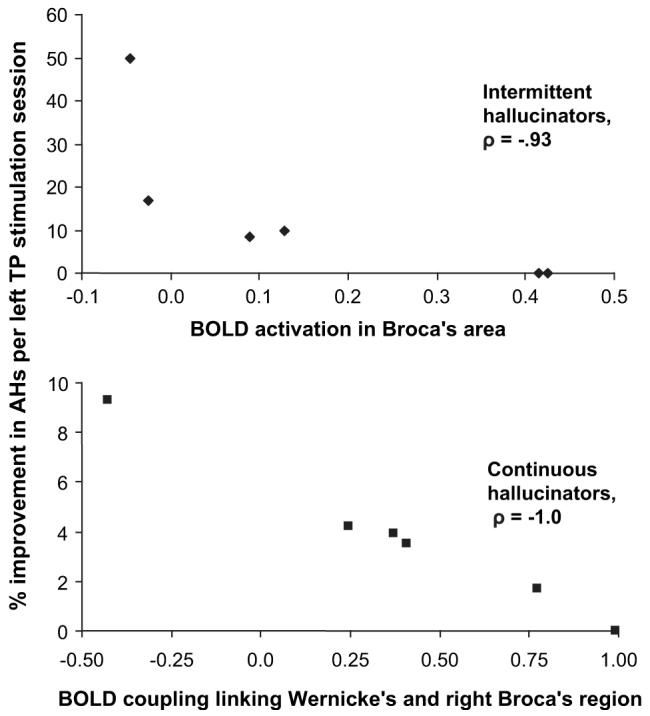
The y axis shows percent improvement in hallucination severity expressed as average hallucination change score reductions per each left temporoparietal (TP) rTMS session. Each plot represents an independent set of 6 patients. Scale of x axis in upper panel corresponds to percent increase in BOLD signal for hallucination periods relative to nonhallucination periods for intermittent hallucinators averaged over all pixels in Broca’s area (BA 44/45). The robust negative correlation suggests that higher levels of activation involving Broca’s region reinforce underlying pathophysiology in this patient group as evidenced by curtailment of effects of rTMS delivered to left temporoparietal cortex, the cortical region appearing to optimize clinical response. Scale of x axis in lower panel is z-transformed BOLD signal correlation values averaged across the right homologue of Broca’s region. The robust negative correlation suggests that higher levels of coupling between Wernicke’s area and the right homologue of Broca’s region reinforce underlying pathophysiology in continuous hallucinators as evidenced by curtailment of effects of left temporoparietal rTMS. These correlational findings, which were derived from 2 distinct patient groups, appear to partially replicate each other because both implicate inferior frontal regions in the pathophysiology of AVHs.
Correlations between temporoparietal rTMS response and functional coupling in ROIs linked to Wernicke’s area were assessed for continuous hallucinators (N = 6). A robust negative correlation was detected between level of Wernicke’s coupling to right Broca’s area and left temporoparietal rTMS response (Spearman rank rho = −1.0, P = 0.001, Fig. 7) but not for coupling to left Broca’s area (Spearman rank rho = −0.60, P = 0.20). Insofar as all continuously hallucinating patients completed an identical number of scan runs (=6), this variable was not a confound influencing these correlational findings. Hallucination frequency in these cases was also identical, that is, continuous.
Patients tolerated active rTMS without significant difficulties. Two patients experienced significant pain during rTMS when stimulating over Broca’s region that required reductions of stimulation strength to 70% motor threshold.
Discussion
Statistically greater rates of improvements in AVHs were observed when rTMS was directed to left temporoparietal sites compared with anterior temporal sites and sham stimulation. Findings did not appear to be accounted for by order of region of stimulation. These data, considered together, implicate left temporoparietal regions in the genesis or expression of AVHs.
The study of patients with AVHs by Schönfeldt-Lecuona et al. (2004) probed an STG site with 1-Hz rTMS corresponding to left primary auditory cortex (BA 41/BA 42) and found improvements at this site compared with control stimulation only at a trend level. Superior temporal sites anterior to Wernicke’s area overall demonstrated no consistent evidence of improvement following rTMS in our study. However, improvement following rTMS delivered to right and left primary auditory cortex was detected in 3/5 patients; in contrast, while more anterior sites in STG elicited no change or worsening AVHs. The significance of these findings, because of the small number of subjects, remains uncertain.
