Abstract
Evidence suggests that the neural system associated with face processing is a distributed cortical network containing both bottom-up and top-down mechanisms. While bottom-up face processing has been the focus of many studies, the neural areas involved in the top-down face processing have not been extensively investigated due to difficulty in isolating top-down influences from the bottom-up response engendered by presentation of a face. In the present study, we used a novel experimental method to induce illusory face detection. This method allowed for directly examining the neural systems involved in top-down face processing while minimizing the influence of bottom-up perceptual input. A distributed cortical network of top-down face processing was identified by analyzing the functional connectivity patterns of the right fusiform face area (FFA). This distributed cortical network model for face processing includes both “core” and “extended” face processing areas. It also includes left anterior cingulate cortex (ACC), bilateral orbitofrontal cortex (OFC), left dorsolateral prefrontal cortex (DLPFC), left premotor cortex, and left inferior parietal cortex. These findings suggest that top-down face processing contains not only regions for analyzing the visual appearance of faces, but also those involved in processing low spatial frequency (LSF) information, decision making, and working memory.
Keywords: top-down processing, psychophysiological interaction (PPI), distributed cortical network, fMRI, face processing
Introduction
The processing of visual information involves not only bottom-up mechanisms, such as the cascade of cortical regions that analyze increasingly complex information based on retinal input, but also top-down mechanisms. Top-down mechanisms use existing knowledge and expectations to recognize or interpret ambiguous sensory information quickly and correctly [25, 26, 37, 38, 42]. A series of studies have been carried out to determine where top-down control signals come from [29]. For example, a recent study proposed that the medial frontal cortex might contain a face template that sends top-down signals to face-sensitive brain areas, which then compare visual input against the template to detect faces [37]. Using a face imagery task, it has been found that posterior cortical regions involved in face processing are modulated by top-down signals originating in prefrontal cortex [20, 28]. Taken together, these findings suggest that the prefrontal cortex may be the source of the top-down mechanisms involved in face processing.
Converging evidence from functional neuroimaging and neuropsychological research has revealed that face processing is mediated by a distributed bottom-up cortical network. This network includes a “core” system associated with processing invariant and dynamic facial information and an “extended” system involved in further processing of faces in concert with other neural systems [7, 11, 12, 14, 18]. However, the neural activation patterns induced by pure top-down mechanism have not been studied in depth and the relationship between top-down face processing and these core and extended face areas is unknown. Therefore, the goal of the present study is to investigate the neural systems for top-down face processing by minimizing bottom-up information. The results of this investigation may complement the previously identified bottom-up distributed neural network for face perception and thus allow for a more comprehensive understanding of the neural mechanisms of face processing.
Because experiments typically involve actual images of faces, most studies of top-down face processing fail to isolate top-down effects from the activations engendered by strong bottom-up visual input. To minimize contamination from bottom-up input, we used a novel experimental paradigm that induces illusory detection of faces while viewing complex noisy images; unbeknownst to participants, the presented images are pure noise, and contain no systematic information [42]. Thus, any particular image for a face detection response looks no different from the images that fail to produce face detection. It is only in averaging thousands of face-detected pure noise images that the subtle physical properties that promote illusory face detection are revealed. Thus, the neural response patterns on face detection trials are almost entirely attributable to top-down face processing. Our recent study using this method revealed that the FFA plays a crucial role in top-down face processing [42]. However, this finding was obtained by simply subtracting the activation patterns on face detection trials from non-detection trials, which is a method that cannot reveal interactions between regions. In the present study, a psychophysiological interaction (PPI) analysis was used to investigate the interregional functional connectivity. By analyzing PPI maps, we sought to identify the complex network involved in the top-down control of face processing.
Materials and Methods
Twelve right-hand healthy subjects (five women, age=23.8 ± 1.4), with normal or corrected-to-normal vision participated in this study. The Human Research Protection Program of Tiantan Hospital approved this study. All participants provided written informed consent prior to their participation in the study.
