Abstract
A central feature of models of associative memory formation is the reliance on information convergence from pathways responsive to the conditioned stimulus (CS) and unconditioned stimulus (US). In particular, cells receiving coincident input are held to be critical for subsequent plasticity. Yet identification of neurons in the mammalian brain that respond to such coincident inputs during a learning event remains elusive. Here we use Arc cellular compartmental analysis of temporal gene transcription by fluorescence in situ hybridization (catFISH) to locate populations of neurons in the mammalian brain that respond to both the CS and US during training in a one-trial learning task, conditioned taste aversion (CTA). Individual neurons in the basolateral nucleus of the amygdala (BLA) responded to both the CS taste and US drug during conditioning. Coincident activation was not evident, however, when stimulus exposure was altered so as to be ineffective in promoting learning (backward conditioning, latent inhibition). Together, these data provide clear visualization of neurons in the mammalian brain receiving convergent information about the CS and US during acquisition of a learned association.
Keywords: Arc, memory, novelty, plasticity, taste aversion conditioning
A central issue in behavioral neuroscience is how alterations in neural activity mediate the durable behavioral changes involved in learning. Current concepts of associative memory formation are based on the premise that plasticity relies on convergence of information from pathways responsive to the conditioned stimulus (CS) and the unconditioned stimulus (US) (1–3). Yet despite impressive progress in defining underlying neuronal circuitry and probable sites of association for several associative learning models (2, 4–6), identification of neuronal populations where convergent activation actually occurs during learning remains elusive. Electrophysiological studies have made inroads toward this goal, but are hampered by limited sampling ability, especially when the targets are convergent neurons, which are likely to be sparsely distributed during any single training trial. In those studies where convergent activation was recorded, animals were either anesthetized or had already reached asymptotic performance on a learning task (5–7).
The imaging method known as cellular compartmental analysis of temporal gene transcription by fluorescence in situ hybridization (catFISH) can circumvent several of the technical limitations that have made it difficult to sample broad regions of the mammalian brain with high cellular and temporal resolution during a learning event (8). In particular, catFISH serves as a functional imager that allows investigators to distinguish neuronal populations activated by two distinct behavioral experiences. CatFISH utilizes Arc (or Arg 3.1), an immediate early gene (IEG) that is expressed in forebrain glutamatergic neurons after periods of enhanced activation (9, 10). The innovative features of catFISH rely on the time course of Arc mRNA localization after its expression in response to a behavioral event: Arc mRNA is seen only in the nucleus 5 min after induction, after which it moves to the cytoplasm where it is exclusively found by 25–30 min (9). Thus by using the subcellular distribution of Arc to determine when a neuron was activated, catFISH has the potential to identify neuronal populations engaged by the CS, the US, and the pairing of the two stimuli during learning.
However, because catFISH analysis requires that the presentation of stimuli be separated by 20–30 min, and associative learning typically requires that CS and US be separated by no more than seconds to a few minutes (11), such efforts have been limited. In contrast, conditioned taste aversions (CTAs), which result in the rejection of a taste because of a learned association with illness, are routinely acquired in a single trial despite delays of ≥30 min between presentation of CS taste and US illness (12–14). Thus CTAs represent an ideal model for catFISH analysis, allowing us to mark individual neurons responding to both CS and US during an actual learning event. Identifying such neurons is critical to further characterization of their network and phenotypic properties. Initial studies in our lab [supporting information (SI) Fig. S1] revealed robust induction of Arc mRNA in response to individual presentation of CS taste (0.5% saccharin solution) and US LiCl (0.15 M 15 ml/kg body weight, i.p.) in the insular cortex (IC) and basolateral nucleus of the amygdala (BLA), two regions believed to be involved in CTA acquisition (15–18), leading us to focus on these regions for evidence of CS–US convergence.
Results
Neurons in Basolateral Amygdala Respond to Convergent CS and US Information During Taste Aversion Conditioning.