We did not find that AVHs were consistently improved by rTMS to Broca’s area, a finding also reported by Schönfeldt-Lecuona et al. (2004). These negative findings appear to challenge the mislabeled inner speech hypothesis for AVHs (Frith and Done 1989; McGuire et al. 1993, McGuire, Silbersweig, Murray et al. 1996; McGuire, Silbersweig, Wright et al. 1996; Shergill et al. 2001, 2003) insofar as inner speech produces Broca’s activation ordinarily (McGuire, Silbersweig, Murray et al. 1996; Shergill et al. 2001). Of note, however, is that targeting Broca’s region with rTMS required stimulation over the anterior aspect of the temporalis muscle, which is thicker than over temporoparietal areas. Two of our patients could not tolerate full stimulation strength during Broca’s TMS, and greater muscle mass over this area will displace the stimulation coil from the skull surface (see Fig. 2B). Reductions in Broca’s gray matter and the underlying insula in this patient group have been reported (Shapleske et al. 2002; Yamasue et al. 2004) that would further increase distance from coil to cortical surface. Insofar, as effective field strength drops off exponentially as distance from the stimulation coil increases, these factors are likely to curtail rTMS effects.
In spite of the absence of detected efficacy to rTMS-delivered Broca’s and right homologous regions for our patients overall, correlations between fMRI data and left temporoparietal rTMS response in our study (Fig. 7) suggest involvement of these inferior frontal regions in AVH pathophysiology. Broca’s and left temporoparietal regions (i.e., Wernicke’s and supramarginal cortex) are reciprocally connected (Matsumoto et al. 2004) and synchronously coactivate when subjects produce and listen to speech ordinarily (Fried et al. 1981; Mesulam 1990; Friederici 2002). That higher levels of hallucination-specific Broca’s activation levels predicted nonresponse to temporoparietal rTMS in our study suggests that functional relationships between Broca’s and temporoparietal regions associated with ordinary language processing also reinforce pathophysiology of AVHs. The right-sided region homologous to Broca’s area appears to play an important though less well-understood role in language processing (Gaillard et al. 2000; Moro et al. 2001). Higher levels of functional coupling linking this region to Wernicke’s cortex appeared to reinforce pathophysiology in the continuous hallucinator group as evidenced by robust correlations with resistance to left temporoparietal rTMS. A less parsimonious hypothesis accounting for the capacity of inferior frontal fMRI data (incorporating Broca’s and the right homologous area) to predict subsequent temporoparietal rTMS response cannot be ruled out, however. Another region, perhaps subcortically located, could activate left and/or right Broca’s area as well as temporoparietal regions and itself induce rTMS resistance—thereby, producing our correlative findings. Of interest is that level of activation within the left temporoparietal region itself was not as robust a predictor of clinical response. These data suggest that rTMS to this region was able to override partially higher activation levels when accessed directly. The robust negative correlations between temporoparietal rTMS response and hallucination-related activation/coupling involving inferior frontal regions were not accounted for by signal acquisition variables that could have influenced robustness of fMRI maps as well as rTMS response in parallel.
Limitations of our methodology should be considered in interpreting findings:
Five patients were reenrolled from previous rTMS studies administered to the TP3 site positioned based on scalp landmarks due to availability of fMRI maps. Three of these patients previously had a significant response to rTMS, whereas 2 patients had no response, which is roughly comparable to the 52% rate of response in our recently completed trial (Hoffman et al. 2005). Four of these patients received rTMS at left temporoparietal sites during the course of this study, which averaged 2.2 cm distant from the parietal TP3 site used in our previous rTMS studies of AVHs. The focality of the magnetic field produced by the figure-8 coil employed (defined as the distance from the coil center producing a 50% drop-off in magnetic field strength) is likely to have radius of ∼0.75 cm when administered at 90% motor threshold (Thielscher and Kammer 2004). Thus, temporoparietal rTMS sites used in this study had little to no overlap with the more posterior, inferior parietal TP3 site used in previous studies for these four reenrolled patients. The inclusion of patients from earlier protocols was prompted by availability of fMRI maps for these patients and the fact that this study focused on spatial specificity and fMRI predictors of rTMS response rather than assessment of overall efficacy.