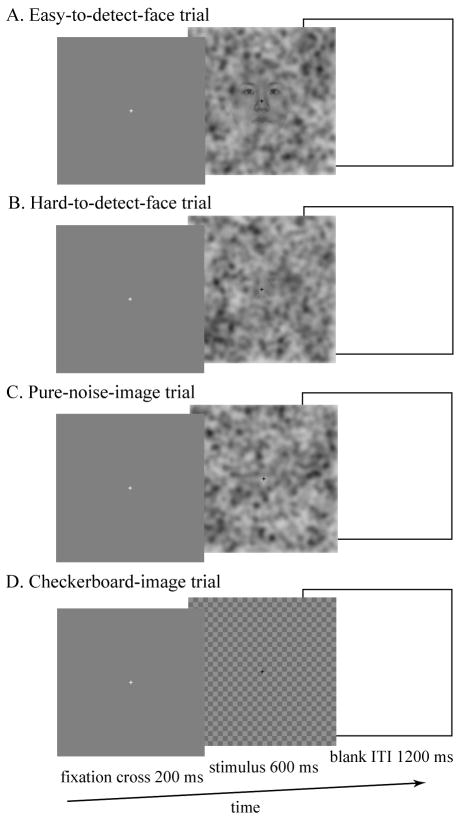
Four types of stimuli were used: face images overlaid with 50% noise (Figure 1, A), face images overlaid with 75% noise (Figure 1, B), pure noise image (Figure 1, C), and checkerboard images (Figure 1, D). The experiment began with a session designed to train participants in the task of progressively more difficult face detection. The training session consisted of 6 56-s blocks, each of which included 8 checkerboard-image null trials and 20 task trials in three phases (2 blocks per phase). In the first phase, half of the task trials presented faces overlaid with 50% noise while the other half presented pure noise images. In the second phase, half of the task trials contained faces overlaid with 75% noise while the other half presented pure noise images. The third phase of training presented pure noise images on every task trial. Participants were instructed that half of all task trials contained faces and that the task would become progressively more difficult. They were told to press a button on a response device with their left or right finger (counterbalanced across subjects) when they detected a face in the image. For each trial, the image was presented for 600 ms after a 200-ms fixation cross, followed by a blank screen for 1200 ms (Figure 1). Following training, four testing sessions ensued, each of which contained 40 checkerboard trials that were used as control null trials and 120 pure-noise-image task trials. The procedure for these test trials was the same as the third phase of training that only contained noise images. During the checkerboard trials, no responses were required.
Fig. 1.
The sequence of displays in a trial (left to right) and examples of the four types of stimuli used in the experiment (A to D). During training, the first phase used image 50% noise image stimuli (A) for face present trials. The second phase used 75% noise image stimuli (B) for face present trials. Finally, by the end of training, all trials were pure noise (C), although participants were instructed that half of the trials still contained faces. Checkerboard images were used as controls (D).
Structural and functional fMRI data were collected using a 3.0 T MR imaging system (Siemens Trio a Tim, German) at Tiantan Hospital. The functional fMRI series was collected using a single shot, T2*-weighted gradient-echo planar imaging (EPI) sequence (TR/TE = 2000/30 ms; 32 slices; 4 mm thickness; matrix = 64×64) covering the whole brain with a resolution of 3.75×3.75 mm. High-resolution anatomical scans were acquired with a three-dimensional enhanced fast gradient-echo sequence, recording 256 axial images with a thickness of 1 mm and a resolution of 1×1 mm.
Spatial preprocessing and statistical mapping of fMRI data analysis were conducted with SPM5 (http://www.fil.ion.ucl.ac.uk/spm/). The first three functional scans of data were discarded to allow for signal saturation. Scans were corrected for differences in slice time acquisition and for motion effects by sinc interpolation, normalized using transformation parameters derived from the high resolution anatomical image co-registered to MNI template, and smoothed using a 6 mm full-width-at-half-maximum (FWHM) Gaussian smoothing kernel. Analysis was based on the AR(1) model. Voxel-by-voxel t-tests were applied on the first level analysis, which used a fixed-effects model. For the second-level analysis, one-sample t-tests were used to explore the brain connectivity pattern.
