Abstract
Background
Iodine deficiency disorders (IDD) constitute significant public health problems in parts of the world with poor iodine nutrition, but have been eradicated in North America and other regions. We herein report three cases of iodine deficiency disorders (IDD) which occurred in women living in iodine-replete environments.
Methods
The clinical presentation, biochemical findings, and radiological features of the patients were analyzed and presented in three case reports. The radiological features are illustrated in sonographic and scintigraphic images. A literature review and discussion which highlight the risk factors, pathogenesis, ancillary investigations and rational treatment of iodine deficiency goiter and hypothyroidism are provided.
Results
All of our three patients were young females, aged 24–38 years, who had goiter. Two of them presented with goitrous hypothyroidism. Radioactive iodine scintigraphy showed a characteristic finding of diffusely increased uptake (in the absence of clinical and biochemical evidence of hyperthyroidism). This scintigraphic pattern was found to be pathognomonic. Dietary iodine supplementation alone resulted in complete remission of IDD in the subjects, including the two patients with hypothyroidism.
Conclusion
IDD can occur in iodine replete-environments. A high index of suspicion is needed to recognize these cases. It is pertinent that the correct diagnosis be made to avoid unwarranted life-long thyroxine therapy in patients presenting with goiter and hypothyroidism, which is easily treatable with iodized salt. These cases underscore the need for considering iodine deficiency in the etiologic diagnosis of goiter and hypothyroidism, even in iodine sufficient regions.
Keywords: Iodine deficiency, Goiter, Hypothyroidism, Urinary iodine
Introduction
Iodine deficiency disorders (IDD) remain major public health problems in parts of the world where iodine intake is inadequate. Over 1.9 billion people are estimated to be at the risk of IDD worldwide (1,2,3). Fortunately, these conditions were eradicated in North America over 50 years ago (4). Following the pioneering work of David Marine in Ohio (5), iodized salt was introduced in the United States in 1922 to prevent endemic goiter. Iodized salt became widely available in North America in the late 1930s. Although, the US adopts a policy of voluntary iodization, the majority of persons living in the US use iodized salt (5,6,7).
The National Health and Nutrition Examination Surveys (NHANES), determined that iodine nutrition in the U.S. was adequate (6). However, the survey showed more than 50% reduction in the median urinary iodine (UI) concentration (320 to 145 µg/L) between 1971 and 1994 (6,8). A latter study, which showed median UI of 168 µg/L confirmed adequate iodine intake (8). However, there has been concern that subjects who adhere to restricted diets or have increased iodine requirement (pregnant and lactating women) may be at risk of IDD (9,10). Reports of proven cases of IDD in North America are extremely rare, with only one case documented in the literature since the eradication of IDD (5). The subject, a 6-year-old Canadian boy with multiple food allergies developed iodine deficiency goiter and hypothyroidism due to dietary restriction (5). In a previous brief communication, we highlighted 2 cases of IDD (11). In this report, we present 3 patients (including a fuller description of the two patients in reference 11), 2 of whom were lifetime US residents.
Case Reports
Case 1
A 34 year-old African-American woman was referred to the endocrine clinic for hypothyroidism. She complained of cold intolerance, constipation and tiredness. The referring physician confirmed hypothyroidism biochemically (Table 1), however, a radioactive iodine (RAI) study he ordered showed increased uptake, which was reported as “consistent with hyperthyroidism” (Figure 1). The perplexing finding of hypothyroidism and increased radioiodine uptake triggered the referral to endocrinology. Dietary history revealed that patient avoided iodized salt for several years “to prevent hypertension”. Also, she rarely ate fish, or used dairy products. She denied any dietary allergies. She had a normal pregnancy and delivery a year prior to presentation. She gave no past or family history of thyroid disease.
Table 1.
