Abstract
Differentiation of murine T-helper (Th) 17 cells is induced by antigenic stimulation and the sequential action of the cytokines IL-6, IL-21, and IL-23, along with TGFβ. Current dogma proposes that IL-6 induces IL-21, which, in a STAT3-dependent manner, amplifies its own transcription, contributes to IL-17 production, and, moreover, promotes the expression of the IL-23 receptor. This, in turn, prepares cells for IL-23-mediated stabilization of the Th17 phenotype. Here we demonstrate that these effects of IL-21 on Th17 differentiation are completely dependent on IFN regulatory factor 4 (IRF4). After culturing in the presence of IL-21 plus TGFβ, IRF4-deficient (Irf4−/−) Th cells showed a profound intrinsic defect in IL-17 production and in the autocrine IL-21 loop. Likewise, the levels of IL-23 receptor and the lineage-specific orphan nuclear receptors RORα and RORγt were diminished, whereas the T regulatory (Treg) transcription factor forkhead box P3 (Foxp3) was strongly up-regulated, consistent with the reciprocal relationship between Th17 and Treg development. Despite this loss of IL-21 functions, IL-21-induced STAT3 activation was unimpaired and induced normal Socs3 expression. Forced expression of Foxp3 in WT cells inhibited IL-21-mediated IL-17 production, suggesting that the increase in Foxp3 contributes to the Irf4−/− phenotype. Additionally, the low levels of RORα and RORγt are also partially responsible, because simultaneous overexpression of both proteins restored IL-17 production in Irf4−/− cells to some extent. These data highlight IRF4 as a decisive factor during the IL-21-mediated steps of Th17 development by influencing the balance of Foxp3, RORα, and RORγt.
Keywords: Foxp3, orphan nuclear receptors
Following activation by antigen, the local cytokine milieu drives the differentiation of naïve T-helper (Th) cells into cytokine-secreting effector cells with specialized functions. Depending on their specific cytokine profile, effector Th cells can be classified into four main subsets termed Th1, Th2, Th17, and T regulatory (Treg) cells. Whereas Th1 cells produce IFNγ, which regulates cellular immunity, Th2 cells secrete IL-4, IL-5, and IL-13 to control humoral and anti-parasite immunity. Because of their critical functions in a variety of autoimmune diseases in humans and mice, Th17 and Treg cells have recently received much attention. Th17 cells produce IL-17A, IL-17F, IL-21, and IL-22, which are highly pro-inflammatory and induce severe autoimmunity, e.g., during experimental autoimmune encephalomyelitis (EAE), the mouse model for multiple sclerosis. In contrast, Treg cells control effector T cell responses and suppress autoimmunity (1, 2).
In mice, differentiation of both Th17 and Treg cells requires TGFβ. By itself, this cytokine induces the Treg-specific transcription factor forkhead box P3 (Foxp3) and generates inducible Treg cells (3, 4). Conversely, a combination of TGFβ with IL-6, which blocks TGFβ-driven Foxp3 expression, triggers Th17 development (5–7). Two additional cytokines, IL-21 and IL-23, are also critically involved in the differentiation of Th17 cells (8). IL-21 belongs to the common γ-chain family, signals mainly via STAT3, and is highly expressed by Th cells after stimulation via the TCR in the presence of IL-6 (9, 10). In combination with TGFβ, IL-21 induces and amplifies Th17 development independently of IL-6 (11–13). IL-21 further induces its own expression in an autocrine manner and additionally up-regulates IL-23 receptor (IL-23R) (11–13). Hereby, IL-21 prepares Th cells for the signals provided by IL-23, which facilitates the maintenance and stabilization of the Th17 phenotype (14, 15). Consistent with the reciprocal relationship between Th17 and Treg development, IL-21 inhibits the expression of Foxp3 (11, 13). Thus, IL-21 plays a critical role in a positive feedback loop, which facilitates de novo induction, expansion, and stabilization of differentiating Th17 cells.
The retinoic acid receptor-related orphan receptor (ROR) γt and the recently described RORα are two STAT3-regulated lineage-specific transcription factors that orchestrate the development of Th17 cells. These transcription factors are sufficient to cooperatively augment the expression of IL-17A, IL-17F, and IL-23R (16). Accordingly, loss of function in either RORα or RORγt partially reduces, and combined mutation profoundly impairs, Th17 differentiation in vitro and in vivo (16, 17). Another transcription factor regulated by TCR signal transduction, IFN regulatory factor 4 (IRF4), is also essential for Th17 development (18). IRF4 belongs to the IRF transcription factor family, which is important for type I IFN production, Toll-like receptor signaling, and Th cell differentiation (19, 20). We have previously shown that, apart from its previously reported role during Th2 development (21, 22), IRF4 is absolutely required for IL-6 and TGFβ-mediated Th17 generation in vitro (18). Remarkably, IRF4-deficient (Irf4−/−) mice are totally resistant to EAE, a phenotype accompanied by complete lack of Th17 cells in the brain and spleen. This observation suggests that not only the conventional IL-6- and TGFβ-mediated pathway of Th17 differentiation, but also the alternative IL-21-mediated pathway, might be dependent on IRF4.
