Abstract
Development of clinically relevant regenerative medicine therapies using human embryonic stem cells (hESCs) requires production of a simple and readily expandable cell population that can be directed to form functional 3D tissue in an in vivo environment. We describe an efficient derivation method and characterization of mesenchymal stem cells (MSCs) from hESCs (hESCd-MSCs) that have multilineage differentiation potential and are capable of producing fat, cartilage, and bone in vitro. Furthermore, we highlight their in vivo survival and commitment to the chondrogenic lineage in a microenvironment comprising chondrocyte-secreted morphogenetic factors and hydrogels. Normal cartilage architecture was established in rat osteochondral defects after treatment with chondrogenically-committed hESCd-MSCs. In view of the limited available cell sources for tissue engineering applications, these embryonic-derived cells show significant potential in musculoskeletal tissue regeneration applications.
Keywords: cartilage, hydrogel, tissue engineering, differentiation
Human embryonic stem cells (hESCs) remain an exciting prospective cell source for regenerative medicine applications because of their strong proliferative potential and multilineage differentiation capability (1, 2). The potential application of these cells has been further enabled by recent advances in creating embryonic-like stem cells from adult skin cells that can easily be harvested from a patient (3, 4). However, the complex orchestra of signals required to control hESC behavior still has not been fully elucidated, particularly for de novo engineering of robust, functional tissues. For translation to a clinical scenario, functional 3D tissues must be formed or maintained in an in vivo environment of tissue loss. Thus, practical use of hESCs depends on the development of simple and efficient protocols to generate “easy to grow” cell populations that can survive in vivo transplantation and produce functional tissue without the risk of tumor formation. By manipulating the culture conditions in which ES cells differentiate, it has been possible to control and restrict the differentiation pathways and thereby generate cultures enriched in lineage-specific precursors (5, 6). In addition, recent advances in ES cell biology have identified relevant mesenchymal progenitor cell populations that can undergo multilineage differentiation into mesenchymal tissues such as cartilage, fat, and bone (5–9). However, strategies for derivation of these progenitors are too complex for practical translation, and limited studies have been performed with ES cells in tissue-specific in vivo environments.
There are a number of approaches to induce tissue formation, including exposure to growth factors, small molecules, and 3D biomaterials (10). In addition to the initial problem of maintaining cell viability after transplantation, controlling differentiation and tissue-forming cues in vivo remains a significant challenge. In the case of cartilage tissue formation, the standard cues to produce tissue from differentiated cells (chondrocytes) or adult stem cells did not translate to the embryonic precursor population (9). Previous evidence supportsthe potential for direct chondrogenic differentiation of stem cells by coculturing with a terminally differentiated chondrocyte cell population (11, 12). The autocrine and paracrine factors secreted by the differentiated cells may readily interact with stem cells to lead a direct and efficient transduction of molecular signals that may result in expression of tissue-specific markers. To generate a more complex instructive microenvironment for cartilage induction, we differentiated mesenchymal stem cells (MSCs) from hESCs (hESCd-MSCs) into the chondrogenic lineage by using morphogenetic factors from chondrocytes (13). Morphogenetic factors from chondrocytes induced long-term survival and commitment of hESCd-MSCs encapsulated in poly-(ethylene-glycol)-based hydrogels in vivo.
In this study, we generated and characterized an embryonic-derived mesenchymal progenitor population without cell sorting, hESCd-MSCs, and demonstrated the capacity for significant cell expansion and multilineage differentiation. Furthermore, to address the challenges and inefficiencies found in current in vivo differentiation protocols for hESCs, we demonstrate that chondrocyte-secreted morphogenetic factors may be a promising method for inducing in vivo commitment and cartilaginous tissue formation for the successful treatment of articular cartilage defects devoid of growth factors or gene transduction. The simplicity and efficiency of these derivation and differentiation strategies enable the potential clinical translation of these cells for numerous musculoskeletal applications, including the regeneration of the articular joint where we implanted human embryonic-derived cells.
Results
Isolation and Characterization of Mesenchymal Precursor Cells from hESCs.
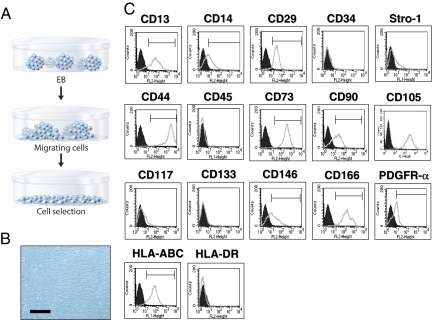
hES-derived MSCs were generated by selecting cells that are migrating from embryoid bodies (EBs). EBs were formed via suspension culture for10 days and plated on gelatin-coated tissue culture plates (Fig. 1A). After 10 days of plating, outgrowth of cells that were sprouted from EBs were mechanically isolated with a cell scraper and subsequently expanded in mesenchymal stem cell (MSC) growth medium (MSCM). After culturing at high density (2 × 104 cells per cm2), cells displayed a fibroblastic morphology (Fig. 1B) and expressed MSC surface markers including CD13, CD14, CD29, CD44, CD73, CD90, CD105, CD146, CD166, Stro-1, and PDGFR-α (Fig. 1C). These cells did not express CD34, CD45, CD117, and CD133. Furthermore, HLA-ABC was expressed, whereas HLA-DR was absent.
