Abstract
The integration of targeted therapies such as Cetuximab to radiation therapy has revolutionized the management of head and neck cancers in the last decade. However, the use of targeted therapies raised several clinically relevant questions that have yet to be answered. These questions include the optimal patient and tumor profile for biologically targeted therapy, the optimal radiation fractionation to use with targeted therapies, how to integrate them into standard or new chemo-radiation regimens, their schedule and duration of administration, their toxicity and which direction to consider for novel targeted treatment. In this review, we will highlight several of these important issues, the clinical trials that are designed to address these issues and introduce some novel targeted therapies that may contribute to the improvement of the therapeutic ratio for head and neck cancer therapy.
Introduction
The success of the phase III randomized study by Bonner et al that showed a significant improvement in survival with the addition of Cetuximab to radiotherapy (RT) in patients with locally advanced head and neck squamous cell carcinoma (HNSCC) has been hailed as a landmark study for integrating targeted therapy with standard radiation treatment.1,2 However, the results of this randomized trial raised several important questions about targeted therapy that have yet to be addressed. These questions include: (1) what is the optimal radiation fractionation to use with biologically targeted treatment? (2) What is the optimal patient and tumor profile for such treatment? (3) What is the optimal way to integrate these therapies with the standard chemoradiotherapy (CRT)? (4) What is the optimal duration and schedule for targeted therapies like cetuximab? (5) What acute and late toxicity profile is considered acceptable for biologically targeted treatment? (6) Where should we go from here? (7) What are other molecular pathways that should be considered as we build towards combining targeted drugs for specific molecular profile of an individual HNSCC? In this review, we will use Bonner’s study as an example of successful biologically targeted therapy to dissect these important issues and discuss the relevant on-going studies attempting to address some of these dilemmas. We will also discuss new combinations of targeted therapies that are being introduced into the clinics.
Radiation fractionation
The role of altered fractionation in combination with systemic therapy is still being defined. Randomized studies to date have shown that both accelerated fractionation and hyperfractionation, when administered without chemotherapy, can improve local control and disease-free survival in locally advanced HNSCC patients.3,4 A meta analysis using individual data also showed that pure hyperfractionation regimens with dose escalation conferred an absolute overall survival benefit of 8%, which is the same level of improvement noted for concurrent chemotherapy with conventionally fractionated irradiation, without an increase in late toxicity.5 When combining radiation with concurrent chemotherapy, the benefits of altered fractionation over once daily radiation remain unproven. RTOG 99-14, a phase II study evaluating a concomitant boost radiation schedule with concurrent cisplatin, demonstrated encouraging 3-year survival rates; however, acute toxicity was considerable.6 Both the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) have conducted randomized trials to compare accelerated fractionation to standard fractionation when delivered with concurrent chemotherapy for locally advanced HNSCC (RTOG 0129 and EORTC 22962). However, the results of these large studies have not yet matured to provide us guidance on the optimal fractionation to use with concurrent chemotherapy.
Scrutiny of the Bonner trial reveals that 74% of the patients received altered fractionation radiotherapy with 56% treated with accelerated fractionation using the concomitant boost approach and 18% with hyperfractionation. In addition to performance status, nodal involvement and tumor classification, radiation fractionation regimen (concomitant boost vs. once daily vs. twice daily) was a specified stratification factor for randomization. Interestingly, when survival data was presented according to the fractionation schedule, the largest benefit favoring Cetuximab was noted for the concomitant boost approach (hazard ratio [HR] = 0.62), followed by hyperfractionation (HR = 0.74). There was no apparent difference between the 2 arms for the conventionally fractionated group (HR = 1.01).
Does this finding mean that cetuximab should be used only with altered fractionation to achieve its maximal efficacy? The purist would say that these subset analyses are only hypothesis generating and should be interpreted with caution. However, the majority of the patients in this study received altered fractionation concomitantly with cetuximab, and it is therefore reasonable to consider such fractionation regimens when this drug is contemplated. Of interest is the reported relationship between pretreatment tumoral EGFR expression and locoregional control benefit from accelerated fractionation. Bentzen et al reported that only HNSCC patients with high pretreatment EGFR levels achieved a locoregional control benefit from continuous hyperfractionated accelerated radiation therapy (CHART) whereas those with low levels did not.7 One might hypothesize that altering the EGFR signaling pathway with EGFR inhibitors might impact tumor proliferation and negate the advantage of accelerated fractionation in tumors with high EGFR levels. However, until more data are available, the relationship between EGFR targeting, radiation fractionation and EGFR expression remained undefined.
With the integration of biologically targeted therapy into concurrent CRT, there is even less data to guide us on the optimal radiation fractionation schedule. The RTOG had decided to employ accelerated fractionation, delivered via either a concomitant boost approach or the DAHANCA style (6 fractions/week at 2 Gy/fraction to 70 Gy), in RTOG 0522, which is a large randomized study comparing concurrent cisplatin-based CRT to the same regimen plus Cetuximab in patients with locally advanced HNSCC.8 However, if the results of RTOG 0129 do not demonstrate a benefit for altered fraction chemoradiation, then RTOG 0522 may fall back to once daily CRT +/− cetuximab. Nevertheless, RTOG 0522 will provide critical information as to the efficacy of adding an EGFR inhibitor to a CRT regimen in patients with locally advanced HNSCC.
Patient Selection
Eligibility criteria for Bonner’s trial required that the patients have non-metastatic and measurable stage III–IV SCC of the oral cavity (OC), oropharynx (OP), larynx (LX) and hypopharynx (HP), be suitable for definitive radiation treatment, Karnofsky performance status (KPS) ≥60, and normal hematologic, hepatic and renal function. In addition, patients must not have undergone surgery prior to treatment.1 In other words, these patients were relatively healthy and would have qualified for most modern aggressive CRT studies. In fact, the median age of the patients in the cetuximab arm was 56 and only 3% had KPS of 60. Although performance status was a predefined stratification factor (KPS 60–80 vs. 90–100), the benefit of cetuximab for each KPS group was not reported in the paper. It would be important to see if patients in the poor KPS group derived the same benefit from cetuximab as those with higher KPS. Yet, because of its relative low rate of hematologic and mucosal toxicities, the cetuximab-RT treatment has been widely used in the community for elderly patients and for those with poor performance status or organ dysfunction. Unfortunately, most of these patients would not have met the eligibility criteria for the Bonner study, and both the efficacy and the toxicity of such treatment in patients with poor performance status are unknown.