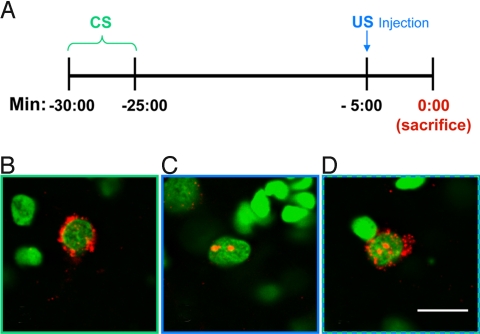
To identify and localize neurons that respond to convergent CS and US information, rats were exposed to a CS–US conditioning trial, using parameters amenable to catFISH analysis and that are known to yield robust taste aversions in a single trial (16), (Fig. 1A). Under these parameters, cells responsive to the earlier stimulus (CS taste) showed strong Arc mRNA signal restricted to the cytoplasm (Fig. 1B), whereas cells responsive to the later stimulus (US LiCl) showed Arc signal in the form of dense foci restricted to the nucleus (Fig. 1C). Neurons responsive to both CS and US (e.g., cells receiving convergent input) were marked by both nuclear and cytoplasmic signal (Fig. 1D).
Fig. 1.
Timing of CS/US presentation and resulting Arc localization. (A) A schematic timeline outlining presentation of the CS (onset 30 min before killing) and US (onset 5 min before killing) during a conditioning trial. (B–D) Representative images from BLA showing Arc localization following stimulus presentation under these timing parameters. Neurons responding only to the earlier CS event show Arc signal (in red) in the cytoplasm surrounding the nucleus (counterstained green) (B). Neurons responding only to the later US event show dense Arc foci confined to the nucleus (C). Neurons responding to both the CS and US show cytoplasmic and nuclear staining (D). (Scale bar, 10 μm.)
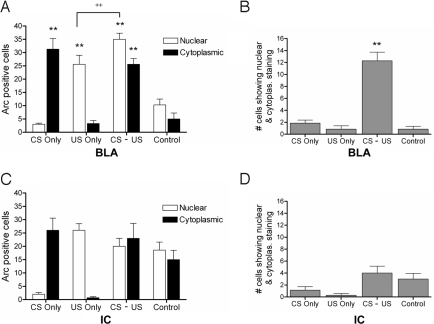
Table 1 presents the total number of neurons sampled in the BLA along with the number and percentage of neurons that showed Arc positive staining in the cytoplasmic and/or nuclear compartments. Significantly more neurons expressed Arc following CS and/or US exposure than after water and saline injection (Fig. 2A), indicating that the Arc response in this area is sensitive to novel taste and illness, but not to the nonspecific effects of drinking, handling, and injection. Most importantly, BLA demonstrated strong evidence of CS–US convergence, with a significantly greater number of neurons showing dual Arc activation in paired (CS–US) animals compared to all other groups (Fig. 2B). Although paired animals had higher overall levels of Arc activation than the other groups because of exposure to two Arc-inducing stimuli, chance factors alone cannot account for the high number of convergent cells. Expected frequencies based on experimentally observed population responses to the CS (8.4% of sampled neurons, Table 1) and US (11.4%) in animals that received the CS–US pairing predict that 0.96% of neurons should show convergent activation. However, the observed frequency of 4.2% was four times higher than the expected value (χ2 = 74.35; df = 2; P < 0.001).
Table 1.
