Abstract
To investigate the circuitry involved in detecting odorants in the rodent brain, we developed a method using manganese-enhanced MRI (MEMRI) to map the flow of neural information from the olfactory sensory neurons (OSNs) to the central layers of the olfactory bulb. Studies have shown that Mn2+ enters active neurons and is transported anterogradely to axon terminals where it can cross synapses to functionally trace neural networks. Thus, by delivering MnCl2 directly into the nasal cavity of mice and then exposing them to defined odorants, Mn2+ is preferentially taken up by activated OSNs. Using the time course of the MRI signal, we generated maps of Mn2+ accumulation in the olfactory bulb for both glomerular and mitral cell layers. Results demonstrated that overlapping yet distinct enhancement patterns were produced by exposure to either octanal, acetophenone, or carvone. Notably, areas of Mn2+ accumulation in the mitral cell layer were similar to those in the glomerular layer consistent with neural information that passes from specific OSNs to specific mitral cells. Finally, by correlating specific Mn2+ signal peaks to genetically labeled glomeruli that are known to be activated by the odorant octanal, we show that MEMRI maps can be resolved at the level of individual glomeruli.
Introduction
Olfaction plays important roles in reproduction and learning in many mammalian species and understanding how odorants are processed and encoded in the central nervous system is critical to determine the neural basis of these behaviors. The olfactory system of mammals represents a challenge for brain imaging because it is sensitive to many odorant molecules. A given odorant is selectively recognized by a subset of about 1000 different odor receptors (ORs) in the nasal epithelium (Buck and Axel, 1991) and is thus encoded by a combination of activities initiated through those ORs (Malnic et al., 1999). Since olfactory sensory neurons (OSNs) expressing the same ORs form synapses with mitral/tufted (M/T) cells and interneurons in olfactory glomeruli in the glomerular layer of the main olfactory bulb (MOB) in a stereotypic fashion (Mombaerts et al., 1996; Ressler et al., 1994), activation of OSNs elicited by odorants is transformed to a spatial pattern, i.e., an odor map, in the glomerular layer (Buck, 1996). Information is refined by M/T cells involving lateral and feedback inhibitions and then transmitted to the primary olfactory cortex where it is relayed to deeper brain structures such as amygdala (Shipley et al., 1995).
To fully understand coding of odorants in these higher-order brain regions, methods for revealing odorant representation in MOB and olfactory cortex must be improved (for reviews, see Kauer and White, 2001; Korsching, 2002). Several imaging methods have been pursued to map odorant activation in the glomerular layer, such as, 2-deoxyglucose (2-DG) autoradiography (Sharp et al., 1975), c-Fos mRNA expression (Guthrie et al., 1993), optical imaging of intrinsic signals (Rubin and Katz, 1999), calcium indicator dye (Friedrich and Korsching, 1997), voltage-sensitive dye (Friedrich and Korsching, 1998), and functional MRI (Xu et al., 2003; Yang et al., 1998). However, due to the limited spatial resolution and/or field-of-view, these methods either cannot resolve responses of individual glomeruli or cannot detect activity throughout the olfactory system such as in deeper layers beyond the glomerular layer.
Manganese-enhanced MRI (MEMRI) is a new method to map neuronal function and connections (for review, see Koretsky and Silva, 2004). Manganese ion (Mn2+)enters neurons through voltage-gated calcium channels (Drapeau and Nachshen, 1984; Narita et al., 1990) and can be transported anterogradely along axons and can cross synapses (Pautler et al., 2003; Pautler et al., 1998; Sloot and Gramsbergen, 1994; Tjalve et al., 1995). Mn2+ transport across a synapse relies on presynaptic release and postsynaptic uptake, therefore, the amount of Mn2+ transported may change depending on the strength of connections if there is plasticity in a neural system (Van der Linden et al., 2004; van der Zijden et al., 2006). If so, it should be possible to produce quantitative indices of Mn2+ movement through a neural system after an activity-based representation is initiated, and hence map the strongest functional connections through that system. This would supply unique information about neural circuits. A first step in this direction was taken when it was demonstrated that Mn2+ can be transported from the nose of a rodent to the olfactory bulb and the tracing to the olfactory bulb could be modulated by odorants (Pautler and Koretsky, 2002).
Here, we have developed a method using MEMRI which capitalizes on the activity dependent uptake and trans-synaptic transport of Mn2+ to generate maps of functional circuitry. These maps are shown to be specific for different odorants and can be generated simultaneously from the glomerular and mitral cell layers of the mouse MOB. Through comparison with optical images of green fluorescent protein (GFP)-labeled glomeruli we show that this Mn2+ mapping technique can detect individual glomeruli. These results demonstrate the potential of using the unique properties of MEMRI to produce maps at layer and column specific resolution.
Materials and methods
Transgenic mouse line
To test the sensitivity to individual glomeruli, a genetically altered mouse strain, rI7→M71, was used because the rI7 odorant receptor is known to respond to the odorant octanal. The mouse was created by replacing the mouse M71 odorant receptor coding sequence with the rat I7 odorant receptor coding sequence and engineered to co-express both GFP and β-galactosidase as markers using tandem IRES sequences (for details see Bozza et al., 2002). All mice were of mixed (129 x C57BL/6) background. The mice were bred in-house with males removed from breeder cages prior to parturition.
Animal preparation
All animal experiments followed the NIH guidelines and were approved by the Animal Care and Use Committee of the National Institute of Neurological Disorders and Stroke, National Institutes of Heath (Bethesda, MD, USA). Adult male C57BL/6 mice and rI7→M71 transgenic mice (body weights 20 – 32 g) were used. The C57BL/6 mice were divided into four groups: control (N = 7), octanal stimulation (N = 8), carvone stimulation (N = 5) and acetophenone stimulation (N = 6). The rI7→M71 transgenic mice (N = 6) were only stimulated by octanal.
The animal was anesthetized by 5% isoflurane (in 1:1:1 mixture of air: nitrogen: oxygen), removed from the induction chamber, and fixed in an upright position. Before it woke up, 7-μL aqueous solution of 10 mM MnCl2 (Sigma-Aldrich Co, MO, USA) was rapidly injected into each nostril using a 20-μL micropipette. After the mouse awoke (usually about 20 sec after Mn2+ injection), it was put into an empty clean cage. For the odor stimulation groups, 7-μL of 1:10 diluted octanal, carvone, or acetophenone were dropped at each of the four corners in the cage. For the control group, nothing was given in the clean cage. After exposure to the odor or air for 20 min, the mouse was removed from the cage and anesthetized by isoflurane for MRI.
