Abstract
The mechanisms of malignant cell transformation caused by the oncogenic, chimeric nucleophosmin (NPM)/anaplastic lymphoma kinase (ALK) remain only partially understood, with most of the previous studies focusing mainly on the impact of NPM/ALK on cell survival and proliferation. Here we report that the NPM/ALK-carrying T cell lymphoma (ALK+TCL) cells strongly express the immunosuppressive cell-surface protein CD274 (PD-L1, B7-H1), as determined on the mRNA and protein level. The CD274 expression is strictly dependent on the expression and enzymatic activity of NPM/ALK, as demonstrated by inhibition of the NPM/ALK function in ALK+TCL cells by the small molecule ALK inhibitor CEP-14083 and by documenting CD274 expression in IL-3-depleted BaF3 cells transfected with the wild-type NPM/ALK, but not the kinase-inactive NPM/ALK K210R mutant or empty vector alone. NPM/ALK induces CD274 expression by activating its key signal transmitter, transcription factor STAT3. STAT3 binds to the CD274 gene promoter in vitro and in vivo, as shown in the gel electromobility shift and chromatin immunoprecipitation assays, and is required for the PD-L1 gene expression, as demonstrated by siRNA-mediated STAT3 depletion. These findings identify an additional cell-transforming property of NPM/ALK and describe a direct link between an oncoprotein and an immunosuppressive cell-surface protein. These results also provide an additional rationale to therapeutically target NPM/ALK and STAT3 in ALK+TCL. Finally, they suggest that future immunotherapeutic protocols for this type of lymphoma may need to include the inhibition of NPM/ALK and STAT3 to achieve optimal clinical efficacy.
T cell lymphomas (TCL) that express the anaplastic large cell lymphoma tyrosine kinase (ALK) comprise a distinct category of lymphomas (1, 2). Ectopic expression of ALK results in the affected CD4+ T lymphocytes from chromosomal translocations involving the ALK gene and several different partners, most frequently the nucleophosmin (NPM) gene (3, 4). The NPM/ALK chimeric protein is not only constitutively expressed but is also chronically activated through autophosphorylation (4, 5). NPM/ALK displays potent cell-transforming properties as demonstrated both in vitro (6, 7) and in vivo (8, 9). NPM/ALK mediates its oncogenicity by activating a number of signal transduction proteins, including STAT3 (1, 2, 10). The continuous activation of these signal transmitters leads to the persistent expression of genes and the protein products of which are involved in key cell functions, such as the promotion of cell proliferation and protection from apoptosis.
CD279, or programmed cell death 1 (PD-1), is an immunosuppressive cell-surface receptor expressed by a subset of normal, activated CD4+ and CD8+ T lymphocytes (11–13). CD279 transduces the inhibitory signal when engaged simultaneously with the antigen T-cell receptor (TCR)-CD3 complex. CD279 has two known ligands: CD274 (also called PD-L1 or B7-H1) and CD273 (PD-L2 or B7-DC). Interactions between CD279 and its ligands control the induction and maintenance of peripheral T-cell tolerance during normal immune responses. They are also involved in immune evasion in malignancy, as cells of various tumor types have been shown to aberrantly express CD274 and, seemingly to a lesser degree, CD273.
Here we report that ALK+TCL cells universally express CD274. The CD274 expression is induced in these malignant cells by the NPM/ALK tyrosine kinase. NPM/ALK triggers the expression by activating STAT3, which in turn acts as a transcriptional activator of the CD274 gene. These findings identify a unique role of NPM/ALK and STAT3 in inducing tumor immune evasion, and demonstrate the direct role of an oncogenic protein in controlling the expression of an immunosuppressive cell-surface protein. These observations also provide a different rationale to therapeutically target NPM/ALK and STAT3 in ALK+TCL, and suggest that NPM/ALK inhibition may become a part of future vaccination-based therapies.
Results
ALK+TCL Cells Express CD274.
