Abstract
Patterns of behavior exhibited by mice in their home cages reflect the function and interaction of numerous behavioral and physiological systems. Detailed assessment of these patterns thus has the potential to provide a powerful tool for understanding basic aspects of behavioral regulation and their perturbation by disease processes. However, the capacity to identify and examine these patterns in terms of their discrete levels of organization across diverse behaviors has been difficult to achieve and automate. Here, we describe an automated approach for the quantitative characterization of fundamental behavioral elements and their patterns in the freely behaving mouse. We demonstrate the utility of this approach by identifying unique features of home cage behavioral structure and changes in distinct levels of behavioral organization in mice with single gene mutations altering energy balance. The robust, automated, reproducible quantification of mouse home cage behavioral structure detailed here should have wide applicability for the study of mammalian physiology, behavior, and disease.
Keywords: circadian, ingestion, obesity, phenotyping
Molecular genetic approaches for manipulating gene expression and neural activity in mice, combined with the elucidation of the mouse genome, provide unprecedented opportunities for the investigation of diverse behavioral processes in the context of a mammalian system. While substantial insights have been gained through the application of existing behavioral assays, many of these examine behavior over a limited time window and focus on a single behavioral domain (1, 2). To complement such approaches, we developed an automated, readily-standardized quantitative approach for elucidating the complex organization of diverse behaviors exhibited by mice in their home cages.
We focused on mice in their home cages because the organization of behavior in freely acting animals provides a window into the central integration of numerous behavioral and physiological systems (e.g., energy balance, thermal status, osmotic/volume status, sleep, reproduction, defense, and environmental entrainment). The functions and interactions of these systems result in the coordinated organization of multiple behaviors (3–5). Although several sophisticated approaches for automated behavioral data collection and home cage monitoring exist, they do not employ algorithms that quantitatively capture the rich temporal and spatial structure of diverse behaviors that occur over multiple time scales (1, 6–19).
As a first step in examining this structure, we made use of the observation that in natural environments animals typically alternate between two major discrete states, active and inactive (20–22). During active states (ASs), animals engage in behaviors such as foraging and patrolling within a regularly traversed home range. During inactive states (ISs), animals return to a refuge (nest, burrow, or home base) and engage in behaviors such as rest and sleep (23–26). These two states are thus distinct in terms of their spatial properties, emitted behaviors, energetic costs, and risks of predation (22, 27). Because transitions between these states are critical to the fitness of animals, we predicted that state (AS/IS) regulation would shape home cage behavior.
The capacity to identify ASs and ISs also provided a foundation for evaluating behavioral patterns over shorter time scales. Within ASs, episodes of feeding and drinking appear to be clustered into discrete bouts. However, robust automated quantification of such bouts has been difficult to achieve (28–32). We addressed this by developing an approach for intake bout identification that integrates both temporal and spatial information. In addition, we developed a supervised learning algorithm that identifies bouts of home cage locomotion. We anticipated that the simultaneous quantification of multiple levels of behavioral organization encompassing multiple behaviors would provide unique insights into behavioral regulatory mechanisms and the influences of genetic mutations.
To examine the utility of this approach, we investigated the impact of 2 single gene mutations with distinct effects on energy balance regulation. We examined ob/ob (OB) mice that lack the adipocyte hormone leptin and display marked early onset obesity, diminished physical activity, and an early life hyperphagia that declines with age (33, 34). We also examined mice bearing a targeted null mutation of the htr2c gene encoding the serotonin 5-HT2C receptor (2C mice) that exhibit hyperactivity, increased food intake, and the development of obesity by 6 months of age (35, 36). The comprehensive nature and sensitivity of this approach provide insights into the organization of behavior in mice and reveal unique genetic influences on behavioral organization even in these extensively studied mutant mouse lines. The reproducibility and robustness of this approach can be examined by viewing the structure of home cage behavioral patterns for all animals used in this study at http://mousehouse.ucsf.edu.
Results
Classification of Active and Inactive States.
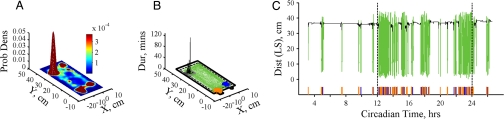
To develop a method for identifying ASs and ISs, we first assessed the spatial features of home cage behavior and uncovered a robust spatial structure revealed by position probability density estimates. These density estimates exhibited single prominent peaks corresponding to the observed nest location with additional smaller peaks at the feeder, water spout, and occasionally at other locations (Fig. 1A). This robust spatial structure (see http://mousehouse.ucsf.edu) allowed us to classify ISs by identifying spatially clustered positions where an animal exhibited the longest visit times (Fig. 1B, details in Materials and Methods). As expected, the location at which the IS positions clustered (home base) typically corresponded to the observed nest location [2 ± 1 cm (mean ± SD) from nest center to home base center; Fig. 1B, see SI Methods]. However, a few mice exhibited a distinct home base despite the absence of a readily observed nest, demonstrating that our IS classification does not require nest building. To assess the temporal structure of behavior, IS onset and offset times were extracted by grouping sequential IS positions (Fig. 1C). Finally, ASs, which could include feeding, drinking, and/or locomotion, were identified as the temporal intervals between ISs (Fig. 1C).