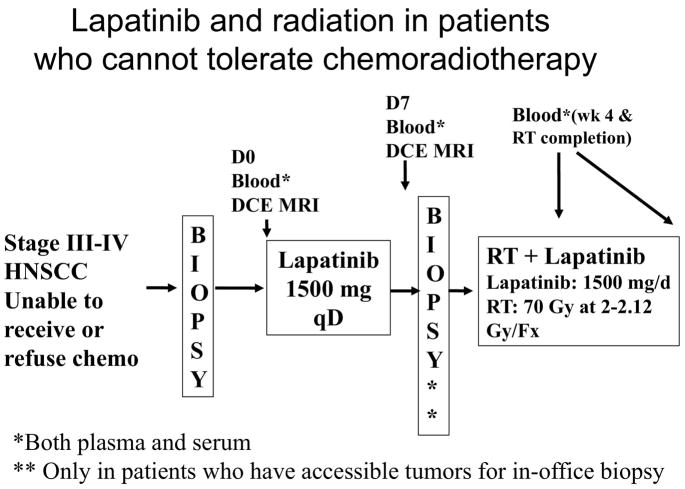
To address the role of EGFR pathway inhibition in elderly patients and those who are medically unfit for concurrent CRT, we are conducting a multi-institutional phase II study, combining radiation with Lapatinib, a dual EGFR and Her-2 tyrosine kinase Inhibitor (TKI). The schema for this study is shown in Figure 1. Enrollment criteria require that the patient must not be able to tolerate concurrent cisplatin-based chemotherapy due to either poor performance status (ECOG ≥2) or have significant medical comorbidities, including renal dysfunction, hepatic dysfunction, immunocompromise, significant hearing loss, or severe baseline neuropathy. This study will provide insight into the toxicity and efficacy of RT combined with EGFR pathway inhibitors in these frail patients.
Figure 1.
Study schema for a multi-institutional study evaluating the feasibility and efficacy of combining lapatinib, a dual EGFR and Her-2 tyrosine kinase inhibitor, with radiation therapy in patients who cannot tolerate chemoradiation treatment for locally advanced HNSCC
Another area with sparse information concerns the efficacy of EGFR inhibition in the adjuvant postoperative setting. RTOG 0234 is a recently completed study that examined the feasibility and efficacy of combining Cetuximab with different CRT combinations (either concurrent weekly cisplatin or concurrent weekly docetaxel) in high-risk postoperative patients (positive primary site surgical margin and/or ≥ 2 involved nodes, and/or extracapsular extension).9 Preliminary toxicity data were reported at the 2007 American Society of Therapeutic Radiology and Oncology (ASTRO) meeting and suggested an acceptable toxicity profile with either cisplatin or docetaxel. Grade 3–4 mucositis was reported at 36.4% for the cisplatin and 32.1% for the docetaxel group. The acute grade 4/5 non-hematologic toxicity rate was less than expected based on prior post-operative CRT trials; 10.6% for the cisplatin arm and 6.2% for the docetaxel arm. Outside of a clinical trial, however, this approach should be considered experimental.
Does Tumor Location Matter?
Approximately 60% of the patients in Bonner’s trial had oropharynx (OP), 25% had larynx (LX) and 15% had hypopharynx (HP) carcinomas. This tumor distribution pattern is similar to those found in several international phase III CRT trials.10,11 Although tumor site was not a predefined stratification factor in this study, subset analyses were performed and reported for individual tumor sites. The hazard ratios suggested that OP tumors derived the most benefit from cetuximab while HP tumors derived the least. These data should be interpreted cautiously, as this is a subset analysis from a non-predefined factor. A follow-up subgroup analysis of the patients with HP and LX tumors revealed that there was a trend for better larynx preservation in cetuximab treated patients with a hazard ratio of 0.62 and an absolute improvement of 8% at 3-years (from 80% for the RT alone arm to 88% for the RT + Cetuximab arm), though the study was not powered for this endpoint, and the confidence interval included 1.0.12 Clearly, LX and HP cancers behave differently than OP primaries. Separate clinical trials should be conducted for individual tumor sites and should incorporate their molecular profiles and clinical behaviors into the study design.
One of the most significant findings in the last decade is the association between the human papillomavirus (HPV) and the development of OP tumors.13,14 The increasing knowledge regarding HPV-related tumors, specifically their different molecular profiles and prognosis, require re-interpretation of past trials and should be utilized in the design of new studies. Both retrospective and prospective studies have shown that HPV (+) tumors fare significantly better than HPV (−) tumors when treated with RT, chemotherapy and even surgery alone.13,15–18 The Eastern Cooperative Group (ECOG) conducted a phase II study, E2399, in which patients with locally advanced LX and OP cancers received two cycles of induction paclitaxel and carboplatin chemotherapy, followed by concomitant weekly intravenous paclitaxel and standard fractionation RT. Initial reports revealed that patients with OP tumors had a 20% greater 2-year survival than those with LX cancers (83% vs. 63%).19 However, when they evaluated the association between HPV tumoral status and treatment outcomes in these patients, the main improvement in survival was related to HPV status rather than the tumor site. Sixty-three percent of the OP tumors were HPV (+), but none of the LX cancers were. At a median follow-up of 39.1 months, patients with HPV (+) tumors had a 2-year overall survival of 95% compared to 62% for HPV (−) tumors (P =.005).20
What are the underlying reasons for the better outcome in HPV+ patients? An intact apoptotic response to chemoradiation due to fewer p53 mutations and functional p16INK4a may provide a partial explanation. 21 Yet, this phenomenon cannot explain the better prognosis when these tumors receive surgery alone.15 Other postulated reasons for the improved outcomes for HPV (+) tumors include the lack of field cancerization and enhanced immune surveillance.21 To date, no definitive explanation has been given for this observed better outcome.
We have evaluated the relationship between HPV status and other molecular prognostic markers, including tumor oxygenation, EGFR levels, phospho-EGFR expression and intratumoral lymphocyte infiltration in a group of 82 HNSCC patients. We found that HPV (+) tumor in general had significantly lower total EGFR and phospho-EGFR expression by immunohistochemistry and higher level of intratumoral lymphocytes (manuscript under review). The relationship between HPV status and EGFR expression and activation suggest that HPV (+) tumors may respond differently to EGFR targeted therapy than HPV (−) tumors. Work is currently on going in our laboratory to test this hypothesis.