For better visualization and segmentation of the glomerular and mitral cell layers of the MOB, 120-mM isotonic MnCl2 solution was infused into the tail veins by a syringe pump (Cole-Parmer Instrument, IL, USA) in 2 mice with a dosage of 88 mg/kg and an infusion rate of 250 μL/h (Aoki et al., 2004; Lee et al., 2005). After that, mice were returned to their cages with free access to food and water. High-resolution MRI was performed 24 h after MnCl2 infusion.
MRI data acquisition
Images were acquired on an 11.7 T/31 cm horizontal magnet (Magnex Scientific Ltd., Abingdon, UK) equipped with a 9-cm gradient set (Resonance Research Inc, Billerica, MA, USA) that can provide 60-G/cm strength and 80-μs rise time, and interfaced to a Bruker Avance console (Bruker BioSpin, Billerica, MA, USA). A homemade 9-cm birdcage volume coil was used for RF transmission and a 1-cm surface coil, which was placed right above the MOB, was used for signal reception. The mouse was placed in a custom designed plastic stereotaxic holder with a tooth bar and ear bars to immobilize the head. The anesthesia (1 – 1.5% isoflurane mixed with air) was delivered through a nose-cone and the body temperature was maintained by a temperature-controlled water bath. Time series, T1-weighted MRI covering the MOB were acquired by 3D rapid acquisition with relaxation enhancement (RARE) sequence. With a repetition time/echo time = 300/10 ms, matrix size = 128 × 128 × 64, and RARE factor of 2, 3D volumes of 100-μm isotropic spatial resolution were obtained every 20 min 30 s. The 3D imaging usually began 40 to 60 min after MnCl2 injection and lasted for 1.5 to 2.5 h, corresponding to 4 to 8 volumetric images in each mouse. The mice received systemic MnCl2 infusion were imaged by T1-weighted 3D RARE (TR/TE = 450/11 ms) with 60-μm isotropic resolution.
Fluorescent imaging
Fluorescent images were collected using a Zeiss LSM-510-Meta confocal attached to an Axioscope 2 microscope (Carl Zeiss Inc., NY, USA). Olfactory epithelium and bulb sections were imaged with an Achroplan 20X/0.45 objective. Fluorophores used were: GFP, excitation 488, emission 507; Alexa-488, excitation 495, emission 519; Cy3, excitation 552, emission 570. Brightfield whole mount images were collected using a Leica MZFL3 microscope (Leica Microsystems Inc., IL, USA) equipped with a Spot-RT cooled CCD camera (Diagnostic Instruments Inc., MI, USA).
Odor map
MRI data were processed and analyzed by custom written software running in Matlab (MathWorks Inc, MA, USA) and public domain tools. To reduce possible residual sub-voxel movement, the time series 3D images of each mouse were realigned to the last image by a 6-parameter rigid-body transformation using SPM2 to minimize the sum of squared difference between two images (Friston et al., 1995). To reduce possible variation caused by system instability, for each time frame, the signal of each voxel was normalized by the signal in a reference area. Since the deep regions of the MOB should not have much manganese enhancement, the signal there was chosen as an internal reference. The intensity normalization also eliminated the difference in signal gain among mice.
Because neurons activated by an odorant would take up MnCl2 faster than other neurons, the projections of these active olfactory neurons into the glomerular layer of the bulb should have a higher signal increase within a certain period of time. Therefore, the integral of the signal time course of each voxel can be used to represent the level of Mn2+ in a specific region:
where Si is the signal intensity of the ith scan and N is the total number of scans. Then an odorant functional circuitry map of individual animal can be obtained by thresholding the area map by a certain positive value.
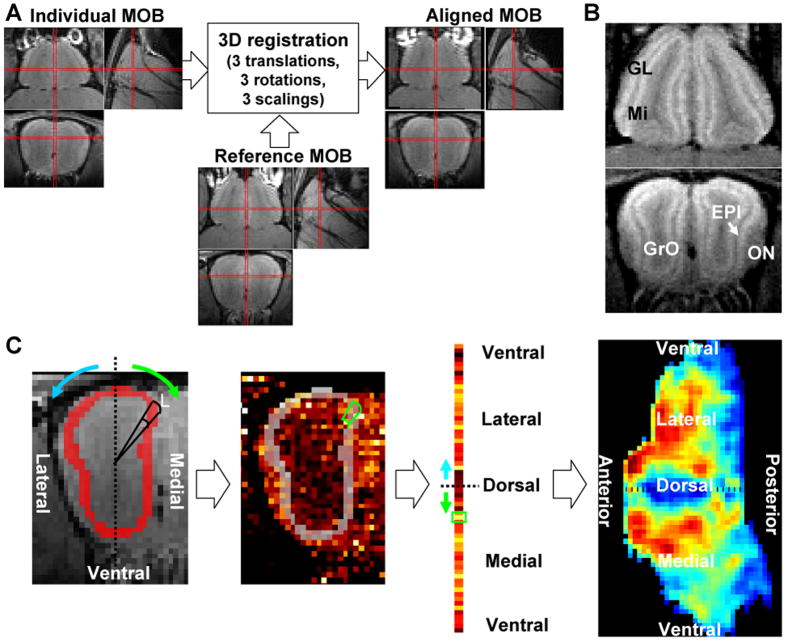
Group averaging
To generate an averaged odor map from animals in the same group and to compare odor maps from different groups, images of the MOB from different animals have to be normalized to the same spatial dimension. We used 9-parameter 3D affine transform, including 3 translations, 3 rotations, and 3 scalings, to co-register the averaged MOB image of each mouse to the image of a chosen mouse. The co-registration was performed by automated image registration (AIR, version 5.2.5) to minimize the standard deviation of ratios between two 3D volumes (Woods et al., 1998). Then, the spatial transformation was applied to the enhancement map using sinc interpolation. To reduce the residual spatial variations after the registration, the data was smoothed by a 3D Gaussian filter with a full-width-at-half-maximum of 100 μm. The group odor map was calculated by averaging the individual maps in each group together. Further more, a statistical map of an odorant was computed voxel-by-voxel using Student t-test with respect to the control maps.
