Abstract
The SLC39A (solute carrier 39A) [ZIP (Zrt-Irt-like protein)] family consists of 14 members which are thought to control zinc uptake into the cytoplasm. Among these, ZIP4 is known to be particularly important for zinc homoeostasis. Mutations in this gene cause acrodermatitis enteropathica, a rare recessive-lethal human genetic disorder. In the present paper, our studies of the regulation and function of the mouse Zip4 gene are briefly reviewed. Mouse Zip4 is expressed at highest levels in tissues involved in absorption of dietary or maternal zinc, and the gene and protein are dynamically regulated by multiple post-transcriptional mechanisms in response to zinc availability. ZIP4 accumulates at the apical surface of enterocytes and endoderm cells when zinc is deficient, because of increased stability of the mRNA and stabilization of the protein. In contrast, when zinc is replenished, the mRNA is destabilized and the protein is internalized and degraded rapidly. The critical importance of ZIP4 in zinc homoeostasis is revealed in mice with targeted deletions of this gene. Homozygous Zip4-knockout embryos die during early morphogenesis and heterozygous offspring are significantly underrepresented and display an array of developmental defects, including exencephalia, anophthalmia and severe growth retardation. Mice heterozygous for Zip4-knockout are hypersensitive to zinc deficiency, which suggests that humans heterozygous for this gene may also be very sensitive to zinc deficiency.
Keywords: acrodermatitis enteropathica, homoeostasis, solute carrier 39A4 (SLC39A4), teratogenesis, transporter, zinc
Introduction
Zinc deficiency can cause growth retardation, immune system dysfunction, male hypogonadism, skin lesions and neurological disorders in humans [1–3]. Maternal zinc deficiency impairs embryonic, fetal and postnatal development [4–6]. In humans, the rare autosomal recessive disorder AE (acrodermatitis enteropathica) is thought to be caused by the inability to absorb sufficient intestinal zinc [7]. Symptoms of severe nutritional zinc deficiency often develop soon after birth in bottle-fed infants or after weaning in breastfed infants [8]. However, zinc supplementation reverses many of the symptoms of this otherwise lethal disease.
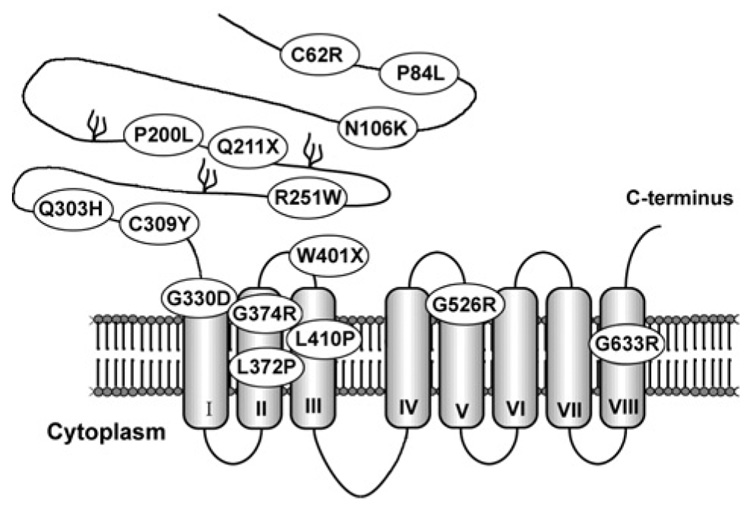
In 2002, the gene responsible for AE was mapped to human chromosomal region 8q24.3 and was shown to be a member of the SLC39A (solute carrier 39A) superfamily [9,10], historically named the ZIP (Zrt-Irt-like protein) family [11]. ZIP family members have eight predicted transmembrane domains and function to transport zinc, and perhaps other metals, into the cytoplasm. To date, 14 members have been identified in mammals [11,12], which group into four subfamilies on the basis of sequence homology [12]. The AE gene is Zip4 (Slc39a4) which is a member of the LIV-1 subfamily (nine members).Multiple amino acid substitutions due to point mutations have been identified in AE patients [9,10,13–15] (Figure 1). Almost all occur at residues that are also conserved in the mouse protein [16]. Other Zip4 mutations in AE patients involve premature termination codons (Figure 1), insertions, deletions or rearrangements of the gene that must cause a complete loss of function.
Figure 1. Mutations in ZIP4 (SLC39A4) cause acrodermatitis enteropathica.