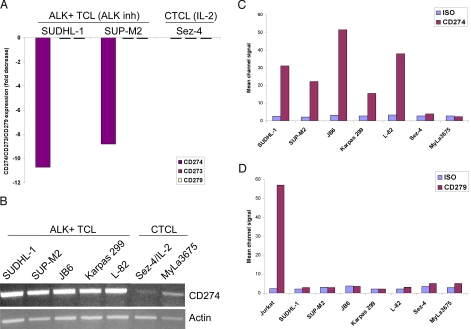
To better understand the mechanisms of NPM/ALK-induced malignant cell transformation, we screened ALK+TCL cells for changes in gene expression in response to a unique small molecule ALK inhibitor, CEP-14083 (14), using DNA oligonucleotide array-based genome-scale gene-expression profiling. When two well-characterized ALK+TCL-derived cell lines, SUDHL-1 and SUP-M2, (10, 15–19) were analyzed, one of the most strongly suppressed genes was the CD274/PD-L1 gene (11- and 9-fold decrease in the mRNA expression as compared to the drug vehicle-treated cells) (Fig. 1A). No CD274 mRNA expression could be detected in the control, IL-2-dependent and ALK-negative Sez-4 cell line derived from a cutaneous T-cell lymphoma (CTCL), either in the presence or absence of IL-2. In contrast to CD274, no modulation or, for that matter, expression of the CD274 receptor CD279/PD-1 and the CD274-related ligand CD273/PD-L2, was detected in the ALK+TCL cell lines SUDHL-1 and SUP-M2 cells, either treated or untreated with the ALK inhibitor. The CTCL cell line Sez-4 cells also did not express CD279 and CD273, regardless of the IL-2 stimulation status.
Fig. 1.
CD274 expression by ALK+TCL cells. (A) Expression of CD274 (PD-L1) and the functionally-related CD273 (PD-L2) and CD279 (PD-1) as determined by cDNA oligonucleotide hybridization in ALK+TCL cell lines (SUDHL-1 and SUP-M2), exposed for 6 h to 175 nM of an ALK inhibitor, CEP-14083 or the compound's vehicle. IL-2-dependent, CTCL-derived Sez-4 cell line depleted of IL-2 for 16 h and subsequently exposed for 4 h to IL-2 or medium (20), served as an additional control. The results are depicted as a fold change in the hybridization signal upon cell treatment with the ALK inhibitor or IL-2, as compared to the untreated cells. (B) Expression of CD274 mRNA in the depicted five ALK+TCL and two CTCL cell lines detected by RT-PCR. Expression of actin served as a positive control. Expression of CD274 protein (C) and CD279 (D) at the cell surface of the ALK+TCL and CTCL cell lines detected by flow cytometry. Staining with an isotype-matched antibody of unrelated specificity served as a negative control. Jurkat cell line served as a positive control of CD279 expression.
To confirm and expand our finding of CD274 expression by ALK+TCL cells using a different method, we performed RT-PCR using primers specific for the CD274 cDNA. As shown in Fig. 1B, all five examined ALK+TCL cell lines strongly expressed CD274 mRNA, with only traces of the message seen in the two control ALK- and CTCL-derived cell lines. CD274 was also strongly expressed by the ALK+TCL but not CTCL cell lines on the protein level, as demonstrated by flow cytometry analysis (Fig. 1C, a diagram). The primary results are depicted in supporting information (SI) Fig. S1. Of note, none of the cell populations expressed CD279 (Fig. 1D). This finding excludes a potential, autocrine CD279-CD274 receptor-ligand interaction within the ALK+TCL cell population. To demonstrate that CD274 expression also occurs in the uncultured, primary ALK+TCL cells, we examined tissue samples from 18 cases of ALK+TCL by immunohistochemistry. Results of this evaluation from a representative case are shown in Fig. 2. In all cases examined, the malignant anaplastic lymphoma cells (see Fig. 2A) strongly expressed not only the ALK kinase (see Fig. 2B) but also CD274 (see Fig. 2C).
Fig. 2.
Expression of CD274 in ALK+TCL tissues. Section of lymph nodes were examined microscopically using an intermediate (100×, large images) and high (500 and 400×, insets) power magnification. (A) H&E showed a predominance of large, frequently highly atypical cells. Immunohistochemical examination revealed strong, selective staining of the atypical cells by both anti-ALK (B) and anti-CD274 antibodies (C). The depicted images are representative for the 18 ALK+TCL cases examined.
Expression of CD274 is Induced by NPM/ALK.