Fig. 1.
State classification. (A) Position probability density for a single WT mouse during 1 day. (Back Left) Maximum position probability peak at nest. (Front) Smaller peaks at feeder (Left) and lick spout (Right). (B) Inactive-state location (home base) revealed by clustering of inactive-state positions (black) at observed nest (small gray box). Forest green, active-state positions; orange, feeding events; blue, drinking events. Dashed black lines, cage floor; solid black lines, cage lip; small box at Front Left, feeder; circle at Front Right, water bottle. (C) Position variation and intake events for a single day. Position as the distance from lick spout (LS) on y axis with inactive states in black and active states in forest green. Feeding (orange) and drinking (blue) event rasters shown below position data. Dashed lines, dark cycle onset and offset.
Intake Bout Classification.
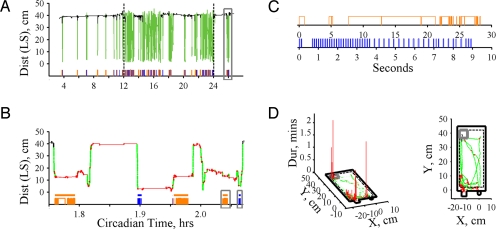
Having characterized behavioral structure at the level of ASs/ISs, we examined behavioral organization within ASs. To quantify the apparent clustering of ingestive events during individual ASs (Fig. 2 A and B),we developed an automated algorithm for grouping feeding or drinking events into bouts. Our algorithm incorporates assessment of both spatial and temporal properties of ingestion. It segregates intervals between ingestive events [inter-event intervals (IEIs)] into distinct populations: those occurring within bouts [within-bout intervals (WBIs)] and those occurring between bouts [inter-bout intervals (IBIs)] (see details in Materials and Methods). The use of spatial information enabled the detection of short IBIs during which an animal leaves an intake device. These would have been misclassified as WBIs (26% of IBIs) using approaches relying solely on temporal data (28, 29, 31, 32). More importantly, spatial information was essential for robustly automating this segregation, overcoming known problems arising from inter-individual variability in temporal properties of IEIs (28, 31, 37). Having separated IEIs into WBIs and IBIs, intake bout onsets and offsets were identified by grouping sequential WBIs (Fig. 2B).
Fig. 2.
Bout classification. (A) Position variation and intake events for single WT mouse with IS positions (black), AS positions (forest green), feeding events (orange), and drinking events (blue). (B) Single light cycle AS indicated by gray box in B. Neon green, locomotion bout positions; red, “other” positions. Bars above feeding and drinking events indicate bout onset and offset. (C) Intake events during feeding (Upper) and drinking (Lower) bouts from left and right gray boxes in B. (D) Position durations (Left) and path taken (Right) for AS shown in B.
Movement Bout Classification.
Movement around the cage within ASs exhibited a characteristic pattern in which rapid movement between locations alternated with pauses and small movements in local areas (Fig. 2 B and D). To quantitatively characterize such patterns, we developed a supervised learning algorithm that used the speeds and turning angles of movements occurring during ISs and intake bouts as a template for nonlocomotor movement (see SI Methods). This allowed us to classify movement events as either locomotor or nonlocomotor events. Uninterrupted strings of locomotor events were then used to define the onset and offset times of locomotion bouts (Fig. 2 B and D). Finally, behaviors occurring during ASs that were not classified as intake or locomotion bouts were classified as “other” behavior (Fig. 2 B and D).
Behavioral Structure at Multiple Time Scales.
The application of our classification methods to the behavioral record enables assessment of behavioral organization over a wide range of time scales. An example is shown in Fig. 2, where a single light cycle AS for a C57BL/6J mouse is selected and plotted on an expanded time scale (Fig. 2 A and B). Bars above feeding and drinking events indicate intake bouts identified by our methods (Fig. 2B). These bouts may also be examined at a higher temporal resolution allowing observation of individual feeding and drinking events (Fig. 2C). Locomotion bouts revealed by our methods (green) exhibit episodes of rapid movement between locations, while “other” behaviors (red) are characterized by movements with low speeds and high turning angles and occur most frequently at the feeder, lickometer and nest (Fig. 2 B and D). Our automated classification approach thus allowed us to extract the structure of behavioral patterns occurring over multiple time scales (i.e., the day, within a day, within a state, within a bout).