In the present study we report that the entire range of IL-21 effects on Th17 development is compromised in Irf4−/− Th cells. This includes IL-21-induced production of IL-17 and IL-21 itself, as well as expression of IL-23R, RORα, and RORγt. As we show, these effects of IRF4 are mediated at least in part by an influence on the balance of Foxp3, RORα, and RORγt.
Results
IL-21-Mediated Th17 Differentiation Is Abolished in Irf4−/− Th Cells.
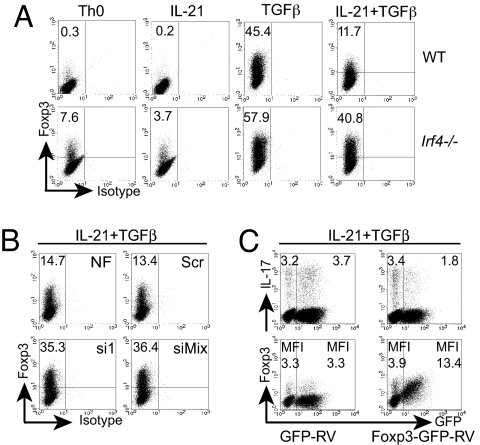
Because IL-21 plays a critical role in IL-6-independent Th17 development (11–13), we compared the responsiveness to IL-21 of purified CD62LhiCD4+ naïve Irf4−/− and WT T cells. The cells were stimulated with plate-bound anti-CD3 and soluble anti-CD28 antibodies in the presence or absence of IL-21 or TGFβ alone or in combination for 3 days. After re-stimulation, IL-17-producing cells were assessed by intracellular staining and flow cytometry. Consistent with previous data (11–13), a high percentage of IL-17-producing WT cells were induced by the combination of IL-21 and TGFβ, whereas IL-21 alone also induced some IL-17-positive cells (Fig. 1A). No such cells were detectable under neutral (i.e., Th0) conditions or in the presence of TGFβ alone. In the absence of IRF4, we observed a complete block in IL-17 production in response to IL-21 or IL-21 plus TGFβ. Apart from an intrinsic function of IRF4 in Th cells, this effect could result from the increased expression of an inhibitory molecule by Irf4−/− Th cells or from the presence of contaminating suppressor cells. To exclude these possibilities, we mixed and co-cultured CD45.2+ Irf4−/− and CD45.2− WT cells in the presence of IL-21 or TGFβ for 3 days. After restimulation, CD45.2 expression and intracellular IL-17 levels were determined. In these mixed cultures, WT cells still produced IL-17 (Fig. 1B), indicating that Irf4−/− cells could not block Th17 differentiation in WT cells and thus arguing against any extrinsic suppressive mechanism involved. Conversely, the presence of WT Th cells could not abolish the Irf4−/− Th cell defect in IL-17 production. These data establish that Irf4−/− Th cells possess an intrinsic defect to differentiate into IL-17 producers in the presence of IL-21 plus TGFβ.
Fig. 1.
IRF4 deficiency abrogates IL-21-mediated Th17 differentiation. (A) Flow cytometry of naïve Irf4−/− or WT CD4+ cells differentiated in the presence of indicated cytokines, re-stimulated, and stained intracellularly by anti-IL-17PE (y axis) or isotypeFITC (x axis). (B) Flow cytometry of naïve CD45.2+/+ Irf4−/− or CD45.2−/− WT CD4+ T cells, which were mixed, differentiated, and stained for CD45.2 (x axis) and IL-17 (y axis). (A and B) the frequencies of IL-17+ cells are indicated. (C and D) Real-time RT-PCR for Il23r, Rora, and Rorc mRNA in naïve Irf4−/− (filled columns) or WT (open columns) CD4+ cells cultured for 48 h under the indicated conditions. The expression level in WT (for Il23r) or Irf4−/− (for Rora and Rorc) cells under Th0 conditions was set to 1. Data (±SD) represent duplicate determinations. Representative data from three experiments are shown.
Recent data indicate that Th17 cells require IL-23 to maintain their pathogenic function during EAE (15). Furthermore, it is known that the expression of Il23r is dependent on IL-21, because IL-21-deficient cells do not express Il23r (12). These considerations and the complete resistance of Irf4−/− mice to the induction of EAE prompted us to analyze Il23r expression in Irf4−/− and WT Th cells. Consistent with previous data (12, 23), real-time RT-PCR measurement revealed robust Il23r mRNA expression in WT Th cells after 48 h of treatment with IL-21 or IL-6 alone or together with TGFβ (Fig. 1C). In striking contrast, Irf4−/− Th cells did not express any Il23r mRNA in response to IL-21 or IL-6 alone, and even when TGFβ was added, the expression of this gene was severely compromised. These data indicate that IRF4 is essential for Il23r expression in Th17 cells.