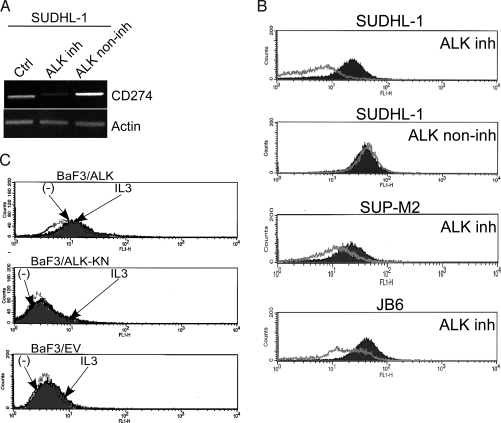
Our observation that CEP-14083, the highly specific inhibitor of ALK (14), suppressed CD274 mRNA expression in the ALK+TCL cells, as determined by the DNA oligonucleotide array analysis (see Fig. 1A), indicated that NPM/ALK is responsible for induction of the CD274 expression. To confirm this finding by a more standard method, we performed RT-PCR using cDNA extracted from cells treated with CEP-14083, a structurally closely related derivative of CEP-14083, designated CEP-11988, which is devoid of ALK-inhibitory activity (14), or the drug vehicle alone. Treatment of the ALK+TCL SUDHL-1 cell line with CEP-14083 at a preselected low, yet strongly ALK-inhibitory dose of 175 nM (data not presented), profoundly suppressed the expression of CD274 mRNA (Fig. 3A). In contrast, treatment with the same dose of CEP-11988 had no effect on CD274 expression. The suppression of CD274 expression in SUDHL-1 cells by CEP-14083 but not CEP-11988 was also detected on the protein level (Fig. 3B). Not surprisingly, CEP-14083 was also effective in inhibiting CD274 protein expression in two other ALK+TCL cell lines, JB6 and SUP-M2.
Fig. 3.
Expression of CD274 is induced by NPM/ALK. (A) Expression of CD274 mRNA in the ALK+TCL SUDHL-1 cell line before and after treatment with 175 nM of the ALK inhibitor CEP-14083 or its structural analog, CEP-11988, noninhibitory for ALK (ALK non-inh) (14). (B) Expression of CD274 protein in the depicted ALK+TCL cell lines before and after treatment with the ALK inhibitor CEP-14083. Treatment of the SUDHL-1 cell line with the ALK noninhibitory analog CEP-11988 served as a control (lane two). (C) Expression of CD274 examined by flow cytometry in the IL-3-dependent BaF3 cells transfected with the intact, enzymatically active NPM/ALK, kinase-activity negative K210R NPM/ALK mutant (ALK-KN), or empty vector after culture for 48 h in medium with IL-3 or without IL-3 (-).
Because even the most specific kinase domain inhibitors tend to inactivate more than one kinase, we next determined if induction of NPM/ALK expression, similar to inhibition of its enzymatic activity, also promotes CD274 expression. To achieve this goal, we examined an IL-3-dependent lymphoid BaF3 cell line after its transfection, with a vector containing either the intact NPM/ALK gene, NPM/ALK kinase activity-deficient K210R mutant, or no insert (10, 17, 18). Only the BaF3 cells carrying the intact NPM/ALK gene strongly expressed CD274 in the presence of IL-3 or after depletion of the cytokine for either 48 h (Fig. 3C) or 24 h (Fig. S2). In contrast, BaF3 cells transfected with either empty vector or the kinase-inactive NPM/ALK mutant displayed essentially no expression of CD274, regardless of the presence or absence of IL-3.
Induction of CD274 Expression by NPM/ALK is Mediated by STAT3.
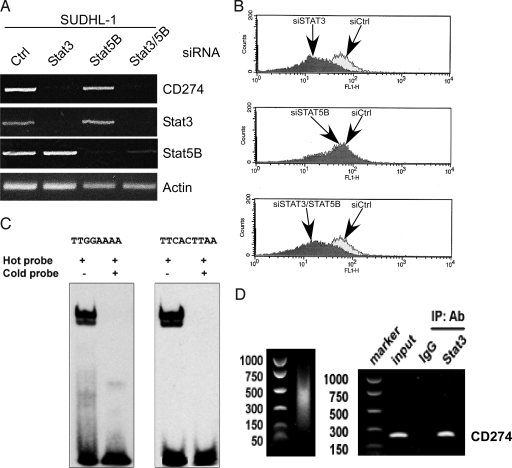
Because NPM/ALK transforms cells by activating several key signal transducing pathways (1, 2), we decided to examine next which of these cell-signaling pathways is directly responsible for the induction of CD274 gene transcription. Treatment of ALK+TCL SUDHL-1 cells with inhibitors of several kinases known to be down-stream of NPM/ALK—rapamycin (mTORC1 inhibitor), wortmanin (PI-3K), U0126 (MEK1/2), or Jak3 inhibitor—all used at the preselected profoundly inhibitory doses, as shown by us previously (15, 16, 18, 20), had no detectable impact on CD274 expression either on the protein or mRNA level (Fig. S3). Faced with this outcome, we focused next on the other potent effectors of the NPM/ALK oncogenicity, STAT3 and STAT5, using the siRNA depletion strategy, given the current lack of small molecule inhibitors genuinely selective for STAT3 or STAT5. Depletion in the SUDHL-1 cells of STAT3 but not STAT5, or more specifically STAT5B, because SUDHL-1 and other ALK+ TCL cells do not express STAT5A (19), profoundly diminished CD274 expression on both the mRNA (Fig. 4A) and protein (Fig. 4B) levels.