A predicted eight-transmembrane domain model of human ZIP4, indicating amino acid substitutions (one letter code) and nonsense mutations (X) identified in patients with AE. Three potential glycosylation sites are indicated in the extracellular N-terminal half of the protein.
Much of what we understand about Zip4 regulation and function comes from recent studies of mice and transfected cells in culture [16–21]. The mouse and human ZIP4 proteins are well conserved. ZIP4 functions as a zinc transporter in transfected cells, and, although several of the amino acid substitutions found in AE patients appear to abolish its activity by causing retention in the endoplasmic reticulum (e.g. G526R), others apparently diminish its zinc transporter activity (e.g. P200L) [16,17] and must be hypomorphic alleles. The extracellular domain of ZIP4 represents approx. 50% of the protein and is rich in histidine and cysteine residues, suggesting that this domain may bind zinc. Several AE mutations are found in this region.
How ZIP4 transports zinc, the precise topology of this protein within the apical membrane and the structure and potential metal-binding activity of the extracellular domain all remain to be determined.
Regulation of Zip4 by zinc
Zip4 gene expression is dynamically regulated by several unique and poorly understood post-transcriptional mechanisms in response to zinc availability. Zip4 gene expression is most active in mouse tissues involved in nutrient uptake (e.g. intestine and embryonic visceral yolk sac). The abundance of Zip4 mRNA and the cellular localization and turnover of this protein are regulated by zinc availability in these tissues [16,18–20,22]. RNA and protein synthesis inhibitor studies and run-on transcription assays revealed that zinc deficiency causes the stabilization of Zip4 mRNA, but has little effect on transcription of this gene [22]. ZIP4 accumulates at the apical surfaces of enterocytes in the intestine and visceral endoderm cells in the embryonic visceral yolk sac when dietary zinc is deficient [16,19]. However, zinc repletion destabilizes this mRNA while causing the rapid endocytosis and degradation of ZIP4 in vivo [22] and in vitro [18,20]. The mechanisms underlying this control are not well understood. A recent study of human ZIP4 suggests that a histidine-rich region within the large intracellular loop between putative transmembrane domains III and IV plays a role in regulating endocytosis and ubiquitination of ZIP4 in response to zinc [20].Western blot analyses of ZIP4 in the intestine and visceral yolk sac during zinc deficiency revealed that the major form of ZIP4 under these conditions is a 37–40 kDa peptide [16,19,22]. This suggests that ZIP4 undergoes proteolytic processing in these cells during zinc deficiency. Studies of that phenomenon are in progress.
ZIP4 function
The dynamic post-transcriptional control of ZIP4 in response to zinc is consistent with its important role in zinc homoeostasis. To examine the functions of ZIP4 directly,we created mice in which the Zip4 gene was mutated, leading to a complete loss of function [21]. Crossing Zip4+/− mice failed to yield homozygous offspring, and heterozygous offspring were significantly underrepresented in the population. Homozygous implantation sites were represented in the expected (25%) frequency at day 10 of gestation, but these embryos had not progressed past the egg cylinder stage. Thus, in mice, Zip4 is an essential gene for development of the early embryo. The early post-implantation death of homozygous Zip4-knockout mouse embryos was unexpected since AE patients generally present symptoms after birth and often benefit greatly from zinc therapy [23].Analyses of the patterns of expression of Zip4 in the early mouse embryo suggest that the expression of this gene is restricted to the visceral endoderm cells of the visceral yolk sac. The visceral endoderm cells are the third cell type to differentiate from the inner cell mass, and, from day 7.5 of pregnancy to late in gestation, they surround the developing mouse conceptus. In humans, the visceral yolk sac is more of a vestigial organ, which may explain in part the difference in phenotypes between these species.
The embryonic lethality caused by the complete loss of ZIP4 function in mice could not be rescued by providing excess zinc to the mother, suggesting the lack of an adequate backup system for zinc uptake through the mother and into the embryonic environment at this critical stage of development. In addition to Zip4, the copper transporter Ctr1, the iron transporter ferroportin and the zinc efflux transporter ZnT1 are also essential genes at this stage of development in mice (reviewed in [24]). The mouse embryo apparently requires the development of functional systems to maintain homoeostasis of essential metals at the egg cylinder stage. Interestingly, in zebrafish, a member of the ZIP superfamily (zLIV-1) is essential during early morphogenesis [25] and the fear-of-intimacy gene in Drosophila encodes a zinc transporter that is essential during early development of the fly [26]. Zip4 is the first example of a mammalian Zip gene shown to have an essential function.