As already mentioned, IL-17 production and IL-23R expression are both dependent on the lineage-specific transcription factors RORα and RORγt (encoded by Rora and Rorc, respectively) (16, 17). Accordingly, mice doubly deficient in RORα and RORγt function fail to generate a Th17 response and are completely resistant to EAE (16). Because Irf4−/− mice display a very similar phenotype (18), we next determined if these transcription factors are expressed by Irf4−/− Th cells. As expected, the combination of IL-21 or IL-6 plus TGFβ synergistically up-regulated Rora and Rorc mRNA expression in WT Th cells (Fig. 1D). In Irf4−/− Th cells, the mRNA levels of both transcription factors were significantly diminished, although the level of Rorc mRNA was more strongly reduced than that of Rora. This result is consistent with the abolished IL-17 production and greatly compromised Il23r expression observed in Irf4−/− Th cells, and reveals that both Rora and Rorc are expressed in an IRF4-dependent manner.
siRNA-Mediated IRF4 Knock-Down Impairs IL-21-Induced Th17 Differentiation in WT Cells.
The inability of Irf4−/− Th cells to react appropriately to IL-21 could be secondary to developmental alterations occurring in these mice rather than to the direct effect of IRF4 on signaling pathways involved in Th17 cell differentiation. To exclude this possibility, we examined the role of IRF4 in WT cells during IL-21-mediated Th17 differentiation after transient knock-down using siRNA. To achieve this, we nucleofected purified CD4+ WT cells with either one siRNA molecule (si1) or a mix of three different siRNA molecules (siMix) targeting IRF4. As a control, we used scrambled siRNA or we nucleofected cells without siRNA to exclude any unspecific influence of the nucleofection protocol on the development of Th17 cells. Twenty-four hours after nucleofection, immunoblotting was performed, which demonstrated lower IRF4 protein amounts in cells nucleofected with si1 or siMix compared with control cells nucleofected with or without scrambled siRNA (Fig. 2A). Consistent with the IRF4 protein levels, nucleofection with both si1 and siMix caused a significant reduction in the frequency of IL-17-producing cells compared with control nucleofection with or without scrambled siRNA (Fig. 2B). Furthermore, the expression of Rorc mRNA was significantly down-regulated by both types of siRNA against IRF4 (Fig. 2C). These data clearly establish that IRF4 is important for IL-21-mediated Th17 differentiation in WT cells, and that the defect of Irf4−/− Th cells is not secondary to developmental alterations.
Fig. 2.
IRF4 regulates IL-21-mediated Th17 development in WT cells. CD4+ WT cells were nucleofected without any siRNA (NF), with scrambled (Scr) or one (si1) or three different (siMix) siRNA molecules targeting IRF4 and further cultured. (A) Immunoblot of IRF4 and β-actin 24 h after nucleofection. (B) Flow cytometry of nucleofected cells after priming for 72 h with IL-21 plus TGFβ and re-stimulation. The percentages of IL-17+ cells are indicated. (C) Real-time RT-PCR for Rorc mRNA in nucleofected cells 24 h after nucleofection. The expression level in cells nucleofected with si1 was set to 1. Data (±SD) represent duplicate determinations. Representative data from three experiments are shown.
IRF4 Is Essential for IL-21 Expression.
The results presented here show that IRF4 is necessary for the response to IL-21 with respect to Th17 differentiation, but thus far we have not addressed whether IRF4 might be similarly important for the expression of IL-21 itself, which is also a product of cells cultured under Th17 conditions (9, 11–13). IL-21 production can be induced by IL-21 or IL-6 alone or in combination with TGFβ in a STAT3-dependent manner (9, 11–13). In Th cells lacking the function of both RORα and RORγt, but not RORγt alone, IL-21 production was greatly compromised, suggesting that its expression requires the cooperation of both of these transcription factors (16). To analyze the role of IRF4 in IL-21 production, we compared Il21 mRNA expression in Irf4−/− versus WT Th cells in response to IL-21 or IL-6 alone or in combination with TGFβ. IL-21 and IL-6 increased Il21 expression in Th cells from WT mice, but not from Irf4−/− mice, an effect essentially independent of TGFβ (Fig. 3A). Furthermore, transient siRNA knock-down of IRF4 confirmed the essential requirement of IRF4 for Il21 expression in WT cells stimulated with IL-21 and TGFβ (Fig. 3B). Consistent with the mRNA data, IL-21 protein production was also greatly reduced in Irf4−/− cells after stimulation with IL-6 alone or in combination with TGFβ (Fig. 3C). In this setting, we were of course unable to measure the response to IL-21 protein. These data show that IRF4 deficiency gave rise to almost complete loss of Il21 expression in response to IL-21 itself and to IL-6, supporting the finding of greatly diminished levels of Rora and Rorc mRNA.
Fig. 3.