Fig. 4.
NPM/ALK induces CD274 expression through STAT3. (A) The effect of siRNA-mediated STAT3 and STAT5B depletion on CD274 mRNA expression. The ALK+TCL cell line SUDHL-1 was treated with siRNA specific for STAT3 or STAT5, STAT3/STAT5 siRNA combination, or control nonspecific siRNA and evaluated by RT-PCR for expression of mRNA coding for CD274 and the depicted other molecules serving as controls. (B) The effect of the siRNA-mediated STAT3 depletion on expression of the CD274 protein. SUDHL-1 cells treated with the STAT3, STAT5, STAT3/STAT5, or control siRNA were examined for CD274 protein expression by flow cytometry. (C) Binding of STAT3 to the CD274 gene promoter in vitro. The nuclear protein extracts from SUDHL-1 cells were incubated with the hot, biotin-labeled oligonucleotide probes corresponding to either of the two STAT3 binding sites identified within the CD274 gene promoter and analyzed in EMSA. The extract of the SUDHL-1 cells preincubated with the corresponding unlabeled cold probes served as control. (D) Binding of STAT3 to the CD274 gene promoter in vivo. Protein cell lysates from the SUDHL-1 cell line were analyzed in the ChIP assay using an anti-STAT3 rabbit polyclonal antibody and primer pairs specific for CD274 gene promoter. Non-immunoprecipitated lysates (input) and immunoprecipitates obtained with the STAT3 nonimmune entire IgG rabbit serum fraction served as a positive and negative control, respectively.
To document that STAT3 acts as a direct activator of CD274 gene transcription, we performed three types of experiments. First, in silico analysis of the CD274 gene promoter identified four potential STAT3 binding sites (data not shown). Second, using two labeled (“hot”) DNA oligonuleotide probes corresponding to the promoter domains containing two of the sites, we documented STAT3 binding in the gel electromobility shift assay (EMSA) (Fig. 4C). The specificity of binding of both probes was confirmed by inhibition of their binding by excess of unlabeled, “cold” corresponding probes. Of note, two closely located bands were identified, indicating binding of both fast and slow migrating forms of STAT3 (21). Finally, to demonstrate STAT3 binding to the CD274 gene promoter in vivo, we performed a chromatin immunoprecititation (ChIP) assay using the PCR primer set capable of amplifying the promoter's region containing the STAT3 binding sites. As shown in Fig. 4D, STAT3 did indeed demonstrate strong binding to the CD274 gene promoter.
Discussion
Here we report that ALK+TCL cells express a highly immunosuppressive protein, CD274. Further multifaceted analysis revealed that CD274 expression is induced in malignant cells by the chimeric NPM/ALK tyrosine kinase, whose expression resulting from a chromosomal translocation represents the critical oncogenic event in the pathogenesis of ALK+TCL (1–9). We also showed that NPM/ALK induces the CD274 gene activation by activating its well-known key signal-transduction transmitter, the transcription factor STAT3. These findings identify a unique function for NPM/ALK as a promoter of evasion of immune response by inducing CD274 expression and documenting the central role of STAT3 in the induction of the immunosuppressive phenotype. These observations also provide a different rationale to therapeutically target NPM/ALK and STAT3, and suggest that potential future therapy aimed at boosting immune response against ALK+ TCL cells may require inhibition of NPM/ALK or STAT3.
CD274 plays a key role in induction and maintenance of immune tolerance to self-antigens and limits normal immune response against microorganisms, to protect the involved tissues from excessive damage incurred during such a response and to prevent its potential autoimmune complications (11, 12). While CD274 has been identified in the whole spectrum of normal hematopoietic and nonhematopoietic cells, including macrophages, dendritic cells, activated T and B lymphocytes, endothelial, muscle, and glial cells, as well as a large variety of epithelial cells, its expression in such cells is transient and tightly controlled in regard to the exact localization, timing, and extent. Several different cytokines produced by immune cells, including IFN-α, -β, and -γ, TNFα, IL-2, and IL-17 have been shown to induce or enhance CD274 expression. CD274 is also very commonly expressed by a multitude of malignant cell types of epithelial and hemaptopoietic cell origin but, in contrast to the normal cells, malignant cells express CD274 in a persistent fashion. Abundant indirect and less plentiful direct evidence indicate that CD274 plays a key role in the induction and maintenance of tolerance toward the malignant cells (11–13). However, the mechanisms of CD274 induction in such cells remain essentially unknown, including the lack of any links to genetic changes underlying the very nature of malignant cell transformation.