In light of earlier studies that examined the main effect of faces (activation evoked by face-detection compared to non-detection responses), this study focused on face-sensitive regions in the fusiform and occipital regions. Many previous studies have identified the fusiform face area (FFA) and the occipital face area (OFA) [3, 10, 21, 39]. Following analysis of these areas to define the volume of interest (VOI), the current study used Psychophysiological interaction (PPI) to identify physiological activity from other areas involved in the process of illusory face detection [9, 36]. Thus, PPI computed whole-brain connectivity between the time series of the seed VOI and the time series of all other voxels. As documented in previous studies, the right FFA plays a crucial role in perception of face processing [7, 21, 22]; therefore the right FFA was used as a seed region in the PPI analysis in this illusory face detection task to identify the neural network for top-down processing. In the present study, the seed region was a 4-mm-radius sphere centered on the most significant right FFA voxel in the main effect analysis, and the interaction term (face-detection vs. non-detection) was used as a regressor or explanatory variable to test significance by a conventional SPM analysis. PPI analysis was carried out for each subject and the resulting images of contrast estimates were entered into a random effect group analysis to determine the regions that showed significant functional connectivity to the seed region. The functional connectivity map was obtained with a threshold of P = 0.005 (uncorrected; T = 3.11) and minimum cluster = 15 voxels and the threshold was determined based on previous studies [4, 37].
Results
First, with regard to the main effect of illusory face detection as measured in fusiform and occipital regions of all 12 subjects after applying a height threshold of P = 0.005 (uncorrected for multiple comparisons), a right fusiform face area (FFA) was identified for all subjects (Talairach coordinate (TAL) = (43±5 −53±4 −13±5)); a left FFA was found for 9 subjects (TAL = (−40±5 −51±6 −13±3)); a right occipital face area (OFA) was found for 8 subjects (TAL = (38±5 −77±7 −9±1)) and a left OFA was found for 5 subjects (TAL = (−35±4 −74±6 −6±3)). The results of this illusory face detection task are consistent with those of previous studies of face processing that contained actual face stimuli. This finding suggests that these posterior regions may be involved not only in bottom-up face processing but also in top-down face processing [33, 35].
Second, we focused on the regions showing significant connectivity to the right FFA based on the activation difference between the face-detection trials and the non-detection trials (P <0.005, uncorrected, k cluster ≥ 15 voxels, see table 1 and Fig. 2). The right FFA showed increased face-detection interactions with several occipito-temporal regions, as well as with the left superior temporal sulcus (STS), bilateral amygdala, left inferior frontal gyrus (IFG), right hippocampus, bilateral orbitofrontal cortex (OFC), left anterior cingulate cortex (ACC), left dorsolateral prefrontal cortex (DLPFC), left inferior parietal lobule (IPL) and left premotor cortex. Most of these same regions have been implicated in previous studies on face processing that used actual face images [7, 11, 12, 14, 18], although in the current case there were no faces, and face detection was illusory.
Table 1.
Brain regions showing significant connectivity to the right FFA.
| Regions | BA | voxel | Z | Talairach
|
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Right Fusiform gyrus | 37 | 201 | 3.43 | 34 | −78 | −12 |
| 2.96 | 44 | −59 | −13 | |||
| L. Fusiform gyrus | 19 | 52 | 2.94 | −28 | −84 | −13 |
| 37 | 60 | 2.81 | −44 | −59 | −13 | |
| L. Inferior Occipital Gyrus | 18 | 25 | 2.99 | 22 | −91 | 8 |
| L. Anterior Cingulate Cortex | 32 | 128 | 3.41 | −16 | 21 | 21 |
| L. Orbitofrontal Cortex | 47 | 65 | 3.61 | 40 | 30 | −11 |
| R. Orbitofrontal Cortex | 47 | 49 | 2.98 | −34 | 21 | −13 |
| L. Superior Temporal Sulcus | 13 | 309 | 3.09 | −51 | −48 | 17 |
| R. Amygdala | 34 | 113 | 3.59 | 28 | 3 | −12 |
| L. Amygdala | 466 | 4.56 | −30 | 1 | −10 | |
| L. Inferior Frontal Gyrus | 9 | 62 | 2.88 | −50 | 3 | 24 |
| L. DLPFC | 9 | 125 | 3.78 | −38 | 27 | 26 |
| L. Inferior Parietal Lobule | 40 | 142 | 2.89 | −46 | −36 | 51 |
| L. Premotor cortex | 6 | 112 | 3.03 | −30 | −9 | 50 |
| L. Caudate | 23 | 3.78 | −12 | 3 | 22 | |
| L. Postcentral Gyrus | 43 | 27 | 3.62 | −55 | −15 | 17 |
| R. Hippocampus | 30 | 3.31 | 28 | −16 | −11 | |
| L. Superior Frontal Gyrus | 9 | 24 | 3.6 | −14 | −52 | 22 |
Coordinates of the peak voxel are shown for each cluster. All activations are significant at p < 0.005 (uncorrected); k cluster ≥ 15 voxels; L, left hemisphere; R, right hemisphere; BA, Brodmann’s area; voxel size is 2 × 2 × 2 mm 3.