Clinical, biochemical and radiographic characteristics of patients.
| Case | Age (years) | Sex | TSH (µIU/ml) | Thyroxine | TPO (iu/ml) | Neck exam | Thyroid Ultrasound | Thyroid RAI* uptake and scan. | Urinary iodine (µg/24hr)** |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 34 | F | 52.9 | Total ¶ 3.4. µg/dl | <10 | Nontender diffuse goiter | N/A | Enlarged,diffuse gland. Uptake 42% | 31 |
| 2 | 38 | F | 0.74 | Free 1.09 ng/dl | <10 | Nontender, diffuse | Diffusely thyromegaly | N/A | undetectable |
| 3 | 24 | F | > 50 | Free 0.41ng/dl | <10 | Nontender, diffuse goiter | N/A | Thyromegaly with uniformly increased uptake. | N/A |
RAI – Radioactive iodine.
Reference ranges: Urinary iodine- 100– 460 µg/24hr.
Total T4 5–12 µg/dl
Free T4 0.7–2.0 ng/dl
TSH 0.5–5.0
N/A Not available.
Figure 1.
Radioactive iodine uptake of Patient #1 showing thyromegaly with diffuse tracer uptake of 42%.
Physical examination showed a well-nourished woman, with normal vital signs and diffuse, firm, non-tender thyromegaly without bruit. The rest of physical examination was unremarkable. Her serum thyroxine level was 3.4 µg/dl, TSH was 52.9 µIU/ml; thyroid peroxidase antibody (TPO) was not detectable. A review of the accompanying radioiodine study showed diffuse and increased 24-hour uptake of 42% (Figure 1). The absence of clinical and biochemical features of hyperthyroidism made Graves’ disease an untenable explanation for the diffuse goiter and scintigraphic finding. Thus, IDD was considered as a plausible explanation. A 24-hour UI level, measured in a commercial laboratory, using inductively coupled plasma-mass spectrometry (Mayo Clinic, Rochester, MN), showed an extremely low value of 31 µg/day (Table 1).
She was advised to use iodized salt and to eat seafood. On follow-up visit two months later, the patient felt improved. Symptoms of hypothyroidism and goiter had resolved; repeat thyroid panel showed normal results: the serum thyroxine level was 6.4 µg/dl, TSH was 2.0 µIU/ml.
Case 2
A 38 year-old, African-American woman who was referred to the endocrine clinic, for evaluation of goiter. She had presented with tiredness, hair loss, cold intolerance, a fifty pound weight gain over the preceding 2 years, and chronic constipation requiring daily use of a stool softener. The past medical history was significant for a stroke seven years before presentation. She had drastically decreased salt intake and eliminated iodized salt from her diet following the stroke. She rarely consumed seafood or dairy products. She gave no history of allergies or family history of thyroid disease, and had not used antithyroid agents. Her menstrual cycle was normal.
Physical examination showed a well-developed woman, who had normal vital signs but was obese (body mass index of 31). Her thyroid gland was diffusely enlarged to three times normal size, non-tender, firm in consistency and without bruit (figure 2A). There was no cervical lymphadenopathy. Other aspects of systemic examination were normal. A suspicion of IDD was made based on her dietary history. Serum levels of thyroid hormones and TSH were normal; TPO was undetectable. A 24-hour urine specimen was sent for iodine measurement, which was reported as undetectable (Table1). The diagnosis of iodine deficiency goiter was made and the patient was advised to use iodized salt and to seafood. On follow-up visit after two months, UI excretion had increased to 167 µg/24hr, and goiter had regressed (figure 2B).
