Abstract
In fear extinction, an animal learns that a conditioned stimulus (CS) no longer predicts a noxious stimulus [unconditioned stimulus (UCS)] to which it had previously been associated, leading to inhibition of the conditioned response (CR). Extinction creates a new CS–noUCS memory trace, competing with the initial fear (CS–UCS) memory. Recall of extinction memory and, hence, CR inhibition at later CS encounters is facilitated by contextual stimuli present during extinction training. In line with theoretical predictions derived from animal studies, we show that, after extinction, a CS-evoked engagement of human ventromedial prefrontal cortex (VMPFC) and hippocampus is context dependent, being expressed in an extinction, but not a conditioning, context. Likewise, a positive correlation between VMPFC and hippocampal activity is extinction context dependent. Thus, a VMPFC–hippocampal network provides for context-dependent recall of human extinction memory, consistent with a view that hippocampus confers context dependence on VMPFC.
Keywords: fear conditioning, extinction, context, hippocampus, ventromedial prefrontal cortex, extinction memory
Introduction
Learning to disregard a conditioned stimulus (CS) that no longer predicts an unconditioned stimulus (UCS) is important for adaptive behavior in a changing environment. An observation, made by Pavlov (1927), that extinguished conditioned responses (CRs) can recover is widely interpreted as indicating that extinction does not erase a CS–UCS association (“fear memory”) but creates a competing CS–noUCS association (“extinction memory”) (Myers and Davis, 2002; Bouton, 2004; Delamater, 2004). Context appears to be a critical regulatory factor in the expression of this putative competition (Bouton, 2004). Thus, after extinction training, the subsequent recall of an extinction memory with CS presentation (i.e., the inhibition of the CR) shows a relative specificity to contexts that resemble those present during extinction training (“extinction context”). In contrast, non-extinction contexts favor recall of fear memory. This may reduce the probability that a “true” danger cue is disregarded, which may be substantially more costly (e.g., lethal) than reacting with fear to a “false” danger cue.
The ventromedial prefrontal cortex (VMPFC) is involved in the storage and recall of extinction memories (Morgan and LeDoux, 1995; Milad and Quirk, 2002; Phelps et al., 2004; Milad et al., 2005). VMPFC activation is necessary for extinction recall in a time window of ∼24–74 h after extinction training (Lebron et al., 2004). The VMPFC may contribute to CR inhibition via suppression of the amygdala (Quirk et al., 2003; Rosenkranz et al., 2003). A current theoretical model (Hobin et al., 2003) predicts that CS-evoked VMPFC activation after extinction is restricted to extinction contexts (is context dependent). Alternatively, the VMPFC may be engaged whenever an extinguished CS is presented (be context independent), and a context dependence in the recall of extinction memory may only be expressed in some other interconnected region [e.g., in the amygdala (Hobin et al., 2003)]. Therefore, the primary goal of this study was to examine whether VMPFC activation is context dependent.
The hippocampus is a region that is classically associated with contextual memory functions, including context-driven recall (Hirsh, 1974; Kennedy and Shapiro, 2004). Thus, the hippocampus is required for the recall of recent context-evoked fear memories (Anagnostaras et al., 2001). Not surprisingly, it has long been hypothesized that the hippocampus contributes to context-dependent recall of extinction memory (Frohardt et al., 2000; Hobin et al., 2003; Sotres-Bayon et al., 2004). In its simplest form, this contribution may take the form of rendering an extinction memory accessible (Delamater, 2004) through activation of associations linking a current context with the previous experience of extinction. At a neural level, the hippocampus may support CS-evoked VMPFC engagement while in an extinction context. Such contextual gating by the hippocampus of CS inputs into the VMPFC should be evident in correlated CS-evoked activity in both areas during recall of extinction memory. An additional goal of this study was therefore to test whether CS-evoked hippocampus activation after extinction is also (extinction) context dependent and, if so, whether this activity positively correlates with CS-evoked VMPFC activity.
A variant of a hippocampus-based model of context-dependent recall of extinction memory was proposed by Hobin et al. (2003). The model takes into account the observation that pretest lesions of the dorsal hippocampus in rats lead to a failure to produce tone-evoked CRs, but only when the CR has been extinguished previously (Corcoran and Maren, 2004; Corcoran et al., 2005; Ji and Maren, 2005). Hobin et al. (2003) thus argued that, after extinction, the hippocampus inhibits the VMPFC and thus allows for CS-evoked amygdala activation (leading to a CR). This block on the VMPFC would only be lifted where contextual information signals it is more appropriate to inhibit the CR, such as in the extinction context. In these instances, the VMPFC would act to inhibit the amygdala, preventing a CR. This model differs from the simpler model described above in that it predicts CS-evoked dorsal (or, in humans, posterior) hippocampus activation in test contexts that differ from the extinction context. It further predicts that CS-evoked hippocampus activation negatively correlates with CS-evoked VMPFC activation in those contexts. A final goal of this study was thus to test both the predictions derived from the model by Hobin et al. (2003).
Materials and Methods
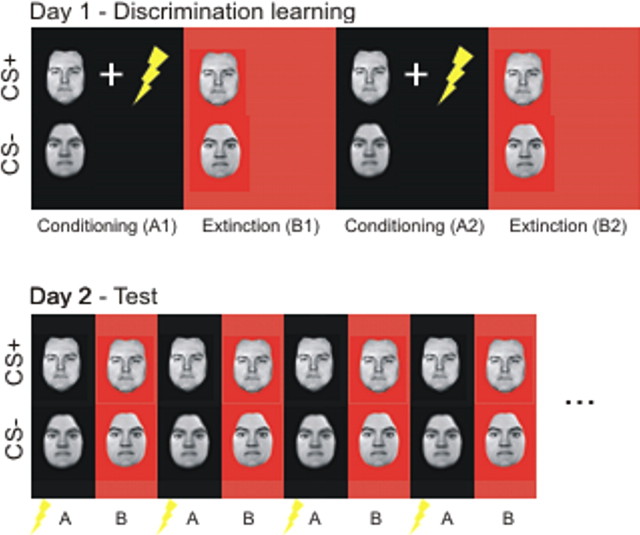
In brief, the experiment used a within-subject AB–AB design consisting of pavlovian fear conditioning in context A and extinction in context B on day 1. This was followed by testing of CS-evoked responses in both the conditioning (A) and the extinction (B) contexts on day 2 (see Fig. 1).
Figure 1.
AB–AB design. On day 1, subjects were fear conditioned to a CS+ (a face) through multiple pairings with a UCS (electric shock) in context A (conditioning context, block A1). Fear responses were extinguished in context B (extinction context, block B1) through multiple CS+ presentations in the absence of the UCS. This procedure was repeated in additional blocks (blocks A2, B2), leading to robust context discrimination. As a control for nonassociative effects, we also used a nonpredictive CS− (a face of opposite gender) that was never paired with the UCS and presented intermixed with the CS+. Contexts were defined by screen color (black in one context, changing between red and orange in the other) and auditory input (two sounds, changing synchronously with the color of the screen in the red–orange context only). These marked physical differences between the two contexts were intended to facilitate context discrimination. On day 2, subjects were tested for CS+-induced recall of fear and extinction memories in both contexts, each context being presented 16 times in alternating order. Recall of fear memory in context A on day 2 was facilitated by additionally presenting one unpaired shock at the beginning of each context A block, thus again firmly associating context A with the UCS. The task was a speeded gender decision task in response to the face stimuli. Gender of faces, conditioning, and extinction contexts and the order of those contexts on day 2 were counterbalanced across subjects. Lightning bolt, Electric shock.