The findings regarding HPV-related tumors have generated several provocative questions that need to be addressed. Can HPV (+) OP tumors be treated with radiation alone or in combination with biologically targeted therapy without the need for chemotherapy? Do HPV (+) cancers in different head and neck locations behave the same or differently? Do patients with HPV (+) tumors have a different metastatic profile than HPV (−) ones and thus require a less intensive systemic treatment? Conversely, should therapeutic intensification trials be restricted to patients with HPV (−) disease? Future clinical trial design will need to segregate HPV (+) and (−) tumors in order to shed some light on these important issues.
Duration of Targeted Therapy
Most studies to date have integrated biologically targeted therapy concurrently with RT or CRT. Several studies initiated the targeted treatment several days before the onset of definitive therapy based on the pharmacokinetics of the individual drug in order to achieve a stable drug level in the blood and the tumor and to facilitate correlative studies to assess biological changes to individual drugs (pharmacogenomic studies). However, preclinical data suggest that continuing certain targeted therapy, such as cetuximab, following completion of RT may be of benefit, as these drugs can target other relevant biological pathways such as the DNA repair pathway.22 ECOG has recently completed a phase II study of RT + cisplatin + cetuximab delivered concurrently, followed by adjuvant cetuximab for up to 6 months in patients with unresectable HNSCC. The result of this study will be compared to that reported by Adelstein et al to determine whether the addition of concurrent and adjuvant cetuximab is beneficial.11 An ongoing international phase III study of patients with high-risk postoperative features randomizes them to receive RT + cisplatin +/− Lapatinib (EGF102988). In this study, the concurrent CRT is followed by either lapatinib or placebo maintenance for one year (personal communication Iman El-Hariry, GSK). Chen et al studied the feasibility of maintenance gefitinib for 2 years (250 mg daily) in a phase I study, evaluating toxicity and outcomes in patients treated with gefitinib and RT or CRT for locally advanced HNSCC.23 Fifteen patients started maintenance gefitinib, and eight (53%) experienced grade 1–2 acne-like skin rash and diarrhea, but no grade 3 or 4 toxicity occurred. Six patients completed 2 years of maintenance gefitinib, whereas 9 discontinued for various reasons (three for disease progression and six for personal reasons or toxicity).
The sobering results recently reported by the Southwest Oncology Group (SWOG) for patients with stage III non-small cell lung cancers (NSCLC) remind us that adjuvant treatment with targeted therapy should be proven in a randomized setting and not based on encouraging preclinical and phase I–II results. In this large trial, patients were treated with standard concurrent CRT followed by 3 cycles of adjuvant docetaxel, then randomized to receive either gefitinib or placebo until disease progression, intolerable toxicity, or 5 years.24 Surprisingly, the overall survival of the gefitinib group was significantly lower than that of the placebo group (median survival of 23 months for Gefitinib and 35 months for placebo, p=0.013). Most of the deaths were related to tumor recurrence or progression. No increase in fatal toxicity was observed in the gefitinib patients so the results remain perplexing. These data strongly indicate that the role of maintenance treatment with biologically targeted therapy is anything but proven and requires further study in controlled settings.
Targeted Therapies and Induction Chemotherapy
Is there a role for biologically targeted therapies as part of an induction or neoadjuvant treatment for locally advanced HNSCC? Induction chemotherapy is under active investigation again based on the positive survival data reported in the US and Europe using a taxane-platinum-5Fluorouracil (TPF) platform prior to definitive RT.10,25 Kies et al have reported early results incorporating cetuximab with induction weekly paclitaxel and carboplatin for 6 cycles, showing an 83% complete response rate (CR) observed prior to initiation of local therapy in a phase II trial.26 The majority of the 47 patients entered had OP primaries. The CR rate in the neck was only 27% with a 70% partial response (PR). Concepts under discussion at the RTOG include phase II trials that would evaluate induction chemotherapy followed by concurrent CRT or cetuximab plus RT. A similar design is being tested by the EORTC using their successful TPF induction platform combined with cetuximab prior to CRT for locally advanced HNSCC patients. Combining gefitinib with induction chemotherapy prior to CRT has also been investigated with encouraging early outcomes at 1 year.27 The results that emerge from these trials will need to be viewed within the context that the benefit of adding induction chemotherapy to a concurrent chemoradiation platform has not been proven. Several randomized phase III trials comparing induction chemotherapy followed by concurrent chemoradiation to chemoradiation are ongoing.
What are the Toxicities of EGFR Targeted Therapies?
As the use of cetuximab in the management of solid cancers becomes more widespread, more is becoming known about its toxicity. Higher rates of cetuximab-related anaphylactic reactions have been reported from different parts of the country.28,29 Chung et al reported that 25 of 76 Cetuximab treated patients at their institution developed hypersensitivity reactions based on the National Cancer Institute Common Toxicity Criteria (NCI CTC version 3): 13 grade 1–2 and 12 grade 3–4.29 This rate is much higher than the 3% reported rate on the drug’s product label. Further analyses revealed that 17/25 patients with Cetuximab hypersensitivity reaction have IgE antibodies against a specific sugar moiety (galactose-α-1,3-galactose) on the Fab portion of Cetuximab heavy chain compared to 1/51 patients without reaction (p < 0.001).29 The prevalence of this IgE antibody appears to be region specific with 21% rate found in samples from Tennessee compared to 6% in samples from Northern California and < 1% in samples from Boston. These data suggest that testing for this specific IgE levels should be carried out in patients considered for Cetuximab, especially if they are from the southern US. Such rare reactions to targeted therapy are unlikely to be seen in smaller phase I-II or even in large phase III studies and are often only noted when patients are monitored vigorously post drug approval. These reactions should be reported through established regulatory channels such as Medwatch.
Predictors of Response to Targeted Therapy
Data from randomized trials showed that only a subset of HNSCC patients benefited from EGFR targeted therapy either as monotherapy or in combination with RT or chemotherapy.1,30,31 Consequently, it is critical to develop biomarker assays to help identify those who would benefit from anti-EGFR therapies. Such biomarkers would facilitate the selection of optimal treatment regimens with maximal clinical benefit and reduced toxicities. The development of a rash to Cetuximab has been consistently reported to predict for better responses and survival in HNSCC patients.30–32 However, this clinical predictor requires that all patients be started on the drug; patients with a rash would then continue on the same schedule whereas those without would be switched to a different regimen or receive dose escalation until rash develops. Such an approach can be quite costly from both monetary and toxicity standpoints. A better approach is needed.