Flattened odor map
To better visualize the enhancement patterns in the glomerular or mitral cell layers, 2D odor maps were created by segmenting and flattening these layers from the averaged 3D odor maps. These layers were identified and manually segmented from the high-resolution layer-enhanced MOB images. The method for flattening each layer is similar to Liu et al. (Liu et al., 2004). Briefly, in each coronal section, the central axis was defined. Starting from the dorsal center toward the ventral center, each layer was partitioned into sectors at every 120-μm arc length along the outer boundary. The averaged intensity in a sector was calculated and arranged on a linear grid. By repeating the same processing slice-by-slice, from anterior-to-posterior, a dorsal-centered 2D odor map can be created. It should be noted that after flattening, the resolution was anisotropic with 100-μm in anterior-posterior and 120-μm in dorsal-ventral directions.
Comparison of odor maps
To compare the enhancement patterns of different odors, the flattened odor maps were thresholded at p < 0.005 (one-tail, uncorrected for multiple comparison). The boundaries of the enhancement were drawn. The distinct enhancement regions between odors were also identified by t-test between the odor maps. Additionally, the flattened glomerular and mitral cell layers were divided into various clusters in a serpentine way from anterior-to-posterior. The averaged signal integral and t-score in each cluster were calculated. To compare the similarity of enhancement patterns between the glomerular and mitral cell layers, the correlation coefficient of the averaged signal enhancement and t-scores between the corresponding clusters in the two layers were calculated. Since the shape and location of clusters in the two layers were slightly different, in a few regions, two clusters were merged to match the closest cluster in the other layer.
Results
Detecting Odorant Responses in the MOB by MEMRI
Figure 1A shows time-series images of a coronal section of a mouse MOB acquired after MnCl2 injection and exposure to 10% octanal for 20 min. The signal intensity in certain regions of the glomerular layer increased continuously from 1 to 2 h and then remained elevated. After enhancement was seen in the glomerular layer, the mitral cell layer also became enhanced. Comparing the signal time courses in the glomerular layer and the central region of the MOB shows that the signal intensity in the glomerular layer increased during the first 2 h after the injection (Figure 1B) while the signal in the center of MOB stayed at a constant level, showing no Mn2+ enhancement in this interval in deep layers of the MOB. Hence its signal can be used as an internal reference for signal intensity normalization. When maps were generated looking for pixels that had significant negative going signal, very few pixels (data not shown) above statistical significance were detected indicating excellent stability of the MRI images and that the reference normalization was working.
Figure 1.
Detecting odor-dependent Mn2+ enhancement in mouse MOB by MRI. (A) Time series images crossing a coronal section of the 3D MRI of a mouse MOB acquired every 20 min after injecting MnCl2 to both nostrils and exposing to octanal. Images show gradual enhancement in the glomerular layer 60 min after the injection. The white rectangle in the sagittal section indicates location of the coronal slice. (B) Signal intensity in a region-of-interest from the glomerular layer (red box in A) increases while signal in a deeper region of the MOB (green box in A) stays at similar level. (C) MEMRI maps after stimulated by acetophenone, carvone, octanal, and control in four mice, respectively, show distributed enhancement in the glomerular layer with each odorant having its own distinct spatial pattern. High signal change at the interface between the olfactory nerve layer and olfactory turbinates (arrow) indicates where Mn2+ flowed in. Scale bars represent 1 mm.
Figure 1C shows the functional circuitry maps calculated from four mice that were exposed to acetophenone, carvone, octanal, and control, respectively. While few enhancement was seen in the control, all three odorants induced high enhancements at the interface between the olfactory nerve layer and olfactory turbinates (arrow in Figure 1C). This is where axons from OSNs enter the MOB. The different odorants elicited a variation in the pattern of glomerular layer enhancement. For example, as shown in Figure 1C, acetophenone enhanced more dorsal medial and lateral areas; carvone enhanced more lateral and ventral medial areas; and octanal enhanced more lateral areas.
Comparison of Odorant Responses in the Glomerular Layer
Figure 2 demonstrates the steps that were taken to register to a common template, segment the glomerular and mitral layers based on anatomical MEMRI, flatten each layer, and generate group average functional circuitry maps. The affine transform effectively reduced the differences in positions and sizes of the MOBs among mice. The glomerular and mitral cell layers enhanced at 24 hours after intravenous administration of MnCl2 was readily detected (Figure 2B) to enable segmentation either manually or semi-automatically.
Figure 2.
Procedures for creating a flattened odor map in the glomerular layer: (A) co-registration, (B) segmentation, and (C) flattening of the segmented layer (Liu et al., 2004). The glomerular layer was manually segmented from high-resolution MRI after systemic infusion of Mn2+ to enhance the layers in the MOB. Both the glomerular layer (Gl) and mitral cell layer (Mi) were enhanced while other layers such as olfactory nerve layer (ON), external plexiform layer (EPl), and, granular cell layer (GrO) were darker. Please see materials and methods for details on creation of a dorsal centered map in the glomerular layer.