Time Budgets.
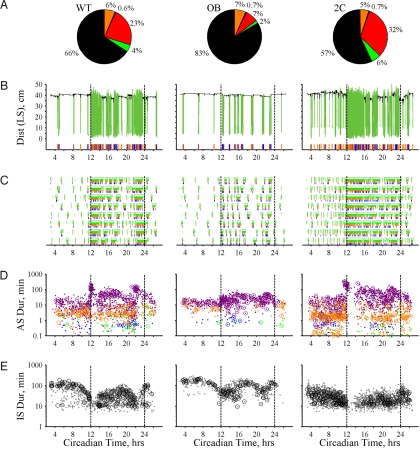
We then determined daily time budgets, which describe the proportions of time allocated to various behaviors (20, 21, 38, 39). OB mice exhibited a striking increase in time spent in the IS along with marked reductions in time spent engaged in locomotion and “other” behavior (Fig. 3A and Table S1). In addition, the percentage of the AS devoted to feeding and drinking doubled in OB mice (feeding: OB 41 ± 2%, WT 19 ± 1%, P = 1.7 × 10−7; drinking OB 4.3 ± 0.2%, WT 1.9 ± 0.1%, P = 6.7 × 10−8). This alteration of within-AS behavior compensated for the global reduction in AS time such that the total time engaged in ingestion did not differ between WT and OB mice (Table S1). In contrast, OB mice decreased time spent in locomotion (Fig. 3A) and decreased locomotion bout speeds (Table S1) resulting in a striking decrease in movement (17% of WT levels). Unlike OB mice, 2C mice decreased IS time while increasing time engaged in “other” behavior (Fig. 3A and Table S1). In addition, 2C mice exhibited increased daily movement and food intake along with increased locomotion and feeding bout intensity (see Table S1).
Fig. 3.
Daily patterns. (A) Daily time budgets. Black, inactive state; orange, feeding; blue, drinking; neon green, locomotion; red, “other.” (B) Position variation and intake events over 1 day for WT (Left), OB (Center), and 2C (Right) mice. AS positions (forest green lines). (C) Feeding (orange), drinking (blue), and locomotion (neon green) events and ASs (forest green lines above the events) over 8 days (y axis) for mice in B. (D) AS onsets (x axis) and log durations (y axis) for days and mice in C (circles) and 64 randomly selected mouse days from each group (dots). Shown are ASs with feeding and drinking (purple), feeding-alone (orange), drinking-alone (blue), and no intake (green). (E) IS onsets and log durations for days and mice in C (black circles) and 64 randomly selected mouse days for each group (gray dots).