Number of Arc+ neurons in sampled area of BLA
| Group | n | Total no. of neurons | No. of neurons that showed Arc+ staining in: |
||
|---|---|---|---|---|---|
| Cytoplasm | Nucleus | Nucleus + cytoplasm | |||
| CS only | 7 | 306 ± 4.3 | 31.3 ± 3.9 | 3.00 ± 0.4 | 1.8 ± 0.5 |
| (10.2%) | (1.0%) | (0.6%) | |||
| US only | 7 | 304 ± 4.1 | 3.28 ± 1.1 | 25.4 ± 3.4 | 0.9 ± 0.5 |
| (1.1%) | (8.4%) | (0.3%) | |||
| CS–US | 7 | 306 ± 2.1 | 25.6 ± 2.2 | 35.0 ± 2.5* | 13 ± 1.5* |
| (8.4%) | (11.4%) | (4.2%) | |||
| Control | 7 | 309 ± 3.2 | 5.00 ± 2.2 | 10.2 ± 2.5 | 0.9 ± 0.5 |
| (1.6%) | (3.3%) | (0.3%) | |||
Values in parentheses indicate percentage of total neurons sampled in BLA that show Arc in the nucleus, cytoplasm, or both. Percentages in the CS–US group were used in χ2 analysis.
* indicates values that are significantly greater than all other groups (P < 0.05, ANOVA with Tukey's post hoc analysis).
Fig. 2.
Number of Arc positive neurons showing sensitivity to the CS and US in IC and BLA. (A) Total number of cells in the BLA staining positively for Arc in cytoplasm and nucleus across groups (n = 7 rats/group). Cytoplasmic responses correspond to the CS event and nuclear responses correspond to the US event. The total number of neurons responding to the CS and US is significantly higher than for water and an injection of isotonic saline (controls), indicating that the Arc response in BLA is selective for the taste- and illness- related aspects of the conditioning stimuli (**, P < 0.001). Moreover, CS–US paired animals show a significantly greater number of neurons responding to the US compared to US-only controls (++, P < 0.05). (B) Number of neurons showing Arc in both the nucleus and cytoplasm across groups. Number of double-labeled cells is significantly higher in CS–US paired animals than in all other groups (**, P < 0.001). (C) Total number of cells in the IC staining positively for Arc in cytoplasm and nucleus across groups (n = 7 rats/group). In IC, responses of animals receiving the CS and/or US do not differ from control animals, indicating that Arc reactivity in this area is not selective for the specific qualities of CS taste or US illness, but rather is nonspecific. (D) Number of neurons showing both nuclear and cytoplasmic staining in IC does not differ significantly across groups. All comparisons were made via analysis of variance (ANOVA) followed by Tukey's post hoc analysis. Data are represented as means ± SEM.
In addition, there is evidence that pairing of CS with US increases the number of cells that respond to the US. Arc activation to the US was significantly higher in paired (CS–US) animals than in those exposed to the US only (Fig. 2A). This enhanced response was not evident in the Arc response to the CS, which was comparable in conditioned and CS-only groups.
Unlike BLA, IC not only failed to show evidence for strong CS–US convergence, but showed positive staining for water and an injection of saline (Fig. 2 C and D), indicating that the Arc response in this area is relatively nonspecific. Overall, these data provide clear evidence that neurons in the lateral amygdala receive convergent information about the CS and US during CTA acquisition and that neurons in IC do not show convergence, as indexed by Arc staining.
Reversing the Order of CS and US Presentation Diminishes Coincident Activation.
Reversing presentation of the CS and US exposes subjects to identical stimuli, but while forward pairing of CS and US leads to associative learning, reversing the order of presentation (backward conditioning) greatly diminishes, or altogether abolishes, learning (11, 19, 20). Thus if convergence of CS and US information onto individual neurons characterizes the acquisition of an association, the number of cells displaying coincident activation should be significantly lower in backward-conditioned animals.
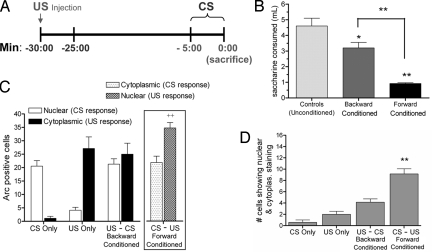
Behavioral testing of forward- and backward-conditioned animals confirmed previous reports (19) that order of presentation strongly influenced strength of CTA learning. Backward-conditioned animals drank less than unconditioned controls (P = 0.04), but significantly more than forward-conditioned animals (P < 0.005), indicating markedly weaker aversions when tested 24 h after conditioning (Fig. 3B).