IRF4 is essential for IL-21 production. (a and b) Real-time RT-PCR analysis for Il21 mRNA: (A) in naïve Irf4−/− (filled columns) or WT (open columns) CD4+ cells differentiated for 48 h under the indicated conditions. The expression level in WT cells under Th0 conditions was set to 1. (B) CD4+ WT cells nucleofected and cultured as described in Fig. 2. The expression level tested with si1 RNA was set to 1. (C) ELISA of IL-21 in culture supernatants of naïve Irf4−/− (filled columns) or WT (open columns) CD4+ cells differentiated in the presence of the indicated cytokines. Culture supernatants were collected at the indicated time points. Data (±SD) represent duplicate determinations. Representative data from three experiments are shown.
IL-21-Induced Signaling in Irf4−/− Th Cells.
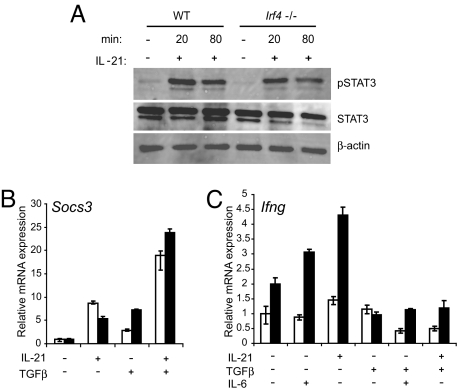
Like other type I cytokines, IL-21 signals via the Jak-STAT pathway and can activate STAT1, STAT3, STAT5a, and STAT5b (24). However, during Th17 development, the activation of STAT3 appears to be most important for IL-21 signaling, because the expression of RORα and RORγt is dependent on STAT3 (9, 16, 25), and consequently, the Th17 developmental program is greatly compromised in STAT3-deficient cells (9, 12, 25). The similarity between the phenotypes of Stat3−/− and Irf4−/− Th cells prompted us to compare IL-21-dependent STAT3 activation in Irf4−/− and WT cells. As shown in Fig. 4A, phosphorylation of STAT3 at tyrosine 705 was equivalent in primed Irf4−/− and WT Th cells after addition of IL-21 for 20 or 80 min. We also examined the possibility that IRF4 might influence STAT3 translocation to the nucleus. Again, we found no differences of Irf4−/− versus WT Th cells here with respect to levels of phosphorylated STAT3 in nuclear extracts after activation with IL-21 plus TGFβ [supporting information (SI) Fig. S1]. Thus, IRF4 is not involved in IL-21-induced STAT3 phosphorylation and nuclear localization.
Fig. 4.
IL-21 induces normal STAT3 activation but increased Ifng expression in Irf4−/− Th cells. (A) Immunoblot of pSTAT3, total STAT3, and β-actin in whole-cell lysates prepared from naïve Irf4−/− or WT CD4+ cells primed for 24 h under Th0 conditions, washed, rested for 2 h, and stimulated with IL-21 for the indicated times. (B and C) Real-time RT-PCR analysis for Socs3 and Ifng mRNA in naïve Irf4−/− (filled columns) or WT (open columns) CD4+ T cells differentiated for 48 h in the presence of the indicated cytokines. The expression level in WT cells under Th0 conditions was set to 1. Data (±SD) represent duplicate determinations. Representative data from three experiments are shown.
As the expression of IL-21 is promoted by nuclear factor of activated T cells cytoplasmic 2 (NFATc2) (26), which interacts with IRF4 (22), we also analyzed whether the absence of IRF4 impairs the translocation of NFATc2 to the nucleus. However, we found comparable amounts of this protein in nuclear extracts of Irf4−/− and WT Th cells (Fig. S2), indicating that the strongly compromised IL-21 production in Irf4−/− cells is not mediated through defective NFATc2 translocation. Consistent with the data in Fig. 4A and Figs. S1 and S2, Il21r expression was not influenced by IRF4 deficiency (Fig. S3).
Because Irf4−/− cells display normal activation of the transcription factors STAT3 and NFATc2 in response to IL-21, but greatly impaired expression of genes directly involved in Th17 development, we asked whether IL-21 is able to influence transcription of other genes in Irf4−/− cells. One of the direct consequences of signaling by many type I cytokines is the “downstream” activation of suppressors of cytokine signaling (SOCS) proteins, and it has been suggested that STAT3 induces the expression of SOCS3 (27). Furthermore, IL-21 induces Socs3 mRNA in CD8+ T cells (28) and macrophages (29). Therefore, we compared Socs3 mRNA expression in Irf4−/− and WT Th cells. Stimulation with IL-21 alone or in combination with TGFβ up-regulated Socs3 mRNA expression to a similar extent in Irf4−/− and WT Th cells (Fig. 4B), illustrating the existence of IL-21-mediated gene induction not influenced by IRF4 deficiency.