Our finding that NPM/ALK oncoprotein induces CD274 expression represents a unique example of such a direct link. By its feature of being constitutively expressed and activated, NPM/ALK secures a persistent, steady expression of the CD274 protein by the ALK+TCL cells. Considering our previous findings that NPM/ALK also induces expression of IL-10 and TGF-β (17), although not FoxP3, as we have clarified recently (22), these combined observations indicate that inhibition of immune response against ALK+TCL cells is an important component of the NPM/ALK-mediated oncogenicity. It is also very interesting that NPM/ALK induces expression of these immunosuppressive proteins through STAT3. Given that STAT3 can be activated by a variety of quite diverse tyrosine kinases (23), that it is persistently activated in a large spectrum of malignancies and, finally, that the STAT3 activation plays a key role in oncogenesis (24–26), it is likely that STAT3 is involved in inducing immune evasion of a substantial number of tumors. Of note, STAT3 has also been implicated in down-regulation of immune response in tumors by indirectly inhibiting activation of tumor-infiltrating antigen-presenting cells (27) and directly inducting anergy in such cells (28). However, the exact molecular mechanisms of this immunosuppression are currently unknown and the potential role of CD274, IL-10, and TGF-β in the process remains to be determined.
A few different signaling pathways have recently been implicated in the control of CD274 expression in various types of cells. Accordingly, the PI3K/AKT pathway has been found to induce CD274 in the glioma cells by activating mTOR/S6K1 signaling (29). However, neither we (see Fig. S3) nor Lee et al. (30), who studied lung and hepatocellular carcinoma cell lines, were able to document the effect of PI3K or mTOR, as well as of MEK/ERK inhibition on the expression of CD274. These findings suggest the existence of alternative signaling pathways, possibly receptor- and tissue-type specific, involved in the control of this important and broadly expressed immunosuppressive protein. The observation that INFγ and Toll-like receptors enhance persistent expression of CD274 in malignant plasma cells by acting via the MyD88, TRAF6, and MAPK signaling pathways (31) supports this conclusion. Finally, the IRS-1 transcription factor has been found to activate the CD274 gene in the lung carcinoma cell line (30). Whether IRS-1 and STAT3 act independently or can cooperate in inducing CD274 expression in at least some types of normal and malignant cells remains to be determined.
Our findings that NPM/ALK induces via STAT3 the expression of the CD274, as well as of the immunosuppressive cytokines IL-10 and TGFß (17), provide new rationale for therapeutic inhibition of the kinase or the transcription factor, with the former being currently a much more attractive target, given the proven effectiveness of kinase inhibitors in general and the beneficial effects of NPM/ALK inhibition already documented in the preclinical models (14, 15, 32). Of note, ALK+TCL patients develop rudimentary humoral (33) and cellular (34) immune responses against NPM/ALK, but they are clearly insufficient per se to control tumor growth. In the NPM/ALK-transgene syngeneic mouse transplant model, DNA vaccination with plasmids encoding portions of the cytoplasmic domain of ALK displayed protective effect and significantly enhanced the impact of chemotherapy on the survival of the recipient mice (35). Therefore, it can be argued that pharmacological targeting of NPM/ALK or STAT3 may drastically increase immunogenicity of the ALK+TCL cells and, hence, markedly boost the host immune response against the lymphoma cells. Moreover, it may dramatically improve the efficacy of any vaccination protocols targeting ALK or other lymphoma-related antigens. It seems relevant in this context that in the mouse model of renal cell carcinoma, the irradiated cancer-cell vaccine combined with an antibody-induced blockade of CD274, and depletion of regulatory cell-rich CD4+ T cells resulted in complete tumor regression (36). This outcome indicates that combination therapy may be required to achieve long-lasting therapeutic effects in human malignancies including ALK+TCL.
Materials and Methods
ALK+ALCL and CTCL Cell Lines and Patients.