Fig. 1.
Isolation and characterization of hESCd-MSCs. (A) EBs were formed from dissociated hESCs via liquid suspension methods for 10 days. EBs were then plated onto gelatin-coated (0.1% wt/vol) tissue culture plates, and migrating cells from EBs were subcultured with MSCM. (B) Inverted light microscopy of hESCd-MSCs pictures fibroblastic cell morphology. (Scale bar = 200 μm.) (C) Flow cytometry analysis of hESCd-MSCs indicates that these cells express MSC surface markers (at passage 7).
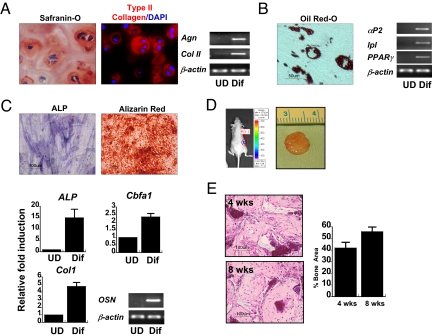
The hESCd-MSCs were functionally characterized by their ability to differentiate into multiple mesenchymal tissues (Fig. 2 A–C). Gene expression and matrix production confirmed that the embryonic-derived cells differentiated toward cartilage, fat, and bone. Cartilage formation was achieved in hydrogels containing adhesion peptides in chondrogenic conditions, resulting in aggrecan and type II collagen production (Fig. 2A). Adipogenic differentiation was validated by the presence of adipocyte fatty acid binding protein 2 (αP2), lipoprotein lipase (lpl), peroxisome proliferator-activated receptor (PPAR-γ) gene expressions, and lipid production observed from Oil red-O staining (Fig. 2B). Finally, to confirm multilineage differentiation capability, osteogenic differentiation was evaluated in vitro by using standard monolayer culture and in vivo in 3D scaffolds after osteogenic stimulation with medium containing β-glycerophosphate and dexamethasone. Strong staining for alkaline phosphatase (ALP) after 7 days and subsequent deposition of calcium after 14 days of monolayer culture indicate that these cells have osteogenic potential (Fig. 2C). Osteogenesis was confirmed by up-regulation of type 1 collagen, osteocalcin, Cbfa1, and ALP genes (Fig. 2C). For evaluation of 3D osteogenic differentiation and in vivo survival of cells, hESCd-MSCS were transduced with lentivirus vector containing a luciferase reporter and seeded on commonly-used biodegradable polyester composite scaffolds containing hydroxyapatite (HA; 5% wt/vol) (14). Detection of luciferase expression in the constructs after 8 weeks of implantation confirmed that the implanted cells survived and maintained vascular connection with host tissue (Fig. 2D). After 8 weeks, neo-bone formation with no evidence of teratoma or marked inflammation around the site of implant was observed in all scaffolds. Histological examination of the implants revealed formation of connective tissue in the scaffold with increasing bone area (Fig. 2E).
Fig. 2.
Developmental potential of hESCd-MSCs. (A) Chondrogenic differentiation in RGD-modified PEG hydrogels with TGF-β1 and ascorbic acid. Safranin-O staining (red) for negatively charged proteoglycans and type II collagen (type II collagen = red, nucleus = blue) in the pericellular signifies cartilaginous matrix production. RT-PCR confirmed chondrogenic differentiation of hESCd-MSCs with up-regulation of aggrecan and type II collagen. (B) The clusters of lipid-containing adipocytes were also detected by Oil red-O staining within 3 weeks of culture in adipogenic medium. Adipogenic differentiation was confirmed by the expression of αP2, lpl, and PPAR-γ by RT-PCR. (C) Bone-specific ALP was detected after 7 days, and mineralized deposits were evident with the alizarin red staining after 2 weeks of culture in osteo-inductive conditions. Osteogenic differentiation was confirmed by up-regulation of ALP, Cbfa1, and type I collagen by real-time PCR, and the expression of osteocalcin was confirmed by RT-PCR. (D) Gross image of in vivo-engineered bone tissues after 8 weeks of implantation. Microvascular perfusion and implant survival was assessed by luciferase detection of recombinant lentiviral vector-transduced hESCd-MSCs after 8 weeks of implantation. (E) H&E staining of the implanted scaffolds after 4 and 8 weeks. Histomophometric assessments showed increased bone area from 4 to 8 weeks (n = 4). UD, undifferentiated hESCd-MSCs; Dif, differentiated hESCd-MSCs.
