Abstract
Specific pathogen-free IL-10 KO mice failed to develop inflammatory bowel disease (IBD), whereas IL-10/vitamin D receptor (VDR) double KO mice developed fulminating IBD. WT CD4 T cells inhibited experimental IBD, while VDR KO CD4 T cells failed to suppress IBD. VDR KO mice had normal numbers and functions of regulatory T cells. The percentages of IL-17- and IFN-γ-secreting T cells in the gut of mice reconstituted with WT and VDR KO CD4 T cells were also not different. Instead, there were twice as many CD8αα intraepithelial lymphocytes (IEL) in mice that were reconstituted with WT CD4 T cells than in mice reconstituted with VDR KO CD4 T cells. Furthermore, VDR KO mice had reduced numbers of CD8αα IEL, absent CD4/CD8αα populations, and as a result low IL-10 production in the IEL. The lack of CD8αα IEL was due in part to decreased CCR9 expression on T cells that resulted in the failure of the VDR KO T cells to home to the small intestine. We conclude that the VDR mediates T cell homing to the gut and as a result the VDR KO mouse has reduced numbers of CD8αα IEL with low levels of IL-10 leading to increased inflammatory response to the normally harmless commensal flora.
Keywords: inflammatory bowel disease, mucosal immunity, regulatory T cell
Inflammatory bowel diseases (IBD) that encompass Crohn's disease and ulcerative colitis are chronic inflammatory conditions of the gastrointestinal tract. Factors that predispose individuals to the development of IBD include genetic, microbial, immunological, and poorly defined environmental factors (1). The high prevalence of IBD in North America and Europe where the availability of vitamin D is low, especially during the winter when sunlight exposure is reduced, implicates vitamin D status as a possible environmental factor that contributes to IBD development (2).
The intestinal epithelial layer not only forms a physical barrier to protect the body from invading bacterial pathogens but also contains a highly specialized innate and adaptive immune system (3). Several types of T cells are important for regulation of homeostasis in the gastrointestinal tract and either induce or suppress IBD. Forkhead box (Fox)P3/CD4/CD25 regulatory T (T reg) cells induce apoptosis of effector cells, produce the inhibitory cytokines IL-10 and TGF-β1, and inhibit inflammation in experimental mouse models of IBD (4). IL-17-producing CD4 T cells or Th-17 cells are proinflammatory T cells that are associated with IBD and mice deficient in Th-17 cells are resistant to experimental IBD (5, 6). Intraepithelial lymphocytes (IEL) contain a population of regulatory T cells that express a homodimeric form of CD8, CD8αα (7). Although these CD8αα T cells are self-reactive, they are not self-destructive and have been shown to be inhibitors of inflammation in the gastrointestinal tract (7). One specific subset of IEL, CD4/CD8αα T cells are regulatory and prevent T cell transfer models of IBD (8). These CD8αα T cells in the gut spontaneously produce a number of cytokines including IL-10 that is known to be important for inhibition of experimental IBD (8). Together these T cell subsets maintain gastrointestinal homeostasis.
The discovery of the vitamin D receptor (VDR) in cells of the immune system and the presence of the 1α 25(OH)vitamin D3 hydroxylase in dendritic cells and macrophages suggests that locally produced 1,25(OH)2D3 has regulatory autocrine and paracrine properties at the site of inflammation (9). Synthesis of active vitamin D requires the 1α hydroxylase, which catalyzes the conversion of 25(OH)D3 to 1,25(OH)2D3. The actions of 1,25(OH)2D3 are mediated by its binding to the VDR, which acts as a transcription factor to modulate the expression of specific genes in a tissue-specific manner. The VDR is a member of the steroid/hormone superfamily of nuclear transcription factors and is constitutively expressed in a variety of immune cells (10). Resting T cells express low levels of VDR, which are upregulated following activation (11).
The active form of vitamin D [1,25(OH)2D3] has been recognized as an immunosuppressive agent that ameliorates the pathogenesis of Th1-autoimmune diseases including IBD (2). Furthermore, vitamin D deficiency and VDR deficiency have been shown to exacerbate experimental IBD in the IL-10 KO mouse, the T cell (CD4/CD45RBhigh) transfer model, and dextran sodium sulfate-induced colitis (12–14). The increase in T reg cells caused by 1,25(OH)2D3 both in vitro and in vivo has been suggested as a mechanism underlying the ability of 1,25(OH)2D3 to suppress autoimmunity (15, 16). In addition, genome wide screening techniques suggest that VDR polymorphisms are associated with increased susceptibility to both Crohn's disease (17) and ulcerative colitis (18) in humans.
Mice lacking the VDR do not develop overt symptoms or present histological evidence of IBD even when they are housed in conventional facilities. IBD is not a vitamin D deficiency disease. However, increased expression of IL-1β and TNF-α in the colon of young (5 weeks) and old (9 months) VDR KO mice when compared to age-matched WT mice suggests that VDR deficiency results in chronic and low-grade inflammation in the gastrointestinal tract (13). T cells from VDR KO mice have been shown to express an inflammatory phenotype, to respond two times higher in a mixed lymphocyte reaction, and to induce a severe form of T-cell-induced IBD than their WT counterparts (13). VDR KO mice have heightened immune responses and inflammation in the colon, which suggest that the absence of the VDR predisposes to the development of IBD. Here we sought to determine the effect of the VDR on regulatory T cell populations that would explain the increased susceptibility of the VDR KO mice to multiple models of experimental IBD (12–14).