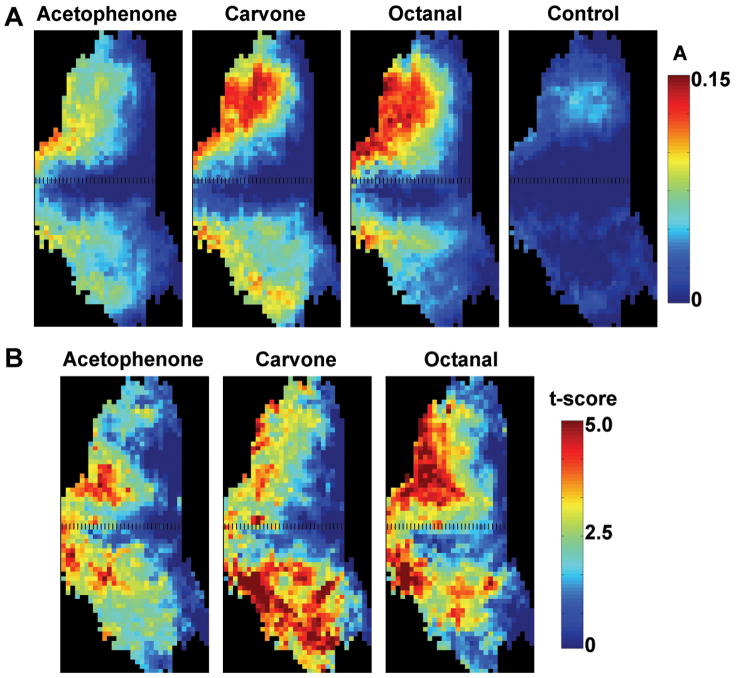
Figure 3A shows the dorsal-centered (indicated by dashed lines), group-averaged enhancement maps in the glomerular layer under acetophenone, carvone, octanal, and clean air (control), respectively. The generally low signal increase in the control group indicates that without odorant stimulation, Mn2+ uptake and transport to the bulb was at a much lower level. The small signal change detected on the lateral side of the control bulbs was likely due to the fact that this is where most of the axons from the OSNs enter into the bulb causing a detectible elevation in Mn2+. By comparison with the control group, the three odorants produced signal changes in different regions, mainly in the anterior, dorsal, lateral, and medial parts of the glomerular layer.
Figure 3.
Group odor maps in the glomerular layer. (A) The dorsal-centered (black dashed lines) glomerular maps of acetophenone (n = 6), carvone (n = 5), octanal (n = 8), and control (n = 7) by averaging the maps in each group. (B) T-score maps of acetophenone, carvone, octanal show significantly enhanced regions common to each odorant.
To eliminate possible bias in the averaged odor map, the t-score between odorant stimulation and the control was calculated (Figure 3B). These maps clearly showed patterns specific to each odorant: acetophenone enhanced more dorsal and medial regions; carvone enhanced more ventral and anteriolateral regions; and octanal enhanced more lateral, medial posterior and some dorsal posterior regions. Furthermore, the symmetrical enhancement patterns in the medial and lateral sides are consistent with the bilateral arrangement of similar glomeruli based upon the molecular map (Lodovichi et al., 2003).
The spatial extents of the significantly enhanced regions (p < 0.005; one-tail, uncorrected) by the three odorants were compared (Figure 4A). Acetophenone enhanced a smaller area than the other two odorants. Acetophenone and octanal had significant overlap in the dorsal regions. Carvone and octanal shared a similar distribution of enhancement pattern in the medial area. Carvone was the only odorant that enhanced a large portion of the ventral area. Comparing the averaged t-score in the clusters in the glomerular layer (Figure 4B) showed that, in general, the enhancement levels of these odorants decreased gradually from anterior toward posterior regions. Acetophenone and octanal had similar enhancement patterns in most of the anterior clusters but with different signal changes. In contrast, carvone had higher enhancements in ventral and posterior regions.
Figure 4.
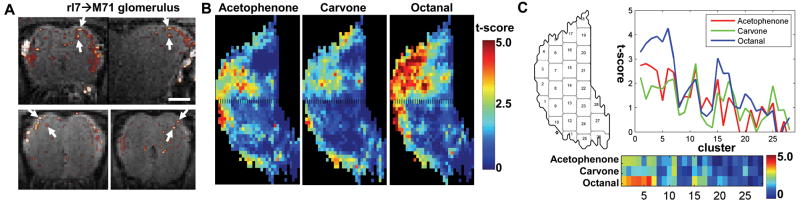
Distributed manganese enhancement in the glomerular layer. (A) Contours of the significantly enhanced regions (p < 0.005, one-tailed t-test) by acetophenone (red), carvone (green), and octanal (blue). (B) For better comparing regional differences between odorants, the glomerular layer was divided into about 30 clusters that were arranged from anterior-to-posterior in a serpentine way. The averaged t-score in each cluster was plotted against the cluster number. (C) The between-odor t-score maps show significant differences between odorants. (AP: acetophenone; CV: carvone; OCT: octanal.)
The distinctly enhanced regions by each odorant were also identified by t-test between the odor maps (Figure 4C). By thresholding the between-odor t-maps at p < 0.05 (one-tail, uncorrected), the area sizes of the significantly enhanced regions are: 12.0% (carvone > octanal), 9.6% (octanal > carvone), 6.0% (carvone > acetophenone), 0.5% (acetophenone > carvone), 12.3% (octanal > acetophenone), and 0.3% (acetophenone > octanal) of the area of the glomerular layer.
MEMRI Detects Single Glomeruli
Figure 5 shows a side-by-side comparison of individual MEMRI maps overlaid onto a projection of the anatomical images with the fluorescent images obtained from excised bulbs after the MRI experiments in three rI7→M71 mice. Due to the expression of GFP, two or three ectopic glomeruli can be identified on the dorsal surface of the MOB (Belluscio et al., 2002; Bozza et al., 2002). Since rI7→M71 glomeruli are not the only glomeruli that respond to octanal, more glomeruli were detected with MEMRI than fluorescence. Arrows indicate the excellent correspondence between glomeruli detected with MEMRI and those that express GFP. 79% (11 out of 14) of the GFP-expressing glomeruli in six rI7→M71 mice studied were detected in the MEMRI maps. This indicates that MEMRI mapping of functional circuitry can be performed at the level of single glomeruli.
Figure 5.
Detection of single glomeruli confirmed by rI7→M71 mice. (A) Horizontal sections of MEMRI maps of rI7→M71 mice overlaid on the projection of the T1-weighted images to mimic the view of the optical images. (B) Fluorescent images acquired in the same animals show the GFP-labeled rI7→M71 glomeruli. Usually two rI7→M71 glomeruli were observed, with one on each MOB. Images from three mice are shown and each row is from the same mouse. The scale bar represents 1 mm.
Odorant Representation in the Mitral Cell Layer could be Mapped by MEMRI
Mn2+ can cross synapses and be transported into post-synaptic neurons, hence output neurons associated with enhanced glomeruli will also accumulate a larger quantity of Mn2+ enabling the detection of functional circuitry into the mitral cell layer. Figure 6A shows examples of MEMRI enhancement maps collected from three of the rI7→M71 transgenic mice. A focal enhancement was clearly observed at about 200 to 300 μm beneath the identified rI7→M71 glomerulus (arrows in Figure 6A). The location and one-to-one correspondence suggest that the pattern of Mn2+ enhancement represents neural information flow into the mitral cell layer.