Heterozygous Zip4-knockout offspring were underrepresented by mid-gestation inmice fed on a diet with adequate zinc. Examination of embryos at day 10 of pregnancy revealed a wide range of abnormalities. Many embryos appeared to be normal, but, compared with their wild-type littermates, the Zip4+/− embryos often varied greatly in size and morphology. Many were smaller and some were severely growth retarded and exhibited abnormal craniofacial development. Heterozygous Zip4-knockout embryos with exencephalia were occasionally found at mid-gestation. These morphological abnormalities are reminiscent of previously reported teratology of maternal zinc deficiency [27,28], and were never seen in wild-type embryos within these litters.
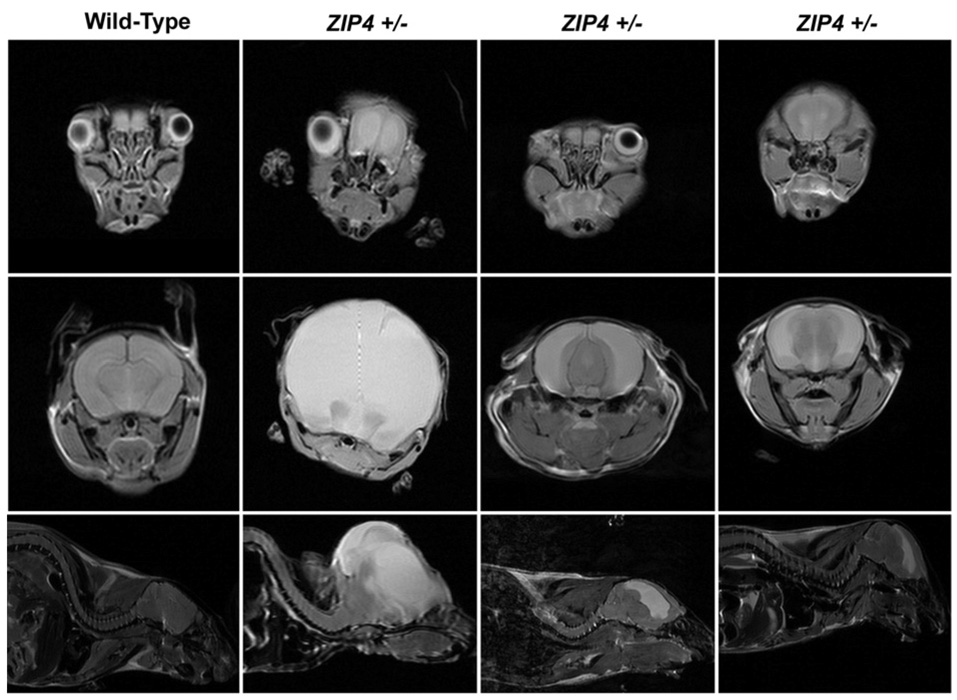
After birth, a few heterozygous Zip4-knockout offspring died before weaning, and approx. 22% of those that survived were morphologically abnormal. Overall, nearly 40% of the heterozygous post-implantation embryos had developed abnormally. Examples of abnormal heterozygous Zip4-knockout mice are shown in Figure 2. High-field MRI (magnetic resonance imaging) revealed abnormal development of the eyes and brain. These heterozygous Zip4-knockout mice had no eyes or only one eye and were hydrocephalic. In addition, abnormal thickening of the ventricular septum and wall of the left ventricle and skeletal abnormalities were present in some of these affected Zip4+/− mice. These abnormalities were never seen among hundreds of wild-type offspring examined.
Figure 2. Zip4 haploinsufficiency is associated with abnormal development in mice.
Wild-type mice and Zip4+/−-knockout mice (Zip4+/−) that were obviously abnormal at weaning were examined using high-field MRI. Top row: axial sections through the face at the plane of the eyes. Second row: axial sections through the midbrain region. Third row: sagittal sections near the midline region. White areas in the brain indicate water accumulation. Modified from Dufner-Beattie, J., Weaver, B.P., Geiser, J., Bilgen, M., Larson, M., Xu, W. and Andrews, G.K. The mouse acrodermatitis gene Slc39a4 (ZIP4) is essential for development and heterozygosity causes hypersensitivity to zinc deficiency. Hum. Mol. Genet., 2007, vol. 16(12), pp. 1391–1399 by permission of Oxford University Press.