Fig. 3.
Reversing the order of CS and US during conditioning. (A) A schematic timeline for the presentation of the US and CS during backward conditioning. (B) Behavioral testing of forward- and backward-conditioned animals shows that the order of presentation affects strength of learning. Backward-conditioned animals (n = 9) drank less than unconditioned controls (n = 8; *, P = 0.04), but drank significantly more than forward-conditioned animals (**, P < 0.005), indicating weaker CTA acquisition. (C) Total number of cells in the BLA staining positively for Arc in cytoplasm and nucleus across groups (n = 7 rats/group). CS responses now correspond to nuclear signal and US responses correspond to cytoplasmic signal in all groups except the forward-conditioned group (Inset box). US responses are enhanced for forward-conditioned (CS–US) animals above US-only and backward-conditioned (US–CS) groups (++, P < 0.01). (D) Forward-conditioned animals show significantly more double-labeled neurons than all other groups that do not differ from each other (**, P < 0.005).
CatFISH analysis demonstrated that temporal order was also crucial to the appearance of cells in BLA responding to convergent input. The number of neurons convergently activated by the CS and US was significantly higher in forward- than in backward-conditioned animals, which were not significantly different from controls (Fig. 3D). General population responses to the CS and US showed a pattern similar to that observed in experiment 1; the CS-induced Arc response was similar across all groups receiving CS access while the US-induced Arc response was significantly elevated in the forward-conditioned group compared to backward-conditioned and US-only groups (Fig. 3C).
Coincident Activation of Neurons in Basolateral Amygdala by CS and US Requires CS Novelty.
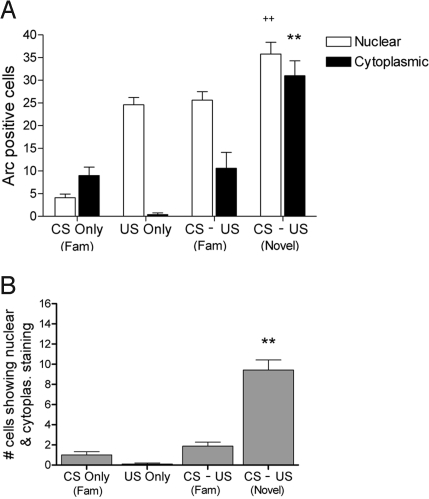
The prior establishment of a “safe” taste memory can completely block development of a significant CTA in this paradigm, a phenomenon known as latent inhibition (14, 16). Thus, when preexposed animals receive identical stimuli during a training trial they do not form a learned association. Indeed, when animals were familiarized with the taste CS before CS–US pairing, catFISH analysis revealed a dramatic alteration in the pattern of Arc induction. Unlike novel CS–US pairing, familiar CS–US pairing was not associated with significant coincident activation (Fig. 4B). Furthermore, there was a marked reduction in cells responsive to the familiar CS (Fig. 4A), and familiar CS–US pairing did not result in a potentiated US response relative to US-only controls. Thus, coincident activation requires that the CS be novel, which clearly parallels the requirement for CS novelty in rapid CTA associations.
Fig. 4.
Number of Arc positive neurons to novel vs. familiar CS–US pairing. (A) Total number of cells in the BLA staining positively for Arc in cytoplasm and nucleus across groups (n = 7 rats/group). Cytoplasmic responses correspond to the CS event and nuclear responses correspond to the US event. Responses to a familiar CS are considerably lower than those to a novel CS. No enhancement of the US response is seen when familiar CS precedes US. Only animals receiving a novel CS before US showed an enhanced Arc response to US (++, P < 0.01; n = 7/group). (B) Number of neurons showing Arc in both the nucleus and cytoplasm across groups. Number of double-labeled cells is significantly higher in novel CS–US paired animals than in all other groups (**, P < 0.005) that were not different from each other. All comparisons were made via analysis of variance (ANOVA) followed by Tukey's post hoc analysis. Data are represented as means ± SEM.