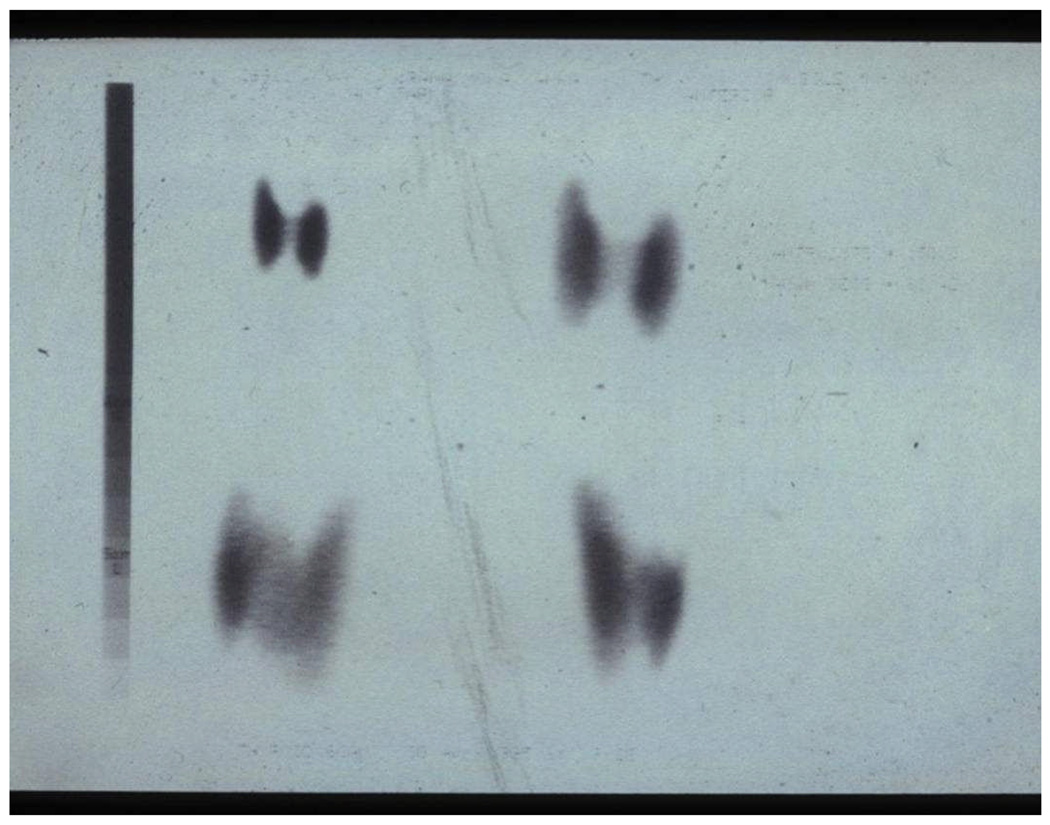
Figure 2.
Figure 2a Thyroid ultrasound of Patient #2 at presentation, showing diffusely enlarged gland. Right lobe: 4.4 cm ×1.6 cm; Left lobe: 4.7 cm × 1.6 cm.
Figure2b. Thyroid ultrasound of Patient #2 after 2 months of iodine supplementation, showing remarkable shrinkage. Right lobe: 3.7 cm × 1.3 cm; Left lobe: 3.6 cm × 2.5 cm.
Case 3
A 24 year-old Sri Lankan-born woman resident in Kuwait was referred to the endocrine clinic for evaluation of incidentally discovered goiter. Patient had no dysthyroid symptoms or significant past medical history. Her only child was born 6 years before presentation; menstrual cycle was normal. Dietary history revealed that she had been avoiding iodized salt for unclear reason but used dairy products infrequently. The patient, who took no medications, had no allergies or family history of thyroid disease.
On physical examination, her vital signs were normal. Neck examination showed a diffusely enlarged, non-tender thyroid gland with a firm consistency and no audible bruit. On laboratory evaluation, the serum free thyroxine level was 0.41ng/dl, TSH was >50 µIU/ml, with undetectable TPO. RAI scan ordered by the referring physician showed an enlarged thyroid gland with uniform tracer uptake (Figure 3) which was reported as being consistent with “hyperfunctioning thyroid gland”. Based on the prior experience, iodine deficiency was suspected, but measurement of UI level was not feasible due to technical reasons. Nonetheless, we reasoned that the co-existence of biochemical evidence of hypothyroidism and a scintigraphic pattern of diffuse radioiodine uptake in a patient who avoided iodized salt was best explained by goitrous hypothyroidism secondary to iodine deficiency. Thus, she was advised to use iodized salt for cooking and to consume three or more servings of seafood weekly. She returned for follow-up in two months. A repeat thyroid panel on that occasion showed TSH level of 3.2 µIU/ml and free thyroxine level of 1.12 ng/dl.
Figure 3.