NPM/ALK-expressing SUDHL-1, JB6, SUP-M2, Karpas 299, and L-82 cell lines were derived from ALK+TCL patients (10, 15–18). IL-2-dependent T cell line Sez-4 and IL-2-independent MyLa3675 were derived from a CTCL patient (21, 22). Jurkat was developed from lymphoblastic T-cell lymphoma. The IL-3-dependent B-cell line was BaF3 transfected with an empty vector or vector containing NPM/ALK, either wild type or K210R kinase-deficient mutant (10, 18). The cell lines were cultured at 37 °C and 5% CO2 in RPMI medium 1640, supplemented with 2-mM L-glutamine, 10% heat-inactivated FBS (FBS), 1% penicillin/streptomycin/fungizone mixture and, where applicable, 200 units of IL-2 (Sez-4) or IL-3 (BaF3).
Microarray Analysis.
The ALK+TCL SUDHL-1 and SUP-M2 cell lines were treated in triplicates with the CEP-14083 ALK inhibitor or the compound's solvent for 4 h. The isolated RNA was reverse-transcribed, biotin-labeled, and hybridized to the U133 Plus 2.0 array chips (Affymetrix) as described (20). Microarray data were normalized using MAS5 algorithm and analyzed using the Partek GS (Partek). Differentially expressed genes were identified using ANOVA. A gene list that was estimated to have a 5% false discovery rate (FDR = 0.05) was used for identification of the NPM/ALK target genes.
RT-PCR.
Total RNA was isolated using RNeasy Mini kit (Qiagen), treated with DNase I (Invitrogen), and reverse-transcribed by using Thermoscript RT-PCR system (Invitrogen) with random hexamers as cDNA synthesis primers. The following primer pairs were used for the cDNA amplification: β-actin, 5′ACCATTGGCAATGAGCGGT and 5′ GTCTTTGCGGATGTCCACGT; CD274, 5′ CCTACTGGCATTTGCTGAACGCAT and 5′ ACCATAGCTGATCATGCAGCGGTA. PCR was performed by using Platium TaqDNA polymerase (Invitrogen) for 21 cycles comprised of the denaturation step for 20 s at 94 °C, annealing for 30 s at 58 °C and elongation for 30 s at 72 °C. The PCR products were visualized by ethium bromide staining in 1.5% agarose gel.
Immunohistochemical Analysis.
Formalin-fixed paraffin-embedded ALK+TCL tissue specimen slides were heat-treated for antigen retrieval in 10-mM citrate buffer. The sections were blocked with the peroxidase blocking system and incubated at room temperature with the rabbit CD274 (B7-H1) antibody (Lifespan Biosciences) at 1:200 dilution for 30 min and an anti-rabbit-HRP polymer for 30 min, washed, exposed to the DABplus chromagen (Dako) for 5 min and counterstained with hematoxylin.
Flow Cytometry.
Cells (0.5 × 106) were washed and stained for 20 min with murine antibodies against CD274 (dilution 1:10, clone MIH1, FITC) or CD279 (dilution 1:10, clone MIH4, APC) or FITC- or APC-labeled mouse IgG1 isotype controls. All antibodies were purchased from BD PharMingen. The stained cells were applied to the flow cytometer (FACSCalibur; Becton Dickinson), and 20,000 events were analyzed. Results of the cell staining are presented as histograms, with cell number on the vertical axis and relative fluorescence on the logarithmic horizontal axis.
Kinase Inhibitors.
A potent ALK inhibitor CEP-14083 and its structurally-related ALK noninhibitory counterpart CEP-11988, both used at the dose of 175 nM, have been described in detail previously (14). Inhibitors of PI3K, wortmannin (Calbiochem) used at 20 nM; MEK1/2, U0126 (Promega) used at 15 μM; mTORC1, rapamycin (Cell Signaling Technology) used at 300 nM; and Jak3 used at 1 μM have also been described in great detail (15, 16, 18, 20).
siRNA Assay.
A mixture of four STAT3- or STAT5B-specific siRNA or nontargeting siRNA (all purchased from Dharmacon) was introduced into cells at 100 nM by lipofection with the new generation Lipofectamine (DMRIE-C; Invitrogen). The procedure was repeated after 24 h and the cells were cultured for an additional 24 h. The cells were harvested at one time-point 48 h after first transfection. The extent of the protein knock-down was examined by flow cytometry and RT-PCR.
Electrophoretic Mobility Shift Assay.
Nuclear proteins were extracted and incubated with biotin-labeled DNA probes, gel-separated, and transferred to nylon membranes as described (10, 19). We used the 5′- CTTTTTTTATTAATAACA-3′ and 5′-CGATTTCACCGAAGGTCAG-3′ probes that correspond to the putative STAT3 binding sites. The blots were developed using the HPR system (Pierce).