State Patterns.
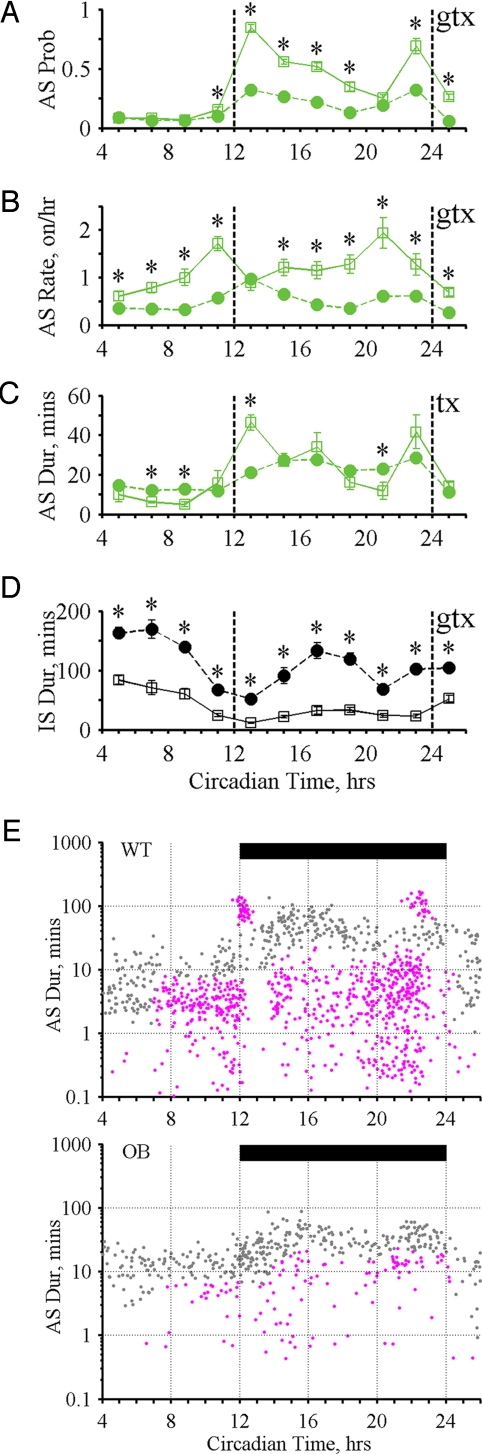
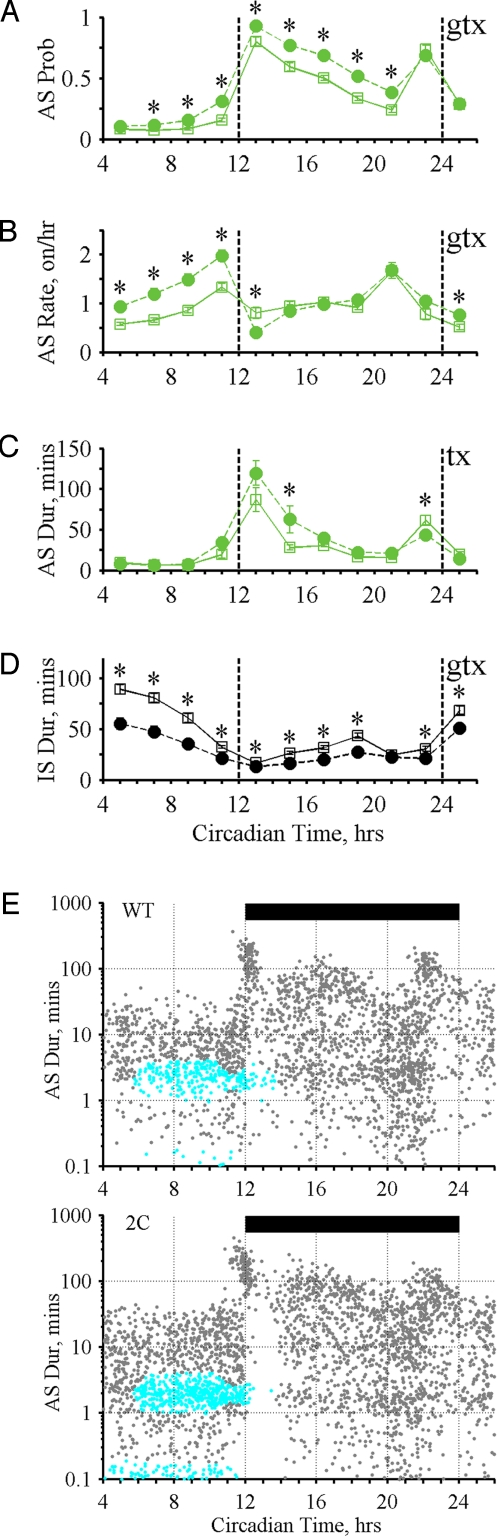
We next examined daily variation in state durations and transitions and the probability of being in the AS (AS probability) (Figs. 3–5). In WT mice, AS probability exhibited clear peaks at dark cycle (DC) onset and offset accompanied by a discrete class of long ASs (1–2 h) that regularly occurred at these times (onset/offset ASs; Figs. 3 B–D; 4 A, C, and E; and 5 A, C, and E). In contrast, IS duration exhibited nadirs at DC onset and offset with broad IS peaks occurring in the middle of the light cycle (LC) and DC (Figs. 3E, 4D, and 5D). Transition rates between states also revealed 2 peaks that preceded the occurrence of the onset/offset ASs (Figs. 4B and 5B).
Fig. 4.
State patterns for WT and OB. (A–D) Effects of genotype (G), time (T), and interaction (GxT) were tested using 2 × 11 RM ANOVA with g (G), t (T), and x (GxT) displayed on each plot for significant effects (4 tests, α = 0.0125). If significant interaction is present, an asterisk indicates significant difference (α = 0.05) by posthoc t test. Daily variation in 2 h bins for WT (open squares) and OB (filled circles): (A) AS Probability (G P = 1.7 × 10−10, GxT P = 2.0 × 10−29) (B) AS Onset Rate (G P = 2.5 × 10−6, GxT P = 1.4 × 10−6) (C) AS Duration (G P = 0.96, GxT P = 2.8 × 10−8) (D) IS Duration (G P = 5.7 × 10−8, GxT P = 1.0 × 10−5) (E) Comparison clustering for AS patterns (Σχ2 = 703, P < 1.6 × 10−4). AS onset time (x axis). AS log duration (y axis). Magenta indicates regions where WT contributed significantly more ASs than OB. Gray indicates regions where significant differences between groups were not detected. Significantly different regions account for 91.2% of total Σχ2. Black bar, dark cycle.
Fig. 5.