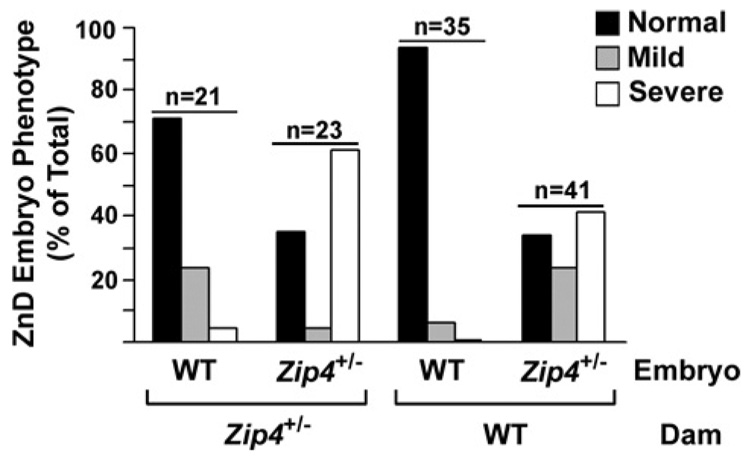
The effects of Zip4 haploinsufficiency were largely ameliorated by feeding pregnant mice and their offspring excess zinc in the drinking water. At weaning, wild-type and heterozygous Zip4-knockout pups were present at the expected Mendelian frequency, and only one heterozygous pup (3%) displayed an overtly abnormal eye phenotype. In contrast, abnormal development of Zip4+/− embryos was exacerbated when Zip4+/− pregnant females were fed on a zinc-deficient diet (Figure 3). At mid-gestation, approx. 50% of the Zip4+/− embryos were lost, and approximately two-thirds of the remaining Zip4+/− embryos were morphologically abnormal. The majority (61%) of those were classified as severely abnormal. In contrast, approx. 71% of the wild-type embryos in these same litters appeared morphologically normal and only a small percentage (4.7%) were severely abnormal. These studies revealed that maternal zinc status modulates the effects of embryonic Zip4 haploinsufficiency on development. Heterozygous Zip4-knockout mouse embryos are clearly hypersensitive to zinc deficiency relative to their wild-type littermates.
Figure 3. Heterozygous Zip4-knockout mice are hypersensitive to zinc deficiency.
Zip4+/−-knockout females (Zip4+/−) or wild-type (WT) females were crossed with Zip4+/−-knockout males. Pregnant females were fed on a zinc-deficient diet during pregnancy, and embryos were examined at mid-gestation. Severe: embryos were dramatically growth retarded and displayed multiple morphological abnormalities. Mild: embryos exhibited delayed limb development and retarded growth. Results are expressed as the percentage of embryos in each genotype of the total number examined (n). Reproduced from Dufner-Beattie, J., Weaver, B.P., Geiser, J., Bilgen, M., Larson, M., Xu, W. and Andrews, G.K. The mouse acrodermatitis gene Slc39a4 (ZIP4) is essential for development and heterozygosity causes hypersensitivity to zinc deficiency. Hum. Mol. Genet., 2007, vol. 16(12), pp. 1391–1399 by permission of Oxford University Press.
We also examined the contribution of maternal Zip4 gene expression to the effects of embryonic Zip4 heterozygosity on development. When wild-type females were mated with Zip4+/− males, heterozygous Zip4-knockout embryos were not underrepresented at mid-gestation (Figure 3) or at weaning, if dietary zinc was adequate. However, 18% (eight pups) of those heterozygous weaned pups display the same types of eye and brain abnormalities as shown in Figure 2. Maternal zinc deficiency also more profoundly affected the development of Zip4+/− embryos in wild-type females than it did wild-type embryos in the same litter. At mid-gestation, approximately two-thirds of the heterozygous Zip4- knockout embryos were morphologically abnormal, and 41% were severely abnormal in dams fed on a zinc-deficient diet. In contrast, almost all (94%) of the wild-type embryos were normal and none was severely abnormal.