We show here that IBD induction in the IL-10/VDR double KO (DKO) mice is severe even when the mice are specific pathogen free (SPF) and antibiotic treated. CD4 T cells from SPF DKO mice induce IBD in T and B cell deficient (recombination activation gene; Rag KO) recipients. Conversely IL-10 single KO mice in our SPF colony did not develop overt IBD and CD4 T cells from SPF IL-10 KO mice did not transfer IBD to Rag KO recipients. In addition, we show that while CD4 T reg cell development and function are normal in the VDR KO mice, unfractionated VDR KO CD4 T cells fail to suppress experimental IBD. The failure of VDR KO CD4 T cells to suppress IBD is not due to increased induction of more pathogenic Th17 or Th1 cells in the gut but is associated with the decreased expression of CCR9 and reduced homing of VDR KO T cells to the IEL. The IEL of the VDR KO mice are missing the CD4/CD8αα T cell population and have half as many CD8αα cells that fail to produce IL-10. These data suggest that the susceptibility of the VDR KO mice to inflammation in the gastrointestinal tract comes as a result of decreased homing of T cells to the gut that limits the number of regulatory CD8αα cells producing IL-10 in the gut. Furthermore, the absence of the VDR and of CD8αα IEL results in inflammation to normally nonpathogenic bacterial flora.
Results
Antibiotic Treatment of SPF DKO Mice Is Ineffective in Preventing IBD.
SPF DKO mice were compared to SPF IL-10 KO mice for IBD development (Table 1). We determined that 100% of the SPF DKO mice developed fulminating IBD and the severity [small intestine (SI)/body weight (BW) and large intestine (LI)/BW] of IBD was the same compared to data we had collected previously from conventional DKO mice (13). Conversely, SPF IL-10 KO mice were free of IBD symptoms (Table 1).
Table 1.
Severe IBD in the DKO mice regardless of the composition of the gut microflora
| Genotype (n) | Facilities | IBD incidence† | SI/BW (%) | LI/BW (%) | Mortality (days) |
|---|---|---|---|---|---|
| IL-10 KO | SPF | 0/20 (0) | 6.3 ± 0.4 | 4.1 ± 0.4 | None‡ |
| IL-10 KO | SPF/antibiotic | 0/4 (0) | 6.3 ± 0.2 | 3.9 ± 0.1 | None |
| DKO | SPF | 28/28 (100) | 8.3 ± 0.9* | 6.9 ± 0.6* | 80 ± 5 |
| DKO | SPF/antibiotic | 11/11 (100) | 8.8 ± 0.1* | 6.5 ± 0.3* | 112 ± 3** |
†IBD incidence was defined as mice showing a decrease in body weight of 10%, rectal bleeding, or rectal prolapse.
‡IL-10 KO mice kept in SPF conditions did not die prematurely.
*, values from DKO mice were significantly different from the values from IL-10 KO mice housed under similar conditions, P < 0.05.
**, SPF/antibiotic-treated DKO mice showed a significant delay in the onset of IBD and resulting mortality compared to SPF DKO mice, P < 0.05.
Ciprofloxacin administration to IL-10 KO mice has been shown to markedly reduce bacteria levels and prevent IBD development (19). Ciprofloxacin was administered to SPF DKO mice from birth onward (19). All of the antibiotic-treated DKO mice manifested a fulminating form of IBD. Microscopic evaluation of the gastrointestinal tract of the antibiotic-treated SPF DKO mice showed severe inflammation and hyperplasia in the SI (histology score 5.0 ± 0.1) and colon (histology score 6.5 ± 0.3). SI/BW and LI/BW ratios in antibiotic-treated SPF DKO mice did not change as a result of the treatment (Table 1). Antibiotic treatment of SPF DKO mice did inhibit the kinetics of IBD development and there was a significant delay in the mortality of the antibiotic-treated SPF DKO mice (Table 1). We concluded that the antibiotic treatment was ineffective for preventing IBD in the SPF DKO mice (Table 1).
CD4 T Cells from DKO Mice Transfer IBD.
Previous work has shown that transfer of whole splenocytes from conventional DKO mice to Rag KO recipients induced a fulminating and fatal form of IBD (13). IL-10 is a key cytokine produced by T reg cells and critical for the suppression of experimental IBD. Because T reg cells from IL-10 KO mice are defective, CD4 T cells from conventional IL-10 KO mice induced intestinal inflammation when transferred to conventionally housed Rag KO recipients (Fig. S1, ref. 20). Rag KO mice receiving CD4 T cells from DKO mice developed chronic inflammation, manifested by weight lost, bloody diarrhea, high ratios of SI/BW (10.4 ± 0.4) and LI/BW (7.1 ± 0.4), rectal prolapse, and death in 8–9 weeks. Histological analysis revealed inflammatory infiltrates in all of the intestinal layers and severe epithelial hyperplasia of the large intestine (Fig. S1). Rag KO recipients of CD4 DKO T cells exhibited significantly more severe disease than the recipients of IL-10 KO CD4 T cells (Fig. S1).