Fig. 2.
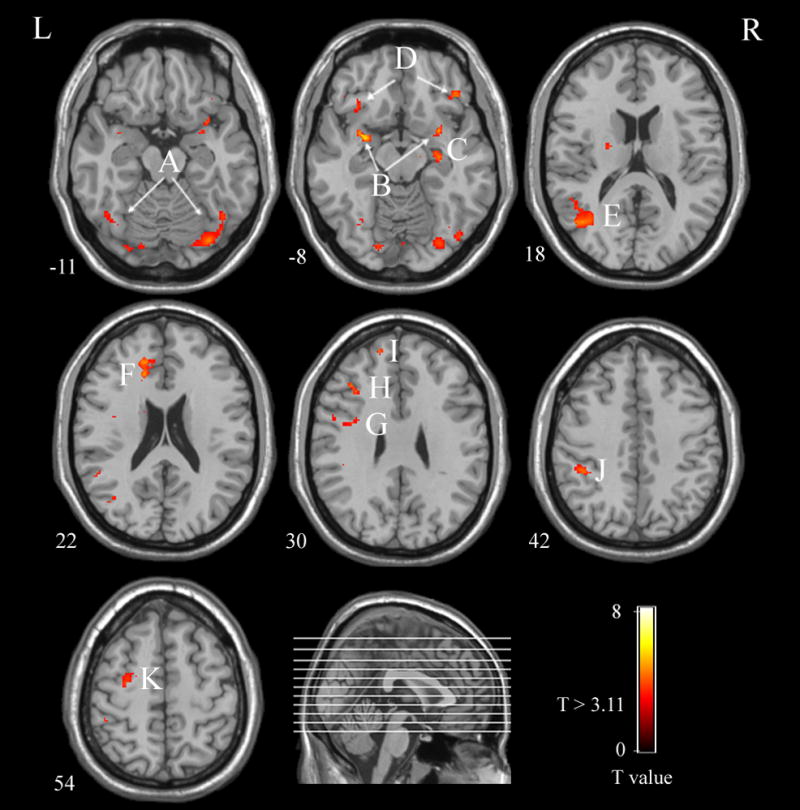
The PPI analysis map. Areas illustrated in this map show a greater covariation with the right FFA activity for illusory face response trials compared to non-face response trials. The threshold was set at T > 3.11 (P < 0.005, uncorrected) and minimum cluster volume = 15 voxels. The color bar represents T values. The numbers beneath each image refer to the z coordinates of Talairach. A, bilateral Fusiform Gyrus; B, bilateral amygdala; C, right hippocampus; D, bilateral Orbitofrontal Cortex; E, left Superior Temporal Sulcus; F, left Anterior Cingulate Cortex; G, left Inferior Frontal Gyrus; H, left Dorsolateral Prefrontal Cortex; I, left Superior Frontal Gyrus; J, left Inferior Parietal Lobule; K, left Premotor Cortex; L, left; R, right;
Discussion
The present study used a novel method that experimentally isolated the influence of the top-down face processing from that of bottom-up input by inducing illusory face detection to pure noise images. Results from a PPI analysis revealed that the brain regions involved in top-down face processing overlapped greatly with the regions reported in previous studies even though previous studies did not control the influence of the bottom-up visual input [7, 11, 12, 14, 18]. For example, the top-down regions identified by our analysis included FFA, IOG and STS, which are considered part of the “core” bottom-up face processing system. In this core system, the fusiform gyrus is responsible for the analysis of invariant features of a face related to the face identity whereas the STS is responsible for the analysis of dynamic features of a face [11, 12, 14, 18]. Our analysis also identified top-down face processing brain regions beyond the ventro-occipital temporal complex, including IFG, hippocampus, and amygdala. Previous studies of face processing that involved actual face images have proposed that these regions are part of the “extended” network of face processing. Furthermore, the IFG has been implicated in semantic aspects of face processing [16, 19, 27] as well as hippocampus mediated memory processing [13, 16, 32]. Others have found that the activation of amygdala is modulated by the valence of faces and facial expressions [5, 8, 17], leading to the conclusion that the amygdala is as a “relevant detector” that serves to provide appraisal of face-related events[34]. In the present study, because all of the regions mentioned above are found to be part of the interregional functional connectivity even though participants only saw pure noise image, the involvement of these regions is attributed to top-down face processing. Thus, the regions previously identified as the core and extended system of faces are not only involved in bottom-up processing in response to actual face images, but also involved in top-down processing such as illusory face detection.
