Abstract
Purpose
To evaluate the difference between radiographic size on computed tomography (CT) and the pathologic size of renal tumors using a contemporary cohort.
Materials and Methods
We retrospectively reviewed the records of 521 patients undergoing surgical resection of a renal mass between 2000 and 2007 who had tumor sizes recorded from both preoperative CT imaging and pathologic evaluation of the tumor specimen. Data regarding histologic tumor type were also extracted. The paired student’s t-test was utilized to compare the mean radiographic size as measured on CT with the mean pathologic size, and p values <0.05 were considered statistically significant.
Results
For all patients, the mean radiographic size and mean pathologic size was 4.79 and 4.69 cm, respectively (p = 0.02). Therefore, on average, radiographic size overestimated pathologic size by 1 mm. In patients with a tumor size of 4 to 7 cm, radiographic size overestimated pathologic size by 0.21 cm (p = 0.007). However, no significant difference was noted in patients with a tumor size of <4 cm or >7 cm.
Conclusions
Using a contemporary cohort of patients, we observed a statistically significant overestimation of renal tumor sizes by CT imaging as compared to pathologic assessment. However, the overall difference between radiographic and pathologic tumor size was 1 mm, suggesting that CT provides an accurate modality with which to estimate renal tumor size.
Keywords: kidney neoplasms/pathology; kidney neoplasms/radiography; retrospective studies; tomography, x-ray computed; nephrectomy/methods
INTRODUCTION
Tumor size has substantial clinical implications for patients with a renal mass. Knowing tumor size permits proper staging, facilitates discussion of prognosis, and guides treatment strategy, specifically the choice between radical nephrectomy, partial nephrectomy, and observation. However, tumor size in the current literature is largely represented by pathologic size, which is routinely recorded during pathologic sectioning.1,2 Thus, when counseling patients in the preoperative setting, the physician must assume that radiographic size is equivalent to pathologic size when addressing expected outcome and specifically when making recommendations for therapy (ie, partial vs radical nephrectomy).
Several previous observations suggest that there is an overestimation of pathologic size of renal tumors when compared with radiographic size.3-5 Thus, if a specific size cutoff (largely determined by the pathologic size in most published series) is used to identify patients for partial nephrectomy,6-8 radiographic overestimation may diminish the number of patients who would otherwise be candidates for a nephron-sparing approach.4 However, most of the literature addressing this issue are limited by small numbers and utilize older imaging techniques.3,4 We examined a contemporary cohort of patients to determine if radiographic size overestimates pathologic size of renal tumors and if so, by how much.
MATERIALS AND METHODS
Upon approval from the institutional review board, we identified 561 patients in the MSKCC kidney cancer database who were treated surgically for a renal cortical tumor and who had both pathologic and radiographic (CT) tumor sizes available. This database is prospectively maintained and includes >150 variables for each patient, including radiographic and pathologic tumor size. Of these patients, 40 were excluded due to preoperative CT scans obtained more than 4 months before surgery. Among the remaining 521 patients, 27 (6%) had multifocal tumors. For these patients, the largest tumor diameter on CT and pathologic size was used for comparison. Patient demographics, including age, sex, histology, type of procedure, and cancer stage, were collected from our database and organized into table 1.
Table 1.
Demographic data
| Feature | Median or Frequency (%) |
|---|---|
| Age, years | 61 (51—69) |
| Sex | |
| Male | 328 (63) |
| Female | 193 (37) |
| Type of procedure | |
| Laparoscopic partial nephrectomy | 58 (11) |
| Laparoscopic radical nephrectomy | 31 (6) |
| Partial nephrectomy | 271 (52) |
| Radical nephrectomy | 161 (31) |
| Histology | |
| Clear cell | 309 (59) |
| Papillary | 56 (11) |
| Chromophobe | 56 (11) |
| RCC other | 33 (6) |
| Oncocytoma | 45 (9) |
| AML | 12 (2) |
| Benign other | 10 (2) |
| Primary tumor classification* | |
| T1a | 224 (49) |
| T1b | 72 (16) |
| T2 | 28 (6) |
| T3a | 66 (15) |
| T3b | 61 (13) |
| T4 | 3 (<1) |
Provided for 454 patients with RCC.
Histological subtypes of each tumor were taken from the official pathologic report and categorized as clear cell, papillary, chromophobe, RCC other, oncocytoma, AML, or benign other. The paired student’s t-test was utilized to compare the mean CT size with mean pathologic size and a p value <0.05 was considered statistically significant. Statistical analyses were performed using Stata 8.2 (Stata Corp., College Station, TX).