Radioactive iodine scan of Patient #4 showing four views of the thyroid gland demonstrating increased uptake in all lobes.
Discussion
About a third of the world’s population live in iodine deficient areas where they are at the risk of IDD including, goiter, hypothyroidism, mental retardation, increased perinatal mortality and retarded physical development (1,2,8,12,13). Although cretinism is the most profound manifestation of (IDD), goiter is the most visible sign. (14,15). Goiter results from increased stimulation of the thyrocytes by thyrotropin, in an attempt to maximize the use of available iodine. This adaptation response maintains euthyroidism in mild cases but hypothyroidism supervenes as iodine stores become severely depleted.
All of our patients were young women, aged between 24 to38 years, who had goiter; while two of them also had hypothyroidism. Pregnancy and lactation are associated with a greater iodine requirement (200µg/day vs. 150 µg/day in other adults), in order to meet the needs of the growing fetus or baby. This may explain the severe hypothyroidism noted in case 1, who had an antecedent history of a full-term pregnancy in the year prior to diagnosis of iodine-deficient hypothyroidism. Both patients who presented with biochemically confirmed hypothyroidism had neither symptoms nor physical signs of thyroid hormone deficiency. This may be a reflection of the chronicity of iodine deficiency which precipitated a very insidious disease. Occasionally, very high TSH levels may be seen in asymptomatic patients with primary hypothyroidism. Dietary practices appeared to predict the risk of iodine deficiency among our patients, who generally tended to avoid using iodized salt. Our patients also did not take enough of dairy products to compensate for the lack of dietary iodine derived from iodized salt. It is noteworthy that 8 0z of cow’s milk provides 50 µg of iodine, which is a third of the recommended daily intake.
Case 3 lived in Kuwait where iodized salt and dairy products are available. The reason she avoided iodized salt remains unclear. Although, a WHO conducted survey in Kuwaiti school children showed that 30% of them had suboptimal urinary iodine excretion, but none of them had goiter (3). Another study in adult diabetic patients reported normal urinary iodine levels (16). Therefore, it would be reasonable to surmise that our Kuwaiti patient, like her American counterparts did not have endemic IDD.
Concomitant deficiency of other nutrients, such as selenium, iron, zinc and vitamin A have been reported to exacerbate the effects of iodine deficiency (17,18). Selenium is a component of the deiodinases, which are selenoproteins involved in the metabolism of thyroid hormones. Therefore, deficiency of this trace element results in the accentuation of the iodine deficient state. In the same vein, iron, which is the core of all heme containing proteins of which thyroid peroxidase is an example, plays a crucial role in thyroid hormonogenesis. Iodine trapping, organification of iodide and the coupling of iodothyronine are all catalized by thyroid peroxidase. Hence, iron deficiency anemia would also worsen the severity of iodine deficiency. Vitamin A supplementation in iodine deficient rats has been shown to reduce TSH secretion by the pituitary and also thyroid size (18). This may have therapeutic implication in humans, given the fact that iodine and vitamin A deficiencies are prevalent in developing nations of the world. In the light of these nutrient interactions, multivitamin preparations which provide iodine and these trace elements in one pill may have an advantage over iodized salt alone in patients with IDD, especially in regions known to have nutritional deficiencies. However, all of our patients were from affluent nations were these nutritional deficiencies are unlikely. Furthermore, the exquisite response to iodized salt alone justifies our hypothesis that these patients had IDD.
In our experience, the paradoxical finding of diffusely increased RAI uptake in a euthyroid, or hypothyroid patient provided the strongest clue to the diagnosis of IDD. However, since RAI study is not routinely indicated for the work up of goiter or hypothyroidism, we estimate that several patients with IDD are probably undiagnosed. Typically, the finding of a high TSH and low freeT4 in a patient with goiter would lead to a secure diagnosis of hypothyroidism and almost immediate, life-long thyroxine replacement. Thus, several patients who are started on life-long thyroxine replacement in current practice, could indeed have IDD, a condition reversible with simple dietary iodine supplementation.