To further characterize the hESCd-MSCs, we performed genomewide expression analysis using oligonucleotide arrays (Affymetrix U133A). The expression profiles of hESCd-MSCs were compared with those of human adult bone marrow-derived MSCs. The analysis of 24,000 genes showed an up-regulation of 318 transcripts and down-regulation of 321 transcripts compared with that of adult MSCs (Dataset S1). Genomewide pathway analysis indicated that hESCd-MSCs have higher transcriptional activity of epithelial-mesenchymal transformation (EMT), matrix metalloproteinase, and reck pathways compared with hMSCs (Fig. S1). These results demonstrate the extracellular matrix remodeling potential of the hESC-derived cells. Moreover, expression of genes known to affect cell proliferation, such as cyclin D2, was up-regulated compared with adult MSCs. However, the large number of relative expression and shared transcript profiles along with morphological and surface markers suggests that the hESCd-MSCs and adult MSCs are highly comparable.
Chondrocyte-Conditioned Medium (C-MSCM) for in Vitro hESCd-MSC Chondrogenesis.
Our previous studies indicated that morphogenetic factors from chondrocytes can be used to direct the differentiation of adult MSCs (13). We investigated whether C-MSCM also has the potential to provide the complex signaling required to direct the commitment of hESCd-MSCs into a chondrocytic lineage and promote generation of functional 3D tissues with therapeutic relevance. hESCd-MSCs cultured in C-MSCM consistently stained positive for type II collagen, which was not detectible in cells cultured in control growth medium (Fig. 3A). Real-time PCR confirmed that the C-MSCM-expanded hESCd-MSCs up-regulated type II collagen expression (Fig. 3B). We further compared the mRNA expression levels of cartilage-specific markers during 10 days of expansion (Fig. 3B). Real-time PCR revealed that the C-MSCM-expanded hESCd-MSCs displayed similar levels of type I collagen and Cbfa1 gene expressions compared with that of control medium expanded cells. However, aggrecan, type II collagen, Sox-9, and link protein gene expression was increased markedly in C-MSCM-expanded hESCd-MSCs, whereas MSCM did not elicit up-regulation of these genes. These results indicate that C-MSCM induced hESC-derived mesenchymal cells to acquire expression of markers associated with the chondrocyte phenotype.
Fig. 3.
Effects of C-MSCM on hESCd-MSCs in vitro. (A) Type I collagen was detected in hESCd-MSCs, and upon expansion of C-MSCM, hESCd-MSCs displayed type II collagen. (Scale bars = 20 μm.) (B) Real-time PCR analysis of hESCd-MSCs in MSCM (○) or C-MSCM (□). Expression of cartilage-specific markers was analyzed for 10 days and normalized to day 1 control.
In Vivo Response of C-MSCM on hESCd-MSC Chondrogenesis.
To investigate whether conditioned medium-expanded cells could be translated to regenerative medicine applications, where survival and maintenance of a stable chondrocytic phenotype in vivo is required, cells were encapsulated in PEG-based hydrogels and subsequently transplanted s.c. into athymic mice. After 12 weeks, gross morphology of C-MSCM-expanded constructs was opaque, suggesting tissue formation, whereas control hydrogels were transparent (Fig. 4A). We did not observe any gross or histological evidence of teratoma formation after 12 weeks in any of the constructs. Histological sections of C-MSCM-expanded constructs exhibited structures typical of neocartilage, including ovoid cells in lacunae surrounded by a dense extracellular matrix (Fig. 4B). The tissue displayed a basophilic ECM characteristic of neo-cartilage, which also included type II collagen (Fig. 4C). To further quantify these morphological observations, the C-MSCM-expanded cells exhibited greater biochemical content and increased mechanical properties compared with controls (Fig. 4 D–G).
Fig. 4.
Chondrogenic differentiation potential of hESCd-MSCs in vivo. Control medium-expanded or conditioned medium-expanded hESCd-MSCs were encapsulated in PEGDA hydrogels and subsequently implanted into the s.c. space of an athymic mouse. (A) Gross image of hydrogels after 12 weeks of implantation. Safranin-O staining and immunostaining for extracellular matrix proteins produced by hESCd-MSCs after 12 weeks of implantation indicated that unstimulated hESCd-MSCs lacked differentiation potential. (B and C) Conditioned medium-expanded hESCd-MSCs produced cartilage-like tissue as indicated by Safranin-O staining (B) and immunostaining for cartilage-specific extracellular matrix molecules (C). (D–G) C-MSCM-expanded hESCd-MSCs resulted in engineered cartilage with enhanced mechanical strength and higher ECM contents. *, P < 0.05; **, P < 0.01. (Scale bars = 100 μm.)