State patterns for WT and 2C. Daily variation in 2 h bins for WT (open squares) and 2C (filled circles). (A) AS probability (G P = 8.9 × 10−5, GxT P = 1.2 × 10−9). (B) AS onset rate (G P = 0.002, GxT P = 4.4 × 10−13). (C) AS Duration (G P = 0.5, GxT P = 1.2 × 10−6). (D) IS duration (G P = 5.0 × 10−8, GxT P = 6.7 × 10−15). (E) Comparison clustering for AS patterns (Σχ2 = 233, P = 0.001). Cyan indicates regions where WT contributed significantly fewer ASs than 2C. Significantly different regions account for 48.3% of total Σχ2.
In addition, we identified 4 AS intake types: no intake, feeding-alone, drinking-alone, and feeding-drinking (Fig. 3D and Table S2). The relative proportions of these AS intake types varied with AS duration such that ASs containing both feeding and drinking dominated ASs with long durations (>10 min), including the onset/offset ASs (Fig. 3D and Fig. S1). In contrast, short ASs (< 1 min) commonly contained only feeding, only drinking, or no intake, while the majority of medium duration ASs (1–10 min) were of the feeding-alone type (Fig. 3D and Fig. S1).
The physiological significance of these AS designations is highlighted by the distinct genetic influences on patterns formed by the variation in AS duration with AS onset time (Figs. 3D, 4E, and 5E). To quantitatively characterize these complex patterns without arbitrarily reducing their rich behavioral features, we developed an algorithm (comparison clustering) that determines whether two patterns are significantly different and identifies aspects of these patterns that contribute most to such differences (details in Materials and Methods). Comparison clustering revealed that OB mice exhibited a statistically significant loss of their onset/offset ASs and most of their medium and short duration ASs (Fig. 4E). These changes were accompanied by an increase in the proportion of feeding-drinking ASs (see Table S2). In contrast, during the 6 h preceding the DC, 2C mice exhibited a significant and specific increase in their medium and short duration ASs, which contained a high proportion of feeding-alone ASs (Fig. 5E and Table S2). The marked sensitivity of AS patterns to genetic alterations highlights the potential of quantitative AS pattern assessment to provide insights into the regulation of behavioral organization in freely acting animals.
Determinants of Circadian Variation.
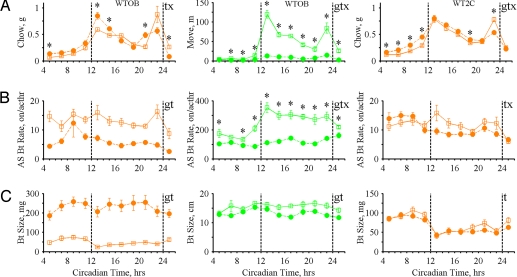
Across all groups of mice, we observed intake and movement peaks occurring at DC onset and offset (Fig. 6A and Fig. S2A) corresponding to the commonly exhibited crepuscular circadian pattern [peaks at dusk and dawn (40)]. Although AS probability exhibited a similar pattern (Fig. 4A, 5A), daily variation in bout size and AS bout rate did not (Fig. 6 B and C and Fig. S2 B and C). This led us to hypothesize that the daily patterns of intake and movement could be better accounted for by variation in the determinants of AS probability than by changes in behaviors occurring within ASs. We used multiple linear regression (41) to determine the extent to which daily patterns of intake and movement were attributable to the predictor variables: AS probability, AS bout rate, and bout size. AS probability accounted for the majority of the daily variation in feeding, drinking and movement across all groups (Table S3 and S4) consistent with our hypothesis that variation in the determinants of AS probability plays a major role in shaping circadian patterns.
Fig. 6.
Feeding and locomotion bout property variation with time of day (2 h bins; 3 tests, α = 0.0167). (A) (Left) WTOB chow intake (G P = 0.1, GxT P = 3.9 × 10−8). (Center) WTOB movement (G P = 2.8 × 10−8, GxT P = 1.6 × 10−36). (Right) WT2C chow intake (G P = 0.01, GxT P = 4.9 × 10−9). (B) (Left) WTOB feeding bouts per AS hour (G P = 4.4 × 10−5, GxT P = 0.2). (Center) WTOB locomotion bouts per AS hour (G P = 1.8 × 10−6, GxT P = 3.3 × 10−13). (Right) WT2C feeding bouts per AS hour (G P = 0.3, GxT P = 0.002). (C) (Left) WTOB feeding bout size (G P = 1.4 × 10−5, GxT P = 0.03). (Center) WTOB locomotion bout size (G P = 0.0167, GxT P = 0.06). (Right) WT2C feeding bout size (G P = 0.4, GxT P = 0.02).
Compensatory Processes Preserve Daily Patterns of Intake in OB Mice.