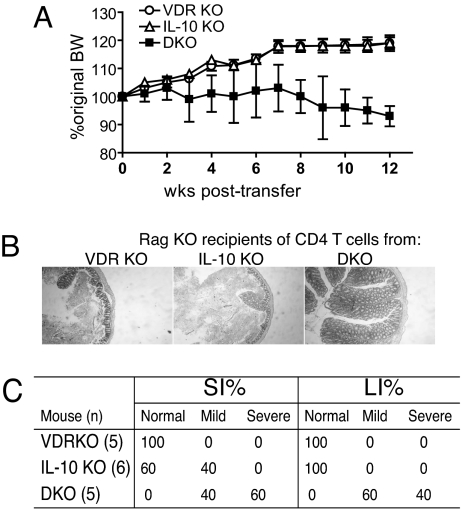
Our conventional mouse facilities are positive for Helicobacter hepaticus and to determine whether the VDR-mediated effects depended on the presence of H. hepaticus, we repeated the reconstitution experiments using SPF donors and recipients (confirmed negative for Helicobacter species). The DKO CD4 T cells from SPF mice transferred colitis to SPF Rag KO mice as was evident by a loss in the BW of Rag KO mice (Fig. 1A). Histopathology scores from the Rag KO recipients of the SPF DKO CD4 T cells were 6.8 ± 0.5 in the small intestine and 6.0 ± 0.4 in the colon and confirmed the development of IBD (Fig. 1 B and C). However, IBD symptoms took longer to develop (only a 10% drop in BW by 12 weeks) when the CD4 DKO T cell transfer was done in SPF vs. conventionally housed mice (Figs. 1A and S1). Conversely, Rag KO recipients of CD4 T cells from SPF VDR KO and SPF IL-10 KO mice increased their BW by ≈10–15% (Fig. 1A), looked healthy, and showed little to no microscopic pathology for 12 weeks posttransfer (Fig. 1 B and C). CD4+ T cells from DKO mice are highly pathogenic and transfer IBD to leukopenic mice even under SPF conditions.
Fig. 1.
CD4 T cells from SPF DKO mice transfer IBD to SPF Rag KO mice. All donors and recipients were SPF. (A) The percentage change in BW over time is plotted ± SEM for each group of recipients. DKO values were significantly different from VDR KO and IL-10 KO values, P < 0.05. (B) Representative colonic sections from the Rag KO recipients of CD4 T cells (scoring system in Materials and Methods). Colon sections shown were rated VDR KO normal, IL-10 KO normal, and DKO mild. (C) The percentage of mice that showed normal, mild, or severe symptoms of IBD is recorded for each group of Rag KO mice receiving CD4 T cells.
Total CD4 T Cells from WT Mice but Not VDR KO Mice Inhibit IBD.
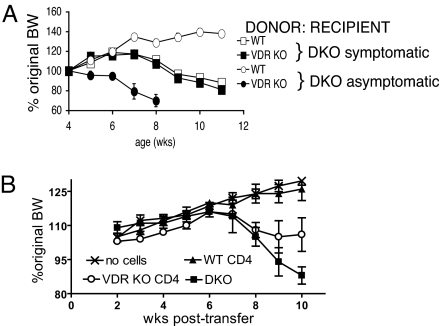
Next, we investigated the regulatory effects of WT or VDR KO CD4 T cells. Purified WT or VDR KO CD4 T cells containing T reg cells were injected into DKO mice either before or after colitis symptoms had developed. Fig. 2A shows that WT CD4 T cells suppressed IBD in DKO mice (increase in BW) if transferred before the mice showed obvious symptoms of intestinal inflammation. Interestingly, injection of VDR KO CD4 T cells into asymptomatic DKO mice had an accelerating effect on intestinal pathology and led to the early death of the mice (Fig. 2A). Histopathological analysis of the DKO mice reconstituted with WT CD4 T cells confirmed attenuated inflammation when compared with untreated or VDR KO CD4 T-cell-injected DKO mice (data not shown). Injection of WT or VDR KO CD4 T cells to DKO mice after they had developed symptoms had no effect on BW or other measures of IBD severity (Fig. 2A).
Fig. 2.
Failure of total CD4 T cells from VDR KO mice to suppress IBD. (A) CD4 T cells were purified from WT or VDR KO mice and injected into DKO recipients that showed symptoms of IBD (squares, n = 5–6) or that were symptom free (circles, n = 3–5). The percentage change in BW over time as a function of age is shown. Symptomatic DKO mice were injected at 4 weeks of age and asymptomatic mice were injected at 2 weeks of age. (B) Changes in BW of recipient Rag KO mice following transfer of no cells, DKO splenocytes, DKO splenocytes plus WT CD4 T cells (WT CD4), or DKO splenocytes plus VDR KO CD4 T cells (VDR KO CD4). Values represent n = 3–4 mice per group and one representative of two independent experiments. At the end of the experiment the VDR KO CD4 group lost significantly more BW than the WT CD4 group and significantly less than the DKO group, P < 0.05.