Ultrastructural analysis (transmission electron microscopy) also supported the requirement for exposure to a cell-derived microenvironment to induce hESCd-MSC chondrogenesis. Conditioned medium-expanded cells displayed a cell morphology characteristic of chondrocytes, surrounded by significant ECM (Fig. 5 A–G). A large spherical nucleus and mitochondria were observed in the cytoplasm. In addition, fibrillar, collagenous-like molecules appeared to be closely associated with the chondrocyte plasma membrane. Cells also exhibited many short filopodia projecting into the surrounding ECM (Fig. 5 D and F) with abundant intercellular ECM (Fig. 5 C and E). In contrast, control cells displayed a cellular phenotype characteristic of apoptotic cells (Fig. 5H). In addition, no ECM was detected in the pericellular regions of the control cells (Fig. 5 I and J).
Fig. 5.
Electron micrographs demonstrated marked differences in cell size, shape, and organization of pericellular extracellular matrix after 12 weeks in vivo. (A–G) hESCd-MSCs expanded in C-MSCM maintained round chondrocyte morphology with the collagen matrix assembled in the pericellular region. (H–J) However, irregular cellular morphology reminiscent of apoptotic cells was observed in the hESCd-MSCs expanded in control medium (H) without any detectable extracellular matrix in the pericellular region (I–J) (n = nucleus, h = hydrogel). (K and L) TUNEL assay confirmed the presence of more apoptotic cells in the hESCd-MSCs expanded in control medium compared with that of C-MSCM. (Scale bars: A, F–I = 6 μm; B, D, E, J = 1 μm; C = 500 nm; K = 50 μm.)
Viability of cells in the hydrogels was confirmed by TUNEL assay (Fig. 5K). Most of the cells exposed to chondrocyte-derived morphogenetic factors maintained survival after 12 weeks, with 25% of cells positive in TUNEL staining (Fig. 5L). However, control cells displayed 97% cell death. These results confirm that the morphogenetic factors from chondrocytes induced long-term in vivo survival and commitment to the chondrocytic phenotype after transplantation.
Repair of Cartilage Defects by Transplantation of hESCd-MSCs.
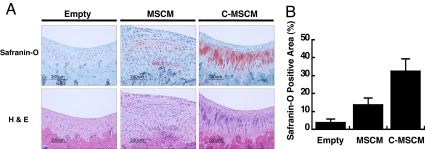
Cartilage tissue formation and repair of critical size defects by hESCd-MSCs in tissue-specific sites was evaluated in a knee joint. Articular cartilage defects (2 mm) were created in the femoropatellar groove of athymic rats. Cells (1 × 106) were pelleted, cultured for 3 days, and transplanted into the critical size defect site. The defects that received conditioned medium-expanded cells demonstrated restoration of a normal condylar appearance after 8 weeks. Gross examination of defects suggested that conditioned medium-expanded hESCd-MSCs contributed to the full repair of the cartilage defects to the original tissue volume (Fig. 6A). Histological evaluation of the treated joints revealed a smooth articular surface and no visible border with the surrounding normal articular cartilage. Architecture of the repaired articular cartilage was similar to that of normal cartilage with respect to cell number and organization. Transplantation of hESCd-MSCs increased the overall percentage of tissue staining for glycosaminoglycans, as determined by histomorphometry, compared with untreated defects (Fig. 6B). Acellular or untreated defects were filled with a fibrocartilage typical of the dysfunctional repair response observed clinically. Histologically, C-MSCM-expanded cells displayed a greater area of tissue staining positive for Safranin-O and reestablished a normal tissue architecture compared with MSCM-expanded hESCd-MSCs.
Fig. 6.
Osteochondral defects created in a rat knee (femorapatellar groove) repaired with hESCd-MSC pellets 8 weeks after transplantation. (A) Defects implanted with cell pellets generated articular cartilage. (B) Histomorphometric analysis of quantified Safranin-O positive area (n = 6). *, P < 0.05; **, P < 0.01.
Discussion
Recent progress in tissue engineering has focused on the possibility of using hESCs, in conjunction with 3D scaffolds, to engineer specialized tissues (9, 15, 16). The rational for using hESC-derived cells is based on their ability to provide large numbers of specialized cell types for regenerative medicine. However, 1 requirement for this application is that differentiated or tissue-restricted cells must be isolated from the hES cells before they can be used safely and effectively in clinical application (5, 7, 8). Furthermore, successful use of hESC-derived cells for clinical application in regenerative medicine requires tight control of the cell differentiation process, resulting in a homogenous population of therapeutically-relevant cell types. Major aspects that need to be taken into consideration for stem cell-based tissue engineering are (i) the mechanisms to deliver the cells to a site of injury, (ii) incorporation of factors that induce differentiation, and (iii) the ability to maintain differentiated phenotype and constitute functional tissues in vivo.