Although OB mice exhibited crepuscular intake patterns similar to WT mice (highest intake at DC onset and offset) (Fig. 6A and Fig. S2A), these patterns were achieved in a markedly different manner. OB mice exhibited large decreases in AS onset rates (Fig. 4B) accompanied by large compensatory increases in food and water intake during their ASs (Fig. S3). (Food: OB, 361 ± 20 mg; WT, 148 ± 14 mg. Water: OB, 310 ± 15 mg; WT, 126 ± 8 mg.) In addition to these striking changes in AS intake, OB mice displayed large increases in intake during bouts (Fig. 6C and Fig. S2C). (Food: OB, 222 ± 74 mg; WT, 38 ± 8 mg. Water: OB, 114 ± 32 mg; WT, 61 ± 6 mg.) These findings indicate that processes regulating state and bout intake can be dissociated from those producing the commonly observed crepuscular intake pattern.
Selective State Alterations Increase Feeding in 2C Mice.
In contrast to OB mice, 2C mice altered their food intake selectively through changes in AS/IS patterns. Moreover, their increased food intake was restricted to the LC (Fig. 6A) when AS onset rate and AS probability were increased (Fig. 5 A and B). During this time neither food consumed per AS (Fig. S4B), feeding AS bout rate (Fig. 6B), nor feeding bout size (Fig. 6C) were altered. Thus, the increased AS probability accounted for their increased food intake. In fact, comparison clustering revealed that preceding DC onset, 2C mice selectively increased short and medium duration ASs (Fig. 5E) likely accounting for their increase in feeding-alone ASs (Table S2).
Within-Active-State Structure.
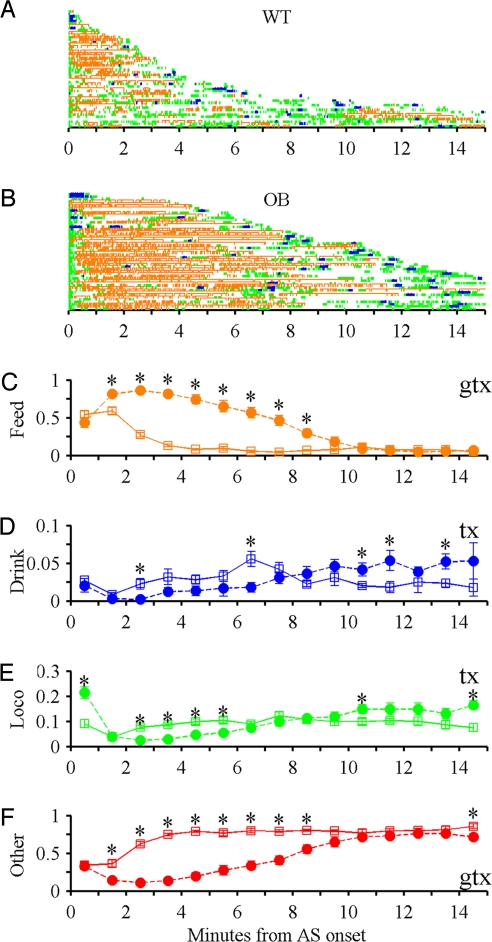
The simultaneous classification of ASs and bouts allowed us to investigate behavioral regulation within the AS. To examine organization on this shorter time scale, we aligned AS onsets and determined how the probability of engaging in particular behaviors varied with the time from AS onset. This revealed a clear sequential structure (Fig. 7). The probability of feeding was highest early in the AS then as the feeding probability declined the probabilities of engaging in drinking, locomotion, and “other” behavior increased. Interestingly, OB mice maintained a strikingly high probability of feeding longer than WT mice consistent with a high priority for feeding in OB mice (Fig. 7).
Fig. 7.
Within active state structure. (A and B) Onsets and offsets of feeding (orange), drinking (blue), and locomotion (green) events occurring during 50 randomly selected light cycle ASs from WT (A) and OB (B) mice. Each (C–F) line displays a single AS (y axis). Time during ASs (x axis) with time 0 at AS onset. Variation in probability during ASs for WT (open squares) and OB (filled circles) mice in 1 minute bins for (4 tests, α = 0.0125); (C) Feeding (G P = 4.6 × 10−6, GxT P = 6.2 × 10−42). (D) Drinking (G P = 0.6, T P = 0.005, GxT P = 0.0002). (E) Locomotion (G P = 0.2, GxT P = 4.2 × 10−13). (F) Other (G P = 9.9 × 10−6, GxT P = 7.9 × 10−34).
Discussion
This quantitative characterization of behavioral organization in freely acting mice provides a powerful approach for assessing the impact of genetic and other experimental manipulations on whole animal physiology and behavior. We developed an algorithm allowing automated identification of a major feature of behavioral organization: the clustering of multiple behaviors into ASs alternating with ISs that occur at discrete home base locations. The demonstration that ASs and ISs occur in caged inbred mice emphasizes the fundamental importance of these states and provides a framework for studying behavioral organization.