EGFR expression by IHC has not directly correlated with Cetuximab responses or survival. In fact, in an ECOG study that randomized patients with recurrent/metastatic HNSCC to Cisplatin +/− Cetuximab, those with low-to-moderate tumoral EGFR IHC expression appeared to have a higher response rates (27%) than those with high staining intensity and density (9%).30 Moreover, a difference in responses between the 2 treatment arms was observed only for EGFR low-to-moderate patients but not for EGFR-high patients and survivals did not differ by EGFR expression. Increased EGFR gene copy number by gene amplification or high polysomy by fluorescence in situ hybridization (FISH) has been shown to associate strongly with worse recurrence-free survival and overall survival in HNSCC patients treated with conventional therapy; however, these parameters have not been tested in patients treated with EGFR inhibitors.33 Although activating mutations in the catalytic domain of EGFR are relatively common in a subset of lung cancer patients, they are rare in HNSCC patients and are not a useful predictor of response to anti-EGFR monoclonal antibody therapies.10
At the present time, investigators are actively seeking biologic factors that may predict for response and resistance to anti-EGFR therapies. One potential factor is the epithelial-mesenchymal transition (EMT), which is a cellular process involved in development of distant spread. As cancer cells undergo EMT progression, the loss of proteins involved in cell junctions such as E-cadherin and the claudins occur, and the expression of mesenchymal markers such as vimentin increase. Chung et al showed that the genes involved in EMT and nuclear factor-kappaB (NF-κB) signaling deregulation are the most prominent molecular characteristics of the high-risk HNSCC by gene array studies.33 Frederick et al have shown that increased protein expression of vimentin combined with the loss of E-cadherin, claudin 4, and claudin 7 by immunoblotting correlated with gefitinib resistance in both HNSCC and NSCLC cell lines.34 Related was the loss of Ca2+-independent cell-cell adhesion molecules EpCAM and TROP2 in resistant cells. Similar findings have been reported by Iwata et al in relation to erlotinib sensitivity in preclinical studies.35 The tumor specimens from RTOG 0234 will provide a valuable opportunity to validate these findings on tumor specimens from patients who have received Cetuximab.
Active efforts are also being focused on directly imaging the in situ distribution of anti-EGFR antibodies in tumors. Our group at Stanford has imaged cetuximab and panitumamab in HNSCC xenografts using Dota-conjugated antibodies. Preliminary data suggested that the distribution of these antibodies in tumors does not correlate directly with EGFR expression but is more a function of microvessel density distribution. Work is ongoing to determine whether the different patterns of antibody distribution in HNSCC xenografts can predict cetuximab and radiation treatment response.
Novel Directions for Targeted Therapy
The inhibition of EGFR signaling to enhance radiation cytotoxicity in HNSCC has laid the foundation for a paradigm shift in our approach to this disease. Challenges, however, still exist. Local failure in the cetuximab trial was approximately 35–40% at 5 years, and it is not clear why some patients do not respond to this combination. Where do we go next? One approach is to build on the EGFR inhibition platform by adding additional biologically targeted agents. A logical pathway to exploit is the angiogenic-signaling pathway. Vascular endothelial growth factors (VEGFs) are critical secreted molecules that can stimulate tumoral angiogenesis. High levels of VEGF and COX-2 have been associated with enhanced tumor dissemination and worse survival.36,37 In addition, enhanced VEGF expression was correlated with resistance to anti-EGFR inhibitors.38 These data provided mechanistic support for targeting the VEGF pathway in combination with either conventional therapy or anti-EGFR therapy. Earlier clinical studies attempting to bypass angiogenic signaling with EGFR inhibition included a phase I trial by Wirth et al, combining COX-2 inhibition with EGFR-TKI in patients with recurrent and/or distant metastatic SCCHN. Patients were treated with escalating doses of gefitinib 250 mg – 500 mg once daily and celecoxib at 200 – 400 mg BID. The regimen was safe with dose limiting toxicities (DLTs) and common toxicities included acneform rash, diarrhea, dyspepsia and anemia. Of the 18 evaluable patients, 4 achieved confirmed PRs (22%, 95% CI 2% to 42%).39
More recently, positive phase III clinical trials for bevacizumab, a monoclonal antibody against VEGF, in colorectal, NSCLC and renal cell carcinomas have spurred interests in its application to HNSCC.40–42 A phase I clinical trial of bevacizumab, 5-Flourouracil, hydroxyurea and concomitant hyperfractionated RT was performed in 43 patients with locally advanced or recurrent HNSCC.43 A dose level of 10 mg/m2 every 2 weeks with reduced dose chemotherapy was found to be tolerable and a randomized phase II study is on going to evaluate the efficacy of bevacizumab in a lower risk HNSCC patient population. The combination of bevacizumab and erlotinib has also been tested in patients with recurrent/metastatic HNSCC in phase I and II studies.44 Since no DLTs was noted, a phase II randomized study was conducted using the highest dose level of 15 mg/kg of bevacizumab every 3 weeks concurrently with oral daily erlotinib (150 mg/day); patients were randomized to receive bevacizumab on either day 1 or day 15 of the daily scheduled erlotinib dose. Although 3 serious hemorrhages were noted, only one was fatal and it appeared that the death was not related to the study drug. These studies suggest that targeting the VEGF pathway either with traditional CRT or anti-EGFR therapy is feasible.
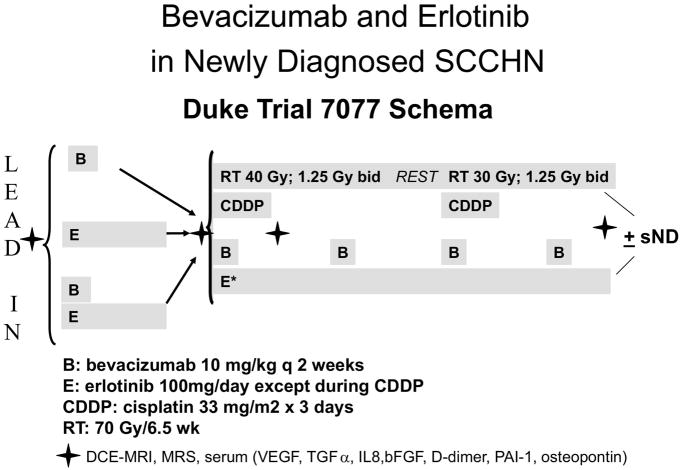
Brizel et al at Duke University are therefore conducting a Phase II study of erlotinib, bevacizumab and concurrent cisplatin-based CRT in patients with locally advanced HNSCC (Figure 2). Preliminary results suggested that this approach is feasible with no unexpected toxicity (personal communication David Brizel). This study also investigates the role of dynamic contrast enhanced (DCE) MRI and several circulating biomarkers including VEGF, TGFα, IL8, βFGF, D-dimer, PAI-1 and osteopontin as potential predictors for anti-VEGF and anti EGFR therapy and will provide important biomarker data for future patient selection to such targeted therapies. The RTOG has just completed a scheduled initial toxicity evaluation of RTOG 0615, a phase II trial in which bevacizumab is added to concurrent Cisplatin and RT in patients with locally advanced nasopharyngeal carcinoma. No undue toxicity was observed and the study has been reopened for patient enrollment (personal communication, Nancy Lee).