MSCs are defined as cells that self-renew and are able to give rise to multiple mesenchymal tissues. Adult MSCs are currently in clinical trials for orthopedic and cardiac applications. However, isolating bone marrow-derived MSCs requires invasive surgical procedures for harvesting marrow, and the resulting cells may have limited or variable ability to proliferate and differentiate into functional mesenchymal tissues because of the age of the donor and culture time (17, 18). hMSCs are being applied allogeneically in a clinical setting today, but it is unclear whether embryonic-derived MSCs would have similar immunological properties. Although cartilage and the joint space is often considered immunopriviledged, the recent advances in inducing a pluripotent state in adult cells to create patient-specific ES cells alleviates many of the challenges related to an allogeneic cell therapy (3). Barberi et al. (5) and Olivier et al. (8) reported the derivation of MSCs from hESCs; however, their methods were limited by the requirement of coculture with OP cells (mouse stromal cells, which may have resulted in contamination with mouse antigens) and limited bipotent differentiation potential, respectively). Recently, Barberi et al. (6) bypassed the need for coculture to derive mesenchymal cells but used FACS-mediated isolation of CD73+ cells. Lian et al. (7) also demonstrated clinically compliant MSCs from hESCs; however, they did not demonstrate functional cartilage tissue formation in vivo. Our approach to generating mesenchymal precursor cells from differentiating EBs is based on the observation of enhanced chondrogenic condensation from cells migrating out of EBs (19, 20). Furthermore, without requiring FACS-mediated isolation, we produced a mesenchymal cell population where all cells expressed CD73+.
In the embryo, epithelial-mesenchymal transition (EMT) occurs in a population of epithelial cells that gives rise to mesenchymal cells. Our 1-way signaling pathway comparison with human MSCs showed activation of EMT in the hESCd-MSCs (Fig. S1), which indicates the possibility of cell selection and transformation into a mesenchymal cell phenotype because of the culture conditions used in this study. An important component of the EMT pathway is its involvement in activation of key transcription factors, which may regulate expression of genes that sustain the mesenchymal cell phenotype (21). Cell plating density played a significant role in controlling cell morphology and proliferation. Subculturing at a relatively high cell density resulted in a near homogeneous population of cells expressing MSC surface markers (2 × 104 cells per cm2) and maintained multilineage differentiation potential even after 60 population doublings. However, a relatively low plating density (<1 × 103 cells per cm2) resulted in a slower proliferation rate and a heterotypic cellular morphology. The hESCd-MSCs expressed significantly higher levels of proliferation-related genes compared with hMSCs and secreted greater amounts of calcium compared with hMSCs during osteogenic differentiation (Fig. S1). The greater proliferative capacity of hESCd-MSCs compared with human MSCs, homogenous tissue production, and the lack of teratoma formation in vivo highlight their significant potential for tissue engineering and regenerative medicine applications.
Articular cartilage cannot repair when damaged and is therefore an interesting target for developing new repair strategies. Hydrogels can serve as the delivery and encapsulation device for in vivo s.c. implantation of hESCd-MSCs. These materials are ideal for nonadhesion-dependent cell types such as chondrocytes and are amenable to minimally invasive injection into the joint environment. A hydrogel was not used for implanting cells in the rat because of the small size of the critical defect in this animal model. However, a biomaterial is useful for maintaining cells in larger tissue defects. Maintaining stable cell lineage commitment in vivo is also a significant challenge in tissue engineering with stem cells. Previous studies have even indicated that in vitro predifferentiation is not sufficient to guarantee stable lineage commitment and differentiation in vivo (22, 23). Indeed, the cartilage-like phenotype induced in vitro was not stable in vivo environment (23). Our results demonstrate that morphogenetic factors from chondrocytes were sufficient to induce a stable phenotype in 3D hydrogels and repair cartilage defects. Transplanted cells were viable even after long-term in vivo culture, and homogenous cartilage-like tissue was present throughout the hydrogels. However, nondifferentiated hESCd-MSCs produced no detectable ECM, and most of the cells displayed an apoptotic morphology, which was further supported by TUNEL assay. Because a similar number of cells was encapsulated in all groups, the increased cell number and reduced apoptosis is likely caused by the extracellular matrix that is produced around the cells that are undergoing chondrogenesis. The PEG-diacrylate (PEGDA) hydrogel used in our study is bio-inert and does not provide the anchorage sites that are required by most cells for survival but are not required by chondrocytes (24). Nuttelman et al. (25) demonstrated low cell viability of hMSCs in PEGDA hydrogels devoid of cell–matrix interaction. Our data indicate that chondrocyte-secreted factors induced the differentiation of hESCd-MSCs into chondrocytic cells. This differentiation promoted the secretion of ECM to which chondrocytes must bind to survive in the PEGDA hydrogels and ultimately produce tissue. This method of using chondrocyte-secreted factors may provide an efficient methodology for inducing commitment of stem cells and generate clinically applicable hyaline cartilage. It still remains to be answered which specific components or combination of the chondrocyte-secreted factors stimulated chondrogenic differentiation of stem cells. Similar to hESCd-MSCs, adult hMSCs expanded in conditioned medium also displayed basophilic ECM characteristic of neocartilage and were viable in vivo (Fig. S2).