Chromatin Immunoprecipitation Assays.
Soluble chromatin-containing lysates obtained from formaldehyde-fixed and sonicated cells were incubated with STAT3 antibody (Santa Cruz) as described (17, 19). Next, the DNA-protein immunocomplexes were precipitated with protein A-agarose beads and the DNA was extracted with phenol/chloroform, precipitated with ethanol and PCR-amplified (17, 19), using primers specific for the CD274 gene promoter: 5′- CAAGGTGCGTTCAGATGTTG -3′ and 5′- GGCGTTGGACTTTCCTGA- 3′.
Supplementary Material
Acknowledgments.
This study was supported in part by National Cancer Institute Grants R01-CA89194 and R01-CA96856.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810958105/DCSupplemental.
References
- 1.Li R, Morris SW. Development of anaplastic lymphoma kinase (ALK) small-molecule inhibitors for cancer therapy. Med Res Rev. 2008;28:372–412. doi: 10.1002/med.20109. [DOI] [PubMed] [Google Scholar]
- 2.Chiarle R, Voena C, Ambrogio C, Piva R, Inghirami G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer. 2008;8:11–23. doi: 10.1038/nrc2291. [DOI] [PubMed] [Google Scholar]
- 3.Morris SW, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science. 1994;263:1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 4.Shiota M, et al. Hyperphosphorylation of a novel 80 kDa protein-tyrosine kinase similar to Ltk in a human Ki-1 lymphoma cell line, S3. Oncogene. 1994;9:1567–1574. [PubMed] [Google Scholar]
- 5.Morris SW, et al. ALK, the chromosome 2 gene locus altered by the t (2; 5) in non-Hodgkin's lymphoma, encodes a novel neural receptor tyrosine kinase that is highly related to leukocyte tyrosine kinase (LTK) Oncogene. 1997;14:2175–2188. doi: 10.1038/sj.onc.1201062. [DOI] [PubMed] [Google Scholar]
- 6.Fujimoto J, et al. Characterization of the transforming activity of p80, a hyperphosphorylated protein in a Ki-1 lymphoma cell line with chromosomal translocation t (2; 5) Proc Natl Acad Sci USA. 1996;93:4181–4186. doi: 10.1073/pnas.93.9.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bischof D, Pulford K, Mason DY, Morris SW. Role of the nucleophosmin (NPM) portion of the non-Hodgkin's lymphoma-associated NPM-anaplastic lymphoma kinase fusion protein in oncogenesis. Mol Cell Biol. 1997;17:2312–2325. doi: 10.1128/mcb.17.4.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuefer MU, et al. Retrovirus-mediated gene transfer of NPM-ALK causes lymphoid malignancy in mice. Blood. 1997;90:2901–2910. [PubMed] [Google Scholar]
- 9.Chiarle R, et al. NPM-ALK transgenic mice spontaneously develop T-cell lymphomas and plasma cell tumors. Blood. 2003;101:1919–1927. doi: 10.1182/blood-2002-05-1343. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Q, et al. Multilevel dysregulation of STAT3 activation in anaplastic lymphoma kinase-positive T/null-cell lymphoma. J Immunol. 2002;168:466–474. doi: 10.4049/jimmunol.168.1.466. [DOI] [PubMed] [Google Scholar]
- 11.Dong H, Chen L. B7–H1 pathway and its role in the evasion of tumor immunity. J Mol Med. 2003;81:281–287. doi: 10.1007/s00109-003-0430-2. [DOI] [PubMed] [Google Scholar]
- 12.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Intern Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 13.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wan W, et al. Anaplastic lymphoma kinase activity is essential for the proliferation and survival of anaplastic large-cell lymphoma cells. Blood. 2006;107:1617–1623. doi: 10.1182/blood-2005-08-3254. [DOI] [PubMed] [Google Scholar]
- 15.Marzec M, et al. Inhibition of ALK enzymatic activity in T-cell lymphoma cells induces apoptosis and suppresses proliferation and STAT3 phosphorylation independently of Jak3. Lab Invest. 2005;85:1544–1554. doi: 10.1038/labinvest.3700348. [DOI] [PubMed] [Google Scholar]
- 16.Marzec M, et al. Oncogenic tyrosine kinase NPM/ALK induces activation of the MEK/ERK signaling pathway independently of c-Raf. Oncogene. 2007;26:813–821. doi: 10.1038/sj.onc.1209843. [DOI] [PubMed] [Google Scholar]