Subjects
Seventeen normal healthy volunteers [mean (M) age, 25 years; age range, 18–34 years; nine males; right-handed] participated in the study. Subjects were preassessed to exclude those with a previous history of neurological or psychiatric illness, including anxiety disorders. All subjects gave informed consent, and the study was approved by the Joint Ethics Committee of the National Hospital for Neurology and Neurosurgery.
Stimuli
Unconditioned stimulus.
The UCS consisted of brief electric shocks to the right hand. Shocks were applied using a Digitimer DS7A electrical stimulator (Digitimer, Welwyn Garden City, UK) delivering electrical pulses of up to 20 mA and 1 or 2 ms duration through a silver chloride electrode. Stimulation parameters were individually adjusted before the experiment to achieve maximum tolerable pain. To this end, subjects were given a series of shocks, starting at a very low current level and slowly increasing in amplitude, until the subject indicated he or she did not want to receive any higher stimulation. The subject was explicitly asked whether the reached level was tolerable and could be used during the subsequent experiment. Note that fear-related areas show lateralized CS responses based on where the source of danger is located in space (Blair et al., 2005; Kalisch et al., 2005). To increase the probability of finding CS-evoked activation, we therefore applied the UCS to the same hand (right) in all subjects. This prohibits inference about lateralization of CS-evoked responses, such as observed in VMPFC and hippocampus (see Results).
Conditioned stimuli.
The two CSs (one CS, or CS+, which was occasionally paired with the UCS, and one CS, or CS−, which was never paired) consisted of one male and one female face from the Ekman series (Ekman and Friesen, 1976) whose hair was removed in view of the gender decision task (see below). Mildly (20%) angry faces were chosen based on previous studies by our group showing successful conditioning and amygdala activation with mildly angry-face CSs (Morris et al., 1998; Critchley et al., 2002). The two same faces were used for all subjects. In 8 of the 17 subjects, the CS+ was the male face, and, in the remaining nine subjects, it was the female face.
Contexts.
Conditioning and extinction occurred in two different contexts that were distinguished by background screen color and auditory input. The screen color was either black or rhythmically changing between red and orange. The fixation mark was a white cross in the black context and a white dot in the red–orange context. There was no auditory input in the black context, whereas in the red–orange context, subjects heard two sounds of different pitch, presented over headphones, that changed synchronously with the color of the screen. In 10 subjects, the black context was the context in which conditioning occurred (conditioning context or A) and the red-orange context was the context in which extinction occurred (extinction context or B). In seven subjects, the red–orange context was the conditioning context, and the black context was the extinction context.
Task
Subjects were told that the study would examine attentional performance under stress and were only debriefed at the end of the study. The task was a speeded gender decision task for which subjects signaled the gender of the face by pressing the left (for female) or the right (for male) button on a keypad with the index or middle finger, respectively, of the right hand as soon as they saw the face.
Design
Day 1 (discrimination learning).
Subjects were first habituated to the CSs and contexts by presenting each CS three times in each context before the actual experiment. Subjects then learned to discriminate the two CSs on the basis of how they predicted danger. In conditioning block A1, the CS+ and the CS− were each presented 10 times in a randomized order in the center of the screen. The duration of CSs ranged from 2 to 8 s, with a mean duration of 5.7 s per CS type (one CS of 2 and 3 s each, and two CSs of 5, 6, 7, and 8 s each per CS type). At 250 ms before the offset of the CS+, the UCS was applied. Those two CS+ presentations, which were shorter than 5 s, were not coupled with a UCS, resulting in a reinforcement ratio of 80%. Varying delays between CS+ and UCS onset were meant to introduce additional uncertainty that would make conditioning somewhat more extinction resistant and therefore increase the likelihood of recall of fear memory on day 2 (see below). A minimum delay of 5 s allowed us to measure conditioned skin conductance responses (SCRs) to the CS+ without a confound from the subsequent unconditioned SCRs to the shock. CSs were separated by an interstimulus interval (ISI) of 9 s, during which subjects saw a central fixation mark (low-level baseline). The length of the ISI was chosen to avoid complete masking of conditioned SCRs by preceding unconditioned SCRs to the shock.
In the following extinction block B1, which was different from the preceding conditioning block in terms of screen color and auditory input (see above), conditioned fear responses were extinguished by presenting the same 20 CSs in the same manner but without shock. This was followed by another conditioning block (A2) and another extinction block (B2), each in their respective contexts. This design allowed subjects to learn to discriminate between two different contexts on the basis of whether the CS+ was (conditioning context) or was not (extinction context) associated with the UCS. Each block lasted 6 min and the corresponding context was already present at the beginning of each block, 9 s before CS presentation started. Blocks were separated by a break of 30 s, during which scanning continued but subjects were allowed to close their eyes if they wanted.
At the end of day 1, the subjects were asked whether they had noticed any relationship between the shock and the gender of the face and between the shock and the screen color.
Day 2 (test).
Each context was again presented 18 times in an alternating order for a duration of 29 s each, separated by 5 s breaks. In nine subjects, the experiment started with the conditioning context (ABABAB…), and, in eight subjects, it started with the extinction context (BABABA…). At the beginning of each block, the context was present without any CS for 10 s. In 16 the 18 blocks of A and B each, one CS+ and one CS− were presented in random order for a duration of 5 s each, followed by an ISI of 4 s each. In those 16 A blocks, subjects received a shock 3 s after the beginning of the block. This was meant to facilitate recall of fear memory in context A, an effect that is difficult to achieve because of ongoing extinction of CRs as a consequence of unreinforced CS+ presentation on the test day.
Autonomic monitoring
Skin conductance measurements were acquired at a sampling rate of 1000 Hz from electrodes on the middle and ring finger of the left hand using an AT64 SCR apparatus (Autogenic Systems, Wood Dale, IL).
Imaging
Subjects were scanned on both days to maximize context identity across days. Only data from day 2 are reported here. A 3 tesla MR head scanner (Magnetom Allegra; Siemens, Erlangen, Germany) was used to acquire gradient echo T2*-weighted echo-planar images (EPIs) with blood oxygenation level-dependent contrast (echo time, 30 ms; repetition time, 1.43 s; flip angle, 70°; slice tilt, 30°; z-shim gradient prepulse, −1 mT · m−1 · ms−1). Each volume comprised 22 oblique axial slices of 2 mm thickness and 3 × 3 mm2 in-plane resolution with a slice gap of 1 mm. The slice package excluded the dorsal frontal, parietal, and occipital cortices. These parameters produced EPIs in which signal dropout because of susceptibility-induced field inhomogeneities was minimized for amygdala and orbitofrontal cortex (Deichmann et al., 2003). Subjects were placed in a light head restraint within the scanner to limit head movement during acquisition. A total of 980 (day 1) and 895 (day 2) volumes were acquired continuously throughout the task, at 1.43 s intervals, starting 14.3 s before onset of the experiment. As a result of the above timings, there was no systematic temporal relationship between the onsets of slices and stimuli, thus allowing for sampling, over the course of the experiment, the entire length of the stimulus-driven hemodynamic responses in each of the 22 slices.