It should be noted that the OFC and ACC had increased functional connectivity to the right FFA during the face-detection trials compared to non-detection trials. OFC has been consistently found to be involved in face processing such as mediating the perception of attractive and sexually relevant faces [15, 24, 30]. With regard to the top-down object processing, recent studies have suggested that OFC also guides a matching process to determine the most probable interpretation of an image based on the global and low spatial frequency (LSF)-based properties of the visual input [1, 2, 25, 26]. A similar mechanism may be at work in the present study where the participants attempted to detect faces in pure noise. With regard to the ACC, many studies have shown that the ACC plays a crucial role in decision-making tasks [6, 23, 41]. The co-activation of the ACC and the OFC in the present study suggests that the ACC is engaged when deciding whether a face is present or absent based on the response provided by the OFC.
The patterns of activation in the DLPFC, premotor cortex and IPL are remarkably similar to those found in previous studies of working memory. As reported in a meta-analysis of working memory neuroimaging studies, DLPFC and premotor cortex respond to the continuous updating of working memory and the maintenance of temporal order memory. In addition, IPL is described as a “buffer for perceptual attributes” [31, 40]. In the present study, these regions, along with the hippocampus, might have been engaged to continuously update and store the face features generated from the existing knowledge via the top-down mechanisms and features extracted from the visual input from the pure noise images to assist the decision of whether one had seen a face.
Conclusions
By using a novel experimental method that induced illusory face detection, the present study examined the neural system involved in the top-down face processing while minimizing the influence of bottom-up perceptual input. We identified a distributed cortical network of top-down face processing by analyzing the functional connectivity patterns of the right fusiform face area (FFA). The identified regions included the “core” and “extended” systems for face processing that were previously identified using actual face images. The identified regions also included left anterior cingulate cortex (ACC), bilateral orbitofrontal cortex (OFC), left dorsolateral prefrontal cortex (DLPFC), left premotor cortex, and left inferior parietal cortex. These findings suggest that the cortical network of the top-down face processing contains not only the regions for analyzing the visual appearance of faces but also those involved in processing low spatial frequencies (LSF) visual information, decision making, and working memory.