Two different fMRI maps were employed that produced different positioning information. Activation maps of hallucination events were used because they can be directly linked conceptually to known effects of 1-Hz rTMS, which are thought to be inhibitory in nature. Given that fMRI activation maps require very high hallucination frequencies (at least one event every other minute) with intervening “silent” periods, this method can be applied to less than 5% of hallucinators, which limited recruitment and prompted us to reenroll patients from earlier protocols for whom these maps had been successfully generated previously. Consequently, we sought to develop a second, correlation-based method for mapping involved neurocircuitry.
For mapping purposes, patient selection was biased toward those with especially severe AVHs. For intermittent hallucinators, average hallucination frequency, as noted above, was required to be very high. We have found that continuous hallucinators had historically demonstrated minimal responses to pharmacological treatment as well as to rTMS delivered to a standard TP3 location (Hoffman et al. 2000, 2005). Overall, our hope was that studying hallucinating patients with especially severe syndromes would shed light on more standard populations. However, it remains possible that patients with less severe hallucination syndromes suffer from somewhat different pathophysiology.
Two different scanners were used in the study. However, there is no suggestion that we obtained improved clinical results due to the higher resolution of the 3T scanner used later in the study.
Total “dose” of rTMS to “best” sites was relatively modest, averaging 3.7 sessions for Wernicke’s region and 4.8 sessions for the left supramarginal cortex. It remains possible that protocols designed so that more stimulation is given to these sites might be able to generate symptoms reductions even in the face of pronounced inferior frontal coactivation/coupling.
Carryover effects arising from the crossover nature of this study cannot be ruled out, which would add noise to region-specific rTMS response data.
The strength of magnetic fields generated by TMS coils drops off exponentially relative to distance from the coil surface with effective physiological penetrance extending only 1–2 cm (Cohen et al. 1990; Thielscher and Kammer 2004). Consequently, rTMS can only access cortex adjacent to the skull surface. Our fMRI data appear to demonstrate significant involvement in medially displaced brain areas (see, for instance, sites in BA 21 and 22 in Fig. 2A and sites in BA 45 and 22 in Fig. 3A). There is some evidence that 1-Hz rTMS can produce indirect effects that are transmitted transsynaptically (Wassermann et al. 1998; Rossi et al. 2000). However, propagated effects are likely to be more limited than direct effects. This limitation may impose a ceiling on overall clinical response to rTMS.
Our method for assessing cortical coupling in the continuous hallucinator group relies on the assumption that Wernicke’s area is involved in the genesis of AVHs. We had no independent way to verify this assumption in individual cases. It therefore remains possible that our positioning method missed identifying promising cortical sites if Wernicke’s involvement was indeed minimal in some continuous hallucinators.
We assumed a relatively brief hemodynamic delay of 3.5 s when mapping activity associated with hallucinations. By comparison, a recent study by Shergill et al. (2004), mapping time course of AVHs assumed a hemodynamic delay between 4–8 s. Our intent was to focus on activation occurring early in and just prior to onset of hallucination events. Along these lines, 2 reports have shown detectable activation that appears prior to hallucination onset that may be of special mechanistic significance (Lennox et al. 1999, Shergill et al. 2004). However, the assumption of a 3.5-s hemodynamic delay may have reduced our statistical power to detect activation emerging later in hallucination events.
In summary, by demonstrating topographic specificity in rTMS response left temporoparietal sites, this study provides evidence that Wernicke’s area and/or the adjacent supramarginal cortex play a direct role in generating or expressing AVHs in dextral patients. Correlations between fMRI data and rTMS response data also implicate involvement of Broca’s area and its right homologous area in pathophysiology even though rTMS did not produce consistent improvements when directed to these sites. Whether brain mechanisms responsible for generating AVHs reflect a distributed property across a network incorporating these brain regions or a focal disturbance (cf. David 1994) that secondarily propagates across this network—analogous perhaps to some forms of epilepsy (see, for instance, Badier and Chauvel 1995)—remains an important unanswered question.