A T1-weighted structural image was also acquired (Deichmann et al., 2004).
Data analysis
Skin conductance data were downsampled to 100 Hz, mean filtered, and then visually inspected for artifacts. Three subjects on day 1 and four subjects on day 2 did not show any apparent SCR to the UCS and were therefore excluded from additional skin conductance analysis, reducing sample size to n = 14 (day 1) and n = 13 (day 2). In the following, an SCR was defined as the maximum skin conductance in a time window of 5 s after CS onset minus skin conductance at the time of CS onset (Buchel et al., 1998). Data were z transformed to account for interindividual differences in physiological reactivity (Buchel et al., 1998).
As SCRs, reaction time (RT) data from the gender decision task were z transformed. Response accuracy on both days was not significantly influenced by either context or CS type (CS+ vs CS−) (repeated-measures ANOVA) and ranged between 91.5 and 100% in the different conditions.
Significance of behavioral effects was assessed using paired t tests. Because the previous literature provided directed hypotheses for both learning (day 1) and test (day 2) effects on SCRs and RT (see Results), a one-tailed threshold of p = 0.05 was used throughout. RT data were also analyzed using a nonparametric Wilcoxon's signed rank test, which gave substantially the same results (data not shown).
Imaging data were analyzed using SPM2 (http://www.fil.ion.ucl.ac.uk/spm) (Ashburner et al., 2004). The 10 initial images were discarded to account for T1 equilibration. To correct for motion artifacts, images were realigned to the 11th volume. Images were unwarped to correct for movement-by-distortion interactions, spatially normalized to a standard EPI template, spatially smoothed using a Gaussian kernel with a full-width at half-maximum (FWHM) of 4 mm, temporally high-pass filtered (cutoff, 128 s), and corrected for temporal autocorrelations using first-order autoregressive modeling. Statistical analysis was performed using a standard approach for functional magnetic resonance imaging (fMRI), involving a general linear convolution model at the single-subject level and a random-effects analysis at the group level (for details, see Friston et al., 1994, 1995; Holmes and Friston, 1998; Penny and Holmes, 2004). First, for each subject, condition-specific regressors were defined that modeled the time course of the experimental events and, after convolution with a canonical hemodynamic response function, served as predictors of the fMRI signal time courses at each voxel in the brain. CSs were modeled as a series of events (i.e., a series of delta functions, separately for CS+ in A, CS− in A, CS+ in B, and CS− in B). Additionally, these four categorical regressors were parametrically modulated using trial-by-trial z-transformed RTs as an index of the magnitude of the evoked CR. Shocks were also modeled as events, whereas A and B blocks were modeled as two separate boxcar regressors (0 for “off” and 1 for “on”). As mentioned above, each regressor was convolved with a canonical hemodynamic response function. Using these regressors in a general linear model (multiple regression) of brain activation at each voxel yields parameter estimates of the contribution of each regressor to the fMRI signal measured in each voxel. Contrasts, i.e., linear combinations of these parameter estimates, were then calculated voxelwise to produce within-subject estimates of effects of interests (e.g., the contrast CS+ – CS− in context A, etc.). Statistical inference is obtained using a t statistic that takes into account the magnitude of the contrast value and its SD. This yields single-subject statistical parametric maps (SPM T maps) for each contrast of interest. For the random-effects group analysis, the subject-specific contrast images were spatially smoothed (FWHM, 10 mm) to account for intersubject variation in the exact location of activations and compared across the 17 subjects. Group effects were tested for significance using voxelwise one-sample one-tailed t tests. To illustrate group effect sizes in selected voxels (insets in the figures), group-level contrast estimates were used. For planned post hoc t tests and regression analysis, the subject-specific contrast estimates were extracted from the individual smoothed contrast images. Correlation coefficients were compared with each other using the test developed by Fisher (1921).
Voxels activated at a statistical threshold of p ≤ 0.001 are reported, unless indicated otherwise. Correction for multiple comparisons following Gaussian random field theory was limited to four predefined small search volumes (left and right hippocampus, left and right VMPFC). These were manually delineated on the mean structural image. The VMPFC search volume excluded the subgenual cingulate cortex and was dorsally delimited by a straight horizontal line between the anterior tip of the genu and the cortical surface. These search volumes, as well as additional left and right amygdala masks, were also used to mask activation images (SPM T maps) in Figures 3 and 4. Structural images were coregistered onto functional images and spatially normalized using the nonlinear transformation estimated from the functional EPIs. Anatomical localization was performed with reference to the atlas of Duvernoy (1999). Hippocampal subregions along the rostrocaudal axis were named according to the convention proposed by Amaral (1999). Coordinates are described in the standard space defined by the Montreal Neurological Institute (MNI).
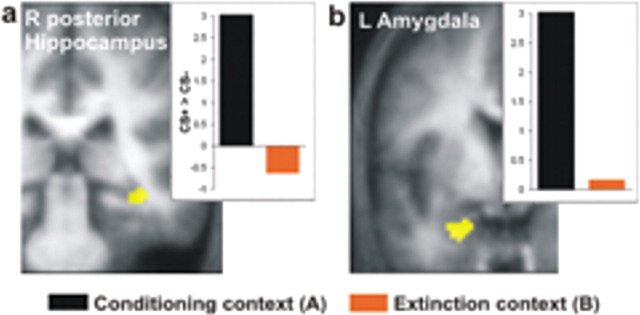
Figure 3.
Recall of fear memory. Activations associated with recall of fear memory on day 2. a, Right (R) posterior hippocampus [MNI coordinates (38, −32, −12)]. b, Left (L) amygdala [coordinates (−28, −2, −32)]. Images show group-level estimates for the contrast (CS+ > CS−)A, modulated by RT, display threshold p ≤ 0.01. Activations superimposed on mean structural image, masked for hippocampus and amygdala. Insets, Group estimates for CS+ > CS− contrasts in both contexts.
Figure 4.