Therefore, we suggest that iodine deficiency be considered in the differential diagnosis of diffuse goiter, or hypothyroidism, or the finding of diffusely increased RAI uptake in the absence of Graves’ disease (or a TSH-secreting adenoma). The suspicion will be heightened if such a patient also exhibits the dietary restrictions (e.g., avoidance of iodized salt or seafood) shown by our patients. Therefore, we suggest that a careful dietary history be obtained as part of the routine evaluation of patients presenting with goiter and/or hypothyroidism, regardless of the regional classification for iodine status. In patients with a suspicious dietary history, measurement of 24-hour urine iodine excretion would be warranted. Serum TSH and thyroid hormones correlate very poorly with UI concentration (19), therefore measurement of UI is the only reliable way to establish a definitive diagnosis of iodine deficiency.
Intrathyroidal iodine exerts a natural anti-goitrogenic effect through the stimulation of local synthesis of the antimitogenic transforming growth factor (TGF)-beta (20). Iodine-induced augmentation of TGF-beta also inhibits the goitrogenic effects of TSH and a variety of autocrine growth factors within the thyroid (20,21,22). Proper diagnosis and correction of iodine deficiency thus offers the best chances of cure for iodine-deficient goiter and any associated hypothyroidism. As is well known, thyroxine replacement seldom results in complete regression in patients with sporadic goiter, and is even less likely to do so in patients with IDD.
Table 2.
Clues to Iodine deficiency related thyroid disease.
| • | History of avoiding iodized salt. |
| • | History of avoiding seafood. |
| • | History of recent pregnancy or lactation. |
| • | Goiter. |
| • | Hypothyroidism |
| • | Thyroid function test consistent with euthyroidism or hypothyroidism with elevated radioactive iodine uptake |
Acknowledgements
Dr. Dagogo-Jack is supported in part by NIH Grants R01 DK067269 and MO1 RR00211.
Footnotes
Disclaimer: An abstract based in part on data in this manuscript was communicated at the annual scientific meeting of the Southern Society for Clinical Investigation in February 2007 (Nyenwe EA, Dagogo-Jack S. Recognition of Iodine Deficiency Disorders in the Iodine-Replete Environment. Journal of Investigative Medicine. 2007; 55 (suppl 1):S297). Also, a brief account of two of the cases described in our manuscript appeared as a Letter to the Editor in the September 2007 issue of the New England Journal of Medicine (Nyenwe EA, Dagogo-Jack S. N Engl J Med 357:1263, 2007).
References
- 1.de Benoist B, Anderson M, Takkouche B, Egli I. Prevalence of iodine deficiency worldwide. Lancet. 2004;362:1859–1860. doi: 10.1016/S0140-6736(03)14920-3. [DOI] [PubMed] [Google Scholar]
- 2.de Benoist B, Anderson M, Egli I, Takkouche B, Allen H, editors. Geneva: World Health Organization; Iodine status worldwide: WHO global database on iodine deficiency. 2004
- 3. [May 6, 2006];Geneva: World Health Organization; WHO database on iodine deficiency. Assessed online at WWW3.who.int/whosis/micronutrient/
- 4.Pacaud D, Vliet VG, Delvin E, Garel L, Chad Z, Delange, Deal C. A third world endocrine disease in a 6-year-old North American boy. J Clin Endocrinol Metab. 1995;80:2574–2576. doi: 10.1210/jcem.80.9.7673397. [DOI] [PubMed] [Google Scholar]
- 5.Dunn JT. What’s happening to our iodine? J Clin Endocrinol Metab. 1998;83:3398–3400. doi: 10.1210/jcem.83.10.5209. (Editorial) [DOI] [PubMed] [Google Scholar]