Table 1.
Demographic and clinical characteristics of the 2 subgroups of hallucinating patients
| Intermittent hallucinators (N = 8) | Continuous hallucinators (N = 8) | |
|---|---|---|
| Agea | 33.7 ± 13.7 | 35.9 ± 6.5 |
| Gender (F/M) | 2/6 | 4/4 |
| Education level (grades)a | 14.4 ± 1.8 | 14.1 ± 1.7 |
| Diagnosis | Schizophrenia, paranoid (5); schizoaffective disorder, depressed type (3) |
Schizophrenia, paranoid type (3); schizoaffective disorder, bipolar type (2); schizophrenia, depressed type (1); schizophrenia, undifferentiated type (2) |
| Number of psychiatric hospitalizationsa | 5.3 ± 5.5 | 5.1 ± 3.9 |
| Age of first onset of AVHs | 24 ± 5.8 | 20.9 ± 4.8 |
| Months since last remission of auditory hallucinationsa,b | 118 ± 122 | 180 ± 95 |
| Medication resistance of AVHsc | 7 (88) | 7 (88) |
Data reported as mean ± standard deviation.
Remission defined as AVHs absent for at least 4 weeks.
Medication resistance defined as daily AVHs occurring in the face of at least 2 adequate trials of antipsychotic medications including at least one atypical antipsychotic medication. An adequate medication trial was defined as a minimum of at least 6 weeks at a dose of 1000 chorpromazine equivalents for standard neuroleptics (Davis 1976) and published recommendations of therapeutic dosing for atypical neuroleptics (minimum dose of risperidone 6 mg/day, olanzapine 15 mg/day, quetiapine 500 mg/day, clozapine 400 mg/day). Data in parentheses correspond to percent of sample.
Acknowledgments
This study was supported by a Dana Foundation grant (R.E.H.), a National Alliance for Research on Schizophrenia and Depression Independent Investigator Award, Peterson 50th Anniversary Research Partner (R.E.H.), National Institute of Mental Health grants R21MH63326 (R.E.H.), R01MH067073 (R.E.H.), KO2 AA 00261-01 (J.K.), the Department of Veterans Affairs Schizophrenia Biological Research Center (J.K.), National Institutes of Health/National Center for Research Resources/General Clinical Research Center Program grant RR00125, and the Department of Mental Health and Addiction Services of the State of Connecticut through its support of the Abraham Ribicoff Research Center at the Connecticut Mental Health Center. We also extend our thanks to the patients who participated in this study.
Footnotes
Conflict of Interest: None declared.
References
- Andreasen NC, Flaum M. Schizophrenia: the characteristic symptoms. Schizophr Bull. 1991;17:27–50. doi: 10.1093/schbul/17.1.27. [DOI] [PubMed] [Google Scholar]
- Badier JM, Chauvel P. Spatio-temporal characteristics of paroxysmal interictal events in human temporal lobe epilepsy. J Physiol (Paris) 1995;89:255–264. doi: 10.1016/0928-4257(96)83642-4. [DOI] [PubMed] [Google Scholar]
- Benson RR, Whalen DH, Richardson M, Swainson B, Clark VP, Lai S, Liberman AM. Parametrically dissociating speech and nonspeech perception in the brain using fMRI. Brain Lang. 2001;78:364–396. doi: 10.1006/brln.2001.2484. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Prager A, Muelibacher W, Cohen LG. Reduction of human visual cortex excitability using 1-Hz transcranial magnetic stimulation. Neurology. 2000;54:1529–1531. doi: 10.1212/wnl.54.7.1529. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Chibbaro G, Daniele M, Alagona G, Di Pasquale C, Cannavo M, Rapisarda V, Pennisi G. Repetitive transcranial magnetic stimulation in schizophrenic patients reporting auditory hallucinations. Neurosci Lett. 2005;383:54–57. doi: 10.1016/j.neulet.2005.03.052. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Roth BJ, Nilsson J, Dang N, Panizza M, Bandinelli S, Friauf W, Hallett M. Effects of coil design on delivery of focal magnetic stimulation. Technical considerations. Electroencephalogr Clin Neurophysiol. 1990;75:350–357. doi: 10.1016/0013-4694(90)90113-x. [DOI] [PubMed] [Google Scholar]