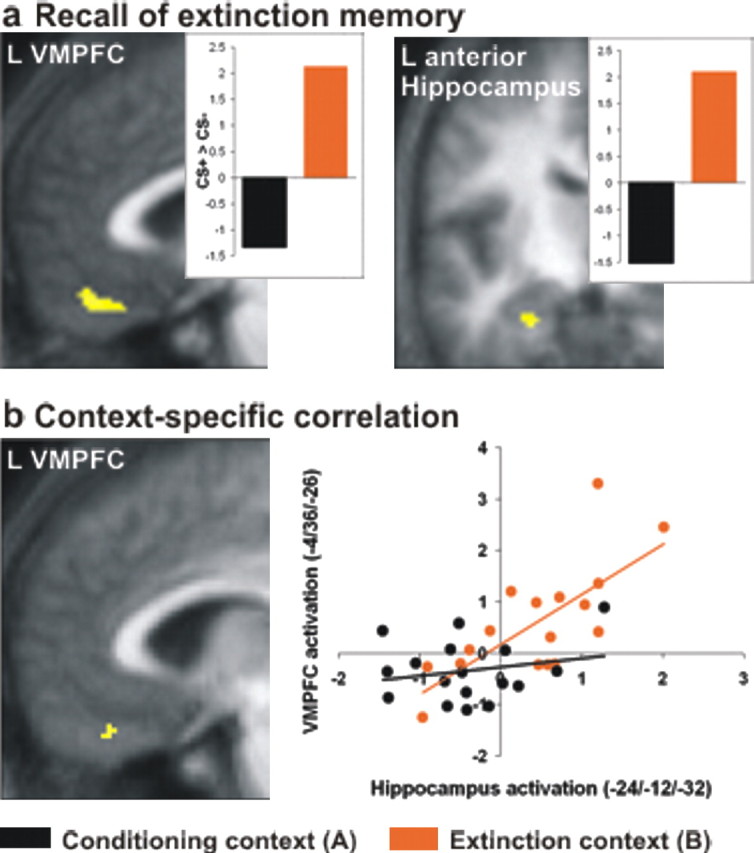
Recall of extinction memory. a, Activations associated with context-dependent recall of extinction memory on day 2. Images show contrast (CS+ > CS−)B > (CS+ > CS−)A, display threshold p ≤ 0.001, masked for VMPFC and hippocampus. Activations are superimposed on mean structural image. Insets, Group-level estimates for CS+ > CS− contrasts in both contexts. b, CS+-evoked left VMPFC activity is positively correlated with CS+-evoked left anterior hippocampus activity in the extinction context only. Left, Correlation in VMPFC in the extinction context, display threshold p ≤ 0.001, masked by VMPFC activation in a. Right, An extinction context-specific correlation is apparent from the subject-specific estimates for the two CS+ > CS− contrasts (black: conditioning context, r = 0.212, p = 0.207, one-tailed; orange: extinction context, r = 0.715, p = 0.0005, one-tailed). L, Left.
Results
Context-dependent recall of extinction memory was studied using a novel within-subject AB–AB design (Fig. 1), developed specifically for purposes of fMRI. The design consists of pavlovian fear conditioning in context A and extinction in context B on day 1, with testing of CS-evoked responses in both the conditioning (A) and the extinction (B) contexts on day 2. We predicted positively correlated CS-evoked brain activation in anatomically predefined search volumes in (left and right) VMPFC and hippocampus during test on day 2 in the extinction context B but not in the conditioning context A. We also tested the hypothesis of CS-evoked posterior hippocampus activation during test in the conditioning context A, but not the extinction context B, and whether this activation was negatively correlated with VMPFC activation.
Context-dependent recall of extinction memory
Twelve of 17 subjects reported awareness of the CS+–UCS contingency, and 16 of 17 reported awareness of the context A–UCS contingency when interviewed after the experiment on day 1. SCRs from day 1 confirmed successful conditioning and context discrimination (Fig. 2a). SCRs to the CS+ were larger than to the CS− in A [(CS+ > CS−)A: M(CS+) of 0.26; M(CS−) of −0.03; t(13) = 2.61; p = 0.011, paired t test, one-tailed] but not in B [(CS+ >CS −)B: M(CS+) of −0.09; M(CS−) of −0.14; t(13) = 0.92; p = 0.188], resulting in a significant context discrimination effect [(CS+ > CS−)A > (CS+ > CS−)B: M(]CS+ > CS−]A) of 0.28; M([CS+ > CS−])B = 0.05; t(13) = 2.0; p = 0.033]. There were no significant differential RT effects in the incidental gender decision task (as described in Fig. 1) (Fig. 2b).
Figure 2.
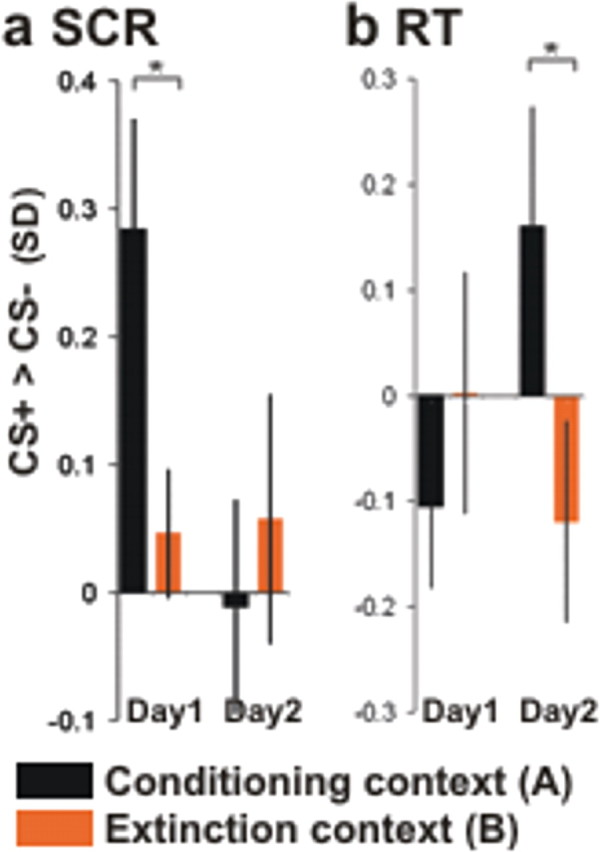
Successful discrimination learning and context-dependent recall. a, SCR. Larger SCRs to the CS+ versus CS− during fear conditioning in context A compared with the extinction context B on day 1 indicate learning of the CS+–UCS contingency (fear memory) in context A and of the CS+–noUCS contingency (extinction memory) in context B on day 1 (left). b, RTs. Slower RTs to the CS+ versus CS− in the conditioning context A compared with the extinction context B on day 2 indicate recall of fear memory in context A and recall of extinction memory in B on day 2 (right). Scale: z scores (unit, SDs). z scores were used instead of raw values (micro-ohms, seconds) to account for interindividual differences. *p < 0.05, one-tailed.