Discussion
The experiments presented here use catFISH as a functional imager to visualize patterns of neuronal activation in response to the CS and US in animals that are awake and acquiring a one-trial learned association. They provide evidence that, during CTA acquisition, CS and US information converge on a subset of cells in the BLA when presentation of the stimuli is effective in promoting learning (novel CS–US pairing) but not when the same stimuli are presented in an ineffective manner (familiar CS–US pairing; backward US–CS pairing). The development of a behavioral and analytical protocol that pinpoints neurons receiving convergent stimulus information during learning provides a critical tool for subsequent characterization of the neurochemical and neuroanatomical characteristics of these neurons and ultimately for the identification of downstream signaling events that depend on coincident activation.
In the present studies, the finding that backward pairing does not yield coincident activation despite exposure to identical stimuli raises fundamental questions about underlying mechanisms. We propose a model in which potentiation of US responses by novel CS presentation is key to coincident activation and its sensitivity to temporal order. Specifically, the model proposes that the elevation observed in US responding is the result of a subset of neurons in BLA that receive strong CS input and weak US input. When the US is presented alone, the stimulus fails to excite these neurons. However when a novel CS precedes the US, these neurons become more sensitive to subsequent US input and convergent activation is seen. This model is supported by the consistent observation that the number of neurons responding to the US is enhanced when it is preceded by a novel CS but not when it was preceded by a familiar CS (or followed by a novel CS). Indeed, this enhancement in US responding is correlated with the appearance of convergently activated neurons, suggesting that the same process may be responsible for both. Enhancement of neural response systems by recent exposure to novel stimuli has been reported to occur in the hippocampus, where exposure to a novel context can enhance induction and maintenance of long-term potentiation (LTP) and long-term memory (LTM) for an avoidance task (21, 22). Our evidence supports a similar process in the amygdala during CTA acquisition.
The unusual temporal parameters of CTA learning have raised questions about whether this learning involves unique signaling mechanisms, which might imply that the patterns of coincident activation in the amygdala revealed here are unique to the CTA paradigm (23, 24). Arguing against this notion is an accumulating literature which shows that CTA learning has fundamental similarities with other associative learning paradigms, including a short-term and long-term memory phase (25, 26) and heavy reliance on signaling pathways such as cAMP response element-binding (CREB) protein (27) and protein kinase A (PKA) (28) in the amygdala. Interestingly, the lateral amygdala has been proposed as the site of CS–US association in another adaptive, defensive associative learning task, cued fear conditioning (2, 6, 29, 30), suggesting that a population of glutamatergic neurons in this region of the amygdala has specialized properties that support rapid neuronal plasticity.
In the present studies CS–US pairing was associated with strong, prolonged activation of Arc in convergently activated neurons. This IEG has been implicated in stabilizing the plasticity that underlies memory formation (10, 31). Disruption of Arc by antisense or knockout technologies leads to a disruption of long-term, but not short-term memory. In fact, Arc null mutant mice are severely impaired in their ability to learn CTAs, indicating that Arc expression may be necessary for CTA acquisition (32). Here we used Arc principally as a visualization technique and demonstrated that dual activation of Arc emerges only when novel stimuli are delivered in a sequence supportive of learning. The clear demonstration of coincident activation of neurons in the amygdala during associative conditioning in animals that were awake and learning a task provides a significant advance as the actual demonstration of such convergence has heretofore been rather elusive.
Materials and Methods
Subjects.