Figure 6.
Odor maps in the mitral cell layer. (A) Coronal and sagittal sections of MEMRI maps from three rI7→M71 mice (the top two views are from one mouse, the lower left view from another, and the lower right view from the other). Focal enhancements were identified at about 300 μm beneath the rI7→M71 glomeruli (arrows). (B) Dorsal-centered, flattened t-score maps in the mitral cell layer comparing the odorant stimulation groups (acetophenone, carvone, and octanal) with the control group in the wild-type mice. (C) Averaged t-scores in clusters arranged from anterior to posterior. The scale bar represents 1 mm.
The flattened t-score maps of the odorants in the mitral cell layer in the wild-type mice (Figure 6B) shows that although the signal change in the mitral cell layer was generally lower than that in the glomerular layer, the two layers shared similar patterns of enhancement: acetophenone enhanced more anterior dorsal and medial portions; carvone enhanced more laterally and ventrally; and octanal enhanced dorsal and lateral areas. Detailed regional differences between odorants were compared in clusters (Figure 6C).Acetophenone and octanal had similar distributions while octanal had higher signal change in the anterior and dorsal regions. Carvone had less enhancement in regions where octanal showed higher enhancements such as the dorsal regions, while causing greater enhancement in the ventral and posterior area.
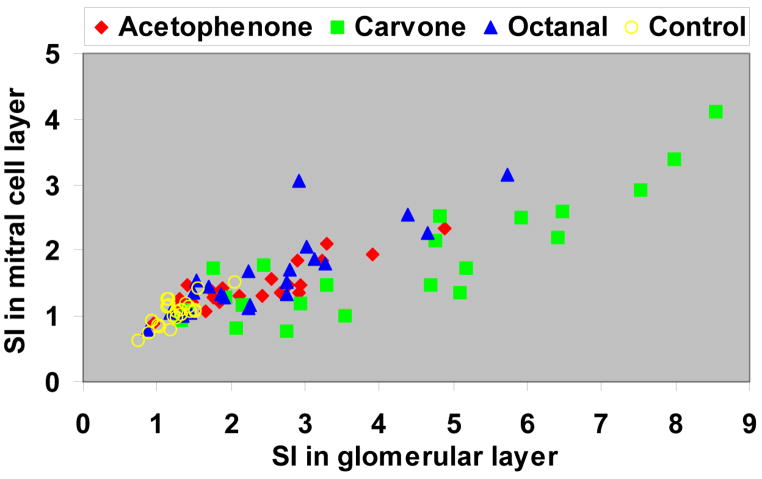
The similarity between the glomerular layer and mitral cell layer maps was further evaluated by correlating the profiles in the corresponding clusters shown in Figure 4B and 6C. High correlation coefficients between the two layers were found under the same odorant (0.56, 0.63, and 0.53 for acetophenone, carvone, and octanal, respectively (p < 0.008)), but not between different odorants. In addition, the enhancement between the corresponding clusters of the two layers showed similar linear trend among all the groups (Figure 7) with a fitted slope of 0.29 (R2 = 0.60).
Figure 7.
The scatter plot of average signal integrals (SI) in the corresponding clusters between the glomerular layer and mitral cell layer.
Discussion
Detection of Individual Glomeruli with MEMRI Functional Circuitry Maps
In this study, we demonstrated that Mn2+ can be used as a functional contrast agent for MRI to generate detailed maps of functional circuitry from both the glomerular and mitral cell layers of the mouse MOB non-invasively. Mn2+ enhanced loci detected after exposure to octanal were highly co-localized with the GFP-expressing rI7→M71 glomeruli. These data clearly show that MEMRI maps can be obtained at the resolution of specific layers and individual glomeruli in the mouse MOB. This represents the first direct comparison, within the same brain, of an MRI functional map with a marked anatomical structure that is known to be activated.
A single glomerulus, which is only about 50 – 100 μm in diameter in mice, is the smallest functional unit to be detected by MRI techniques. Previously, it was shown that fMRI can resolve activation of a single whisker barrel in rats (Yang et al., 1996), orientation columns in cat visual cortex (Kim et al., 2000), ocular dominance columns in human visual cortex (Cheng et al., 2001; Menon et al., 1997), and layers in rat somatosensory cortex (Silva and Koretsky, 2002). However, because of the relatively low resolution and low contrast to noise combined with limitations on resolution associated with vascular regulation, none of these studies have successfully resolved neural structures below ~200 μm isotropic resolution. Moreover, they all relied on averaging the spatial extent of a neural structure either across the entire cortex or along a specific layer.
Many imaging methods have been pursued to detect glomerular activity in rodents while only a few of them can identify single glomeruli (Guthrie et al., 1993; Wachowiak and Cohen, 2001). Studies using fMRI reported the detection of glomerular layer activation (Xu et al., 2000; Xu et al., 2003) but the resolution of these maps and extent of activation indicated that information was from groups of glomeruli. Using the known responding odorant, octanal, of rI7→M71 glomeruli and their known loci under fluorescent microscope in the transgenic mice, we validated the detection of single glomeruli and the detection sensitivity was estimated to be about 0.8. The specificity was not estimated because many other glomeruli were also activated by octanal (for example, it was estimated that 40–90 different receptors can respond to an odorant (Ma and Shepherd, 2000)) but their loci could not be identified. In some maps, only one of the paired rI7→M71 glomeruli was detected. This could be due to difficulties in displaying the curved surface of the bulb as horizontal slices and simply missing the enhanced rI7→M71 glomerulus. Alternatively, it may be that the odorant presentation did not activate all glomeruli. The ability to image functional information form individual glomeruli from the entire bulb raises the very challenging problem of how to analyze and compare this information from animal to animal. It is well known that glomeruli shift from animal to animal and this was clearly seen from the GFP labeled glomeruli in the rI7→M71 mice. Therefore, for further analysis of group averaged maps smoothed data were used.