Although STAT3 phosphorylation and nuclear localization are normal, the entire IL-21-mediated Th17 developmental program is compromised in Irf4−/− Th cells to a very similar extent as that observed in Stat3−/− Th cells. In Stat3−/− cells, but not in WT cells, stimulation with IL-21 or IL-6 up-regulated the Th1 cytokine IFNγ instead of IL-17 (12). When we analyzed Ifng expression, it became apparent that Irf4−/− Th cells again reacted similarly to Stat3−/− cells, in that IL-21 and IL-6 up-regulated the Th1 cytokine in these cells, but not in WT cells (Fig. 4C). This result shows that Irf4−/− Th cells are not completely unresponsive to IL-21 and IL-6, but change aspects of their transcriptional program in response to these cytokines.
IRF4 Is Involved in IL-21-Mediated Foxp3 Suppression.
IL-21 is important in the developmental relationship between Th17 and Treg cells, and acts by impairing TGFβ-mediated Foxp3 up-regulation. Thereby, IL-21 promotes RORγt function and consequently Th17 cell differentiation (11, 13, 23). Because IL-21-mediated Th17 differentiation was abolished in Irf4−/− cells, we analyzed whether, under these conditions, Foxp3 expression was also affected. Therefore, we stimulated Irf4−/− and WT Th cells, as described earlier, in the presence or absence of IL-21 and/or TGFβ. After 72 h, we re-stimulated the cells and analyzed Foxp3 expression by flow cytometry. As previously described, IL-21 efficiently inhibited TGFβ-induced Foxp3 expression in WT cells (Fig. 5A) (11, 13). In contrast, in Irf4−/− Th cells, IL-21-mediated Foxp3 suppression was minimal. Again, IRF4 reduction by siRNA led to a similar result in WT cells, as shown by up-regulated Foxp3 expression under IL-21 plus TGFβ conditions (Fig. 5B). Thus, IRF4 is required for IL-21-mediated Foxp3 inhibition in WT cells as well.
Fig. 5.
IRF4 is important for IL-21-mediated Foxp3 suppression. Flow cytometry of naïve Irf4−/− or WT CD4+ T cells differentiated as described in Fig. 1A and stained for intracellular Foxp3 (A) and of CD4+ WT cells nucleofected, cultured as described in Fig. 2B, and stained for Foxp3 (B). (A and B) Frequencies of Foxp3+ cells are indicated. (C) CD4+ T WT cells transduced with retroviruses expressing Foxp3-GFP or GFP, treated with IL-21 plus TGFβ, and stained for intracellular IL-17 or Foxp3. (Top) The percentages of GFP−IL-17+ (Left) or GFP+IL-17+ cells (Right) or the mean fluorescence intensity (MFI) of GFP− or GFP+ cells stained for Foxp3 are indicated. Representative data from three experiments are shown.
To analyze whether increased Foxp3 can influence IL-21-mediated IL-17 production, we overexpressed Foxp3 by retroviral transduction in WT cells stimulated with IL-21 plus TGFβ. Compared with infection with control retrovirus, transduction with Foxp3-encoding virus substantially decreased the percentage of IL-17-producing cells (Fig. 5C). By intracellular staining, Foxp3 levels correlated with the relative amount of GFP expression, and GFP-negative cells in this same culture did not exhibit any decrease in IL-17 expression compared with cells transduced with an empty virus, indicating a cell-intrinsic effect of Foxp3. Consistent with a recently published antagonistic effect of Foxp3 on RORγt function (23), our results suggest that increased Foxp3 levels are involved in the massive impairment of IL-21-induced Th17 differentiation in Irf4−/− cells.
RORα and RORγt Deficiency Contributes to the Impaired Th17 Phenotype of Irf4−/− Th Cells.
Previously, we have shown by retroviral transduction that RORγt over-expression only partially restored the IL-6- and TGFβ-induced Th17 phenotype in Irf4−/− cells (18). Because the mRNA levels for RORα and RORγt were both greatly impaired in Irf4−/− cells (Fig. 1D), we examined whether the co-expression of these lineage-specific transcription factors could rescue IL-17 production in these cells. Therefore, naïve Irf4−/− and WT Th cells were co-infected with two types of viruses expressing (i) either RORγt-GFP or GFP or (ii) either RORα-Thy1.1 or Thy1.1. After infection, the cells were cultured either under neutral conditions or with IL-21 plus TGFβ or IL-21 alone. After re-stimulation, IL-17-producing cells were assessed by intracellular staining and analyzed on a GFP+Thy1.1+ gate. Under neutral conditions, RORα or RORγt alone but not the control viruses were sufficient to promote some IL-17 production in WT cells (Fig. 6A). In striking contrast, over-expression of either transcription factor alone induced very low frequencies of IL-17-positive Irf4−/− cells. Co-expression of RORα and RORγt led to significantly enhanced IL-17 production in WT as well as in Irf4−/− cells, although the percentage of IL-17-producing cells was still markedly higher in WT (26.3%) than in Irf4−/− (3.8%) cells (Fig. 6A). Under IL-21 plus TGFβ conditions, the co-expression of RORα and RORγt also enhanced Th17 differentiation in WT and Irf4−/− cells (Fig. 6B), although the percentage of IL-17-positive Irf4−/− cells was lower (1.4%) than after culture of these cells under neutral conditions. This finding was probably caused by the RORα- and RORγt-antagonizing function of Foxp3, which is expressed at a high level under IL-21 plus TGFβ conditions in Irf4−/− cells (Fig. 5A). Indeed, after incubation of co-transduced cells with IL-21 alone, the percentage of IL-17-positive cells was greatly enhanced in WT cultures (65.9%) and much less but still substantially increased in Irf4−/− cultures (6.8%; Fig. 6C). The IL-17 detection was specific because staining with the isotype control was minimal (Fig. 6B).