In the present work, we demonstrated direct commitment of the hESCd-MSCs for cartilage repair by using morphogenetic factors from chondrocytes. Unlike conventional hESC differentiation protocols requiring growth factors, coculture, or genetic manipulation, our methodology uses culture techniques to derive a mesenchymal cell population from hESCs. These developments have enabled microenvironment-dependent commitment and efficient cartilage tissue formation from hESCd-MSCs in clinically relevant in vivo environments.
Materials and Methods
Cell Culture and Mesenchymal Cell Derivation.
hESCs (Hues9) were cultured as reported (26). To generate mesenchymal cell lines, hESCs were dissociated into small clumps by incubating at 37 °C for 30 min with 1 mg/ml collagenase IV (GIBCO) and cultured to form EBs for 10 days. The EBs were transferred onto gelatin (0.1% wt/vol)-coated plates and cultured for 10 additional days until the outgrowth from the EBs occurred as described (9). The outgrowth cells were selectively isolated by using cell scrapers and subcultured at an initial cell density of 2 × 104 cells per cm2 in MSCM consisting of DMEM (GIBCO) supplemented with 10% FBS (HyClone), 2 mM l-glutamine (GIBCO), 100 units/ml of penicillin, and 100 μg/ml of streptomycin (GIBCO). For C-MSCM, DMEM was incubated with primary bovine chondrocytes for 48 h, passed through a 0.22-μm filter, and supplemented with 10% FBS, 2 mM l-glutamine, 100 units/ml of penicillin, and 100 μg/ml of streptomycin. In vitro chondrogenic differentiation was induced in arginine-glycine-aspartic acid (RGD)-modified PEG-based hydrogels as described (9). For adipogenic differentiation, cells were exposed to adipogenic induction medium (1 μM dexamethasone, 200 μM indomethacin, 10 μg/ml insulin, and 0.5 mM methylisobutylxanthine; Cambrex Biosciences) for 3 weeks. For osteogenic differentiation, cells were cultured in osteogenic differentiation medium (50 μM ascorbic acid-2-phosphate, 10 mM β-glycerophosphate, and 100 nM dexamethasone) for 2 weeks.
Chemical Staining.
To stain lipids, cells were fixed in 10% formalin, rinsed with deionized (DI) water and then 60% isopropanol, stained with 30 mg/mL of Oil red O (Sigma) in 60% isopropanol, and washed in DI water. Calcium deposition was detected by Alizarin red S (Sigma) following the manufacturer's instructions. ALP was stained by using Sigma kit 85 following the manufacturer's instructions.
Flow Cytometry.
hESCd-MSCs (P7) were harvested and resuspended to ≈3 × 105 cells in 50 μL of PBS containing 0.1% BSA. Cells were separately labeled with FITC-conjugated rat anti-human antigen CD105, CD45, HLA-ABC (Becton Dickinson), phycoerythrin (PE)-conjugated rat anti-human CD13, CD29, CD73, CD146, CD90, CD166, CD34, CD44, PDGFR-α, HLA-DR (Pharmingen), CD117 (Biolegends) CD133 (Miltenyi Biotec), and CD14 (Chemicon) on ice for 30 min. Cells were also incubated with purified mouse anti-human Stro-1 (Becton Dickinson) and then stained with FITC-conjugated goat anti-mouse IgM (Jackson ImmunoResearch Laboratories). An isotype-matched mAb was used as a control (Becton Dickinson). Data were analyzed with the FACSCalibur cytometer and CellQuest software (Becton Dickinson).
RT-PCR and Real-Time PCR.
Total RNA was extracted with TRIzol and reverse-transcribed into cDNA by using the SuperScript First-Strand Synthesis System (Invitrogen). RT-PCR and real-time PCR were performed as previously described (27). The PCR primers are listed in Table S1.
Cell Staining, Histology, and Immunostaining.