- Copolov DL, Seal ML, Maruff P, Ulusoy R, Wong MT, Tochon-Danguy HJ, Egan GF. Cortical activation associated with the experience of auditory hallucinations and perception of human speech in schizophrenia: a PET correlation study. Psychiatry Res. 2003;122:139–52. doi: 10.1016/s0925-4927(02)00121-x. [DOI] [PubMed] [Google Scholar]
- David AS. The neuropsychological origin of auditory hallucinations. In: David AS, Cutting JC, editors. The neuropsychology of schizophrenia. Erlbaum; Mahwah (NJ): 1994. pp. 269–313. [Google Scholar]
- Dierks T, Linden DEJ, Jandl M, Formisano E, Goebel R, Lanfermann H, Singer W. Activation of Heschl’s gyrus during auditory hallucinations. Neuron. 1999;22:615–621. doi: 10.1016/s0896-6273(00)80715-1. [DOI] [PubMed] [Google Scholar]
- Dunlap WP, Cortina JM, Vaslow JB, Burke MJ. Meta-analysis of experiments with matched groups or repeated measures designs. Psychol Methods. 1996;1:170–177. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I disorders—patient edition. Version 2.0 New York Psychiatric Institute, Biometrics Research Department; 1995. [Google Scholar]
- Fitzgerald PB, Benitez J, Daskalakis JZ, Brown TL, Marston NA, de Castella A, Kulkarni J. A double-blind sham-controlled trial of repetitive transcranial magnetic stimulation in the treatment of refractory auditory hallucinations. J Clin Psychopharmacol. 2005;25:358–362. doi: 10.1097/01.jcp.0000168487.22140.7f. [DOI] [PubMed] [Google Scholar]
- Fried I, Ojemann GA, Fetz EE. Language related potentials specific to human language cortex. Science. 1981;212:353–356. doi: 10.1126/science.7209537. [DOI] [PubMed] [Google Scholar]
- Friederici AD. Toward a neural basis of auditory sentence processing. Trends Cogn Sci. 2002;6:78–84. doi: 10.1016/s1364-6613(00)01839-8. [DOI] [PubMed] [Google Scholar]
- Frith CD, Done DJ. Towards a neuropsychology of schizophrenia. Br J Psychiatry. 1989;153:437–443. doi: 10.1192/bjp.153.4.437. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Hertz-Pannier L, Mott SH, Barnett AS, LeBihan D, Theodore WH. Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology. 2000;54:180–185. doi: 10.1212/wnl.54.1.180. [DOI] [PubMed] [Google Scholar]
- Hampson M, Peterson B, Skudlarski P, Gatenby C, Gore J. Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp. 2002;15:247–262. doi: 10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwig U, Satrapi P, Schoenfeldt-Lecuona C. Using the international 10-20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr. 2003;16:95–99. doi: 10.1023/b:brat.0000006333.93597.9d. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Boutros NN, Hu S, Berman RM, Krystal JH, Charney DS. Transcranial magnetic stimulation of left temporoparietal cortex in schizophrenic reporting auditory hallucinations. Lancet. 2000;355:1073–1075. doi: 10.1016/S0140-6736(00)02043-2. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Cavus I. Slow transcranial magnetic stimulation, long-term depotentiation, and brain hyperexcitability disorders. Am J Psychiatry. 2002;159:1093–1102. doi: 10.1176/appi.ajp.159.7.1093. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Gueorguieva R, Hawkins KA, Varanko M, Boutros NN, Wu Y-T, Carroll K, Krystal JH. Temporoparietal transcranial magnetic stimulation for auditory hallucinations: safety, efficacy and moderators in a fifty patient sample. Biol Psychiatry. 2005;58:97–104. doi: 10.1016/j.biopsych.2005.03.041. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Hawkins KA, Gueorguieva R, Boutros NN, Rachid F, Carroll K, Krystal JH. Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinations. Arch Gen Psychiatry. 2003;60:49–56. doi: 10.1001/archpsyc.60.1.49. [DOI] [PubMed] [Google Scholar]