Adult male Long Evans rats (Charles River Laboratories) were individually housed and maintained on a 12:12 light/dark cycle with ad libitum access to food and water until 1 week before the experiment. All animals were then adapted to a water restriction schedule with two daily drinking sessions at 9:00 a.m. (30 min initially, gradually reduced to 5 min) and at 3:30 p.m. (for 1.5 h). Animals that were familiarized to the CS before conditioning (experiment 3) received access to the CS tastant during the morning drinking session; all other groups received water for both drinking sessions. Subsequent conditioning, testing, and stimulus exposure occurred at ≈9:00 a.m. Animals were treated in accordance with guidelines approved by the Institutional Animal Care and Use Committee at the University of Washington.
Conditioning.
Conditioned rats received a pairing of a 0.5% saccharin solution (CS) with an i.p. injection of 0.15 M LiCl (15 ml/kg body weight) to induce transient nausea (US). In experiment 1, animals were given access to the saccharin solution for 5 min, followed 25 min later with an injection of LiCl (Fig. 1A). Five minutes after the drug injection, animals were killed and brain tissue was collected for in situ analysis. CS-only and US-only groups were included to define population response patterns to the individual stimuli. Additional controls were given water in place of the CS and an injection of isotonic saline (0.15 M; 15 ml/kg body weight) instead of the US to account for nonspecific effects of drinking and handling. All controls received identical parameters for exposure and killing as the conditioned animals.
In experiment 2 (backward conditioning), rats in the backward-conditioned group were exposed to a US–CS conditioning trial, with an injection of 0.15 M LiCl (US) presented 30 min before, and CS presentation 5 min before killing (Fig. 3A). Controls included CS-only and US-only conditions with identical exposure and killing parameters as backward-conditioned animals, and a forward-conditioned group with identical exposure and killing parameters as conditioned animals from experiment 1. Intakes were yoked to ensure that backward-conditioned animals consumed the same amount of the CS as other groups.
In experiment 3 (novel vs. familiar CS), conditioning was done with the same parameters as experiment 1, with the inclusion of animals (familiar groups) that were preexposed to the tastant for 5 days before conditioning.
Behavioral Testing.
Separate animals underwent forward- and backward-conditioning procedures and were tested the next day to determine the behavioral effects of conditioning. A nonconditioned control group was given access to the CS tastant for 5 min followed 25 min later with an injection of isotonic NaCl. Animals were tested for the strength of learning 24 h later using a one-bottle taste test as described elsewhere (16).
Fluorescent in Situ Hybridization and Analysis.
After conditioning, brains were rapidly extracted, flash frozen, and stored at −80 °C. Forebrain tissue was sectioned into 20-μm coronal slices using a cryostat and mounted onto slides. Regions containing agranular IC (+1.1 ± 0.1 mm from bregma) and BLA (−2.7 ± 0.1 mm from bregma) were selected for in situ hybridization (Fig. S2; coordinates from ref. 33). Although disgranular IC was also analyzed, it failed to show robust Arc activation in response to US and these data are not discussed further (Fig. S3). Other regions of the putative CTA pathway, such as central nucleus of the amygdala (CeA) and parabrachial nucleus (PBN) were not analyzed because these regions either failed to show an Arc response to the CS (CeA) or else do not express the Arc gene (PBN) (34). Digoxigenin-labeled Arc riboprobes were generated from a modified cDNA plasmid (kindly provided by P. Worley) and fluorescent in situ hybridization for Arc was carried out as described elsewhere (8, 9). Arc signal was visualized using the Cyanine 3 TSA system (Perkin–Elmer); nuclei were counterstained with Sytox Green (Invitrogen). One section corresponding to each of the above coordinates was analyzed per rat. To avoid bias, image capture and subsequent analysis was carried out by an experimenter blind to group membership. Images were acquired using a Leica SL microscope with a 20× objective lens using GrHe/Ne and Argon lasers. Z-series stacks (1-μm-thick optical sections) were constructed and analyzed on the MetaMorph 6.0 program. Careful optical dissecting was done to ensure that only neurons with fully intact nuclei were scored. In particular, nuclei had to be present in the median plane of the z-stack and fully present throughout the stack without damage or bisecting to be scored. Neurons were scored as being positive for cytoplasmic staining if a “halo” of signal was found to be encircling at least 75% of the nucleus. For neurons to be scored as positive for nuclear staining, robust foci with high saturation (≥230 on the red channel of the Metamorph program) were required. Statistical analyses (analysis of variance (ANOVA) and appropriate post hocs) were carried out on SPSS software.