Maternal Zip4 haploinsufficiency clearly sensitized the developing embryo (wild-type and Zip4+/−) to the effects of zinc deficiency. Furthermore, the combination of embryonic and maternal Zip4 heterozygosity dramatically increased the chances of abnormal embryonic development and diminished the chances of survival of the embryo to parturition. These effects were exacerbated by dietary zinc deficiency during pregnancy and were largely ameliorated by providing excess zinc. Some of the abnormal phenotypes noted at weaning in heterozygous Zip4-knockout offspring reflect the diminished function of the embryonic Zip4 gene, since they were noted in mice delivered from wild-type females.
Neurogenesis was particularly affected by embryonic Zip4 haploinsufficiency. Exencephalia, hydrocephalus, anophthalmia and anopia each were noted even under normal dietary zinc conditions. These types of abnormalities have been associated with zinc deficiency in the rat [4,6,29,30]. Zinc is essential for brain development and function in monkeys as well [31].Diminished DNA and RNA synthesis in neurons during zinc deficiency may cause abnormal neurogenesis [32,33]. The retina is also rich in zinc, concentrated in photoreceptors and retinal pigment epithelial cells [34],where it may function as a neuromodulator [35,36]. Depletion of intracellular zinc induces caspase-dependent apoptosis in retinal cells [37].
There are no published studies indicating that ZIP4 haploinsufficiency in humans is associated with effects on embryonic development, but our studies suggest that dietary zinc availability as well as gene dosage may modulate the severity and type of symptoms associated with AE mutations. Given the rare occurrence of this disease gene (~1 in 125 000 adults are heterozygous), the pleiotropic effects of zinc deficiency and the recent identification of this gene as the cause of AE, it is not surprising that the affects of Zip4 heterozygosity in humans has not been examined. It should be noted, however, that gene dosage for Atp7a, the Menkes disease gene, controls the sensitivity of the developing zebrafish notochord to copper deficiency [38], and a transient neonatal zinc deficiency during breastfeeding has been associated with heterozygosity in the maternal ZnT2 (SLC30A2) gene [39]. Our studies suggest that zinc nutritional status, interacting with mutations in human ZIP4 may be associated with a wide range of developmental abnormalities.
Acknowledgments
This work was funded, in part, by NIH (National Institutes of Health) grant DK063975.
Abbreviations used
- AE
acrodermatitis enteropathica
- MRI
magnetic resonance imaging
- Slc39a
solute carrier 39A
- ZIP
Zrt-Irt-like protein
References
- 1.Prasad AS. Zinc in human health: an update. J. Trace Elem.Exp. Med. 1998;11:63–87. [Google Scholar]
- 2.Prasad AS. The role of zinc in gastrointestinal and liver disease. Clin. Gastroenterol. 1983;12:713–741. [PubMed] [Google Scholar]
- 3.Prasad AS. Zinc deficiency. Br. Med. J. 2003;326:409–410. doi: 10.1136/bmj.326.7386.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hurley LS, Swenerton H. Congenital malformations resulting from zinc deficiency in rats. Proc. Soc. Exp. Biol. Med. 1966;123:692–696. doi: 10.3181/00379727-123-31578. [DOI] [PubMed] [Google Scholar]
- 5.Hurley LS, Shrader RE. Abnormal development of preimplantation rat eggs after three days of maternal dietary zinc deficiency. Nature. 1975;254:427–429. doi: 10.1038/254427a0. [DOI] [PubMed] [Google Scholar]
- 6.Hurley LS, Gowen J, Swenerton H. Teratogenic effects of short-term and transitory zinc deficiency in rats. Teratology. 1971;4:199–204. [Google Scholar]
- 7.Moynahan EJ. Acrodermatitis enteropathica: a lethal inherited human zinc-deficiency disorder. Lancet. 1974;2:399–400. doi: 10.1016/s0140-6736(74)91772-3. [DOI] [PubMed] [Google Scholar]