- Junginger J, Frame CL. Self-report of the frequency and phenomenology of verbal hallucinations. J Nerv Mental Dis. 1985;173:149–154. doi: 10.1097/00005053-198503000-00003. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Lau WK, Polk M. The structural determinants of recovery in Wernicke’s aphasia. Brain Lang. 1993;44:153–164. doi: 10.1006/brln.1993.1010. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kim W, Chung YC, Jung KH, Bahk WM, Jun TY, Kim KS, George MS, Chae JH. A double blind study showing that two weeks of daily repetitive TMS over the left or right temporoparietal cortex reduces symptoms in patients with schizophrenia who are having treatment-refractory auditory hallucinations. Neurosci Lett. 2005;376:177–181. doi: 10.1016/j.neulet.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Lennox BR, Bert S, Park G, Jones PB, Morris PG. Spatial and temporal mapping of neural activity associated with auditory hallucinations. Lancet. 1999;353:644. doi: 10.1016/s0140-6736(98)05923-6. [DOI] [PubMed] [Google Scholar]
- Lennox BR, Park SBG, Medley I, Morris PG, Jones PB. The functional anatomy of auditory hallucinations in schizophrenia. Psychiatry Res. 2000;100:13–20. doi: 10.1016/s0925-4927(00)00068-8. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Matsumoto R, Nair DR, LaPrestor E, Najm I, Bingaman W, Shibasaki H, Luders HO. Functional connectivity in the human language system: a corticocortical evoked potential study. Brain. 2004;127:2316–2330. doi: 10.1093/brain/awh246. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Shah GMS, Murray RM. Increased blood flow in Broca’s area during auditory hallucinations in schizophrenia. Lancet. 1993;342:703–706. doi: 10.1016/0140-6736(93)91707-s. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Silbersweig DA, Murray RM, David AS, Frackowiak RSJ, Frith CD. Functional anatomy of inner speech and auditory verbal imagery. Psychol Med. 1996;26:29–38. doi: 10.1017/s0033291700033699. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Silbersweig DA, Wright I, Murray RM, Frrackowiak RSJ, Frith CD. The neural correlates of inner speech and auditory verbal imagery in schizophrenia: relationship to auditory hallucinations. Br J Psychiatry. 1996;169:148–159. doi: 10.1192/bjp.169.2.148. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Semple D, Tasker K, Harrison LK, Owens DG, Johnstone EC, Ebmeier KP. Transcranial magnetic stimulation for auditory hallucinations in schizophrenia. Psychiatry Res. 2004;127:9–17. doi: 10.1016/j.psychres.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M. Large-scale neurocognitive networks and distributed processing for attention, language and memory. Ann Neurol. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Moro A, Tettamanti M, Perani D, Donati C, Cappa SF, Fazio F. Syntax and the brain: disentangling grammar by selective anomalies. Neuroimage. 2001;13:110–118. doi: 10.1006/nimg.2000.0668. [DOI] [PubMed] [Google Scholar]
- Nayani TH, David AS. The auditory hallucination: a phenomenological survey. Psychol Med. 1996;26:177–189. doi: 10.1017/s003329170003381x. [DOI] [PubMed] [Google Scholar]
- Poulet E, Brunelin J, Bediou B, Bation R, Forgeard L, Dalery J, d’Amato T, Saoud M. Slow transcranial magnetic stimulation can rapidly reduce resistant auditory hallucinations in schizophrenia. Biol Psychiatry. 2005;57:188–191. doi: 10.1016/j.biopsych.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rossi S, Pasqualetti P, Rossini PM, Feige B, Ulivelli M, Glocker FX, Battistini N, Lucking CH, Kristeva-Feige R. Effects of repetitive transcranial magnetic stimulation on movement-related cortical activity in humans. Cereb Cortex. 2000;10:802–808. doi: 10.1093/cercor/10.8.802. [DOI] [PubMed] [Google Scholar]