Fig. 6.
RORα and RORγt co-expression partially restores IL-17 production in Irf4−/− Th cells. Flow cytometry of naïve Irf4−/− or WT CD4+ T cells cotransduced on days 0 and 1 with retroviruses expressing RORα-IRES-Thy1.1 (RORα), RORγt-IRES-GFP (RORγt), or the control empty viruses MSCV-IRES-GFP (MIG) or MSCV-IRES-Thy1.1 (MIT). After transduction, the cells were cultured under the indicated conditions, re-stimulated, and stained intracellularly for IL-17 and extracellularly for Thy1.1. The analyses were performed on a GFP+Thy1.1+ gate and the percentages of IL-17+ are indicated. Representative data from three experiments are shown.
Thus, the combined expression of RORα and RORγt restores the Th17 phenotype in Irf4−/− cells only to a limited extent; therefore, other mechanisms such as the interplay of these factors with Foxp3 are also of relevance.
Discussion
The differentiation of Th17 cells is guided by cytokines derived not only from the environment but also from autocrine production. In the current study, we found that, in the context of Th17 differentiation, IRF4 is absolutely required in a T cell-intrinsic manner for the production of and responsiveness to IL-21. This conclusion is based on the finding that IRF4-deficient Th cells possess a severely compromised reactivity to IL-21 and TGFβ with respect to production of IL-17 and expression of IL-23R, IL-21, and the lineage-specific transcription factors RORα and RORγt. As for IL-17, synchronous overexpression of RORα and RORγt cooperatively rescued its production by Irf4−/− cells to some extent. Thus, the defective expression of these both master regulators is causally linked to the impaired Th17 development in Irf4−/− cells. However, the Th17 phenotype was not completely re-established by co-expression of RORα and RORγt, indicating that additional factors regulated by IRF4 must also be involved. Indeed, we found that IRF4 affects IL-21-mediated Foxp3 downregulation, which normally occurs after culture in IL-21 plus TGFβ (compared with TGFβ alone) and that retroviral overexpression of Foxp3 inhibited IL-21-mediated IL-17 production in WT cells. These results support the described antagonistic effect of Foxp3 on RORγt and RORα function (23, 30), but also point out that IRF4 is another player in this system that acts by balancing the levels of RORα, RORγt, and Foxp3. However, expression of Foxp3, RORα, and RORγt per se is unable to totally explain the devastating effect of IRF4 deficiency on Th17 cell differentiation. That view is supported by the finding that, even under reduced Foxp3 level (i.e., IL-21 conditions), the co-expression of RORα and RORγt did not completely rescue the IL-17 production in Irf4−/− cells. Either posttranslational modification of these factors or the participation of further factors has to be considered.
Although the genetic and biochemical programming of Th17 lineage differentiation in response to IL-6, IL-21, or IL-23 is not well understood, we tried to elucidate the mechanism by which IRF4 regulates the levels of RORα, RORγt, and Foxp3. Because the expression of RORα and RORγt has been shown to be STAT3-dependent (9, 16, 25) and, moreover, the suppression of Foxp3 expression by IL-21 is also at least in part mediated by STAT3 (11), we analyzed activation of STAT3 in Irf4−/− cells. Notably, phosphorylation as well as nuclear translocation of STAT3 in response to IL-21 was normal in Irf4−/− cells, indicating that IRF4 regulates the level of RORα, RORγt, and Foxp3 independently of STAT3. In line with unimpaired STAT3 activation, the induction of Socs3, which has been described to be STAT3-dependent (27), was equivalent to that in WT cells. This result confirmed that IL-21-induced STAT3 activation was not only normal in Irf4−/− cells, but also successfully led to transcription of STAT3-dependent genes. In addition, IL-21 enhanced Ifng expression in Irf4−/− cells but not in WT cells, revealing a change in the IL-21-mediated gene expression pattern in the absence of IRF4. In summary, our results suggest that IRF4 regulates the balance of Th17- and Treg-specific transcription factors independently of STAT3 phosphorylation and nuclear translocation.
Apart from STAT3, NFATc2 was an attractive candidate to explain the effects of IRF4, because (i) IRF4 interacts with NFATc2 (22), (ii) NFATc2 has been shown to regulate the expression of IL-21 (26), and (iii) Irf4−/− cells produce IL-21 at a greatly diminished level. Therefore we analyzed whether the absence of IRF4 influences the nuclear translocation of NFATc2. However, as our data show unimpaired NFATc2 translocation in Irf4−/− cells, the molecular basis of IRF4-mediated regulation of RORα, RORγt, and Foxp3 levels is unclear at this time and requires further investigation.