- 6.Hollowell JG, Staehling NW, Hannon WH, et al. Iodine nutrition in the United States.Trends and public health implications: iodine excretion data from National Health and Nutrition Examination Surveys I and III (1971–1974 and 1988–1994) J Clin Endocrinol Metab. 1998;83:3401–3408. doi: 10.1210/jcem.83.10.5168. [DOI] [PubMed] [Google Scholar]
- 7.Utiger RD. Iodine nutrition- more is better. N Engl F Med. 2006;354(26):2819–2821. doi: 10.1056/NEJMe068092. [DOI] [PubMed] [Google Scholar]
- 8.Caldwell KL, Jones R, Hollowell JD. Urinary iodine concentration: United States National Health and Nutrition Examination Survey 2001–2002. Thyroid. 2005;15:692–699. doi: 10.1089/thy.2005.15.692. [DOI] [PubMed] [Google Scholar]
- 9.Borak J. Adquacy of iodine nutrition in the United States. Conn Med. 2005;69:73–77. [PubMed] [Google Scholar]
- 10.Larsen RP, Davies TF. Hypothyroidism and thyroiditis. In: Larsen RD, Krokenberg HM, Melmed S, Polonsky KS, editors. Williams textbook of endocrinology. 10th edition. Philadelphia: Saunders; 2003. pp. 423–455. [Google Scholar]
- 11.Nyenwe EA, Dagogo-Jack S. Recognizing iodine deficiency in iodine-replete environments. N Engl J Med. 2007 Sep 20;357:1263–1264. doi: 10.1056/NEJMc070196. [DOI] [PubMed] [Google Scholar]
- 12.Breverman LE. Adequate iodine intake- the good far outweighs the bad. European Journal of Endocrinology. 1998;139:14–15. doi: 10.1530/eje.0.1390014. [DOI] [PubMed] [Google Scholar]
- 13.Lombardi-Aghini F, Antonangeli L, Martino E, et al. The spectrum of thyroid disease in an iodine-deficient community: the Pescopagano survey. J Clin Endocrinol Metab. 1999:84561–84566. doi: 10.1210/jcem.84.2.5508. [DOI] [PubMed] [Google Scholar]
- 14.Delenge F. iodine deficiency as a cause of brain damage. Postgrad. Med. J. 2001;77:217–220. doi: 10.1136/pmj.77.906.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bleichrodt N, Born MP. Meta-analysis of research on iodine and its relationship to cognitive development. In: Stanbury JB, editor. The damaged brain of iodine deficiency. New York: Cognizant Communication; 1994. pp. 195–200. [Google Scholar]
- 16.Vladeva S, Gatseva P, Argirova M. Iodine status in patients with diabetes mellitus type 1 and type 2. Trace Elements and Electrolytes. 2007;24:143–145. [Google Scholar]
- 17.Zimmermann MB, Kőhle J. The impact of iron and selenium deficiencies on iodine and thyroid metabolism: biochemistry and relevance to public health. Thyroid. 2002;12:867–878. doi: 10.1089/105072502761016494. [DOI] [PubMed] [Google Scholar]
- 18.Biebinger R, Arnold M, Langhans W, Herrell RF, Zimmermann MB. Vitamin A repletion in rats with concurrent vitamin A and iodine deficiency affects pituitary TSHβ gene expression and reduces thyroid hyperstimulation and thyroid size. J Nutr. 2002;137:573–577. doi: 10.1093/jn/137.3.573. [DOI] [PubMed] [Google Scholar]
- 19.Soldin OP, Tractenberg RE, Pezzullo JC. Do thyroxine and thyroid-stimulating hormone levels reflect urinary iodine concentrations? Ther Drug Monit. 2005;27(2):178–185. doi: 10.1097/01.ftd.0000149954.20089.0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grubeck-Loebenstein B, uchan G, Sadeghi R, Kissonerghis M, Londei M, et al. Transforming growth Factor Beta regulates thyroid growth. J Clin. Invest. 1989;83:764–770. doi: 10.1172/JCI113955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dagogo-Jack S. Dietary iodine affects EGF levels in mouse thyroid and submaxillary glands. Endocrine Res. 1994;20:247–257. doi: 10.1080/07435809409035862. [DOI] [PubMed] [Google Scholar]
- 22.Dagogo-Jack S. A new understanding of goitrogenesis: Role of cytokines in the regulation of normal and aberrant thyroid growth. Afr J Med Sci. 1995;24:211–217. [PubMed] [Google Scholar]