Supplementary Material
Acknowledgments.
We thank the labs of Carol Barnes, Paul Worley, Robert Steiner, and Charles Chavkin for their considerable technical assistance. Sheri Mizumori, Jeansok Kim, Benjamin Land, and Richard Palmiter provided helpful suggestions on the manuscript. This research was supported by National Institutes of Health Grant NS37040 and a Royalty research grant from the University of Washington.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808996106/DCSupplemental.
References
- 1.Thompson RF. The neurobiology of learning and memory. Science. 1986;233:941–947. doi: 10.1126/science.3738519. [DOI] [PubMed] [Google Scholar]
- 2.Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annu Rev Psychol. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- 3.Abrams TW, Karl KA, Kandel ER. Biochemical studies of stimulus convergence during classical conditioning in Aplysia: dual regulation of adenylate cyclase by Ca2+/calmodulin and transmitter. J Neurosci. 1991;11:2655–2665. doi: 10.1523/JNEUROSCI.11-09-02655.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tracy JA, Britton GB, Steinmetz JE. Comparison of single unit responses to tone, light, and compound conditioned stimuli during rabbit classical eyeblink conditioning. Neurobiol Learn Mem. 2001;76:253–267. doi: 10.1006/nlme.2001.4024. [DOI] [PubMed] [Google Scholar]
- 5.Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem. 2003;10:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- 6.Romanski LM, Clugnet MC, Bordi F, LeDoux JE. Somatosensory and auditory convergence in the lateral nucleus of the amygdala. Behav Neurosci. 1993;107:444–450. doi: 10.1037//0735-7044.107.3.444. [DOI] [PubMed] [Google Scholar]
- 7.Rosenkranz JA, Grace AA. Dopamine-mediated modulation of odour-evoked amygdala potentials during pavlovian conditioning. Nature. 2002;417:282–287. doi: 10.1038/417282a. [DOI] [PubMed] [Google Scholar]
- 8.Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Imaging neural activity with temporal and cellular resolution using FISH. Curr Opin Neurobiol. 2001;11:579–584. doi: 10.1016/s0959-4388(00)00252-x. [DOI] [PubMed] [Google Scholar]
- 9.Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- 10.Tzingounis AV, Nicoll RA. Arc/Arg 3.1: linking gene expression to synaptic plasticity and memory. Neuron. 2006;52:403–407. doi: 10.1016/j.neuron.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Rescorla RA. Behavioral studies of Pavlovian conditioning. Annu Rev Neurosci. 1988;11:329–352. doi: 10.1146/annurev.ne.11.030188.001553. [DOI] [PubMed] [Google Scholar]
- 12.Garcia J, Ervin FR, Koelling RA. Learning with prolonged delay of reinforcement. Psychon Sci. 1966;5:121–122. [Google Scholar]
- 13.Garcia J, Hankins WG, Rusiniak KW. Behavioral regulation of the milieu interne in man and rat. Science. 1974;185:824–831. doi: 10.1126/science.185.4154.824. [DOI] [PubMed] [Google Scholar]
- 14.Chambers KC. A neural model for conditioned taste aversions. Ann Rev Neurosci. 1990;13:373–385. doi: 10.1146/annurev.ne.13.030190.002105. [DOI] [PubMed] [Google Scholar]
- 15.Bermudez-Rattoni F. Molecular mechanisms of taste-recognition memory. Nat Rev Neurosci. 2004;5:209–217. doi: 10.1038/nrn1344. [DOI] [PubMed] [Google Scholar]
- 16.Koh MT, Bernstein IL. Mapping conditioned taste aversion associations using c-Fos reveals a dynamic role for insular cortex. Behav Neurosci. 2005;119:388–398. doi: 10.1037/0735-7044.119.2.388. [DOI] [PubMed] [Google Scholar]