Finally, the relevance of defective Th17 development for the in vivo situation of the Irf4−/− mouse should be considered. Work from our group has shown that this mouse is completely resistant to disease models which are considered to be strongly dependent on Th17 cells, namely EAE and inflammatory bowel disease (18, 31). In both disease models, IL-23 signals are relevant for a strong disease phenotype (15, 32). Because IL-21 is obligatory for stabilizing IL-23 signals (12, 15) and Irf4−/− cells produce no IL-21 in response to either IL-21 or IL-6, our data strongly suggest that it is the combination of inadequate responses to IL-21 and IL-6 that makes Irf4−/− mice completely resistant to EAE and inflammatory bowel disease. Therefore, the central function of IRF4 in controlling Th17 differentiation may provide new opportunities for developing therapies aimed to treat autoimmune diseases.
Materials and Methods
CD4+ T Cell Purification, In Vitro Stimulation, and Staining.
WT C57BL/6 mice were purchased from the Jackson Laboratory. Irf4−/− mice were bred at the animal facility of the Biomedical Research Center at the University of Marburg, Germany. Naïve CD4+CD62Lhi T cells were prepared by magnetic cell sorting (MACS; Miltenyi Biotec) from spleens and lymph nodes of 8- to 12-week-old WT or Irf4−/− mice and were primed with plate-bound anti-CD3 (5 μg/ml; 145–2C11) and soluble anti-CD28 mAb (1.5 μg/ml; 37.51) in the presence of recombinant human IL-2 (50 U/ml; Novartis), anti-IL-4 (10% culture supernatant of clone 11B11), and anti-IFNγ (5 μg/ml, XMG1.2; i.e., “Th0 conditions”). Some cultures also received 2 ng/ml recombinant human TGFβ1 (R&D Systems), 50 ng/ml recombinant murine IL-6 (Peprotech), 50 ng/ml recombinant murine IL-21 (R&D Systems), or combinations of these stimuli. Seventy-two hours later (unless otherwise indicated), cells were washed and re-stimulated with 50 ng/ml PMA and 750 ng/ml ionomycin in the presence of 10 μg/ml brefeldin A (all from Sigma) for 4 h, after which IL-17-producing cells (anti-IL-17PE; eBio17B7; eBiosciences) were analyzed by intracellular staining. For Foxp3 detection (anti-Foxp3PE; FJK-16s; eBiosciences), the Foxp3 staining kit (eBioscience) was used. CD45.2 or Thy1.1 surface staining was done with anti-CD45.2FITC (104; eBioscience) or anti-Thy1.1PerCP (OX-7; BD) for 15 to 20 min at 4 °C before fixation and intracellular staining. Cells were analyzed with a FACSCalibur system and CellQuest Pro software (BD). IL-21 in culture supernatants was detected by using the mouse IL-21 DuoSet assay (R&D Systems) according to the manufacturer's instructions.
Quantitative Real-Time PCR.
Total RNA was isolated from cell pellets with the High Pure RNA isolation kit (Roche). cDNA was synthesized with oligo(dT) primers using the RevertAid First Strand cDNA Synthesis Kit (MBI Fermentas), and gene expression was examined with an ABI Prism 7700 Sequence Detection System (Applied Biosystems) using the qPCR Core Kit for Il21, Ifng, Socs3, and Hprt1 (hypoxanthine-guanine phosphoribosyl transferase) or the SYBR Green I qPCR core kit (both from Eurogentec) for IL21r, Il23r, Rorc, Rora, and Hprt1. Levels of each gene were normalized to Hprt1 expression using the ΔΔCt method. The primer sets and probes for Ifng, Il21, Il23r, Rora, Rorc, and Socs3 were previously described (7, 12, 16, 18, 33).
Nucleofection.
WT CD4+ T cells were nucleofected with IRF4-specific si1, a mixture of three different IRF4-specific siRNA constructs, or scrambled siRNA. The siRNAs were designed as described (34) and prepared by IBA. Nucleofection was performed as described (33), then the cells were cultured under the conditions indicated in the experiments. The sequences of the siRNAs were previously described (18).
Immunoblotting.
For the phospho-STAT3 (pSTAT3) and IRF4 immunoblots, whole-cell lysates were prepared as described (33). To study pSTAT3 and NFATc2 translocation, nuclear extracts were made as described (35). Proteins were fractionated by SDS/PAGE, transferred to nitrocellulose membrane, immunoblotted with pSTAT3 Tyr-705 (9131; Cell Signaling Technology), IRF4 (M-17; sc6059; Santa Cruz Biotechnology), or NFATc2 (IG209; ImmunoGlobe) antibodies, then re-probed with antibodies to total STAT3 (124H6; 9139; Cell Signaling Technology), Sp3 (D20; sc644; Santa Cruz Biotechnology), or β-actin (Sigma-Aldrich).