Figure 2.
Study schema for single institutional study (Duke 7077) evaluating the feasibility of combining bevacizumab and erlotinib in combination with cisplatin-based chemotherapy and split course hyperfractionated radiotherapy in patients with locally advanced HNSCC.
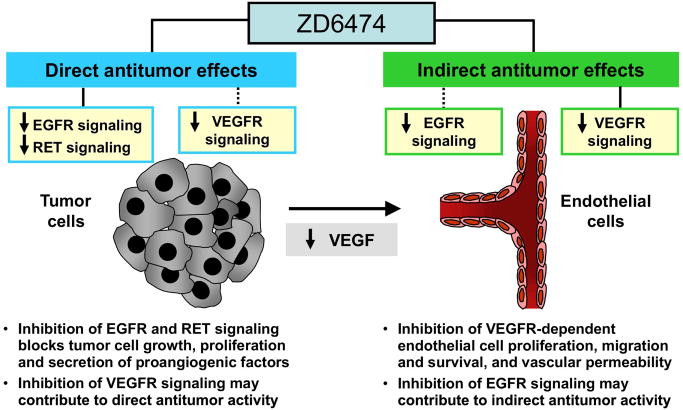
The University of Colorado group, in collaboration with the MD Anderson Cancer Center and the University of Chicago, are conducting a Phase I trial of ZD6474 (Vandetanib, Zactima™), an oral TKI with activity against both EGFR and VEGFR (Figure 3), in combination with either RT alone or RT plus weekly cisplatin (30 mg/m2) in patients with stage III/IVB HNSCC. ZD6474 has shown promising progression free survival data in NSCLC when combined with docetaxel versus docetaxel alone.45 Similarly, the RTOG has submitted a proposal to test ZD6474 in combination with CRT in a randomized Phase II study of HNSCC patients with high-risk post-operative features (involved surgical margin and/or extracapsular extension). For those with intermediate risk features (T3–4 tumor, multiple involved cervical nodes, perineural invasion, lymphovascular invasion), the RTOG is planning a randomized phase II study of RT with an EGFR inhibitor.
Figure 3.
ZD6474: targeting EGFR and VEGFR signalling pathways in cancer
Other Novel Approaches to Consider for HNSCC?
As treatment continues to improve in terms of local-regional control, what can we expect after therapy is completed? What should be done for those patients at highest risk for distant metastasis? Data from the University of Colorado has demonstrated that the distant metastatic rate exceeds 40% in N3 patients.46 Induction chemotherapy is one avenue being explored in the hopes of reducing distant failure; however, this approach can be quite toxic as it adds additional adverse effects above those incurred from CRT.
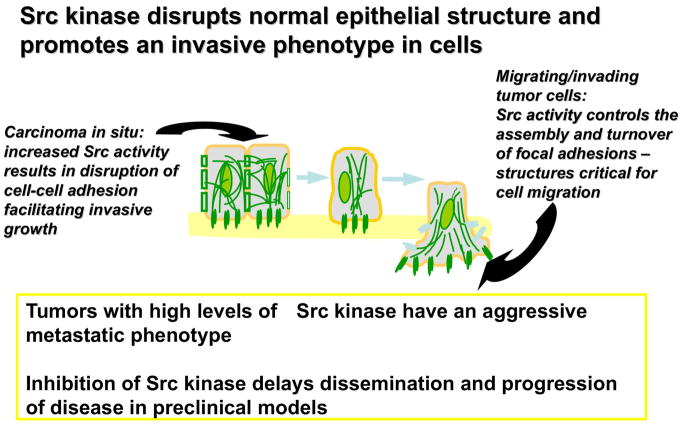
New insights as to mechanisms driving tumor cell invasion and metastasis has prompted investigation of the Src kinase pathway and its role with cancer cell motility and invasion (Figure 4). The Src family of non-receptor tyrosine kinases has been implicated in the development, growth, progression and metastasis of epithelial tumors47 There is also interconnected signaling occurring between Src and EGFR as well as VEGF expression on endothelial cells.48 In HNSCC, Grandis and colleagues have shown that Src activation resulted in constitutive activation of STATs 3 and 5; similarly inhibiting Src activities either pharmacologically or genetically decreased STAT activation and subsequent tumor cell proliferation.49
Figure 4.
The role of Src kinase in cancer progression, invasion and metastasis
Pre-clinical studies provide a rationale for exploring the administration of Src kinase inhibitors in HNSCC. Src kinase inhibition using either a dominant-negative approach or small molecule inhibitors, decreased growth and invasion of HNSCC cells, regardless of EGFR stimulation.50,51 HNSCC invasion is associated in part with the development of invadopodia, a phenomenon whereby cancer cells form plasma membrane-like protrusions that make contact with and facilitate degradation of the extracellular matrix and subsequent invasion invasion2. Dasatinib, an ATP competitive, dual c-Src and Abl kinase inhibitor currently in clinical trials, inhibited migration and invasion in HNSCC and NSCLC cell lines as well as shifting cells into G1 phase.52 The effects of dasatinib on tumor cell migration and invasion correlated with the inhibition of Src and downstream mediators of cell-cell adhesion such as focal adhesion kinase (FAK), p130 and paxillin. Inhibition of c-Src has also been shown to reduce osteoclast activity and reduce bone, nodal and liver metastases in orthotopic pancreatic and ovarian cancer xenograft models.53–56
AZD0530 is another orally bioavailable tyrosine kinase inhibitor with blocking specificity for both Src and Abl kinase. It is currently being evaluated in phase II trials for pancreatic and ovarian cancers. Work from the University of West Virginia and the University of Colorado has demonstrated that AZD0530 can prevent the development of invadopodia. This drug, at the range of 0.01 μM to 10 μM, also inhibited the growth of several HNSCC cell lines in association with less tyrosine phosphorylation of several Src substrates.57
How might this class of agents be best integrated into the management of HNSCC? Src kinase inhibitors may be excellent drugs to study in the adjuvant setting after definitive treatment for locally advanced HNSCC patients, who are at high risk for developing distant metastasis such as those with N2B-N3 nodes. Randomized Phase II studies would be an efficient approach to determine whether these agents can impact tumor spread and distant metastasis. Incorporation of novel serum biomarkers such as cross linked C telopeptides (CTX), a validated marker of bone resorption and a surrogate for osteoclastic activity, might provide early information as to whether the distant metastatic endpoint is being met.