- 17.Kasprzycka M, Marzec M, Liu X, Zhang Q, Wasik MA. Nucleophosmin/anaplastic lymphoma kinase (NPM/ALK) oncoprotein induces the T regulatory cell phenotype by activating STAT3. Proc Natl Acad Sci USA. 2006;103:9964–9969. doi: 10.1073/pnas.0603507103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marzec M, et al. Oncogenic tyrosine kinase NPM/ALK induces activation of the rapamycin-sensitive mTOR signaling pathway. Oncogene. 2007;26:5606–5614. doi: 10.1038/sj.onc.1210346. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Q, Wang HY, Liu X, Wasik MA. STAT5A is epigenetically silenced by the tyrosine kinase NPM1-ALK and acts as a tumor suppressor by reciprocally inhibiting NPM1-ALK expression. Nat Med. 2007;13:1341–1348. doi: 10.1038/nm1659. [DOI] [PubMed] [Google Scholar]
- 20.Marzec M, et al. Differential effects of interleukin-2 and interleukin-15 versus interleukin-21 on CD4+ cutaneous T-cell lymphoma cells. Cancer Res. 2008;68:1083–1091. doi: 10.1158/0008-5472.CAN-07-2403. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen M, et al. Constitutive activation of a slowly migrating isoform of Stat3 in mycosis fungoides: tyrphostin AG490 inhibits Stat3 activation and growth of mycosis fungoides tumor cell lines. Proc Natl Acad Sci USA. 1997;94:6764–6769. doi: 10.1073/pnas.94.13.6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasprzycka M, et al. Gamma c-signaling cytokines induce a regulatory T cell phenotype in malignant CD4+ T lymphocytes. J Immunol. 2008;181:2506–2512. doi: 10.4049/jimmunol.181.4.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu H, Jove R. The STATs of cancer-new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 24.Chan KS, et al. Disruption of Stat3 reveals a critical role in both the initiation and the promotion stages of epithelial carcinogenesis. J Clin Invest. 2004;114:720–728. doi: 10.1172/JCI21032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ling X, Arlinghaus RB. Knockdown of STAT3 expression by RNA interference inhibits the induction of breast tumors in immunocompetent mice. Cancer Res. 2005;65:2532–2536. doi: 10.1158/0008-5472.CAN-04-2425. [DOI] [PubMed] [Google Scholar]
- 26.Chiarle R, et al. Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nat Med. 2005;11:623–629. doi: 10.1038/nm1249. [DOI] [PubMed] [Google Scholar]
- 27.Wang T, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 28.Cheng F, et al. A critical role for Stat3 signaling in immune tolerance. Immunity. 2003;19:425–436. doi: 10.1016/s1074-7613(03)00232-2. [DOI] [PubMed] [Google Scholar]
- 29.Parsa AT, et al. Loss of tumor suppressor PTEN function increases B7–H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 30.Lee SJ, et al. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7–H1 (CD274) FEBS Lett. 2006;580:755–762. doi: 10.1016/j.febslet.2005.12.093. [DOI] [PubMed] [Google Scholar]
- 31.Liu J, et al. Plasma cells from multiple myeloma patients express B7–H1 (PD-L1) and increase expression after stimulation with IFN- {gamma} and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007;110:296–304. doi: 10.1182/blood-2006-10-051482. [DOI] [PubMed] [Google Scholar]
- 32.Galkin AV, et al. Identification of NVP-TAE684, a potent, selective, and efficacious inhibitor of NPM-ALK. Proc Natl Acad Sci USA. 2007;104:270–275. doi: 10.1073/pnas.0609412103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pulford K, et al. Immune response to the ALK oncogenic tyrosine kinase in patients with anaplastic large-cell lymphoma. Blood. 2000;96:1605–1607. [PubMed] [Google Scholar]
- 34.Passoni L, et al. ALK as a novel lymphoma-associated tumor antigen: identification of 2 HLA-A2.1-restricted CD8+ T-cell epitopes. Blood. 2002;99:2100–2106. doi: 10.1182/blood.v99.6.2100. [DOI] [PubMed] [Google Scholar]
- 35.Chiarle R, et al. The anaplastic lymphoma kinase is an effective oncoantigen for lymphoma vaccination. Nat Med. 2008;14:676–680. doi: 10.1038/nm1769. [DOI] [PubMed] [Google Scholar]
- 36.Webster WS, et al. Targeting molecular and cellular inhibitory mechanisms for improvement of antitumor memory responses reactivated by tumor cell vaccine. J Immunol. 2007;179:2860–2869. doi: 10.4049/jimmunol.179.5.2860. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.