DKO splenocyte transfer into Rag KO mice (13) was used as a second model to test the effect of the VDR on CD4 T cells in IBD. Rag KO mice were either left untreated (no cells injected) or given DKO splenocytes only, DKO splenocytes plus WT CD4 T cells, or DKO splenocytes plus VDR KO CD4 T cells. Untreated Rag KO mice grew during the experiment, increasing their BW by 25% (Fig. 2B). Rag KO mice that received only DKO splenocytes developed IBD and lost 12% of their starting BW during the 10 weeks (Fig. 2B). CD4 T cells from WT mice suppressed the weight loss in the Rag KO recipients and the mice were as healthy as the untreated controls, also gaining 25% of their original BW (Fig. 2B). Rag KO mice that received VDR KO CD4 T cells and DKO splenocytes gained weight initially and then subsequently lost weight (Fig. 2B). The Rag KO mice that received VDR KO CD4 T cells plus DKO splenocytes ended up weighing the same at the end of the experiment as at the beginning. In addition, one of the Rag KO recipients of VDR KO CD4 T cells plus DKO splenocytes died of severe IBD at 9 weeks posttransfer. VDR KO CD4 T cells lack regulatory function in two different IBD models.
VDR KO Mice Have Normal Numbers of Functional T Reg Cells.
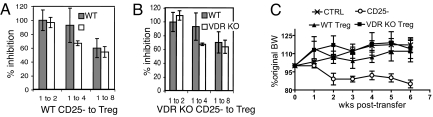
Regulatory T cells that express CD4 and CD25 were purified from WT mice and found to express the VDR by RT–PCR (data not shown). T regs (CD4/CD25) that express FoxP3 have been shown to suppress T cell proliferation in vitro and suppress T-cell-dependent IBD in vivo (4). The percentages of FoxP3+ T reg cells were also determined in the VDR KO and WT mice (Table S1). The numbers of T reg cells were not different in either the spleen or the thymus of VDR KO and WT mice. In addition, the IEL of VDR KO and WT mice had similar numbers of CD4/CD25 double-positive T cells (data not shown). T reg cells were tested in vitro for functional suppression of CD4/CD25− T cell proliferation (Fig. 3). T reg cells from VDR KO mice were as effective as WT T reg cells in suppressing proliferation of both WT CD25− cells and VDR KO CD25− cells (Fig. 3 A and B). T reg cells were sorted from VDR KO and WT mice and tested in vivo for suppression of IBD induced by WT CD4+/CD25− T cell transfers to Rag KO mice. CD25− WT T cells induced IBD in Rag KO recipients and the mice lost 15% of their starting BW by week 6 posttransfer (Fig. 3C). Either WT or VDR KO T regs (CD4+/CD25+) suppressed IBD development when they were transferred at the same time as the CD25− T cells (Fig. 3C). VDR KO T reg cells are phenotypically and functionally normal.
Fig. 3.
T regs from VDR KO mice are functionally normal. CD4+/CD25− and CD4+/CD25+ T cells were isolated from VDR KO and WT mice. (A) WT CD25− proliferation was inhibited equally by WT and VDR KO CD4/CD25+ cells at all ratios tested. (B) VDR KO CD25− proliferation was inhibited equally by WT and VDR KO CD4/CD25+ cells at all ratios tested. (C) VDR KO T regs suppress IBD in the Rag KO mouse. The percentage change in BW of Rag KO mice that did not receive any cells (control, CTRL), received only WT CD4/CD25− T cells (CD25−), received WT CD4/CD25− and WT CD4/CD25+ (WT T reg), or received WT CD4/CD25− and VDR KO CD4/CD25+ (VDR KO T reg) is shown. Only Rag KO recipients of CD25− cells lost significantly more BW by the end of the experiment than the CTRL, WT T reg, or VDR KO T reg Rag KO recipients, P < 0.05.
Reduced Numbers of CD8αα IEL in VDR KO Mice.
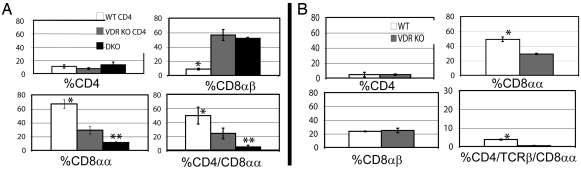
Because FoxP3+ T regs were normal in the VDR KO host, we next examined the composition of the T cells found in the IEL of the Rag KO recipients shown in Fig. 2B. The total numbers of IEL and the percentage of CD4 T cells recovered from the IEL of the three groups of Rag KO recipients were not different (data not shown and Fig. 4A). Rag KO recipients of DKO splenocytes plus WT CD4 T cells had the lowest percentage of CD8αβ cells and the highest percentage of CD8αα single-positive and CD4/CD8αα double-positive cells in the IEL (Fig. 4A). Rag KO recipients of only DKO splenocytes or DKO splenocytes plus VDR KO CD4 T cells had low levels of CD8αα and CD4/CD8αα and high levels of CD8αβ cells in the IEL (Fig. 4A).