Exploiting differences in DNA repair capacities between normal and cancer cells is another worthy strategy to explore in HNSCC. Downstream of the ataxia telangectasia mutated gene (ATM) is poly(ADP-ribose) polymerase (PARP), a nuclear-based enzyme, that is activated by DNA damage from various insults, including ionizing radiation. PARP helps regulate the repair of these damages through homologous and non-homologous recombination pathways, which include BRCA-1 and BRCA-2 dependent repair and XRCC1/DNA ligase III repair. Interfering with the ability of cancer cells to activate PARP-related repair pathways can enhance the cytotoxic effects of both chemotherapy and radiation in pre-clinical models.58 PARP inhibition has been shown to best enhance radiation effectiveness when cells are cycling through S or G2.59 Pre-clinical screening to identify the best PARP inhibitors for clinical trials showed that many screened compounds were radiosensitizers with relatively little toxicity when administered alone in animals.60 A dose enhancement factor of 8 was observed on fibroblasts when INO-1001, a PARP inhibitor was combined with fractionated RT in vitro.61 A variety of PARP inhibitors are being evaluated in clinical trials including ABT-888 and KU-0059436. The latter appears to have excellent activity in patients with BRCA 1–2 mutations.62 Seven of 44 patients entered into a dose escalation study experienced stabilization of disease, 4 had tumor marker decline with 1 experiencing a PR. Even at doses of 600 mg twice a day, this compound elicited minimal toxicity. One can envision combining RT with EGFR inhibitors that have activity against DNA-PK, a DNA repair enzyme, and PARP inhibitors in locally advanced HNSCC as an way of increasing the therapeutic ratio. Animal studies that compare CRT to this type off approach are needed to give investigators confidence that this strategy is feasible and potentially as efficacious as conventional, highly toxic approaches.
Conclusions
We are now at the next crossroads of biologically targeted therapies and radiation in HNSCC. It is an exciting era for this disease as this is the first time that we have an approved targeted agent to be used in concert with RT. Coupled with this is the rapid development of promising new therapeutics to be combined with either traditional CRT or EGFR inhibition. These new discoveries, however, raise new challenges for translation into the clinic. Issues, such as how best to deliver these targeted agents, the optimal dose and timing in relation to conventional treatments, and the optimal patient profile for such therapies, have yet to be defined. Hopefully some of these questions will be addressed by ongoing clinical trials and their companion translational research. We believe that determining the molecular profile of the tumors is the true driving force for individualizing therapy in HNSCC and that there is a critical need to identify the most effective and least toxic combinations that can be used with RT. Realization of these aims can be best accomplished via clinical trials that incorporate serial novel, non-invasive surrogate endpoints such as molecular makers or imaging methods. As oncologists, our responsibility is to develop and support such trials in order to improve efficacy and reduce toxicity in our patients.
Acknowledgments
Grant Support: This work was support by the National Institute of Health, 1 R01 CA118582-01 & PO1- CA67166 (QTL)
Footnotes
Disclosure: Investigator initiated study from GSK (QTL)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Quynh-Thu Le, Department of Radiation Oncology, Stanford University, 875 Blake Wilbur Dr, MC 5847, Stanford, CA 94305-5847, Tel: 650-498-5032, Fax: 650-725-8231, qle@stanford.edu.
David Raben, Department of Radiation Oncology, University of Colorado Denver, Anschutz Medical Campus, 1665 North Ursula Street, Suite 1032, PO Box 6510, Mail Stop F-706, Aurora, CO 80045.
Bibliography
- 1.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 2.Ayala I, Baldassarre M, Caldieri G, et al. Invadopodia: a guided tour. Eur J Cell Biol. 2006;85:159–64. doi: 10.1016/j.ejcb.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Fu KK, Pajak TF, Trotti A, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48:7–16. doi: 10.1016/s0360-3016(00)00663-5. [DOI] [PubMed] [Google Scholar]
- 4.Overgaard J, Hansen HS, Specht L, et al. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet. 2003;362:933–40. doi: 10.1016/s0140-6736(03)14361-9. [DOI] [PubMed] [Google Scholar]
- 5.Bourhis J, Overgaard J, Audry H, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet. 2006;368:843–54. doi: 10.1016/S0140-6736(06)69121-6. [DOI] [PubMed] [Google Scholar]
- 6.Ang KK, Harris J, Garden AS, et al. Concomitant boost radiation plus concurrent cisplatin for advanced head and neck carcinomas: radiation therapy oncology group phase II trial 99–14. J Clin Oncol. 2005;23:3008–15. doi: 10.1200/JCO.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 7.Bentzen SM, Atasoy BM, Daley FM, et al. Epidermal growth factor receptor expression in pretreatment biopsies from head and neck squamous cell carcinoma as a predictive factor for a benefit from accelerated radiation therapy in a randomized controlled trial. J Clin Oncol. 2005;23:5560–7. doi: 10.1200/JCO.2005.06.411. [DOI] [PubMed] [Google Scholar]
- 8.RTOG 0522: a randomized phase III trial of concurrent accelerated radiation and cisplatin versus concurrent accelerated radiation, cisplatin, and cetuximab [followed by surgery for selected patients] for Stage III and IV head and neck carcinomas. Clin Adv Hematol Oncol. 2007;5:79–81. [PubMed] [Google Scholar]
- 9.Harari PM, Harris J, Kies MS. Phase II Randomized Trial of Surgery Followed by Chemoradiation Plus Cetuximab for High-Risk Squamous Cell Carcinoma of the Head and Neck (RTOG 0234) Int J Radiat Oncol Biol Phys. 2007;69:S13. [Google Scholar]