Recall of fear memory (CR recovery) at test is usually evident in augmented SCRs (LaBar and Phelps, 2005; Milad et al., 2005; Vansteenwegen et al., 2005) and/or slower RTs (Dirikx et al., 2004; Hermans et al., 2005) to CS+ compared with CS− stimuli. On day 2, we found no significant differential SCR effects (Fig. 2a). However, as predicted, RTs to the CS+ (relative to the CS−) in context A were significantly slower than in context B [(CS+>CS−)A > (CS+>CS−)B: M([CS+ > CS−]A) of 0.16; M([CS+ > CS−]B) of −0.12; t(16) = 1.86; p = 0.041] (Fig. 2b). This context-discrimination effect was driven by slower RTs to the CS+ versus the CS− in context A and faster RTs to the CS+ versus the CS− in context B, providing behavioral evidence for recall of fear memory restricted to the conditioning context. Supplemental Figure 1 (available at www.jneurosci.org as supplemental material) shows that the effect was not attributable to changes in RTs to the CS− but to slowing of RTs to the CS+ in the conditioning context A and quickening of RTs to the CS+ in the extinction context B. The absence of SCR effects may reflect recall of only some aspects of the CR 1 d after conditioning (see Discussion).
fMRI data from day 2 were in agreement with recall of fear memory in context A. Brain areas mediating CR recovery in A were identified from the contrast (CS+ > CS−)A. Categorical effects were parametrically modulated by trial-by-trial RTs as an index of CR magnitude. We observed activation in areas previously implicated in fear responses [bilateral striatum (Jensen et al., 2003; Phelps et al., 2004; Seymour et al., 2004; Wager et al., 2004), left temporal cortex and cerebellum (Ploghaus et al., 1999; Wager et al., 2004), hypothalamus (Simpson et al., 2001; Boshuisen et al., 2002), right posterior hippocampus (Frohardt et al., 2000; Gray and McNaughton, 2000; Corcoran and Maren 2004; Ji and Maren, 2005); all p ≤ 0.001 uncorrected, one-sample t test, one-tailed)] (Fig. 3a) (supplemental Table 1, available at www.jneurosci.org as supplemental material). We note that, at a more liberal threshold (p = 0.002 uncorrected), ventral left amygdala was also activated (Fig. 3b) (supplemental Table 1, available at www.jneurosci.org as supplemental material). This finding is interesting given the presumed central role of amygdala in fear conditioning and memory (for review, see Buchel and Dolan, 2000; Kim and Jung, 2006). To our knowledge, this is the first indication in the human literature that the amygdala is implicated in recall of a fear memory. The result resonates with findings of amygdala activation in provocation paradigms in patients with certain anxiety disorders in which fear conditioning is a putative etiological mechanism (Cannistraro and Rauch, 2003). Together, the combined behavioral and neural data show that, on day 2, CR recovery was restricted to the conditioning context A. This is consistent with successful recall of extinction memory in context B, preventing expression of a CR.
VMPFC and anterior hippocampus activation during recall of extinction memory
On the basis of the above data, we conjectured that, in our fMRI analysis, areas supporting context-dependent recall of extinction memory would show a contextual modulation of CS+-evoked activation. We examined this by testing for a categorical CS-by-context interaction (CS+ > CS−)B > (CS+ > CS−)A in VMPFC and hippocampus on day 2. In this contrast, no parametric modulation was used. We found a significant interaction in left VMPFC [MNI coordinates (−2, 42, −22); p = 0.048 after small volume correction (SVC)] and left anterior hippocampus, extending into entorhinal cortex [(−24, −12, −32), p = 0.011 SVC; (−26, −18, −26), p = 0.043 SVC) (Fig. 4a) (for uncorrected p values, see supplemental Table 1, available at www.jneurosci.org as supplemental material). The interaction was driven by a relatively greater activation to the CS+ than to the CS− in the extinction context B (p = 0.025, 0.021, and 0.08, post hoc paired t tests, one-tailed, on subject-specific contrast estimates), whereas, in the conditioning context A, activations were greater for the CS− than for the CS+ (p = 0.1, 0.018, and 0.019, one-tailed). Thus, as predicted, a CS+-evoked activation of VMPFC and (left anterior) hippocampus was specifically expressed in an extinction context. Greater activation for the CS− than for the CS+ in the conditioning context may reflect safety signal properties of the CS−, acquired during conditioning, in this context.
Positive anterior hippocampus–VMPFC correlations during recall of extinction memory
We next tested whether CS+-evoked left anterior hippocampal activation during recall of extinction memory was positively correlated with corresponding VMPFC activation. For this purpose, we performed a group-level regression analysis in which subject-specific contrast estimates of the contrast (CS+ > CS−)B from the peak activation in left anterior hippocampus [coordinates (−24, −12, −32)] were used as a regressor on whole-brain (CS+ > CS−)B contrast maps. Using inclusive masking (masking threshold, p = 0.001 uncorrected), the search volume was restricted to the above VMPFC interaction (as in Fig. 4a). There was a positive correlation (r = 0.715; p = 0.0005, one-tailed) at coordinates (−4, 36, −26) (Fig. 3b). That is, individuals with strong extinction-related hippocampal activation also had strong extinction-related activation in VMPFC. This relationship was specific for the extinction context B because the correlation between both voxels in the conditioning context A [contrast (CS+ > CS−)A; r = 0.212; p = 0.207, one-tailed] was significantly smaller (p = 0.036, one-tailed Fisher's test) (Fig. 4b). These data are consistent with a simple model of hippocampal contributions to context-dependent recall of extinction memory, stating that information about the extinction context processed in the hippocampus supports recall of the extinction memory in the VMPFC, possibly through some hippocampal-dependent gating of CS inputs into the VMPFC.
Posterior hippocampus
In contrast, the model of Hobin et al. (2003) predicts that the posterior hippocampus is active specifically during recall of fear memory in non-extinction contexts. In such contexts, the hippocampus would inhibit the VMPFC and thus the recall of the extinction memory outside an extinction context. The right posterior hippocampus activation we observed during recall of fear memory [contrast (CS+ > CS−)A, modulated by RT (Fig. 3a)] survived small volume correction for a right hippocampal search volume (p = 0.045 SVC). Furthermore, as predicted by Hobin, contrast estimates suggested conditioning context-specific activation (Fig. 3a, inset). However, the interaction contrast (CS+ > CS−)A > (CS+ > CS−)B, modulated by RT, did not reach significance (p = 0.008 uncorrected; p = 0.235 SVC). We thus failed to find strong evidence for a (conditioning) context dependence of posterior hippocampus activation.
This failure to observe context dependence in posterior hippocampus may have been attributable to possible incomplete or weak recall of fear memory in the conditioning context, as indexed by a failure to find context-dependent SCRs on day 2 (see above). Hence, to explore this question further, we ascertained whether CS+-evoked right posterior activation during recall of fear memory was negatively correlated with corresponding VMPFC activation. We performed another regression analysis in which subject-specific estimates of the contrast (CS+ > CS−)A, modulated by RT, from the peak activation in right posterior hippocampus [coordinates (38, −32, −12)] were used as a regressor on whole-brain (CS+ > CS−)A contrast maps. Using inclusive masking (masking threshold, p = 0.001 uncorrected), the search volume was again restricted to VMPFC, as in Figure 4a. We failed to find any correlation (p = 0.05 uncorrected), even when lowering the masking threshold to p = 0.05 uncorrected. Thus, we found no evidence that the posterior hippocampus exerts inhibitory action over extinction memories by suppressing the VMPFC. In an analogous regression analysis on whole-brain maps of the contrast (CS+ > CS−)A, modulated by RT, we found no evidence for positive correlations between right posterior hippocampus and amygdala that might support a suggestion that the posterior hippocampus supports recall of fear memory by directly interacting with the amygdala.