- Sartorious N, Shapiro R, Jablonsky A. The international pilot study of schizophrenia. Schizophr Bull. 1974;1:21–35. doi: 10.1093/schbul/1.11.21. [DOI] [PubMed] [Google Scholar]
- Schönfeldt-Lecuona C, Gron G, Walter H, Buchler N, Wunderlich A, Spitzer M, Herwig U. Stereotaxic rTMS for the treatment of auditory hallucinations in schizophrenia. Neuroreport. 2004;15:1669–1673. doi: 10.1097/01.wnr.0000126504.89983.ec. [DOI] [PubMed] [Google Scholar]
- Shapleske J, Rossell SL, Chitnis XA, Suckling J, Simmons A, Bullmore ET, Woodruff PW, David AS. A computational morphometric MRI study of schizophrenia: effects of hallucinations. Cereb Cortex. 2002;12:1331–1341. doi: 10.1093/cercor/12.12.1331. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Brammer MJ, Amaro E, Williams SCR, Murray RM, McGuire PK. Temporal course of auditory hallucinations. Br J Psychiatry. 2004;185:516–517. doi: 10.1192/bjp.185.6.516. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Brammer MJ, Fukuda R, Williams SC, Murray RM, McGuire PK. Engagement of brain areas implicated in processing inner speech in people with auditory hallucinations. Br J Psychiatry. 2003;182:525–531. doi: 10.1192/bjp.182.6.525. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Brammer MJ, Williams SCR, Murray RM, McGuire PK. Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry. 2000;57:1033–1038. doi: 10.1001/archpsyc.57.11.1033. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Bullmore ET, Brammer MJ, Williams SCR, Murray RM, McGuire PK. A functional study of auditory verbal imagery. Psychol Med. 2001;31:241–253. doi: 10.1017/s003329170100335x. [DOI] [PubMed] [Google Scholar]
- Silbersweig DA, Stern E, Frith C, Cahill C, Holmes A, Grootoonk S, Seaward J, McKenna P, Chua SE, Schnorr L, Jones T, Frackowiak RSJ. A functional neuroanatomy of hallucinations in schizophrenia. Nature. 1995;378:176–179. doi: 10.1038/378176a0. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Yuasa S, Minabe Y, Murata M, Kurachi M. Eur Arch Psychiatry Clin Neurosci. 1993;242:257–261. doi: 10.1007/BF02190383. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme Medical Publishers; New York: 1988. [Google Scholar]
- Thielscher A, Kammer T. Electric field properties of two commercial figure-8 coils in TMS: calculation of focality and efficiency. Clin Neurophysiol. 2004;115:1697–1708. doi: 10.1016/j.clinph.2004.02.019. [DOI] [PubMed] [Google Scholar]
- van de Ven VG, Formisano E, Roder CH, Prvulovic D, Bittner RA, Dietz MG, Hubl D, Dierks T, Federspiel A, Esposito F, et al. The spatiotemporal pattern of auditory cortical responses during verbal hallucinations. Neuroimage. 2005;27:644–655. doi: 10.1016/j.neuroimage.2005.04.041. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Wedegaertner FR, Ziemann UI, George MS, Chen R. Crossed reduction of human motor cortex excitability by 1-Hz transcranial magnetic stimulation. Neurosci Lett. 1998;250:141–144. doi: 10.1016/s0304-3940(98)00437-6. [DOI] [PubMed] [Google Scholar]
- Woodruff P, Brammer M, Mellers J, Wright I, Bullmore E, Williams S. Auditory hallucinations and perception of external speech. Lancet. 1994;346:1035. doi: 10.1016/s0140-6736(95)91715-2. [DOI] [PubMed] [Google Scholar]
- Yamasue H, Iwanami A, Hirayasu Y, Yamada H, Abe O, Kuroki N, Fukuda R, Tsujii K, Aoki S, Ohtomo K, et al. Localized volume reduction in prefrontal, temporolimbic, and paralimbic regions in schizophrenia: an MRI parcellation study. Psychiatry Res. 2004;131:195–207. doi: 10.1016/j.pscychresns.2004.05.004. [DOI] [PubMed] [Google Scholar]