Fig. 4.
CD8αα T cell populations in the IEL. (A) The IEL from Rag KO recipients of DKO splenocytes, DKO splenocytes plus WT CD4 T cells (WT CD4), and DKO splenocytes plus VDR KO CD4 T cells (VDR KO CD4) cells (same mice as in Fig. 2B) were isolated and stained for CD4, CD8α, and CD8β. *, values in the WT CD4 group were significantly different from those in the VDR KO CD4 and DKO group, P < 0.05. **, values in the DKO only group were significantly different from those in the WT or VDR KO group, P < 0.05. (B) IEL were isolated from 6–8 VDR KO and WT mice and stained for CD4, CD8α, CD8β, and TCRβ. *, values in the WT group were significantly different from those in the VDR KO group, P < 0.01. Fig. S2 shows the isotype controls for staining and representative histograms.
IEL from WT mice do express the VDR (data not shown). We found that the total number of IEL isolated, the percentage of CD4 T cells, and the percentage of CD8αβ T cells were not different in the VDR KO and WT IEL (data not shown and Fig. 4B). However, the percentage of VDR KO CD8αα and TCRβ/CD8αα IEL was about half of that in the WT IEL (Fig. 4B and data not shown). More importantly, the TCRβ/CD4/CD8αα positive population is missing in the VDR KO IEL (Fig. 4B). To determine whether the CD8αα T cells were ligand responsive, vitamin D deficient and vitamin D sufficient WT mice were generated, and the CD8αα T cells were compared. Vitamin D deficient WT mice had 19.0 ± 1.3% TCRβ/CD8αα and 0.4 ± 0.1% TCRβ/CD4/CD8αα positive IEL vs. 36.7 ± 4.2% TCRβ/CD8αα and 3.7 ± 0.3% TCRβ/CD4/CD8αα positive IEL in the vitamin D sufficient WT mice. There are reduced numbers of CD8αα T cells in the IEL of VDR KO, vitamin D deficient WT, and Rag KO mice reconstituted with VDR KO CD4+ T cells.
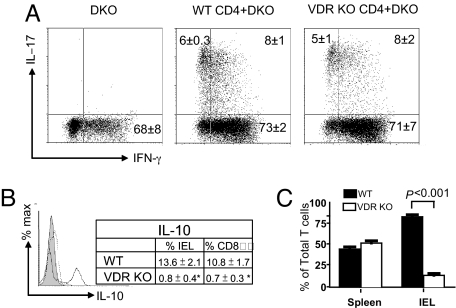
Normal IL-17 but Reduced IL-10 in VDR KO IEL.
IL-17 and IFN-γ production is associated with increased IBD symptoms. IEL from Rag KO recipients of DKO splenocytes produced only IFN-γ and no detectable IL-17 (Fig. 5A). The Rag KO recipients that received either VDR KO or WT CD4 T cells plus DKO splenocytes made IFN-γ and IL-17 (Fig. 5A). There was no difference in the percentage of cells that made IFN-γ, IL-17, or both IFN-γ and IL-17 in the IEL of Rag KO recipients of the VDR KO or WT CD4 T cells (Fig. 5A). IL-10 production is protective in experimental IBD. Intracellular staining for IL-10 in WT IEL showed that 13.6% of the cells produced IL-10 while only 0.8% of the VDR KO IEL produced IL-10 (Fig. 5B). The majority of the IL-10 produced was from the CD8αα IEL in both the WT and the VDR KO mice (Fig. 5B). Therefore, the amount of IL-10 secretion in the IEL corresponds to the numbers of CD8αα cells that are present.
Fig. 5.
Cytokine production and homing to the IEL. (A) Intracellular staining for IL-17 and IFN-γ in IEL from Rag KO recipients of DKO splenocytes, DKO splenocytes plus WT CD4 T cells, and DKO splenocytes plus VDR KO CD4 T cells (same mice as in Fig. 2B). Values from five to six mice per group ± SEM are shown. (B) Intracellular staining for IL-10 in IEL from VDR KO and WT recipients. The histogram shows isotype control staining (shaded) and VDR KO (dotted line) and WT (solid line) IEL staining for IL-10. The values in the table represent percentage of IL-10 secreting in the total IEL and CD8αα IEL of three individual mice per group ± SEM. *, values in the WT group were significantly higher than those in the VDR KO group, P < 0.05. (C) T cell reconstitution of the spleen and IEL of Rag KO mice following injection of a 1:1 mixture of WT (CD45.1) and VDR KO (CD45.2) cells. VDR KO cells are the TCRβ+ and CD45.1− staining cells and WT cells are the TCRβ+ and CD45.1+ cells. *, the percentages of T cells from WT and VDR KO mice in the spleen and IEL of Rag KO mice were compared in seven Rag KO recipients ± SEM and the percentages of T cells from the WT mice were significantly higher than those from the VDR KO mice in the IEL, P < 0.001.
Reduced CCR9 and T Cell Homing of VDR KO T Cells.