Discussion
Our data provide evidence suggesting that the recall of extinction memory in humans is mediated by a network of brain areas, including the VMPFC and the anterior hippocampus. Interestingly, a similar, although right-sided, VMPFC area to the one observed here shows increased cortical thickness in subjects with better extinction recall performance (Milad et al., 2005). There was no activation to the CS+ relative to the CS− in this network in the conditioning context. That is, the network does not activate whenever an extinguished CS+ is presented but only when contextual information signals the appropriateness of inhibiting the CR. This network is therefore likely to form a neurobiological substrate for the context dependence of extinction recall (Bouton, 2004), a hypothesis that existing animal (Sotres-Bayon et al., 2004) and human (Phelps et al., 2004; LaBar and Phelps, 2005; Milad et al., 2005) studies have not tested. The finding of extinction context-specific relative activations in our study [as opposed to the extinction-related deactivations observed previously by others (LaBar and Phelps, 2005)] also supports the general idea that extinction (and its recall) is not simply a process of forgetting the CS–UCS association but consists in creating (and later recalling) a new CS–noUCS memory trace (Myers and Davis, 2002; Bouton, 2004; Delamater, 2004).
Our data are consistent with the hypothesis that, during recall of extinction memory, the hippocampus processes contextual information supporting recall of that memory (Delamater, 2004) and that this may confer (extinction) context dependence to CS+-evoked VMPFC activity. The reported correlations between hippocampus and VMPFC do not allow us to infer causality or directionality. We note, however, that a recent study of recall of extinction memory in which the test context was identical to the conditioning and extinction contexts (“AAA design”) found evidence of VMPFC, but not hippocampal, activation (LaBar and Phelps, 2005). In an AAA design, the context provides ambiguous and thus essentially useless information with regard to the competition between fear and extinction memory, and, hence, the competition may simply be regulated by the relative strength of the two memory traces. As a consequence, recall of extinction memory in an AAA design may not require the hippocampus. The model is also supported by evidence that the hippocampus provides a major excitatory input to the VMPFC (for review, see Sotres-Bayon et al., 2004) and that hippocampus-to-VMPFC projections are considerably stronger than VMPFC-to-hippocampus projections (Cavada et al., 2000). It is noteworthy that, in the rat, hippocampal VMPFC afferents stem from subiculum and CA1 (Sotres-Bayon et al., 2004), which corresponds to the location of the anterior hippocampal activation observed in this study (Fig. 4).
Rat data have shown that lesions of the entire hippocampus or fornix do not impair context-dependent recall of extinction memory (Wilson et al., 1995; Frohardt et al., 2000). A study in human patients yielded similar results (LaBar and Phelps, 2005). However, in these experiments, lesions were present before conditioning and extinction. This leaves open the possibility that redundant systems provided for encoding and recall of context information in the hippocampus-lesioned subjects. We suggest that the VMPFC normally receives contextual information from the anterior hippocampus but can call on inputs from other polymodal areas (Heidbreder and Groenewegen, 2003) when this hippocampus area is dysfunctional.
Our data provide only limited evidence for the alternative model by Hobin et al. (2003) positing a role of the posterior hippocampus in regulating the fear versus extinction memory competition. Although we found the predicted activation of right posterior hippocampus during recall of fear memory, this activation was not conditioning context specific. Also, we did not find the predicted negative correlation with VMPFC that could have been interpreted as indexing suppression of extinction memories in a non-extinction context. Nevertheless, the idea that the hippocampus supports context-dependent recall of fear memory has recently been strengthened by the finding of impaired context-specific reinstatement in two hippocampal patients (LaBar and Phelps, 2005). Unfortunately, no information about the exact anatomy of the lesion was available. Furthermore, the lesions occurred before conditioning and extinction. We thus do not want to exclude a contribution of posterior hippocampus to the inhibition of extinction recall in humans. However, any contribution has to be weighed against an active contribution from more anterior hippocampal areas, supporting the recall of extinction memory.
Any recall test in the absence of paired UCSs is necessarily accompanied by ongoing extinction, usually restricting CR recovery to one or a few initial CS presentations. fMRI design constraints, however, require that conditions are presented repeatedly, forcing us to induce CR recovery over 16 blocks of context A. This was achieved here by combining renewal with reinstatement. In renewal, CS-induced CR recovery is facilitated by presenting the CS in the same context as during initial conditioning. In reinstatement, CS-evoked recall of fear memory is facilitated by unpaired UCS presentations in the same context in which the CS is presented. A limitation of this study is therefore that we are unable to differentiate between renewal and reinstatement effects on recall of fear memory. This makes any conclusions derived about recall of fear memory (such as about a possible role of the posterior hippocampus) less strong than conclusions about context-dependent recall of extinction memory, for which purpose this study was explicitly designed.
Renewal/reinstatement on day 2 was evident from increased reaction times to the CS+ relative to the CS−, although, unexpectedly, there were no skin conductance differences. We suggest that this behavioral dissociation can be understood by considering that conditioning is expressed in a variety of behaviors, making it conceivable that not all behavioral expressions of a CR are produced with each CS presentation. Such a scenario is more likely where CS presentations are non-reinforced, possibly resulting in on-line extinction, such as on day 2 here (see above). Dissociations between behavioral and physiological measures of emotional reactions have also been observed in other studies (Johnstone and Page, 2004). It should be noted that reaction times are a valid and widely used method to measure CRs in humans (Critchley et al., 2002; Gottfried et al., 2002; Dirikx et al., 2004; Gottfried and Dolan, 2004; Hermans et al., 2005). They are also widely used in the assessment of other emotional responses, such as the attention-grabbing effect of aversive stimuli, in which their use has plausible theoretical grounds [namely interference with cognitive processing by aversive stimuli (Mathews et al., 1997)]. Nevertheless, the observed dissociation suggests caution in the interpretation of our data and warrants independent confirmation of our results by future studies.
We did not observe any significant negative correlation between VMPFC and amygdala. This may reflect the fact that VMPFC-dependent suppression of CS+-evoked amygdala output involves both excitation (of direct VMPFC target neurons in lateral amygdala or intercalated cell masses) and inhibition (of amygdala output neurons, possibly by inhibitory VMPFC target neurons) (Quirk et al., 2003; Rosenkranz et al., 2003; Pare et al., 2004). Given the low spatial resolution of fMRI, both effects may cancel each other out.