RESULTS
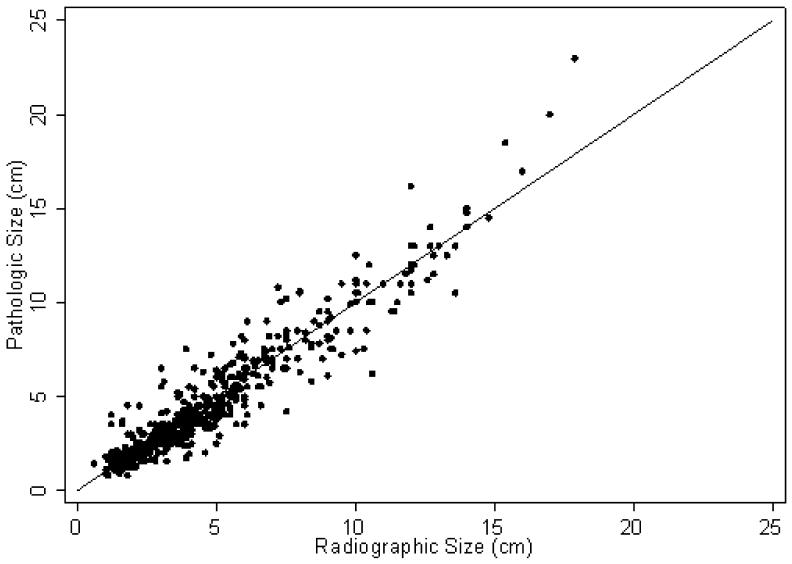
A summary of patient demographics is detailed in table 1 and a scatter plot of radiographic and pathologic size are depicted in figure 1. The patients included 193 (37%) women and 328 (63%) men with an overall median age of 61 years (range 51–69 years). Among the 521 patients, 329 (63%) patients underwent partial nephrectomy and 192 (37%) were treated with radical nephrectomy. Among the 329 patients treated with partial nephrectomy, the mean (standard deviation [SD]) CT size was 3.31 (1.49) and the mean pathologic size was 3.11 (1.53), p <0.001. No significant difference was noted in mean CT size (7.34, SD 3.35 cm) and mean pathologic size (7.38, SD 3.63 cm) for patients treated with radical nephrectomy (p = 0.7).
Figure 1.
Scatter plot of radiographic and pathologic tumor size. For reference, the line of perfect correlation is shown.
The mean CT size and mean pathologic size for all 521 patients was 4.79 and 4.69 cm, respectively. The mean change in size for all tumors examined was 0.10 cm larger in the radiographic assessment vs the pathologic measurement (p = 0.02). All 521 patients with renal tumors had a median change in size (interquartile range) of 0.20 (-0.30 to 0.60) with the smallest change in size of -5.10 and the largest change in size of +4.40.
Table 2 lists the average radiographic and pathologic tumor sizes separated into 1-cm intervals by radiographic size. There were statistically significant overestimations in renal tumor size by CT scan in the 2 to <5 cm range. The largest difference in radiographic size versus pathologic size was found in the 4 to <5 cm CT size category, with an overestimation of 0.32 cm (p = 0.004). The only underestimation by CT scan was found in the 1 to <2 cm size range, where the pathologic size was found to be 0.24 cm larger in size than the CT size (p = 0.02).
Table 2.