Implications of Glomerular Enhancement
Results of the MEMRI odor mapping indicate many aspects of olfactory processing. Distributed but similar signal enhancement patterns were observed in the glomerular layer of different animals after exposure to the same odorant while different kinds of odorants enhanced different glomerular patterns, showing that groups of glomeruli responded to individual odorants. This agrees with current understanding that individual odorants are coded by sets of glomeruli and odorants with similar chemical structure activate similar glomeruli (Korsching, 2002; Meister and Bonhoeffer, 2001; Rubin and Katz, 1999). The relatively symmetric enhancement between the medial and lateral sides of the glomerular layer is also in accordance with the symmetric, stereotypic arrangement of glomerular pairs that receive projections from OSNs expressing the same receptor gene (Lodovichi et al., 2003).
Detection of Enhancement in the Mitral Cell Layer
By receiving excitatory inputs from OSNs and inhibitory inputs from periglomerular cells, M/T cells refine and project odorant information to the primary olfactory cortex. Therefore, the activity in the mitral cell layer represents the output of the MOB and is likely quite different from the glomerular activity. Measuring activity to map odor representations in the mitral cell layer has been very challenging. For example, optical imaging-based methods are limited by light penetration depth as well as interference from the glomerular layer which lies above the mitral cell layer. As shown in Figure 6A, the corresponding foci in the mitral cell layer beneath the rI7→M71 glomeruli indicates that Mn2+ moves very specifically into mitral cells in a manner consistent with the flow of neural information into the mitral cell layer. Inspecting the signal time courses in those foci, the signal in the mitral cells increased almost at the same time as the glomeruli. Mn2+ moves at rates consistent with it being transported by fast axonal transport (Pautler et al., 1998), therefore, the time for Mn2+ to move the short distance from the glomerular layer to mitral cell layer is faster than the temporal resolution used. Faster imaging methods will be needed to resolve the transport between layers.
There was excellent correlation between areas enhanced in the glomerular maps and areas enhanced in the mitral maps. Many specific glomeruli sending manganese to specific portions of the mitral cell layer could be detected. However, it will require higher temporal and spatial mapping to make quantitative conclusions about the specific flow of Mn2+ at this level of resolution. The data indicates that we can detect single glomeruli and the evidence of focal enhancement in the mitral cell layer has us optimistic this can be achieved. Furthermore, it has been shown in olfactory tract tracing experiments that Mn2+ can be transported to the granule cell layer as well as the olfactory cortex when applied locally to the bulb (Chuang and Koretsky, 2006; Pautler et al., 1998). Hence, it should be possible to map the representation beyond the mitral cell layer.
Flow of Manganese Could Indicate Flow of Neural Information
The process used in producing MEMRI odor maps represents an innovative way to make functional maps of neural systems. The ability to load a tract tracer into a specific neural representation based on activity is unique. The ability to quantify the flow of the tracer into anterograde regions and across synapses in a noninvasive manner is also unique. This new type of functional connectivity map leads to several interesting questions about interpretation. We propose that the mapping strategy shows the most probable pathway of neural information even though the stimulus is not continuously applied throughout the mapping period. This is accomplished using odorant induced activity to control where Mn2+ initially enters the system. Once introduced, the assumption is that Mn2+ accumulation in the anterograde direction will preferentially highlight downstream pathways that are most strongly connected. This interpretation is based on the fact that Mn2+ transport requires active synapses and the assumption that this transport will follow the flow of activity. Therefore, the maps neither represent purely anatomical information nor moment-to-moment neural activity but rather a combination of both. Future work will be critical to relate what aspects of neural activity/neuroanatomy control the flow of Mn2+.
A major challenge in neuroscience is the ability to follow the path of information flow throughout a specific neural system. For instance, electrodes can be used to measure local action potentials and conventional tract tracers can be used to infer the paths of neural information flow; however, it is very difficult to identify and record from neurons that are connected even across one synapse in vivo (Sommer and Wurtz, 2004). Molecular genetic techniques can be envisioned that will accomplish some aspects of the MEMRI neural information flow maps by combining techniques like c-Fos-driven fluorescent imaging of activity (Barth et al., 2004) and transneuronal tracer (Zou et al., 2001) under control of specific promoters. However, an advantage of Mn2+ is that it requires functioning synapses to cross and thus has added information about functional neuronal connectivity and can be used in any animal model without requiring genetic manipulation.
Advantages of MEMRI Odor Mapping
Until now, all other imaging methods for mapping glomerular activation typically involve fairly invasive procedures making it difficult to examine the same animal across a long period of time. In this study, injected Mn2+ was easily absorbed by OSNs in the olfactory epithelium. Hence, there is no need to disturb the blood-brain barrier as in previous activity-induced MEMRI experiments (Aoki et al., 2002; Lin and Koretsky, 1997). This noninvasive protocol will provide many possibilities to study the progression of learning and development. Although the temporal information of neural dynamics can not be resolved due to the slow transport and accumulation of Mn2+, the detection of on-going signal enhancement after stimulation has ceased allows experiments to be performed in awake, normal behaving animals outside the MRI magnet. While there are some caveats associated with repeated imaging experiments such as the lifetime of the Mn2+ in the bulb (2 to 3 weeks; data not shown), adjustments can be made to reduce Mn2+ concentrations and shorten this period.
By comparing odorant-exposed groups and controls with a group t-test, the confounding effects of background odorants that are common to both groups, such as anesthetic odor, can be reduced. Isoflurane has a very strong odor even at the low level (1%) used for this study. The smell of the anesthetic will likely activate OSNs and change Mn2+ uptake and enhancement patterns in the bulb. However, since the anesthetic is similar in both the control and stimulation groups, this confounding effect was removed by performing a t-test between groups.
Potential Shortcoming of MEMRI Functional Circuitry Maps
One major concern of using free Mn2+ as a contrast agent is its possible neurotoxicity (Barbeau, 1984). Although the concentration used is low and produced no observable behavioral abnormalities, it is still possible that Mn2+ in the nostrils may cause damage to the OSNs thereby changing the resulting map. One way to ameliorate this problem is to use a lower concentration of Mn2+ together with a more sensitive T1 mapping sequence to compensate for the reduced signal contrast (Chuang and Koretsky, 2006). Another problem is the way of delivering Mn2+ may affect the observed maps because variations in the spread of Mn2+ in the convoluted turbinates make it difficult to ensure that the Mn2+ solution reaches each zone equally. One solution to this issue is to vaporize Mn2+ solution and allow the animal to breathe the contrast agent into the nose (Pautler and Koretsky, 2002).