For histological analysis, tissue samples were fixed in 4% paraformaldehyde, dehydrated in serial ethanol dilutions, and paraffin-embedded. Tissue constructs were cut into 5-μm sections and stained with hematoxylin and eosin or Safranin-O/fast green. For immunostaining, sections were blocked with 5% normal goat serum in PBS for 30 min and incubated with rabbit polyclonal antibodies against types I or II collagen (RDI) with 1:100 dilutions. Sections were incubated with either FITC- or Texas red-conjugated goat anti-rabbit secondary antibody (all 1:100 dilutions) for 1 h (Jackson ImmunoResearch Laboratory). Nuclei were counterstained with DAPI (Chemicon) for 10 min, and images were collected on a Zeiss LSM Metal Confocal microscope. TUNEL was used for detecting apoptotic cells. H&E-stained sections were used for quantification of bone formation.
Biochemical Assays and Mechanical Property Characterization.
The DNA content of tissue constructs was determined by Hoeschst 33258 dye assay as described (27). The GAG content was quantified by using dimethylene blue spectrophotometric assays at A525 as described (27). Total collagen content was determined by measuring the hydroxyproline content of the constructs after acid hydrolysis and reaction with p-dimethylaminobenzaldehyde and chloramine-T as described (27). Mechanical characterization of transplanted hydrogels was performed with an Instron Universal Testing Apparatus (n = 6).
Electron Microscopy.
Implants were retrieved, fixed for 1 h in 2.5% (wt/vol) gluteraldehyde, 3% (wt/vol) paraformaldehyde, and 2.5% (wt/vol) sucrose in 0.1 M sodium cacodylate buffer (pH 7.4) and then postfixed in 1% (wt/vol) OsO4 in veronal-acetate buffer for 1 h. The cells were stained en block overnight with Kellenberger uranyl acetate (pH 6.0), dehydrated, and embedded in Epon resin. Sections were cut on a Leica ULTRACUT UCT ultramicrotome, poststained in uranyl acetate, and observed in a Philips EM420 transmission electron microscope.
Photoencapsulation and Transplantation.
hESCd-MSCs expanded in C-MSCM or MSCM were photo-encapsulated into 10% (wt/vol) PEGDA hydrogels with a concentration of 2 × 107 cells per ml as described (13). The constructs were then implanted s.c. into the dorsal region of 6- to 8-week-old athymic mice for 12 weeks. For articular defect transplantation, conditioned medium or control-expanded hESCd-MSCs (1 × 106 cells) were pellet-cultured for 3 days and transplanted into articular cartilage defects created on the femoral condyle (≈2-mm defect) of athymic nude rats and sealed with fibrin glue (n = 6). After 8 weeks, the femoral condyle was removed and processed according to standard histological analysis. Histomorphometric analysis was used to quantify the area of tissue on the condyle that stained positive for glycosaminoglycans.
Ectopic Bone Formation and Luciferase Detection.
Recombinant lentiviral vector, with the EF-1α promoter driving the luciferase gene and a GFP reporter controlled by the IRES element, was kindly provided by Linzhao Cheng, Johns Hopkins University School of Medicine and transduced as described at passage 2 (28). Transduced hESCd-MSCs were expanded (P9), seeded (3 × 106) onto the polymer scaffold, and cultured for 10 days in osteogenic condition. Polymer scaffold composed of poly(l-lactic acid)/poly(lactide-co-glycolide) (PLLA/PLGA) (1:1) was fabricated as described (15) with HA particles (5% wt/vol). The cell-seeded scaffolds were then implanted s.c. into the dorsal region of 6-week-old athymic nude mice. For luciferase detection, mice were anesthetized after 8 weeks by using an i.m. injection of ketamine and xylazine. Luciferin (200 μL of 5 mg/mL) was injected, and animals were imaged with a Xenogen IVIS device.
Statistical Analysis.
Data are expressed as mean ± SD. Statistical significance was determined by ANOVA single factor.
Supplementary Material
Acknowledgments.
We thank J. M. McCaffery and E. Perkins of the Integrated Imaging Facility (Johns Hopkins University Department of Biology) for help with confocal microscopy and electron microscopy and K. Konstantopoulos (Johns Hopkins University Department of Chemical and Biomolecular Engineering) for flow cytometer access. This work was supported by the Johns Hopkins University–Technion Joint Program and the Whitaker Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809680106/DCSupplemental.