- 10.Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–15. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 11.Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21:92–8. doi: 10.1200/JCO.2003.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Bonner JA, Harari PM, Giralt J, et al. Improved Preservation of Larynx with the Addition of Cetuximab to Radiation for Cancers of the Larynx and Hypopharynx. J Clin Oncol. 2005;23:5533. [Google Scholar]
- 13.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–20. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 14.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 15.Licitra L, Perrone F, Bossi P, et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2006;24:5630–6. doi: 10.1200/JCO.2005.04.6136. [DOI] [PubMed] [Google Scholar]
- 16.Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus--associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24:736–47. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 17.Mellin H, Friesland S, Lewensohn R, et al. Human papillomavirus (HPV) DNA in tonsillar cancer: clinical correlates, risk of relapse, and survival. Int J Cancer. 2000;89:300–4. [PubMed] [Google Scholar]
- 18.Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121:1813–20. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 19.Cmelak AJ, Li S, Goldwasser MA, et al. Phase II trial of chemoradiation for organ preservation in resectable stage III or IV squamous cell carcinomas of the larynx or oropharynx: results of Eastern Cooperative Oncology Group Study E2399. J Clin Oncol. 2007;25:3971–7. doi: 10.1200/JCO.2007.10.8951. [DOI] [PubMed] [Google Scholar]
- 20.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–9. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 21.Fakhry C, Gillison ML. Clinical implications of human papillomavirus in head and neck cancers. J Clin Oncol. 2006;24:2606–11. doi: 10.1200/JCO.2006.06.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milas L, Fang FM, Mason KA, et al. Importance of maintenance therapy in C225-induced enhancement of tumor control by fractionated radiation. Int J Radiat Oncol Biol Phys. 2007;67:568–72. doi: 10.1016/j.ijrobp.2006.09.044. [DOI] [PubMed] [Google Scholar]
- 23.Chen C, Kane M, Song J, et al. Phase I trial of gefitinib in combination with radiation or chemoradiation for patients with locally advanced squamous cell head and neck cancer. J Clin Oncol. 2007;25:4880–6. doi: 10.1200/JCO.2007.12.9650. [DOI] [PubMed] [Google Scholar]
- 24.Kelly K, Chansky K, Gaspar LE, et al. Phase III Trial of Maintenance Gefitinib or Placebo After Concurrent Chemoradiotherapy and Docetaxel Consolidation in Inoperable Stage III Non-Small-Cell Lung Cancer: SWOG S0023. J Clin Oncol. 2008 doi: 10.1200/JCO.2007.14.4824. [DOI] [PubMed] [Google Scholar]
- 25.Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695–704. doi: 10.1056/NEJMoa071028. [DOI] [PubMed] [Google Scholar]
- 26.Kies MS, Garden AS, Holsinger C. Induction chemotherapy (CT) with weekly paclitaxel, carboplatin and cetuximab for squamous cell carcinoma of the head and neck (HN) J Clin Oncol. 5520;24:2006. [Google Scholar]
- 27.Doss HH, Greco FA, Meluch AA. Induction chemotherapy + gefitinib followed by concurrent chemotherapy/radiation therapy/gefitinib for patients (pts) with locally advanced squamous carcinoma of the head and neck: a phase I/II trial of the Miinie Pearl Cancer Research Network. Proc Am Soc Clin Oncol. A5543:2006. [Google Scholar]
- 28.O’Neil BH, Allen R, Spigel DR, et al. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol. 2007;25:3644–8. doi: 10.1200/JCO.2007.11.7812. [DOI] [PubMed] [Google Scholar]
- 29.Chung CH, Mirakhur B, Chan E, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358:1109–17. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burtness B, Goldwasser MA, Flood W, et al. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol. 2005;23:8646–54. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 31.Vermorken JB, Trigo J, Hitt R, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007;25:2171–7. doi: 10.1200/JCO.2006.06.7447. [DOI] [PubMed] [Google Scholar]
- 32.Fracasso PM, Burris H, 3rd, Arquette MA, et al. A phase 1 escalating single-dose and weekly fixed-dose study of cetuximab: pharmacokinetic and pharmacodynamic rationale for dosing. Clin Cancer Res. 2007;13:986–93. doi: 10.1158/1078-0432.CCR-06-1542. [DOI] [PubMed] [Google Scholar]
- 33.Chung CH, Parker JS, Ely K, et al. Gene Expression Profiles Identify Epithelial-to-Mesenchymal Transition and Activation of Nuclear Factor-{kappa}B Signaling as Characteristics of a High-risk Head and Neck Squamous Cell Carcinoma. Cancer Res. 2006;66:8210–8218. doi: 10.1158/0008-5472.CAN-06-1213. [DOI] [PubMed] [Google Scholar]
- 34.Frederick BA, Helfrich BA, Coldren CD, et al. Epithelial to mesenchymal transition predicts gefitinib resistance in cell lines of head and neck squamous cell carcinoma and non-small cell lung carcinoma. Mol Cancer Ther. 2007;6:1683–91. doi: 10.1158/1535-7163.MCT-07-0138. [DOI] [PubMed] [Google Scholar]
- 35.Thomson S, Buck E, Petti F, et al. Epithelial to mesenchymal transition is a determinant of sensitivity of non-small-cell lung carcinoma cell lines and xenografts to epidermal growth factor receptor inhibition. Cancer Res. 2005;65:9455–62. doi: 10.1158/0008-5472.CAN-05-1058. [DOI] [PubMed] [Google Scholar]
- 36.Kyzas PA, Stefanou D, Agnantis NJ. COX-2 expression correlates with VEGF-C and lymph node metastases in patients with head and neck squamous cell carcinoma. Mod Pathol. 2005;18:153–60. doi: 10.1038/modpathol.3800244. [DOI] [PubMed] [Google Scholar]
- 37.Kyzas PA, Cunha IW, Ioannidis JP. Prognostic significance of vascular endothelial growth factor immunohistochemical expression in head and neck squamous cell carcinoma: a meta-analysis. Clin Cancer Res. 2005;11:1434–40. doi: 10.1158/1078-0432.CCR-04-1870. [DOI] [PubMed] [Google Scholar]