Acknowledgments
This paper is supported by the Project for the National Key Basic Research and Development Program (973) under Grant No.2006CB705700, Changjiang Scholars and Innovative Research Team in University (PCSIRT) under Grant No. IRT0645, CAS Hundred Talents Program, CAS scientific research equipment develop program (YZ0642, YZ200766),863 program under Grant No. 2008AA01Z411, the Joint Research Fund for Overseas Chinese Young Scholars under Grant No.30528027, the National Natural Science Foundation of China under Grant No. 30672690, 30600151, 30873462, 30870685, 60532050, 60621001, 90209008, Beijing Natural Science Fund under Grant No. 4071003, and NIH R01 HD046526.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bar M. A cortical mechanism for triggering top-down facilitation in visual object recognition. J Cogn Neurosci. 2003;15:600–609. doi: 10.1162/089892903321662976. [DOI] [PubMed] [Google Scholar]
- 2.Bar M, Kassam KS, Ghuman AS, Boshyan J, Schmid AM, Dale AM, Hamalainen MS, Marinkovic K, Schacter DL, Rosen BR, Halgren E. Top-down facilitation of visual recognition. Proc Natl Acad Sci U S A. 2006;103:449–454. doi: 10.1073/pnas.0507062103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barth H, La Mont K, Lipton J, Dehaene S, Kanwisher N, Spelke E. Non-symbolic arithmetic in adults and young children. Cognition. 2006;98:199–222. doi: 10.1016/j.cognition.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner T, Speck D, Wettstein D, Masnari O, Beeli G, Jancke L. Feeling present in arousing virtual reality worlds: prefrontal brain regions differentially orchestrate presence experience in adults and children. Front Hum Neurosci. 2008;2:8. doi: 10.3389/neuro.09.008.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- 6.Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci U S A. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fairhall SL, Ishai A. Effective connectivity within the distributed cortical network for face perception. Cereb Cortex. 2007;17:2400–2406. doi: 10.1093/cercor/bhl148. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL. Beyond threat: amygdala reactivity across multiple expressions of facial affect. Neuroimage. 2006;30:1441–1448. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 10.Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, Gore JC. Activation of the middle fusiform ‘face area’ increases with expertise in recognizing novel objects. Nat Neurosci. 1999;2:568–573. doi: 10.1038/9224. [DOI] [PubMed] [Google Scholar]
- 11.Gobbini MI, Haxby JV. Neural systems for recognition of familiar faces. Neuropsychologia. 2007;45:32–41. doi: 10.1016/j.neuropsychologia.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 13.Haxby JV, Ungerleider LG, Horwitz B, Maisog JM, Rapoport SI, Grady CL. Face encoding and recognition in the human brain. Proc Natl Acad Sci U S A. 1996;93:922–927. doi: 10.1073/pnas.93.2.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishai A. Let’s face it: It’s a cortical network. Neuroimage. 2008;40:415–419. doi: 10.1016/j.neuroimage.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 15.Ishai A. Sex, beauty and the orbitofrontal cortex. Int J Psychophysiol. 2007;63:181–185. doi: 10.1016/j.ijpsycho.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Ishai A, Haxby JV, Ungerleider LG. Visual imagery of famous faces: effects of memory and attention revealed by fMRI. Neuroimage. 2002;17:1729–1741. doi: 10.1006/nimg.2002.1330. [DOI] [PubMed] [Google Scholar]
- 17.Ishai A, Pessoa L, Bikle PC, Ungerleider LG. Repetition suppression of faces is modulated by emotion. Proc Natl Acad Sci U S A. 2004;101:9827–9832. doi: 10.1073/pnas.0403559101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishai A, Schmidt CF, Boesiger P. Face perception is mediated by a distributed cortical network. Brain Res Bull. 2005;67:87–93. doi: 10.1016/j.brainresbull.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 19.Ishai A, Ungerleider LG, Haxby JV. Distributed neural systems for the generation of visual images. Neuron. 2000;28:979–990. doi: 10.1016/s0896-6273(00)00168-9. [DOI] [PubMed] [Google Scholar]