Having captured the structure of behavior at the level of ASs and ISs, we examined the organization of behaviors within ASs. We developed an approach for intake bout identification using spatial and temporal information to improve the accuracy and robustness of classification. We also developed a supervised learning algorithm to identify bouts of locomotion alternating with pauses and small movements in local areas. Similar patterns of intermittent locomotion have been observed in rodents exploring novel laboratory environments (42) and are also common in natural environments (43). Despite the prevalence of such movement patterns, procedures for capturing their basic features had not previously been developed for home cage behavioral assessment (1, 6–13, 16–18).
These new techniques provide a unique opportunity to examine basic properties of behavioral regulation in freely acting mice. For example, within ASs the probability of feeding is highest near AS onset, raising the possibility that processes regulating food acquisition may be a primary determinant of AS initiation. In addition, a subsequent decline in feeding probability was accompanied by increases in the probabilities of engaging in drinking, locomotion, and “other” behavior. This pattern of within-AS behavior is reminiscent of the satiety sequence: a sequence of behaviors observed in animals provided access to food after food deprivation (44, 45). The detection of such sequences using an automated system provides new opportunities for understanding the regulation of ingestion in ad libitum fed animals.
We also observed previously uncharacterized and distinct features of circadian patterns in C57BL/6J mice, such as the presence of the onset/offset ASs and distinct AS intake types. Our approach thus provides the first opportunity to gain insights into the regulatory processes producing these striking features of AS/IS properties. The importance of understanding these regulatory processes is highlighted by our observation that circadian patterns of feeding and movement are shaped predominantly at the level of AS/IS properties rather than bout properties.
Furthermore, the preservation of crepuscular intake patterns in OB mice despite profound alterations in state properties indicates that the regulatory processes producing crepuscular intake patterns and those governing AS/IS regulation are dissociable. More generally, these findings indicate that genetic influences may dramatically alter some aspects of behavioral organization (e.g., bouts and states), while compensatory changes may preserve other aspects of behavioral organization (e.g., crepuscular patterns). This has implications for behavioral phenotyping, illustrating that dramatic changes in behavior may be missed if only a particular aspect of behavior (e.g., hourly food intake) is characterized.
With regard to OB mice, studies exploring the impact of the Lepob mutation on energy balance will benefit from consideration of the global reorganization of behavioral patterns we observed. Feeding, drinking and locomotor activity patterns were markedly altered at multiple levels of organization. In addition, circadian variation in AS patterns was vastly altered in OB mice as highlighted by the loss of their onset/offset ASs. The loss of these states may reflect a suppression of nonessential behaviors and their accompanying locomotion, consistent with the possibility that signals favoring energy conservation contribute substantially to the global reorganization of behavioral patterns in OB mice (33). It is therefore notable that animals respond to increased costs of food acquisition by reducing the number and increasing the size of feeding clusters (46, 47).
The notion that energy conservation shapes behavioral patterns in OB mice may also be considered in light of the phenomenon known as nonexercise activity thermogenesis (NEAT) (38). In humans, NEAT refers to all physical activity except purposeful exercise. It has been proposed that NEAT levels are innately determined, subject to biological regulation, and highly correlated with obesity susceptibility (38). In this context, the capacity to quantify the time and intensity of distinct behaviors may help elucidate mechanisms contributing to obesity. It is therefore intriguing that the Lepob and htr2c− mutations produce opposing effects on IS time, locomotion and “other” behavior. It is possible that elevations of NEAT in hyperphagic 2C mice might facilitate their maintenance of normal body weights as young adults, whereas reductions of NEAT may exacerbate adiposity in OB mice at this time of life.
The utility of this approach for phenotypic assessment is further highlighted by the selective manner in which the htr2c− mutation increases the frequency of feeding-only ASs at a particular time of day. This occurs during what is typically an inactive phase (light cycle) for these nocturnal animals, and is reminiscent of the clinical condition termed night eating syndrome (NES). NES is found in up to 27% of severely obese persons and is characterized by multiple nocturnal awakenings accompanied by food ingestion (48). The relevance of the 2C feeding pattern to NES is further suggested by the responsiveness of NES to serotonin reuptake blockade (48).
This behavioral assessment approach may also be widely applied to facilitate the development and utility of rodent models of disease. For many common diseases, perturbations of daily patterns of multiple behaviors (i.e., “lifestyle”) are associated with disease susceptibility, progression, and treatment outcome (6, 8, 9, 38, 39). The ability to quantitatively assess the organization of multiple home cage behaviors will be useful for the study of disease processes and drug development relevant to metabolic, affective, addictive, pain, neurodegenerative and other disorders (8–10, 39, 49). Furthermore, since adverse effects of compounds at both central and peripheral sites may impact behavioral regulation (11), this technology can also be applied for toxicological screening.