References
- 1.Lerou PH, Daley GQ. Therapeutic potential of embryonic stem cells. Blood Rev. 2005;19:321–331. doi: 10.1016/j.blre.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 4.Park IH, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barberi T, Willis LM, Socci ND, Studer L. Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med. 2005;2:e161. doi: 10.1371/journal.pmed.0020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barberi T, et al. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med. 2007;13:642–648. doi: 10.1038/nm1533. [DOI] [PubMed] [Google Scholar]
- 7.Lian Q, et al. Derivation of clinically compliant MSCs from CD105+, CD24− differentiated human ESCs. Stem Cells. 2007;25:425–436. doi: 10.1634/stemcells.2006-0420. [DOI] [PubMed] [Google Scholar]
- 8.Olivier EN, Rybicki AC, Bouhassira EE. Differentiation of human embryonic stem cells into bipotent mesenchymal stem cells. Stem Cells. 2006;24:1914–1922. doi: 10.1634/stemcells.2005-0648. [DOI] [PubMed] [Google Scholar]
- 9.Hwang NS, Varghese S, Zhang Z, Elisseeff J. Chondrogenic differentiation of human embryonic stem cell-derived cells in arginine-glycine-aspartate-modified hydrogels. Tissue Eng. 2006;12:2695–2706. doi: 10.1089/ten.2006.12.2695. [DOI] [PubMed] [Google Scholar]
- 10.Hwang NS, Varghese S, Elisseeff J. Controlled differentiation of stem cells. Adv Drug Deliv Rev. 2008;60:199–214. doi: 10.1016/j.addr.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vats A, et al. Chondrogenic differentiation of human embryonic stem cells: The effect of the microenvironment. Tissue Eng. 2006;12:1687–1697. doi: 10.1089/ten.2006.12.1687. [DOI] [PubMed] [Google Scholar]
- 12.Hwang NS, Varghese S, Elisseeff J. Derivation of chondrogenically-committed cells from human embryonic cells for cartilage tissue regeneration. PLoS ONE. 2008;3:e2498. doi: 10.1371/journal.pone.0002498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang NS, Varghese S, Puleo C, Zhang Z, Elisseeff J. Morphogenetic signals from chondrocytes promote chondrogenic and osteogenic differentiation of mesenchymal stem cells. J Cell Physiol. 2007;212:281–284. doi: 10.1002/jcp.21052. [DOI] [PubMed] [Google Scholar]
- 14.Cowan CM, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560–567. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 15.Levenberg S, et al. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci USA. 2003;100:12741–12746. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levenberg S, et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879–884. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 17.Fehrer C, Lepperdinger G. Mesenchymal stem cell aging. Exp Gerontol. 2005;40:926–930. doi: 10.1016/j.exger.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Stolzing A, Scutt A. Age-related impairment of mesenchymal progenitor cell function. Aging Cell. 2006;5:213–224. doi: 10.1111/j.1474-9726.2006.00213.x. [DOI] [PubMed] [Google Scholar]
- 19.Kramer J, et al. Embryonic stem cell-derived chondrogenic differentiation in vitro: Activation by BMP-2 and BMP-4. Mech Dev. 2000;92:193–205. doi: 10.1016/s0925-4773(99)00339-1. [DOI] [PubMed] [Google Scholar]
- 20.Hegert C, et al. Differentiation plasticity of chondrocytes derived from mouse embryonic stem cells. J Cell Sci. 2002;115:4617–4628. doi: 10.1242/jcs.00171. [DOI] [PubMed] [Google Scholar]
- 21.Radisky DC. Epithelial-mesenchymal transition. J Cell Sci. 2005;118:4325–4326. doi: 10.1242/jcs.02552. [DOI] [PubMed] [Google Scholar]
- 22.De Bari C, Dell'Accio F, Luyten FP. Human periosteum-derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis Rheum. 2001;44:85–95. doi: 10.1002/1529-0131(200101)44:1<85::AID-ANR12>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 23.Pelttari K, et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254–3266. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- 24.Lee HJ, et al. Collagen mimetic peptide-conjugated photopolymerizable PEG hydrogel. Biomaterials. 2006;27:5268–5276. doi: 10.1016/j.biomaterials.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Nuttelman CR, Tripodi MC, Anseth KS. Synthetic hydrogel niches that promote hMSC viability. Matrix Biol. 2005;24:208–218. doi: 10.1016/j.matbio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Cowan CA, et al. Derivation of embryonic stem-cell lines from human blastocysts. N Engl J Med. 2004;350:1353–1356. doi: 10.1056/NEJMsr040330. [DOI] [PubMed] [Google Scholar]
- 27.Hwang NS, et al. Effects of three-dimensional culture and growth factors on the chondrogenic differentiation of murine embryonic stem cells. Stem Cells. 2006;24:284–291. doi: 10.1634/stemcells.2005-0024. [DOI] [PubMed] [Google Scholar]
- 28.Cui Y, et al. Targeting transgene expression to antigen-presenting cells derived from lentivirus-transduced engrafting human hematopoietic stem/progenitor cells. Blood. 2002;99:399–408. doi: 10.1182/blood.v99.2.399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.