- 20.Johnson MR, Mitchell KJ, Raye CL, D’Esposito M, Johnson MK. A brief thought can modulate activity in extrastriate visual areas: Top-down effects of refreshing just-seen visual stimuli. Neuroimage. 2007;37:290–299. doi: 10.1016/j.neuroimage.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanwisher N, Yovel G. The fusiform face area: a cortical region specialized for the perception of faces. Philos Trans R Soc Lond B Biol Sci. 2006;361:2109–2128. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kennerley SW, Walton ME, Behrens TE, Buckley MJ, Rushworth MF. Optimal decision making and the anterior cingulate cortex. Nat Neurosci. 2006;9:940–947. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- 24.Kranz F, Ishai A. Face perception is modulated by sexual preference. Curr Biol. 2006;16:63–68. doi: 10.1016/j.cub.2005.10.070. [DOI] [PubMed] [Google Scholar]
- 25.Kveraga K, Boshyan J, Bar M. Magnocellular projections as the trigger of top-down facilitation in recognition. J Neurosci. 2007;27:13232–13240. doi: 10.1523/JNEUROSCI.3481-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kveraga K, Ghuman AS, Bar M. Top-down predictions in the cognitive brain. Brain Cogn. 2007;65:145–168. doi: 10.1016/j.bandc.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leveroni CL, Seidenberg M, Mayer AR, Mead LA, Binder JR, Rao SM. Neural systems underlying the recognition of familiar and newly learned faces. J Neurosci. 2000;20:878–886. doi: 10.1523/JNEUROSCI.20-02-00878.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mechelli A, Price CJ, Friston KJ, Ishai A. Where bottom-up meets top-down: neuronal interactions during perception and imagery. Cereb Cortex. 2004;14:1256–1265. doi: 10.1093/cercor/bhh087. [DOI] [PubMed] [Google Scholar]
- 29.Miller BT, D’Esposito M. Searching for “the top” in top-down control. Neuron. 2005;48:535–538. doi: 10.1016/j.neuron.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 30.O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- 31.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ranganath C, D’Esposito M. Medial temporal lobe activity associated with active maintenance of novel information. Neuron. 2001;31:865–873. doi: 10.1016/s0896-6273(01)00411-1. [DOI] [PubMed] [Google Scholar]
- 33.Rossion B, Caldara R, Seghier M, Schuller AM, Lazeyras F, Mayer E. A network of occipito-temporal face-sensitive areas besides the right middle fusiform gyrus is necessary for normal face processing. Brain. 2003;126:2381–2395. doi: 10.1093/brain/awg241. [DOI] [PubMed] [Google Scholar]
- 34.Sander D, Grafman J, Zalla T. The human amygdala: an evolved system for relevance detection. Rev Neurosci. 2003;14:303–316. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- 35.Steeves JK, Culham JC, Duchaine BC, Pratesi CC, Valyear KF, Schindler I, Humphrey GK, Milner AD, Goodale MA. The fusiform face area is not sufficient for face recognition: evidence from a patient with dense prosopagnosia and no occipital face area. Neuropsychologia. 2006;44:594–609. doi: 10.1016/j.neuropsychologia.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 36.Stephan KE. On the role of general system theory for functional neuroimaging. J Anat. 2004;205:443–470. doi: 10.1111/j.0021-8782.2004.00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Summerfield C, Egner T, Greene M, Koechlin E, Mangels J, Hirsch J. Predictive codes for forthcoming perception in the frontal cortex. Science. 2006;314:1311–1314. doi: 10.1126/science.1132028. [DOI] [PubMed] [Google Scholar]
- 38.Summerfield C, Egner T, Mangels J, Hirsch J. Mistaking a house for a face: neural correlates of misperception in healthy humans. Cereb Cortex. 2006;16:500–508. doi: 10.1093/cercor/bhi129. [DOI] [PubMed] [Google Scholar]
- 39.Tong F, Nakayama K, Moscovitch M, Weinrib O, Kanwisher N. Response properties of the human fusiform face area. Cognitive Neuropsychology. 2000;17:257–280. doi: 10.1080/026432900380607. [DOI] [PubMed] [Google Scholar]
- 40.Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- 41.Walton ME, Croxson PL, Behrens TE, Kennerley SW, Rushworth MF. Adaptive decision making and value in the anterior cingulate cortex. Neuroimage. 2007;36(Suppl 2):T142–154. doi: 10.1016/j.neuroimage.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang H, Liu J, Huber DE, Rieth CA, Tian J, Lee K. Detecting faces in pure noise images: a functional MRI study on top-down perception. Neuroreport. 2008;19:229–233. doi: 10.1097/WNR.0b013e3282f49083. [DOI] [PubMed] [Google Scholar]