Conclusion
Using the unique properties of Mn2+ which is taken up preferentially in active neurons and then transported anterogradely across synapses, enables MRI to produce functional connectivity maps that are consistent with the flow of neural information from the OSNs to the glomerular and mitral cell layers. These maps have sensitivity at the level of single glomeruli and should allow tracing neural representations through different brain structures in a noninvasive manner in individual animals before and after manipulations that effect learning, plasticity, development, or degeneration.
Acknowledgments
We would like to thank Dr. Peter Mombaerts for the rI7→M71 mice. Dr. Hellmut Merkle and Dr. Stephen J. Dodd for MRI hardware support. This research was supported by the Intramural Research Program of the NINDS, NIH.
Footnotes
part of the results had been presented in the International Society for Magnetic Resonance in Medicine 14th Scientific Meeting & Exhibition, Seattle, Washington, USA, 6-12 May 2006
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoki I, Tanaka C, Takegami T, Ebisu T, Umeda M, Fukunaga M, Fukuda K, Silva AC, Koretsky AP, Naruse S. Dynamic activity-induced manganese-dependent contrast magnetic resonance imaging (DAIM MRI) Magn Reson Med. 2002;48:927–933. doi: 10.1002/mrm.10320. [DOI] [PubMed] [Google Scholar]
- Aoki I, Wu YJ, Silva AC, Lynch RM, Koretsky AP. In vivo detection of neuroarchitecture in the rodent brain using manganese-enhanced MRI. Neuroimage. 2004;22:1046–1059. doi: 10.1016/j.neuroimage.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Barbeau A. Manganese and extrapyramidal disorders (a critical review and tribute to Dr. George C. Cotzias) Neurotoxicology. 1984;5:13–35. [PubMed] [Google Scholar]
- Barth AL, Gerkin RC, Dean KL. Alteration of neuronal firing properties after in vivo experience in a FosGFP transgenic mouse. J Neurosci. 2004;24:6466–6475. doi: 10.1523/JNEUROSCI.4737-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluscio L, Lodovichi C, Feinstein P, Mombaerts P, Katz LC. Odorant receptors instruct functional circuitry in the mouse olfactory bulb. Nature. 2002;419:296–300. doi: 10.1038/nature01001. [DOI] [PubMed] [Google Scholar]
- Bozza T, Feinstein P, Zheng C, Mombaerts P. Odorant receptor expression defines functional units in the mouse olfactory system. J Neurosci. 2002;22:3033–3043. doi: 10.1523/JNEUROSCI.22-08-03033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Buck LB. Information coding in the vertebrate olfactory system. Annu Rev Neurosci. 1996;19:517–544. doi: 10.1146/annurev.ne.19.030196.002505. [DOI] [PubMed] [Google Scholar]
- Cheng K, Waggoner RA, Tanaka K. Human ocular dominance columns as revealed by high-field functional magnetic resonance imaging. Neuron. 2001;32:359–374. doi: 10.1016/s0896-6273(01)00477-9. [DOI] [PubMed] [Google Scholar]
- Chuang KH, Koretsky A. Improved neuronal tract tracing using manganese enhanced magnetic resonance imaging with fast T(1) mapping. Magn Reson Med. 2006;55:604–611. doi: 10.1002/mrm.20797. [DOI] [PubMed] [Google Scholar]
- Drapeau P, Nachshen DA. Manganese fluxes and manganese-dependent neurotransmitter release in presynaptic nerve endings isolated from rat brain. J Physiol. 1984;348:493–510. doi: 10.1113/jphysiol.1984.sp015121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich RW, Korsching SI. Combinatorial and chemotopic odorant coding in the zebrafish olfactory bulb visualized by optical imaging. Neuron. 1997;18:737–752. doi: 10.1016/s0896-6273(00)80314-1. [DOI] [PubMed] [Google Scholar]
- Friedrich RW, Korsching SI. Chemotopic, combinatorial, and noncombinatorial odorant representations in the olfactory bulb revealed using a voltage-sensitive axon tracer. J Neurosci. 1998;18:9977–9988. doi: 10.1523/JNEUROSCI.18-23-09977.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Human Brain Mapping. 1995;3:165–189. [Google Scholar]
- Guthrie KM, Anderson AJ, Leon M, Gall C. Odor-induced increases in c-fos mRNA expression reveal an anatomical “unit” for odor processing in olfactory bulb. Proc Natl Acad Sci U S A. 1993;90:3329–3333. doi: 10.1073/pnas.90.8.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JS, White J. Imaging and coding in the olfactory system. Annu Rev Neurosci. 2001;24:963–979. doi: 10.1146/annurev.neuro.24.1.963. [DOI] [PubMed] [Google Scholar]
- Kim DS, Duong TQ, Kim SG. High-resolution mapping of iso-orientation columns by fMRI. Nat Neurosci. 2000;3:164–169. doi: 10.1038/72109. [DOI] [PubMed] [Google Scholar]
- Koretsky AP, Silva AC. Manganese-enhanced magnetic resonance imaging (MEMRI) NMR Biomed. 2004;17:527–531. doi: 10.1002/nbm.940. [DOI] [PubMed] [Google Scholar]
- Korsching S. Olfactory maps and odor images. Curr Opin Neurobiol. 2002;12:387–392. doi: 10.1016/s0959-4388(02)00348-3. [DOI] [PubMed] [Google Scholar]
- Lee JH, Silva AC, Merkle H, Koretsky AP. Manganese-enhanced magnetic resonance imaging of mouse brain after systemic administration of MnCl2: dose-dependent and temporal evolution of T1 contrast. Magn Reson Med. 2005;53:640–648. doi: 10.1002/mrm.20368. [DOI] [PubMed] [Google Scholar]
- Lin YJ, Koretsky AP. Manganese ion enhances T1-weighted MRI during brain activation: an approach to direct imaging of brain function. Magn Reson Med. 1997;38:378–388. doi: 10.1002/mrm.1910380305. [DOI] [PubMed] [Google Scholar]
- Liu N, Xu F, Marenco L, Hyder F, Miller P, Shepherd GM. Informatics approaches to functional MRI odor mapping of the rodent olfactory bulb: OdorMapBuilder and OdorMapDB. Neuroinformatics. 2004;2:3–18. doi: 10.1385/NI:2:1:003. [DOI] [PubMed] [Google Scholar]