Average radiographic and pathologic tumor size by 1 cm radiographic size
| CT Size (cm) | n | Mean CT Size in cm (SD) |
Mean Path Size in cm (SD) |
Change in Size (95% CI) |
p Value |
|---|---|---|---|---|---|
| <1 | 1 | 0.6 (-) | 1.4 (-) | 0.8 (-) | — |
| 1 to <2 | 63 | 1.51 (0.27) | 1.75 (0.76) | 0.24 (0.05, 0.42) | 0.02 |
| 2 to <3 | 90 | 2.42 (0.28) | 2.28 (0.55) | -0.14 (-0.25, -0.03) | 0.01 |
| 3 to <4 | 109 | 3.44 (0.30) | 3.27 (0.90) | -0.17 (-0.34, -0.01) | 0.05 |
| 4 to <5 | 71 | 4.38 (0.31) | 4.06 (0.93) | -0.32 (-0.54, -0.11) | 0.004 |
| 5 to <6 | 58 | 5.40 (0.29) | 5.18 (1.13) | -0.21 (-0.48, 0.06) | 0.1 |
| 6 to <7 | 33 | 6.36 (0.34) | 6.36 (1.30) | 0 (-0.44, 0.44) | 1 |
| 7 or greater | 96 | 10.14 (2.43) | 10.09 (3.10) | -0.05 (-0.38, 0.28) | 0.8 |
| Total | 521 | 4.79 (3.06) | 4.69 (3.25) | -0.10 (-0.20, -0.02) | 0.02 |
Table 3 lists the average radiographic and pathologic tumor sizes divided into clinically significant ranges of <4 cm, 4 to 7 cm, and >7 cm. Patients with a tumor size of 4 to 7 cm were found to have an overestimation in radiographic size versus pathologic size of 0.21 cm (p = 0.007). However, no significant difference was noted in patients with tumor sizes of <4 or >7 cm. Among 258 patients with a CT size >4 cm and thought to have ≥ T1b tumor, 30 (11.6%) ended up with a pathologic size <4 cm. Among 92 patients with a CT scan >7 cm and thought to have ≥T2 tumor, 7 (7.6%) patients ended up with a pathologic size <7 cm.
Table 3.
Average radiographic and pathologic tumor size by clinical stage sizes
| CT Size (cm) | n | Mean CT Size in cm (SD) |
Mean Path Size in cm (SD) |
Change in Size (95% CI) |
p Value |
|---|---|---|---|---|---|
| <4 cm | 263 | 2.62 (0.83) | 2.56 (0.99) | -0.06 (-0.15, 0.03) | 0.2 |
| 4-7 cm | 166 | 5.19 (0.86) | 4.98 (1.41) | -0.21 (-0.37, -0.06) | 0.007 |
| >7 cm | 92 | 10.28 (2.39) | 10.23 (3.10) | -0.05 (-0.39, 0.29) | 0.7 |
| Total | 521 | 4.79 (3.06) | 4.69 (3.25) | -0.10 (-0.20, -0.02) | 0.02 |
Table 4 demonstrates a comparison of histologic subtypes of renal tumors and the difference in size between mean radiographic and mean pathologic size. Among the 309 patients with clear cell histology, tumor size was overestimated by 0.23 cm on CT scan (p <0.001). Among the 56 patients were noted to have papillary renal tumors, an underestimation of 0.37 cm by CT scan was observed (p = 0.02). In the remaining histologic subgroupings no significant difference was seen between radiographic and pathologic tumor sizes.
Table 4.
Average radiographic and pathologic tumor size by histologic subtype
| Histology | n | Mean CT Size (cm) |
Mean Path Size (cm) |
Change in Size (cm) |
p Value |
|---|---|---|---|---|---|
| Clear cell | 309 | 4.94 | 4.71 | -0.23 | <0.001 |
| Papillary | 56 | 4.36 | 4.72 | 0.37 | 0.02 |
| Chromophobe | 56 | 5.68 | 5.62 | -0.05 | 0.7 |
| RCC other | 33 | 4.71 | 4.83 | 0.12 | 0.5 |
| Oncocytoma | 45 | 3.75 | 3.66 | -0.10 | 0.5 |
| AML | 12 | 4.2 | 3.86 | -0.34 | 0.2 |
| Benign other | 10 | 3.25 | 3.54 | 0.29 | 0.4 |
DISCUSSION
To our knowledge, we present the largest comparison of radiographic and pathologic tumor sizes for patients with a renal mass. Consistent with previous observations, we observed that CT size overestimates pathologic size when comparing all patients. However, in our experience the overall difference between the radiographic and pathologic size was only 1 mm. While statistical significance was reached, we do not believe that 1 mm represents a clinically significant difference in tumor size. Thus, we believe our data confirm that contemporary CT imaging adequately estimates the true tumor size. This information should prove useful when counseling patients about risk of metastases and when deciding on an appropriate operative approach.