We have discussed our findings in terms of extinction, but it is important to acknowledge that a conditioned inhibition (CI) framework provides an alternative account of our data. In CI, conditioning to a first CS (“target” stimulus T) is followed by a training phase in which T is presented together with a second CS (“feature” stimulus F) but without the UCS (Pavlov, 1927). Hence, F comes to predict absence of the UCS, resulting in an inhibitory F–noUCS memory trace. In the present experiment, the CS+ could be conceptualized as T and the extinction context B as F. CR inhibition would then be mainly conveyed by a F–noUCS association (i.e., the extinction context itself) and not, as in the case of extinction, by a CS+–noUCS association (the extinction memory). Nevertheless, current models of CI (Pearce and Hall, 1980; Wagner and Brandon, 1989; Nelson and Bouton, 1997) assume that, similar to extinction, T (i.e., the CS+) also acquires some inhibitory properties. Furthermore, in the case of extinction, when one recalls an extinction memory in its extinction context, the context will contribute to the activation of the inhibitory association of the CS+, that is, will contribute to inhibition. Therefore, CI and the type of contextualized extinction investigated here are conceptually hard to distinguish. It should also be noted that current theories do not distinguish between an “extinction”–inhibition and a “CI”–inhibition but treat all inhibition the same, suggesting that either account is appropriate to describe our data.
Little has been known about the context-dependent recall of extinction memory in humans. A key feature of this study is that our design allows delineation of the neural circuitry involved in that function, using a psychological manipulation that engenders recall of extinction memory in the appropriate context. Clinically, contextual restrictions on extinction can considerably complicate anxiety therapy, sometimes resulting in fear recovery in nontherapy contexts even after successful fear extinction. For therapeutic purposes, therefore, it may often be desirable to create noncontextualized extinction memories. Our data suggest that such decontextualization may be achieved by rendering the VMPFC-dependent recall of extinction memories hippocampus independent. New pharmacological treatments facilitating the consolidation of extinction memories (Walker et al., 2002; Ressler et al., 2004) (for review, see Richardson et al., 2004) may also facilitate such context-independent activation of the VMPFC (Ledgerwood et al., 2004).
Footnotes
This work was supported by a Wellcome Trust programme grant (R.J.D.) and the European Union Presencia programme (R.J.D., R.K.). We thank M. Bouton, D. Vansteenwegen, K. Corcoran, H. Critchley, and K. Friston for useful discussions on design and manuscript, K. Wiech and E. Featherstone for help with experiments, and C. Hutton for help with data preprocessing.
References
- Amaral, 1999.Amaral DG. Introduction: what is where in the medial temporal lobe? Hippocampus. 1999;9:1–6. doi: 10.1002/(SICI)1098-1063(1999)9:1<1::AID-HIPO1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Anagnostaras et al., 2001.Anagnostaras SG, Gale G, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Ashburner et al., 2004.Ashburner J, Friston KJ, Penny W. Imaging neuroscience—theory and analysis. In: Frackowiak RS, Friston KJ, Frith C, Dolan RJ, Price CJ, editors. Human brain function. San Diego: Academic; 2004. pp. 599–1104. [Google Scholar]
- Blair et al., 2005.Blair HT, Huynh VK, Vaz VT, Van J, Patel RR, Hiteshi AK, Lee JE, Tarpley JW. Unilateral storage of fear memories by the amygdala. J Neurosci. 2005;25:4198–4205. doi: 10.1523/JNEUROSCI.0674-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshuisen et al., 2002.Boshuisen ML, Ter Horst GJ, Paans AM, Reinders AA, Den Boer JA. rCBF differences between panic disorder patients and control subjects during anticipatory anxiety and rest. Biol Psychiatry. 2002;52:126–135. doi: 10.1016/s0006-3223(02)01355-0. [DOI] [PubMed] [Google Scholar]
- Bouton, 2004.Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Buchel and Dolan, 2000.Buchel C, Dolan RJ. Classical fear conditioning in functional neuroimaging. Curr Opin Neurobiol. 2000;10:219–223. doi: 10.1016/s0959-4388(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Buchel et al., 1998.Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Cannistraro and Rauch, 2003.Cannistraro PA, Rauch SL. Neural circuitry of anxiety: evidence from structural and functional neuroimaging studies. Psychopharm Bull. 2003;37:8–25. [PubMed] [Google Scholar]
- Cavada et al., 2000.Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Corcoran and Maren, 2004.Corcoran KA, Maren S. Factors regulating the effects of hippocampal inactivation on renewal of conditional fear after extinction. Learn Mem. 2004;11:598–603. doi: 10.1101/lm.78704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran et al., 2005.Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci. 2005;25:8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley et al., 2002.Critchley HD, Mathias CJ, Dolan RJ. Fear conditioning in humans: the influence of awareness and autonomic arousal on functional neuroanatomy. Neuron. 2002;33:653–663. doi: 10.1016/s0896-6273(02)00588-3. [DOI] [PubMed] [Google Scholar]
- Deichmann et al., 2003.Deichmann R, Gottfried JA, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. NeuroImage. 2003;19:430–441. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- Deichmann et al., 2004.Deichmann R, Schwarzbauer C, Turner R. Optimisation of the 3D MDEFT sequence for anatomical brain imaging: technical implications at 1.5 and 3 T. NeuroImage. 2004;21:757–767. doi: 10.1016/j.neuroimage.2003.09.062. [DOI] [PubMed] [Google Scholar]
- Delamater, 2004.Delamater AR. Experimental extinction in Pavlovian conditioning: behavioural and neuroscience perspectives. Q J Exp Psychol B. 2004;57:97–132. doi: 10.1080/02724990344000097. [DOI] [PubMed] [Google Scholar]
- Dirikx et al., 2004.Dirikx T, Hermans D, Vansteenwegen D, Baeyens F, Eelen P. Reinstatement of extinguished conditioned responses and negative stimulus valence as a pathway to return of fear in humans. Learn Mem. 2004;11:549–554. doi: 10.1101/lm.78004. [DOI] [PubMed] [Google Scholar]
- Duvernoy, 1999.Duvernoy HM. Wien, Austria: Springer; 1999. The human brain: surface, blood supply, and three-dimensional sectional anatomy. [Google Scholar]
- Ekman and Friesen, 1976.Ekman P, Friesen WV. Palo Alto, CA: Consulting Psychologists; 1976. Pictures of facial affect. [Google Scholar]
- Fisher, 1921.Fisher RA. On the probable error of a coefficient of correlation deduced from a small sample. Metron. 1921;1:3–32. [Google Scholar]
- Friston et al., 1994.Friston KJ, Jezzard PJ, Turner R. Analysis of functional MRI time-series. Hum Brain Mapp. 1994;1:153–171. [Google Scholar]
- Friston et al., 1995.Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Frohardt et al., 2000.Frohardt RJ, Guarraci FA, Bouton ME. The effects of neurotoxic hippocampal lesions on two effects of context after fear extinction. Behav Neurosci. 2000;114:227–240. doi: 10.1037//0735-7044.114.2.227. [DOI] [PubMed] [Google Scholar]
- Gottfried and Dolan, 2004.Gottfried JA, Dolan RJ. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nat Neurosci. 2004;7:1144–1152. doi: 10.1038/nn1314. [DOI] [PubMed] [Google Scholar]