- 8.Prasad AS. Zinc: an overview. Nutrition. 1995;11:93–99. [PubMed] [Google Scholar]
- 9.Wang K, Zhou B, Kuo YM, Zemansky J, Gitschier J. A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. Am. J. Hum. Genet. 2002;71:66–73. doi: 10.1086/341125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kury S, Dreno B, Bezieau S, Giraudet S, Kharfi M, Kamoun R, Moisan JP. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat. Genet. 2002;31:239–240. doi: 10.1038/ng913. [DOI] [PubMed] [Google Scholar]
- 11.Guerinot ML. The ZIP family of metal transporters. Biochim. Biophys. Acta. 2000;1465:190–198. doi: 10.1016/s0005-2736(00)00138-3. [DOI] [PubMed] [Google Scholar]
- 12.Taylor KM, Nicholson RI. The LZT proteins; the LIV-1 subfamily of zinc transporters. Biochim. Biophys. Acta. 2003;1611:16–30. doi: 10.1016/s0005-2736(03)00048-8. [DOI] [PubMed] [Google Scholar]
- 13.Meftah SP, Kuivaniemi H, Tromp G, Kerkeni A, Sfar MT, Ayadi A, Prasad AS. A new mutation in exon 3 of the SCL39A4 gene in a Tunisian family with severe acrodermatitis enteropathica. Nutrition. 2006;22:1067–1070. doi: 10.1016/j.nut.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Nakano A, Nakano H, Nomura K, Toyomaki Y, Hanada K. Novel SLC39A4 mutations in acrodermatitis enteropathica. J. Invest. Dermatol. 2003;120:963–966. doi: 10.1046/j.1523-1747.2003.12243.x. [DOI] [PubMed] [Google Scholar]
- 15.Lehnert T, Kury S, Burk G, Hoepffner W, Schuster V. Acrodermatitis enteropathica (AE) is caused by mutations in the zinc transporter gene SLC39A4. Klin. Padiatr. 2006;218:221–223. doi: 10.1055/s-2005-836465. [DOI] [PubMed] [Google Scholar]
- 16.Dufner-Beattie J, Wang F, Kuo YM, Gitschier J, Eide D, Andrews GK. The acrodermatitis enteropathica gene ZIP4 encodes a tissue-specific, zinc-regulated zinc transporter in mice. J. Biol. Chem. 2003;78:33474–33481. doi: 10.1074/jbc.M305000200. [DOI] [PubMed] [Google Scholar]
- 17.Wang FD, Kim BE, Dufner-Beattie J, Petris MJ, Andrews G, Eide DJ. Acrodermatitis enteropathica mutations affect transport activity, localization and zinc-responsive trafficking of the mouse ZIP4 zinc transporter. Hum. Mol. Genet. 2004;13:563–571. doi: 10.1093/hmg/ddh049. [DOI] [PubMed] [Google Scholar]
- 18.Kim BE, Wang FD, Dufner-Beattie J, Andrews GK, Eide DJ, Petris MJ. Zn2+-stimulated endocytosis of the mZIP4 zinc transporter regulates its location at the plasma membrane. J. Biol. Chem. 2004;279:4523–4530. doi: 10.1074/jbc.M310799200. [DOI] [PubMed] [Google Scholar]
- 19.Dufner-Beattie J, Kuo YM, Gitschier J, Andrews GK. The adaptive response to dietary zinc in mice involves the differential cellular localization and zinc-regulation of the zinc transporters ZIP4 and ZIP5. J. Biol. Chem. 2004;279:49082–49090. doi: 10.1074/jbc.M409962200. [DOI] [PubMed] [Google Scholar]
- 20.Mao X, Kim BE, Wang F, Eide DJ, Petris MJ. A histidine-rich cluster mediates the ubiquitination and degradation of the human zinc transporter, hZIP4, and protects against zinc cytotoxicity. J. Biol. Chem. 2007;282:6992–7000. doi: 10.1074/jbc.M610552200. [DOI] [PubMed] [Google Scholar]
- 21.Dufner-Beattie J, Weaver BP, Geiser J, Bilgen M, Larson M, Xu W, Andrews GK. The mouse acrodermatitis gene Slc39a4 (ZIP4) is essential for development and heterozygosity causes hypersensitivity to zinc deficiency. Hum. Mol. Genet. 2007;16:1391–1399. doi: 10.1093/hmg/ddm088. [DOI] [PubMed] [Google Scholar]