When evaluating the subgroups according to 1-cm intervals, we observed that the largest changes in size occurred in patients with tumors <5 cm. Interestingly, patients with radiographic tumors <2 cm tended to have pathologic tumors that were significantly larger. However the change in size for these patients was slightly more than 2 mm, which we argue is a clinically insignificant artifact. The largest overestimation for CT was observed in patients within the 4- to 5-cm range. This difference, while statistically significant, was again only 3 mm and likely only significant due to our relatively large number of patients. Changes in radiographic and pathologic tumor size were less pronounced for patients with larger tumors. In fact, the CT size and pathologic size for patients in the 6- to 7-cm subgroup were exactly the same (mean 6.36 cm for both). This observation is in agreement with Schlomer et al, who observed similar trends in their cohort of 126 patients.4
Tumor size is an important clinical and pathologic feature for evaluating patients with RCC. For tumors limited to the kidney, the pT1a, pT1b, and pT2 primary classifications are determined based solely on tumor size.9 In our study, of the 258 patients with a CT scan >4 cm and thought to have ≥T1b tumor, 30 ended up with a pathologic size of <4 cm. A similar phenomenon was found among the 92 patients with a CT scan >7 cm and thought to have ≥T2 tumor: 7 patients ended up with a pathologic size <7 cm. Although this discrepancy in tumor stage may seem to have an effect on patient selection for different treatment modalities, we believe that this was true for only a limited number of patients and therefore would not change overall management.
Tumor size is an independent predictor of progression-free survival and cancerspecific survival after surgery.10 Furthermore, tumor size has largely been used when recommending partial nephrectomy for patients in an elective situation. Traditionally, a 4-cm cutoff has been proposed,6 although more recent observations suggest that a cutoff of 7 cm for appropriately selected patients is safe.7,8 Thus, tumor size is widely utilized and represents a key factor to dictate therapy for patients with RCC.
Another important prognostic indicator, which may also have a role in deciding treatment, are the histopathological features of RCC. Because more than 30% of patients have renal tumors with histology other than conventional clear cell carcinoma, studies have looked at the correlation between size, histology, and metastatic potential.11,12 Tumors of histology other than clear cell carcinoma appear to have a better prognosis and might be suitable for partial nephrectomy regardless of size.13,14 We evaluated the difference between mean radiographic tumor size and mean pathologic tumor size within each histologic subgroup. Statistically significant differences were noted in the clear cell and papillary types. However, the changes are small (2–3 mm) and unlikely to be clinically significant. With the current focus on nephron-sparing surgery for renal masses, we anticipate that tumor size will have an expanded role in the preoperative management in patients with a renal mass.
With the increased frequency of cross-sectional imaging, small and incidental renal masses are being diagnosed more often than in the past.15,16 Thus, more patients, especially those with significant medical comorbidities, are being considered for observation.17 In addition, chronic renal failure is a condition that is more prevalent than previously thought among patients with a renal mass.18 More than 25% of all patients with a renal mass have at least Grade 3 chronic kidney disease at presentation.18 Furthermore, a recent retrospective observation suggests that partial nephrectomy is associated with an improved overall survival for patients with small renal masses when compared with radical nephrectomy.19 Thus, we anticipate that partial nephrectomy will become increasingly utilized.
Our data suggest that while CT imaging may overestimate the true tumor size, this overestimation is unlikely to be of clinical importance. While evaluation within the histologic subgroups reveals significant differences between the radiographic and pathologic sizes in clear cell and papillary tumors, these variations are again small. We believe that this study confirms the accuracy of CT as a modality for establishing the size of renal masses.
This study is not without limitations. Our review was a retrospective, singleinstitution analysis of patients and is subject to the number of biases that surround these study types. Further bias may result from routine pathologic processing with formalin fixation, a process that has been shown to reduce tumor volume.20 Another important weakness is that central review of the radiographic studies was not performed.
CONCLUSIONS
Using a contemporary cohort of patients, we observed a statistically significant overestimation of renal tumor sizes when comparing CT scan with pathology. However, the overall difference for tumor sizes is 1 mm, which suggests that CT imaging estimates renal tumor size in a manner that is adequate for clinical decision making.