Mesenteric lymph node (MLN) and IEL cells were stained for the two gut homing receptors integrin α4β7 and CCR9. The level of integrin α4β7 staining was similar in cells from the MLN and the IEL even when TCR/CD4 T cells were gated on (data not shown). CCR9 expression was significantly higher (P = 0.017) on CD4/TCR positive T cells in the WT MLN (8.3 ± 0.3 MFI) than in the VDR KO MLN (6.5 ± 0.3 MFI). CCR9 staining of the CD4/TCR/CD8αα positive IEL was also significantly higher (P = 0.005) in WT (6.8 ± 0.3 MFI) than in VDR KO (5.3 ± 0.1 MFI) IEL. The ability of VDR KO T cells to home to the IEL was tested in vivo. Rag KO mice were injected with a 1:1 mixture of CD45.1 WT and CD45.2 VDR KO cells. Staining for T cells (TCRβ) and donor origin (CD45 isotype) was used to identify T cells from VDR KO mice (TCRβ+ and CD45.1−) or WT mice (TCRβ+ and CD45.1+) in the Rag KO recipients. The results were the same whether MLN or IEL cells were transferred and therefore the results were combined. Reconstitution of the spleens of Rag KO mice was 53% VDR KO T cells and 46% WT T cells and the values were not significantly different (Fig. 5C). Reconstitution of the IEL of the Rag KO mice resulted in only 14% VDR KO vs. 85% WT T cells (Fig. 5C).
Discussion
Microbial antigens are well recognized as inducers of inflammation in experimental models of IBD and there is evidence that a similar process occurs in patients with IBD. However, in this study we show that deletion of the VDR in IL-10 KO mice results in a form of experimental IBD that is of a similar severity in conventionally housed (13), SPF, and antibiotic-treated SPF mice. Conversely, the VDR-expressing IL-10 KO mice show minimal symptoms in SPF conditions and are completely disease free when treated with ciprofloxacin. Although IL-10 KO and DKO mice were maintained in the same cages and fed the same antibiotic-treated water, only DKO mice developed IBD. DKO mice were also established as a germ-free (GF) colony. GF DKO mice were maintained and found to be outwardly disease free (normal weight, no diarrhea or bleeding) up until 175 days at which time bacteria were found to have contaminated the colony (data not shown). Therefore it seems that, like all other models of IBD, bacteria found in the gastrointestinal tract drive the disease in DKO mice. Nonetheless the data show that VDR deficiency renders the IL-10 KO mice hypersensitive to antibiotic resistant but otherwise nonpathogenic microbial flora.
To elucidate the potential cell population(s) involved in the induction of colitis in DKO mice, we performed T cell transfer experiments. We show here that the ability of CD4 T cells from DKO mice to transfer intestinal pathology to Rag KO mice is similar in conventional and SPF conditions. Conversely CD4 T cell transfers from either IL-10 single KOs caused pathology when transferred to Rag KO mice in conventional mice but not in SPF mice. Our findings argue that VDR expression in CD4 T cells is a protective factor that controls bacterial antigen-dependent responses in the gut and suggest that vitamin D status and signaling through the VDR are crucial for controlling inflammation in the gastrointestinal compartment.
T reg cells are important for the maintenance of self-tolerance. Barrat et al. have shown that a combination of 1,25(OH)2D3 and dexamethasone induces IL-10-producing T reg cells in vitro (15). Furthermore, in vivo-1,25(OH)2D3 treatment of experimental autoimmune diabetes induces a population of CD4/CD25 positive T reg cells that correlates with protection of the mice from diabetes (16). Here we show that T reg cells develop and are present in normal numbers in VDR KO mice. Furthermore the T regs from VDR KO mice function to suppress proliferation in vitro and IBD in vivo. Therefore, it seems that expression of the VDR is not required for development or function of T regs. However, in the presence of the VDR, 1,25(OH)2D3 can increase the numbers of T regs that are critical for the maintenance of self-tolerance (16) .
Paradoxically even though the classical T reg (CD4/CD25 and FoxP3 positive) cells are normal in the VDR KO mice, transfer of unfractionated VDR KO CD4 T cells does not suppress experimental IBD. In addition, production of both IFN-γ and IL-17 in the IEL was the same in Rag KO mice that received VDR KO or WT CD4 T cells. VDR expression is not required for the development or function of FoxP3 T regs or pathogenic IFN-γ or IL-17-secreting IEL. However, CD8αα IEL cell numbers were reduced and the CD4/CD8αα T cell population was absent in the VDR KO or vitamin D deficient host. In a similar Rag KO transfer model of IBD, Das et al. have shown that CD4/CD8αα T cells are generated from CD4+ precursors that have been stimulated with IL-4 (Th2 cells) and not Th1 cells (8). However, IL-4 does not directly induce CD8α expression in CD4 T cells (8). It would be interesting to determine whether 1,25(OH)2D3 treatment affected CD8α expression in CD4 T cells. The increased numbers of CD8αβ T cells are likely of DKO origin and in the absence of competition traffic to the gut and contribute to the pathology.