- 22.Weaver BP, Dufner-Beattie J, Kambe T, Andrews GK. Novel zinc-responsive post-transcriptional mechanisms reciprocally regulate expression of the mouse Slc39a4 and Slc39a5 zinc transporters (Zip4 and Zip5) Biol. Chem. 2007;388:1301–1312. doi: 10.1515/BC.2007.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandstrom B, Cederblad A, Lindblad BS, Lonnerdal B. Acrodermatitis enteropathica, zinc metabolism, copper status, and immune function. Arch. Pediatr. Adolesc. Med. 1994;148:980–985. doi: 10.1001/archpedi.1994.02170090094017. [DOI] [PubMed] [Google Scholar]
- 24.Kambe T, Weaver BP, Andrews GK. The genetics of essential metal homeostasis during development. Genesis. 2008;46:214–228. doi: 10.1002/dvg.20382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamashita S, Miyagi C, Fukada T, Kagara N, Che YS, Hirano T. Zinc transporter LIVI controls epithelial-mesenchymal transition in zebrafish gastrula organizer. Nature. 2004;429:298–302. doi: 10.1038/nature02545. [DOI] [PubMed] [Google Scholar]
- 26.Mathews WR, Wang F, Eide DJ, Van Doren M. Drosophila fear of intimacy encodes a Zrt/IRT-like protein (ZIP) family zinc transporter functionally related to mammalian ZIP proteins. J. Biol. Chem. 2005;280:787–795. doi: 10.1074/jbc.M411308200. [DOI] [PubMed] [Google Scholar]
- 27.Dufner-Beattie J, Huang ZL, Geiser J, Xu W, Andrews GK. Generation and characterization of mice lacking the zinc uptake transporter ZIP3. Mol. Cell. Biol. 2005;25:5607–5615. doi: 10.1128/MCB.25.13.5607-5615.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dufner-Beattie J, Huang ZL, Geiser J, Xu W, Andrews GK. Mouse ZIP1 and ZIP3 genes together are essential for adaptation to dietary zinc deficiency during pregnancy. Genesis. 2006;44:239–251. doi: 10.1002/dvg.20211. [DOI] [PubMed] [Google Scholar]
- 29.Sandstead HH, Fosmire GJ, McKenzie JM, Halas ES. Zinc deficiency and brain development in the rat. Fed. Proc. 1975;34:86–88. [PubMed] [Google Scholar]
- 30.Rogers JM, Hurley LS. Effects of zinc deficiency on morphogenesis of the fetal rat eye. Development. 1987;99:231–238. doi: 10.1242/dev.99.2.231. [DOI] [PubMed] [Google Scholar]
- 31.Sandstead HH. Zinc is essential for brain development and function. J. Trace Elem. Exp. Med. 2003;16:165–173. [Google Scholar]
- 32.Terhune MW, Sandstead HH. Decreased RNA polymerase activity in mammalian zinc deficiency. Science. 1972;177:68–69. doi: 10.1126/science.177.4043.68. [DOI] [PubMed] [Google Scholar]
- 33.Sandstead HH, Rinaldi RA. Impairment of deoxyribonucleic acid synthesis by dietary zinc deficiency in the rat. J. Cell. Physiol. 1969;73:81–83. doi: 10.1002/jcp.1040730111. [DOI] [PubMed] [Google Scholar]
- 34.Ugarte M, Osborne NN. Zinc in the retina. Prog. Neurobiol. 2001;64:219–249. doi: 10.1016/s0301-0082(00)00057-5. [DOI] [PubMed] [Google Scholar]
- 35.Chappell RL, Redenti S. Endogenous zinc as a neuromodulator in vertebrate retina: evidence from the retinal slice. Biol. Bull. 2001;201:265–267. doi: 10.2307/1543357. [DOI] [PubMed] [Google Scholar]
- 36.Redenti S, Chappell RL. Localization of zinc transporter-3 (ZnT-3) n mouse retina. Vision Res. 2004;44:3317–3321. doi: 10.1016/j.visres.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 37.Hyun HJ, Sohn J, Ahn YH, Shin HC, Koh JY, Yoon YH. Depletion of intracellular zinc induces macromolecule synthesis- and caspase-dependent apoptosis of cultured retinal cells. Brain Res. 2000;869:39–48. doi: 10.1016/s0006-8993(00)02340-4. [DOI] [PubMed] [Google Scholar]
- 38.Mendelsohn BA, Yin C, Johnson SL, Wilm TP, Solnica-Krezel L, Gitlin JD. Atp7a determines a hierarchy of copper metabolism essential for notochord development. Cell Metab. 2006;4:155–162. doi: 10.1016/j.cmet.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Chowanadisai W, Lonnerdal B, Kelleher SL. Identification of a mutation in SLC30A2 (ZnT-2) in women with low milk zinc concentration that results in transient neonatal zinc deficiency. J. Biol. Chem. 2006;281:39699–39707. doi: 10.1074/jbc.M605821200. [DOI] [PubMed] [Google Scholar]