Abbreviations and Acronyms
- AML
angiomyolipoma
- CT
computed tomography
- MSKCC
Memorial Sloan-Kettering Cancer Center
- Path
pathologic
- RCC
renal cell carcinoma
- SD
standard deviation
- CI
confidence interval
REFERENCES
- 1.Yayciouglu O, Rutman MP, Balasubramaniam M, Peters KM, Gonzalez JA. Clinical and pathologic tumor size in renal cell carcinoma; difference, correlation, and analysis of influencing factors. Urology. 2002;60:33. doi: 10.1016/s0090-4295(02)01668-0. [DOI] [PubMed] [Google Scholar]
- 2.Uzzo RG, Novick AC. Nephron sparing surgery for renal tumors: indications, techniques and outcomes. J Urol. 2001;166:6. [PubMed] [Google Scholar]
- 3.Herr HW, Lee CT, Sharma S, Hilton S. Radiographic versus pathologic size of renal tumors: implications for partial nephrectomy. Urology. 2001;58:157. doi: 10.1016/s0090-4295(01)01173-6. [DOI] [PubMed] [Google Scholar]
- 4.Schlomer B, Figenshau RS, Yan Y, Bhayani SB. How does the radiographic size of a renal mass compare with the pathologic size? Urology. 2006;68:292. doi: 10.1016/j.urology.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Herr HW. Radiographic vs surgical size of renal tumours after partial nephrectomy. BJU Int. 2000;85:19. doi: 10.1046/j.1464-410x.2000.00357.x. [DOI] [PubMed] [Google Scholar]
- 6.Patard JJ, Shvarts O, Lam JS, Pantuck AJ, Kim HL, Ficarra V, et al. Safety and efficacy of partial nephrectomy for all T1 tumors based on an international multicenter experience. J Urol. 2004;171:2181. doi: 10.1097/01.ju.0000124846.37299.5e. [DOI] [PubMed] [Google Scholar]
- 7.Leibovich BC, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Nephron sparing surgery for appropriately selected renal cell carcinoma between 4 and 7 cm results in outcome similar to radical nephrectomy. J Urol. 2004;171:1066. doi: 10.1097/01.ju.0000113274.40885.db. [DOI] [PubMed] [Google Scholar]
- 8.Dash A, Vickers AJ, Schachter LR, Bach AM, Snyder ME, Russo P. Comparison of outcomes in elective partial vs radical nephrectomy for clear cell renal cell carcinoma of 4–7 cm. BJU Int. 2006;97:939. doi: 10.1111/j.1464-410X.2006.06060.x. [DOI] [PubMed] [Google Scholar]
- 9.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. 6th Springer-Verlag; New York: 2002. [Google Scholar]
- 10.Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell carcinoma treated with radical nephrectomy based on tumor state, size, grade and necrosis: the SSIGN score. J Urol. 2002;168:2395. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 11.Skolarus TA, Serrano MF, Berger DA, Bullock TL, Yan Y, Humphrey PA, et al. The distribution of histological subtypes of renal tumors by decade of life using the 2004 WHO classification. J Urol. 2008;179:439. doi: 10.1016/j.juro.2007.09.076. [DOI] [PubMed] [Google Scholar]
- 12.Remzi M, Ozsoy M, Klingler HC, Susani M, Waldert M, Seitz C, et al. Are small renal tumors harmless? Analysis of histopathological features according to tumors 4 cm or less in diameter. J Urol. 2006;176:896. doi: 10.1016/j.juro.2006.04.047. [DOI] [PubMed] [Google Scholar]
- 13.Hafez KS, Novick AC, Campbell SC. Patterns of tumor recurrence and guidelines for followup after nephron sparing surgery for sporadic renal cell carcinoma. J Urol. 1997;157:2067. [PubMed] [Google Scholar]
- 14.Crotty TB, Farrow GM, Lieber MM. Chromophobe cell renal carcinoma: clinicopathological features of 50 cases. J Urol. 1995;154:964. doi: 10.1016/s0022-5347(01)66944-1. [DOI] [PubMed] [Google Scholar]
- 15.Chow WH, Devesa SS, Warren JL, Fraumeni JF., Jr Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 16.Hock LM, Lynch J, Balaji KC. Increasing incidence of all stages of kidney cancer in the last 2 decades in the United States: an analysis of surveillance, epidemiology and end results program data. J Urol. 2002;167:57. [PubMed] [Google Scholar]
- 17.Van Poppel H, Joniau S. Is surveillance an option for the treatment of small renal masses? Eur Urol. 2007;52:1323. doi: 10.1016/j.eururo.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 18.Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, Raj GV, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7:735. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson RH, Boorjian SA, Lohse CM, Leibovich BC, Kwon ED, Cheville JC, et al. Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol. 2008;179:468. doi: 10.1016/j.juro.2007.09.077. [DOI] [PubMed] [Google Scholar]
- 20.Hsu PK, Huang HC, Hsieh CC, Hsu HS, Wu YC, Huang MH, et al. Effect of formalin fixation on tumor size determination in stage I non-small cell lung cancer. Ann Thorac Surg. 2007;84:1825. doi: 10.1016/j.athoracsur.2007.07.016. [DOI] [PubMed] [Google Scholar]