- Gottfried et al., 2002.Gottfried JA, O'Doherty J, Dolan RJ. Appetitive and aversive olfactory learning in humans studied using event-related functional magnetic resonance imaging. J Neurosci. 2002;22:10829–10837. doi: 10.1523/JNEUROSCI.22-24-10829.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray and McNaughton, 2000.Gray JA, McNaughton N. Oxford: Oxford UP; 2000. The neuropsychology of anxiety. [Google Scholar]
- Heidbreder and Groenewegen, 2003.Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Hermans et al., 2005.Hermans D, Dirikx T, Vansteenwegen D, Baeyens F, Van den Bergh O, Eelen P. Reinstatement of fear responses in human aversive conditioning. Behav Res Ther. 2005;43:533–551. doi: 10.1016/j.brat.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Hirsh, 1974.Hirsh R. The hippocampus and contextual retrieval of information from memory: a theory. Behav Biol. 1974;12:421–444. doi: 10.1016/s0091-6773(74)92231-7. [DOI] [PubMed] [Google Scholar]
- Hobin et al., 2003.Hobin JA, Goosens KA, Maren S. Context-dependent neuronal activity in the lateral amygdala represents fear memories after extinction. J Neurosci. 2003;23:8410–8416. doi: 10.1523/JNEUROSCI.23-23-08410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes and Friston, 1998.Holmes AP, Friston KJ. Generalisability, random effects and population inference. NeuroImage. 1998;7:S754. [Google Scholar]
- Jensen et al., 2003.Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40:1251–1257. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Ji and Maren, 2005.Ji J, Maren S. Electrolytic lesions of the dorsal hippocampus disrupt renewal of conditional fear after extinction. Learn Mem. 2005;12:270–276. doi: 10.1101/lm.91705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone and Page, 2004.Johnstone KA, Page AC. Attention to phobic stimuli during exposure: the effect of distraction on anxiety reduction, self-efficacy and perceived control. Behav Res Ther. 2004;42:249–275. doi: 10.1016/S0005-7967(03)00137-2. [DOI] [PubMed] [Google Scholar]
- Kalisch et al., 2005.Kalisch R, Wiech K, Critchley HD, Seymour B, O'Doherty JP, Oakley DA, Allen P, Dolan RJ. Anxiety reduction through detachment: subjective, physiological, and neural effects. J Cogn Neurosci. 2005;17:874–883. doi: 10.1162/0898929054021184. [DOI] [PubMed] [Google Scholar]
- Kennedy and Shapiro, 2004.Kennedy PJ, Shapiro ML. Retrieving memories via internal context requires the hippocampus. J Neurosci. 2004;24:6979–6985. doi: 10.1523/JNEUROSCI.1388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim and Jung, 2006.Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neurosci Biobehav Rev. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar and Phelps, 2005.LaBar KS, Phelps EA. Reinstatement of conditioned fear in humans is context dependent and impaired in amnesia. Behav Neurosci. 2005;119:677–686. doi: 10.1037/0735-7044.119.3.677. [DOI] [PubMed] [Google Scholar]
- Lebron et al., 2004.Lebron K, Milad MR, Quirk GJ. Delayed recall of fear extinction in rats with lesions of ventral medial prefrontal cortex. Learn Mem. 2004;11:544–548. doi: 10.1101/lm.78604. [DOI] [PubMed] [Google Scholar]
- Ledgerwood et al., 2004.Ledgerwood L, Richardson R, Cranney J. D-cycloserine and the facilitation of extinction of conditioned fear: consequences for reinstatement. Behav Neurosci. 2004;118:505–513. doi: 10.1037/0735-7044.118.3.505. [DOI] [PubMed] [Google Scholar]
- Mathews et al., 1997.Mathews A, Mackintosh B, Fulcher EP. Cognitive biases in anxiety and attention to threat. Trends Cogn Sci. 1997;1:340–345. doi: 10.1016/S1364-6613(97)01092-9. [DOI] [PubMed] [Google Scholar]
- Milad and Quirk, 2002.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad et al., 2005.Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci USA. 2005;102:10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan and LeDoux, 1995.Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Morris et al., 1998.Morris JS, Ohman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393:467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- Myers and Davis, 2002.Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Nelson and Bouton, 1997.Nelson JB, Bouton ME. The effects of a context switch following serial and simultaneous feature negative discriminations. Learn Motiv. 1997;28:56–84. [Google Scholar]
- Pare et al., 2004.Pare D, Quirk GJ, LeDoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Pavlov, 1927.Pavlov IP. Oxford: Oxford UP; 1927. Conditioned reflexes: an investigation of the physiological activity of the cerebral cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce and Hall, 1980.Pearce JM, Hall G. A model for Pavlovian learning: variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychol Rev. 1980;87:532–552. [PubMed] [Google Scholar]
- Penny and Holmes, 2004.Penny W, Holmes AP. Random-effects analysis. In: Frackowiak RS, Friston KJ, Frith C, Dolan RJ, Price CJ, editors. Human brain function. San Diego: Academic; 2004. pp. 843–850. [Google Scholar]
- Phelps et al., 2004.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Ploghaus et al., 1999.Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JN. Dissociating pain from its anticipation in the human brain. Science. 1999;284:1979–1981. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- Quirk et al., 2003.Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler et al., 2004.Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of d-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Richardson et al., 2004.Richardson R, Ledgerwood L, Cranney J. Facilitation of fear extinction by d-cycloserine: theoretical and clinical implications. Learn Mem. 2004;11:510–516. doi: 10.1101/lm.78204. [DOI] [PubMed] [Google Scholar]
- Rosenkranz et al., 2003.Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 2003;23:11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour et al., 2004.Seymour B, O'Doherty JP, Dayan P, Koltzenburg M, Jones AK, Dolan RJ, Friston KJ, Frackowiak RS. Temporal difference models describe higher-order learning in humans. Nature. 2004;429:664–667. doi: 10.1038/nature02581. [DOI] [PubMed] [Google Scholar]
- Simpson Jr et al., 2001.Simpson JR, Snyder AZ, Gusnard DA, Raichle ME., Jr Emotion-induced changes in human medial prefrontal cortex. I. During cognitive task performance. Proc Natl Acad Sci USA. 2001;98:683–687. doi: 10.1073/pnas.98.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon et al., 2004.Sotres-Bayon F, Bush DE, LeDoux JE. Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn Mem. 2004;11:525–535. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- Vansteenwegen et al., 2005.Vansteenwegen D, Hermans D, Vervliet B, Francken G, Beckers T, Baeyens F, Eelen P. Return of fear in a human differential conditioning paradigm caused by a return to the original acquisition context. Behav Res Ther. 2005;43:323–336. doi: 10.1016/j.brat.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Wager et al., 2004.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wagner and Brandon, 1989.Wagner AR, Brandon SE. Evolution of a structured connectionist model of Pavlovian conditioning (AESOP) In: Klein SB, Mowrer RR, editors. Contemporary learning theories: Pavlovian conditioning and the status of traditional learning theory. Hillsdale, NJ: Erlbaum; 1989. pp. 149–189. [Google Scholar]
- Walker et al., 2002.Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of d-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson et al., 1995.Wilson A, Brooks DC, Bouton ME. The role of the rat hippocampal system in several effects of context in extinction. Behav Neurosci. 1995;109:828–836. doi: 10.1037//0735-7044.109.5.828. [DOI] [PubMed] [Google Scholar]