- 38.Viloria-Petit AM, Kerbel RS. Acquired resistance to EGFR inhibitors: mechanisms and prevention strategies. Int J Radiat Oncol Biol Phys. 2004;58:914–26. doi: 10.1016/j.ijrobp.2003.09.091. [DOI] [PubMed] [Google Scholar]
- 39.Wirth LJ, Haddad RI, Lindeman NI, et al. Phase I study of gefitinib plus celecoxib in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2005;23:6976–81. doi: 10.1200/JCO.2005.02.4182. [DOI] [PubMed] [Google Scholar]
- 40.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 41.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–34. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 43.Seiwert TY, Haraf DJ, Cohen EE, et al. Phase I study of bevacizumab added to fluorouracil- and hydroxyurea-based concomitant chemoradiotherapy for poor-prognosis head and neck cancer. J Clin Oncol. 2008;26:1732–41. doi: 10.1200/JCO.2007.13.1706. [DOI] [PubMed] [Google Scholar]
- 44.Vokes E, Cohen EE, Mauer A. A phase I study of erlotinib and bevacizumab for recurrent or metastatic squamous cell carcinoma of the head and neck (HNC) Proc Am Soc Clin Oncol. 2005;23:Abstr #5504. [Google Scholar]
- 45.Heymach JV, Johnson BE, Prager D, et al. Randomized, placebo-controlled phase II study of vandetanib plus docetaxel in previously treated non small-cell lung cancer. J Clin Oncol. 2007;25:4270–7. doi: 10.1200/JCO.2006.10.5122. [DOI] [PubMed] [Google Scholar]
- 46.Ballonoff A, Raben D, Rusthoven KE, et al. Outcomes of Patients with N3 Neck Nodes Treated with Chemoradiation. Laryngoscope. 2008 doi: 10.1097/MLG.0b013e31816a7120. [DOI] [PubMed] [Google Scholar]
- 47.Frame MC. Src in cancer: deregulation and consequences for cell behaviour. Biochim Biophys Acta. 2002;1602:114–30. doi: 10.1016/s0304-419x(02)00040-9. [DOI] [PubMed] [Google Scholar]
- 48.Summy JM, Gallick GE. Treatment for advanced tumors: SRC reclaims center stage. Clin Cancer Res. 2006;12:1398–401. doi: 10.1158/1078-0432.CCR-05-2692. [DOI] [PubMed] [Google Scholar]
- 49.Xi S, Zhang Q, Dyer KF, et al. Src kinases mediate STAT growth pathways in squamous cell carcinoma of the head and neck. J Biol Chem. 2003;278:31574–83. doi: 10.1074/jbc.M303499200. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Q, Thomas SM, Xi S, et al. SRC family kinases mediate epidermal growth factor receptor ligand cleavage, proliferation, and invasion of head and neck cancer cells. Cancer Res. 2004;64:6166–73. doi: 10.1158/0008-5472.CAN-04-0504. [DOI] [PubMed] [Google Scholar]
- 51.Thomas SM, Bhola NE, Zhang Q, et al. Cross-talk between G protein-coupled receptor and epidermal growth factor receptor signaling pathways contributes to growth and invasion of head and neck squamous cell carcinoma. Cancer Res. 2006;66:11831–9. doi: 10.1158/0008-5472.CAN-06-2876. [DOI] [PubMed] [Google Scholar]
- 52.Johnson FM, Saigal B, Talpaz M, et al. Dasatinib (BMS-354825) tyrosine kinase inhibitor suppresses invasion and induces cell cycle arrest and apoptosis of head and neck squamous cell carcinoma and non-small cell lung cancer cells. Clin Cancer Res. 2005;11:6924–32. doi: 10.1158/1078-0432.CCR-05-0757. [DOI] [PubMed] [Google Scholar]
- 53.Rucci N, Susa M, Teti A. Inhibition of protein kinase c-Src as a therapeutic approach for cancer and bone metastases. Anticancer Agents Med Chem. 2008;8:342–9. doi: 10.2174/187152008783961905. [DOI] [PubMed] [Google Scholar]
- 54.Yezhelyev MV, Koehl G, Guba M, et al. Inhibition of SRC tyrosine kinase as treatment for human pancreatic cancer growing orthotopically in nude mice. Clin Cancer Res. 2004;10:8028–36. doi: 10.1158/1078-0432.CCR-04-0621. [DOI] [PubMed] [Google Scholar]
- 55.Trevino JG, Summy JM, Lesslie DP, et al. Inhibition of SRC expression and activity inhibits tumor progression and metastasis of human pancreatic adenocarcinoma cells in an orthotopic nude mouse model. Am J Pathol. 2006;168:962–72. doi: 10.2353/ajpath.2006.050570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han LY, Landen CN, Trevino JG, et al. Antiangiogenic and antitumor effects of SRC inhibition in ovarian carcinoma. Cancer Res. 2006;66:8633–9. doi: 10.1158/0008-5472.CAN-06-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lopez-Skinner LA, Kelley L, Gatesman-Ammer A. The novel Src/Abl kinase inhibitor AZD0530 inhibits proliferation, invasion and invadopodia formation in head and neck squamous cell carcinoma. 98th AACR Annual Proceedings; Los Angeles. 2007. p. Abstract # 3247. [Google Scholar]
- 58.Calabrese CR, Almassy R, Barton S, et al. Anticancer chemosensitization and radiosensitization by the novel poly(ADP-ribose) polymerase-1 inhibitor AG14361. J Natl Cancer Inst. 2004;96:56–67. doi: 10.1093/jnci/djh005. [DOI] [PubMed] [Google Scholar]
- 59.Noel G, Godon C, Fernet M, et al. Radiosensitization by the poly(ADP-ribose) polymerase inhibitor 4-amino-1,8-naphthalimide is specific of the S phase of the cell cycle and involves arrest of DNA synthesis. Mol Cancer Ther. 2006;5:564–74. doi: 10.1158/1535-7163.MCT-05-0418. [DOI] [PubMed] [Google Scholar]
- 60.Thomas HD, Calabrese CR, Batey MA, et al. Preclinical selection of a novel poly(ADP-ribose) polymerase inhibitor for clinical trial. Mol Cancer Ther. 2007;6:945–56. doi: 10.1158/1535-7163.MCT-06-0552. [DOI] [PubMed] [Google Scholar]
- 61.Brock WA, Milas L, Bergh S, et al. Radiosensitization of human and rodent cell lines by INO-1001, a novel inhibitor of poly(ADP-ribose) polymerase. Cancer Lett. 2004;205:155–60. doi: 10.1016/j.canlet.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 62.Yap TA, Boss DS, Fong PC. First in human phase I pharmacokinetic (PK) and pharmacodynamic (PD) study of KU-0059436 (Ku), a small molecule inhibitor of poly ADP-ribose polymerase (PARP) in cancer patients (p), including BRCA1/2 mutation carriers. J Clin Oncol. 2007;25:3529. [Google Scholar]