- Lodovichi C, Belluscio L, Katz LC. Functional topography of connections linking mirror-symmetric maps in the mouse olfactory bulb. Neuron. 2003;38:265–276. doi: 10.1016/s0896-6273(03)00194-6. [DOI] [PubMed] [Google Scholar]
- Ma M, Shepherd GM. Functional mosaic organization of mouse olfactory receptor neurons. Proc Natl Acad Sci U S A. 2000;97:12869–12874. doi: 10.1073/pnas.220301797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- Meister M, Bonhoeffer T. Tuning and topography in an odor map on the rat olfactory bulb. J Neurosci. 2001;21:1351–1360. doi: 10.1523/JNEUROSCI.21-04-01351.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon RS, Ogawa S, Strupp JP, Ugurbil K. Ocular dominance in human V1 demonstrated by functional magnetic resonance imaging. J Neurophysiol. 1997;77:2780–2787. doi: 10.1152/jn.1997.77.5.2780. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Narita K, Kawasaki F, Kita H. Mn and Mg influxes through Ca channels of motor nerve terminals are prevented by verapamil in frogs. Brain Res. 1990;510:289–295. doi: 10.1016/0006-8993(90)91379-u. [DOI] [PubMed] [Google Scholar]
- Pautler RG, Koretsky AP. Tracing odor-induced activation in the olfactory bulbs of mice using manganese-enhanced magnetic resonance imaging. Neuroimage. 2002;16:441–448. doi: 10.1006/nimg.2002.1075. [DOI] [PubMed] [Google Scholar]
- Pautler RG, Mongeau R, Jacobs RE. In vivo trans-synaptic tract tracing from the murine striatum and amygdala utilizing manganese enhanced MRI (MEMRI) Magn Reson Med. 2003;50:33–39. doi: 10.1002/mrm.10498. [DOI] [PubMed] [Google Scholar]
- Pautler RG, Silva AC, Koretsky AP. In vivo neuronal tract tracing using manganese-enhanced magnetic resonance imaging. Magn Reson Med. 1998;40:740–748. doi: 10.1002/mrm.1910400515. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. Information coding in the olfactory system: evidence for a stereotyped and highly organized epitope map in the olfactory bulb. Cell. 1994;79:1245–1255. doi: 10.1016/0092-8674(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Rubin BD, Katz LC. Optical imaging of odorant representations in the mammalian olfactory bulb. Neuron. 1999;23:499–511. doi: 10.1016/s0896-6273(00)80803-x. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Kauer JS, Shepherd GM. Local sites of activity-related glucose metabolism in rat olfactory bulb during olfactory stimulation. Brain Res. 1975;98:596–600. doi: 10.1016/0006-8993(75)90377-7. [DOI] [PubMed] [Google Scholar]
- Shipley MT, McLean JH, Ennis M. Olfactory System. In: Paxions G, editor. The Rat Nervous System. Academic Press; 1995. pp. 899–926. [Google Scholar]
- Silva AC, Koretsky AP. Laminar specificity of functional MRI onset times during somatosensory stimulation in rat. Proc Natl Acad Sci U S A. 2002;99:15182–15187. doi: 10.1073/pnas.222561899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloot WN, Gramsbergen JB. Axonal transport of manganese and its relevance to selective neurotoxicity in the rat basal ganglia. Brain Res. 1994;657:124–132. doi: 10.1016/0006-8993(94)90959-8. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. What the brain stem tells the frontal cortex. I. Oculomotor signals sent from superior colliculus to frontal eye field via mediodorsal thalamus. J Neurophysiol. 2004;91:1381–1402. doi: 10.1152/jn.00738.2003. [DOI] [PubMed] [Google Scholar]
- Tjalve H, Mejare C, Borg-Neczak K. Uptake and transport of manganese in primary and secondary olfactory neurones in pike. Pharmacol Toxicol. 1995;77:23–31. doi: 10.1111/j.1600-0773.1995.tb01909.x. [DOI] [PubMed] [Google Scholar]
- Van der Linden A, Van Meir V, Tindemans I, Verhoye M, Balthazart J. Applications of manganese-enhanced magnetic resonance imaging (MEMRI) to image brain plasticity in song birds. NMR Biomed. 2004;17:602–612. doi: 10.1002/nbm.936. [DOI] [PubMed] [Google Scholar]
- van der Zijden JP, Wu O, van der Toorn A, Roeling TP, Bleys RL, Dijkhuizen RM. Changes in neuronal connectivity after stroke in rats as studied by serial manganese-enhanced MRI. Neuroimage. 2006 doi: 10.1016/j.neuroimage.2006.11.001. in press. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Cohen LB. Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron. 2001;32:723–735. doi: 10.1016/s0896-6273(01)00506-2. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. J Comput Assist Tomogr. 1998;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- Xu F, Kida I, Hyder F, Shulman RG. Assessment and discrimination of odor stimuli in rat olfactory bulb by dynamic functional MRI. Proc Natl Acad Sci U S A. 2000;97:10601–10606. doi: 10.1073/pnas.180321397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Liu N, Kida I, Rothman DL, Hyder F, Shepherd GM. Odor maps of aldehydes and esters revealed by functional MRI in the glomerular layer of the mouse olfactory bulb. Proc Natl Acad Sci U S A. 2003;100:11029–11034. doi: 10.1073/pnas.1832864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Hyder F, Shulman RG. Activation of single whisker barrel in rat brain localized by functional magnetic resonance imaging. Proc Natl Acad Sci U S A. 1996;93:475–478. doi: 10.1073/pnas.93.1.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Renken R, Hyder F, Siddeek M, Greer CA, Shepherd GM, Shulman RG. Dynamic mapping at the laminar level of odor–elicited responses in rat olfactory bulb by functional MRI. Proc Natl Acad Sci U S A. 1998;95:7715–7720. doi: 10.1073/pnas.95.13.7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z, Horowitz LF, Montmayeur JP, Snapper S, Buck LB. Genetic tracing reveals a stereotyped sensory map in the olfactory cortex. Nature. 2001;414:173–179. doi: 10.1038/35102506. [DOI] [PubMed] [Google Scholar]