- 17.Morris R, Frey S, Kasambira T, Petrides M. Ibotenic acid lesions of the basolateral, but not the central, amygdala interfere with conditioned taste aversion: evidence from a combined behavioral and anatomical tract-tracing investigation. Behav Neurosci. 1999;113:291–302. doi: 10.1037//0735-7044.113.2.291. [DOI] [PubMed] [Google Scholar]
- 18.Lamprecht R, Dudai Y. Transient expression of c-fos in rat amygdala during training is required for encoding conditioned taste aversion memory. Learn Mem. 1996;3:31–41. doi: 10.1101/lm.3.1.31. [DOI] [PubMed] [Google Scholar]
- 19.Barker LM, Smith JC. A comparison of taste aversions induced by radiation and lithium chloride in CS-US and US-CS paradigms. J Comp Physiol Psych. 1974;87:644–654. doi: 10.1037/h0036962. [DOI] [PubMed] [Google Scholar]
- 20.Abrams TW, Kandel ER. Is contiguity detection in classical conditioning a system or a cellular property? Learning in Aplysia suggests a possible molecular site. Trends Neurosci. 1988;11:128–135. doi: 10.1016/0166-2236(88)90137-3. [DOI] [PubMed] [Google Scholar]
- 21.Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. 2003;6:526–531. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- 22.Moncada D, Viola H. Induction of long-term memory by exposure to novelty requires protein synthesis: evidence for a behavioral tagging. J Neurosci. 2007;27:7476–7481. doi: 10.1523/JNEUROSCI.1083-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Domjan M. Advances in the Study of Behavior. Vol. 11. New York: Academic; 1980. Ingestional aversion learning: unique and general processes; pp. 275–336. [Google Scholar]
- 24.Domjan M. Pavlovian conditioning: a functional perspective. Annu Rev Psychol. 2005;56:179–206. doi: 10.1146/annurev.psych.55.090902.141409. [DOI] [PubMed] [Google Scholar]
- 25.Rosenblum K, Meiri N, Dudai Y. Taste memory: the role of protein synthesis in gustatory cortex. Behav Neural Biol. 1993;59:49–56. doi: 10.1016/0163-1047(93)91145-d. [DOI] [PubMed] [Google Scholar]
- 26.Houpt TA, Berlin R. Rapid, labile and protein synthesis independent short-term memory in conditioned taste aversion. Learn Mem. 1999;6:37–46. [PMC free article] [PubMed] [Google Scholar]
- 27.Lamprecht R, Hazvi S, Dudai Y. cAMP response element-binding protein in the amygdala is required for long- but not short-term conditioned taste aversion memory. J Neurosci. 1997;17:8443–8450. doi: 10.1523/JNEUROSCI.17-21-08443.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koh MT, Thiele TE, Bernstein IL. Inhibition of protein kinase A activity interferes with long-term, but not short-term memory of conditioned taste aversions. Behav Neurosci. 2002;116:1070–1074. [PubMed] [Google Scholar]
- 29.Schafe GE, Doyère V, LeDoux JE. Tracking the fear engram: the lateral amygdala is an essential locus of fear memory storage. J Neurosci. 2005;25:10010–10014. doi: 10.1523/JNEUROSCI.3307-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodrigues SM, Schafe GE, LeDoux JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Guzowski JF, et al. Inhibition of activity-dependent Arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plath N, et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 33.Paxinos G, Watson C. 3rd Ed. San Diego: Academic; 1997. The rat brain in stereotaxic coordinates. Compact. [DOI] [PubMed] [Google Scholar]
- 34.Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.