Retroviral Transduction.
The retroviral vectors containing Foxp3 and RORγt have been described (4, 17). The gene encoding RORα (GenBank accession number XM_903197) was amplified by PCR with the following primers: forward, 5′-ATAAGAATGCGGCCGCAGGATGTATTTTGTGATCGCAGC-3′; reverse, 5′-ACGCGTCGACGCGACATTTACCCATCG-3′. They were digested by NotI and SalI and cloned into the retroviral vector MSCV-IRES-Thy1.1 (gift from Dr. Heissmeyer, Munich, Germany) containing the internal ribosome entry site (IRES)-regulated gene for mouse Thy1.1. WT CD4+ T cells were infected by retroviruses expressing Foxp3-IRES-GFP or control empty vector (containing IRES-GFP) as described previously (18). Co-infections were performed with retroviruses expressing RORα or its control vector (both with IRES-Thy1.1) and RORγt or its control vector (both with IRES-GFP). After infection, the cells were cultured under the conditions indicated in the experiments. On day 5, the cells were re-stimulated and analyzed for GFP, Thy1.1, IL-17, or Foxp3 expression.
Supplementary Material
Acknowledgments.
We thank G. Duncan and M. Conrad for critical reading of the manuscript. This work was supported by Deutsche Forschungsgemeinschaft Grants LO 396–1, GRK 767, and SFB/TR22.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809077106/DCSupplemental.
References
- 1.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 2.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, et al. Conversion of peripheral CD4+ J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 5.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 6.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Mangan PR, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 8.Ivanov II, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Semin Immunol. 2007;19:409–417. doi: 10.1016/j.smim.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei L, Laurence A, Elias KM, O'Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282:34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 11.Nurieva R, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 12.Zhou L, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 13.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 15.McGeachy MJ, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 16.Yang XO, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 18.Brustle A, et al. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8:958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 19.Tamura T, Yanai H, Savitsky D, Taniguchi T. The IRF Family Transcription Factors in Immunity and Oncogenesis. Annu Rev Immunol. 2008;26:535–584. doi: 10.1146/annurev.immunol.26.021607.090400. [DOI] [PubMed] [Google Scholar]
- 20.Lohoff M, Mak TW. Roles of interferon-regulatory factors in T-helper-cell differentiation. Nat Rev Immunol. 2005;5:125–135. doi: 10.1038/nri1552. [DOI] [PubMed] [Google Scholar]
- 21.Lohoff M, et al. Dysregulated T helper cell differentiation in the absence of interferon regulatory factor 4. Proc Natl Acad Sci USA. 2002;99:11808–11812. doi: 10.1073/pnas.182425099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rengarajan J, et al. Interferon regulatory factor 4 (IRF4) interacts with NFATc2 to modulate interleukin 4 gene expression. J Exp Med. 2002;195:1003–1012. doi: 10.1084/jem.20011128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou L, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng R, et al. The molecular basis of IL-21-mediated proliferation. Blood. 2007;109:4135–4142. doi: 10.1182/blood-2006-10-054973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laurence A, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Mehta DS, Wurster AL, Weinmann AS, Grusby MJ. NFATc2 and T-bet contribute to T-helper-cell-subset-specific regulation of IL-21 expression. Proc Natl Acad Sci USA. 2005;102:2016–2021. doi: 10.1073/pnas.0409512102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28:477–487. doi: 10.1016/j.immuni.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gagnon J, Ramanathan S, Leblanc C, Ilangumaran S. Regulation of IL-21 signaling by suppressor of cytokine signaling-1 (SOCS1) in CD8(+) T lymphocytes. Cell Signal. 2007;19:806–816. doi: 10.1016/j.cellsig.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Ruckert R, Bulfone-Paus S, Brandt K. Interleukin-21 stimulates antigen uptake, protease activity, survival and induction of CD4+ T cell proliferation by murine macrophages. Clin Exp Immunol. 2008;151:487–495. doi: 10.1111/j.1365-2249.2007.03581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang XO, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mudter J, et al. The transcription factor IFN regulatory factor-4 controls experimental colitis in mice via T cell-derived IL-61. J Clin Invest. 2008;118:2415–2426. doi: 10.1172/JCI33227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hue S, et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huber M, et al. IL-27 inhibits the development of regulatory T cells via STAT3. Int Immunol. 2008;20:223–234. doi: 10.1093/intimm/dxm139. [DOI] [PubMed] [Google Scholar]
- 34.Mantei A, et al. siRNA stabilization prolongs gene knockdown in primary T lymphocytes. Eur J Immunol. 2008;38:2616–2625. doi: 10.1002/eji.200738075. [DOI] [PubMed] [Google Scholar]
- 35.Chuvpilo S, et al. Alternative polyadenylation events contribute to the induction of NF-ATc in effector T cells. Immunity. 1999;10:261–269. doi: 10.1016/s1074-7613(00)80026-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.