CD8αα T cells are regulatory cells that suppress IBD symptoms in the gut (8). We show reduced CCR9 expression on T cells from VDR KO mice in the periphery as well as in the IEL population. Reduced CCR9 expression corresponded with a failure of VDR KO T cells to home to the gut in vivo. Since homing of T cells occurs fairly quickly (3–6 h), the presence of fewer VDR KO T cells in the gut of mice after 72 h probably reflects differences in homing (based on CCR9), retention, and recirculation of VDR KO T cells compared to WT T cells to the IEL. 1,25(OH)2D3 treatment in vitro has been shown to inhibit retinoic acid-induced CCR9 expression but to have no effect on CCR9 expression when added alone (21). The IEL and CD8αα-expressing IEL from VDR KO mice fail to produce IL-10 that is known to suppress IBD in vivo. Expression of the VDR is important for reacquisition of CD8α expression, local production of IL-10, and homing of the largely CD4+/CD8αα T cells to the gut.
Our data show that normally nonpathogenic bacteria cause IBD in the VDR deficient host. Expression of the VDR is required within the CD4 T cell compartment to prevent gastrointestinal inflammation to the bacteria found there. Classical FoxP3 positive regulatory T cells are normal in the VDR KO mice. Instead our data show that in the absence of the VDR, CD4 T cells fail to home to the gastrointestinal tract, express CD8αα, and produce IL-10. It is therefore the lack of CD4/CD8αα T cells in the gut of VDR KO reconstituted mice that allows inflammation to the normal bacterial flora to go unregulated. We propose that vitamin D is a regulator of the T cell response in the gastrointestinal tract such that the vitamin D available in the environment (either sunshine or diet) is one of the factors, which regulates the T cell response and controls immunity, inflammation, and homeostasis in the gastrointestinal tract.
Materials and Methods
Animals.
All of the mice were on the C57BL/6 background, all originally from The Jackson Laboratory. IL-10 KO, Rag KO, VDR KO, WT, and DKO mice (previously described) were bred and maintained in either SPF or conventional animal facilities at Pennsylvania State University. Conventional animals tested positive for H. hepaticus, but negative for murine viruses, other Helicobacter species, and all other bacterial pathogens. All mice (SPF and conventional) were confirmed negative for H. bilis, H. rodentium, H. trogontum, and H. typhlonius species (data not shown). Vitamin D deficient and sufficient WT mice were generated exactly as we have described previously (12). All procedures were reviewed and approved by the Pennsylvania State University Institutional Animal Care and Use Committee.
Antibiotic Therapy.
Ciprofloxacin (200 mg/liter; Mediatech) was added to the drinking water of pregnant VDR KO/IL-10 +/− mice. The pups received the antibiotic via breast milk (ciprofloxacin is secreted in breast milk) and after weaning the mice received the medication in their drinking water.
Cell Purifications and Adoptive Transfer.
CD4 T cells were isolated from the spleen of mice using Cell Select Columns (Cedarlane). FACS analysis confirmed that the purity of the T cells was at least 95%. A total of 2.5 × 106 CD4 T cells were injected i.p. into syngenic Rag KO mice. For the cotransfer experiments, Rag KO mice were injected with a 1:1 ratio of DKO splenocytes and CD4 T cells (106 cells each). Controls included Rag KO mice that did not receive any cells and Rag KO mice that received only 106 DKO splenocytes. Additional Rag KO mice were injected with 106 CD4/CD25− sorted (Cytopeia Influx) T cells from WT mice. Other Rag KO recipients received 5 × 105 CD4/CD25+ T cells coinjected with 106 CD4/CD25− T cells. The purity of CD4+ CD25+ and CD4+CD25− T cells was >98%. The antibodies were PE-conjugated anti-mouse CD4 (L3T4) and FITC anti-mouse CD25 (BD PharMingen).
Additional Rag KO recipients (CD45.2) were injected with a 1:1 mixture of 106 cells from the MLN or IEL of WT CD45.1 and VDR KO CD45.2 mice. Three days later the IEL and spleens from the Rag KO recipients were stained with PE-Cy5 anti-TCRβ and FITC anti-CD45.1 cells (BD PharMingen).
T Reg Cell Assay.
A total of 2.5 × 105 CD4+CD25− T cells from either WT or VDR KO mice were stimulated with CD3 and CD28 antibodies alone or in the presence of decreasing concentrations (1:2–1:8) of CD25+ T reg cells from WT and VDR KO mice. For proliferation assays, 0.4 μCi H3 thymidine (ICN) was added to the cultures for the last 18 h of the 72-h incubation.
IBD Severity.
Colitis development was monitored by weight curves, observation of stool consistency, SI/BW ratios, LI/BW ratios, histopathology scores, anal bleeding, rectal prolapse, and death. The SI/BW and LI/BW ratios have previously been shown to be objective measures of IBD severity (12). Mice were killed before they lost 20% of their body weight. The SI and colon were removed, fixed in formalin, and sent to the Penn State University Animal Diagnostic Laboratories for paraffin embedding and H&E staining. Sections were scored blindly by two observers on a scale of 0–4 for inflammation and 0–4 for epithelial thickening exactly as we have described previously (13). Total histopathology score ranged from 0 to 8. For cell transfer experiments mice were characterized as normal [indistinguishable from uninjected control Rag KO mice (histopathology score, 1–2)], mild [slight epithelial hyperplasia and increased number of leukocytes in the mucosa (histopathology score, 3–5)], or severe [marked epithelial cell hyperplasia and extensive transmural leukocytic infiltrate, crypt abscesses (histopathology score, 6–8)].
Isolation of IEL.
The SI was removed and flushed with Hank's containing 5% FBS and the Peyer's patches were removed. The intestine was cut into 0.5-cm pieces. The pieces were incubated twice in media containing 0.15 μg/ml dithiothreitol (Sigma) and stirred at 37 °C for 20 min. Supernatants were collected and the IEL were collected at the interface of 40/80% Percoll gradients (Sigma).
Intracellular Staining and Flow Cytometry.
Splenocytes and thymocytes were stained with ECD anti-CD4 and then fixed and permeabilized using a FOXP3 Fix/Perm Buffer set and directions as provided (Biolegend). The Fox P3 antibody was conjugated with Alexa 488. IEL were stained with conjugated antibodies: PE anti-CD8β, ECD anti-CD4, and either FITC anti-CD25 and PE-Cy5 anti-CD8α or FITC anti-CD8α and PE-Cy5 anti-TCRβ and appropriate isotype controls. For intracellular staining the IEL were stimulated for 12 h with PMA (0.1 μg, Sigma), ionomycin (0.5 μg, Sigma), and Brefeldin A (10 μg, Sigma) for the final 6 h. Cells were stained with surface markers and then fixed, permeabilized, and stained with FITC anti-IFN-γ and PE anti-IL-17 or APC anti-IL10. Antibodies were purchased from BD PharMingen. Flow cytometry analyses were performed on a FC500 bench top cytometer (Beckman Coulter). Data were evaluated with WinMDI 2.9 software (Scripps Institute).
Data Analysis.
Results are expressed as the mean ± SE. Statistical analysis was performed using the unpaired t test or ANOVAs (StatView, SAS Institute). A value of P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments.
We thank Dr. Avery August and Dr. Mary Ann McDowell for lively discussion. This work was supported by Crohn's and Colitis Foundation of America, Predoctoral Research Award (to M.F.), and National Institutes of Health Grant R01 DK070781 (to M.T.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808700106/DCSupplemental.
References
- 1.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 2.Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood) 2004;229:1136–1142. doi: 10.1177/153537020422901108. [DOI] [PubMed] [Google Scholar]
- 3.Cheroutre H. Starting at the beginning: new perspectives on the biology of mucosal T cells. Annu Rev Immunol. 2004;22:217–246. doi: 10.1146/annurev.immunol.22.012703.104522. [DOI] [PubMed] [Google Scholar]
- 4.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kullberg MC, et al. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med. 2006;203:2485–2494. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yen D, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheroutre H, Lambolez F. Doubting the TCR coreceptor function of CD8alphaalpha. Immunity. 2008;28:149–159. doi: 10.1016/j.immuni.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Das G, et al. An important regulatory role for CD4+CD8 alpha alpha T cells in the intestinal epithelial layer in the prevention of inflammatory bowel disease. Proc Natl Acad Sci USA. 2003;100:5324–5329. doi: 10.1073/pnas.0831037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kreutz M, et al. 1,25-dihydroxyvitamin D3 production and vitamin D3 receptor expression are developmentally regulated during differentiation of human monocytes into macrophages. Blood. 1993;82:1300–1307. [PubMed] [Google Scholar]
- 10.Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. FASEB J. 2001;15:2579–2585. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
- 11.Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem. 2003;89:922–932. doi: 10.1002/jcb.10580. [DOI] [PubMed] [Google Scholar]
- 12.Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000;130:2648–2652. doi: 10.1093/jn/130.11.2648. [DOI] [PubMed] [Google Scholar]
- 13.Froicu M, et al. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol. 2003;17:2386–2392. doi: 10.1210/me.2003-0281. [DOI] [PubMed] [Google Scholar]
- 14.Froicu M, Cantorna MT. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol. 2007;8:5. doi: 10.1186/1471-2172-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrat FJ, et al. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L. A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51:1367–1374. doi: 10.2337/diabetes.51.5.1367. [DOI] [PubMed] [Google Scholar]
- 17.Simmons JD, Mullighan C, Welsh KI, Jewell DP. Vitamin D receptor gene polymorphism: association with Crohn's disease susceptibility. Gut. 2000;47:211–214. doi: 10.1136/gut.47.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dresner-Pollak R, et al. The BsmI vitamin D receptor gene polymorphism is associated with ulcerative colitis in Jewish Ashkenazi patients. Genet Test. 2004;8:417–420. doi: 10.1089/gte.2004.8.417. [DOI] [PubMed] [Google Scholar]
- 19.Hoentjen F, et al. Antibiotics with a selective aerobic or anaerobic spectrum have different therapeutic activities in various regions of the colon in interleukin 10 gene deficient mice. Gut. 2003;52:1721–1727. doi: 10.1136/gut.52.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davidson NJ, et al. T helper cell 1-type CD4+ T cells, but not B cells, mediate colitis in interleukin 10-deficient mice. J Exp Med. 1996;184:241–251. doi: 10.1084/jem.184.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sigmundsdottir H, et al. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–293. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.