Although the potential value of this system for evaluating a number of behaviors relevant to disease pathophysiology and treatment is clear, future development will more thoroughly delineate the capabilities of the system and expand its utility. For example, it will be of interest to determine the sensitivity of behavioral patterns to genetic background (e.g., inbred strain survey). In addition, the algorithms developed can be used to assess a broader range of behavioral domains by modifying the home cage environment (e.g., running wheels, operant response devices, novel objects, auditory and olfactory stimuli). Video analysis can also be used with this system to enable delineation of behaviors currently grouped into the category “other.” Multiple-animal video tracking could also address the current requirement that animals be individually housed allowing comparison of behavioral patterns in singly- and group-housed animals.
Because the processes of data collection, processing, quality control, and analysis are automated and reproducible, our approach allows substantial scaling, providing the opportunity to develop a large searchable home cage behavioral database. Such a database could be populated by patterns (“behavioral signatures”) associated with inbred strains, genetic manipulations, pharmacological treatments, CNS lesions, diet, stressors, disease models, etc. The utility of such comparisons has been enhanced by the development of novel procedures, such as comparison clustering, that enable detailed assessment of the manner in which complex behavioral patterns differ. Our approach for quantifying the organization of mouse home cage behavior should thus provide new opportunities to examine the neural basis of behavioral regulation, comprehensively study disease models, and assess new therapies. Moreover, the availablity of gps and mobile monitoring technologies such as actimetry (50–53) will enable adaptation of our algorithms for behavioral classification and analysis to the monitoring of humans and animals in the field.
Materials and Methods
Animals.
Mutant male mice (Lepob/Lepob, OB) and control C57BL/6J mice (WT) were obtained from The Jackson Laboratory. Male mice hemizygous for the X-linked htr2c− allele (congenic on a C57BL/6J background) and control WT litter mates were bred at UCSF and genotyped as described in ref. 34. Animals were housed under LC/DC 12-hr with free access to water and a chow diet (PicoLab; mouse diet 20). Experiments were performed in accordance with the guidelines of the University of California San Francisco Committee on Animal Research.
Data Collection.
We collected data from 11- to 14-week-old male mice: WT (from the OB colony; n = 8) and OB (n = 8); WT (from the 2C colony; n = 16) and 2C (n = 16). Because the behavior of WT mice could be influenced by phenotypic effects on maternal care and cagemate behavior, comparisons were made between each line of mutant mice and WT animals derived from the same colony. Mice were individually housed for 14 days in home cage monitoring systems containing paper bedding and nestlets. Validation studies were performed to verify that feeder photobeam break time and lick numbers were reliable indicators of food and fluid consumption, respectively (see SI Methods). The last 10 days of data were used for analysis allowing 4 days for acclimation. The large volumes of behavioral data required methods for maximizing data quality. Algorithms developed in-house used the output of each data collection device to cross-check the performance of other devices in each cage (see SI Methods).
Inactive State Classification.
Animals exhibited visits to a location in the cage (home base) that had properties distinct from visits to other locations: Movements there were small and interspersed with the longest observed periods of immobility. We used this observation to quantify the occurrence of ISs by deriving a pause duration threshold that depended on two parameters (time window and spatial filter) determined by minimizing a state classification error (see SI Methods). The time window accounted for occasional relocation of the home base while the spatial filter smoothed out small movements to define positions and pause durations based on the failure of the animal to move beyond a threshold (WTOB, 2 cm; WT2C, 3 cm). The use of the time window and spatial filter allowed us to examine the relationship between time spent at each position (pause duration) and the distance of each position from the position with the longest pause duration in the position's time window (Fig. S5). Positions were binned with respect to their log pause durations (bin width 0.1 log ms, empty bins excluded), and a pause duration threshold was then established by fitting 3 lines to bin duration and maximum distance in each bin using non-linear least squares regression (Fig. S5). The intersection of the second and third lines was set as the pause duration threshold to identify spatially clustered positions with pauses that were longer than at any other location.
Bout Classification.
Our classification of feeding or drinking bouts (described below with regard to feeding) incorporates assessment of both spatial and temporal properties of ingestion. Spatial information was incorporated to implement the concept that the termination of a feeding bout can be identified by the presence of a distinct behavior, such as locomotion from the food source, occurring between two feeding events (29, 54). We therefore examined the location of the mouse during the interval between the end of each feeding event and the onset of the subsequent feeding event (IEI). This allowed us to split IEIs into 2 groups (at-feeder or not) and estimate the probability that the mouse remained at the feeder during each IEI (see SI Methods).
The termination of a feeding bout can also be identified by the occurrence of a long IEI between two feeding events. Standard approaches for the identification of feeding bouts have typically used such a criterion by categorizing two distinct types of IEI durations: short WBIs and longer IBIs (31, 32). To derive such a criterion, we used both temporal and spatial information to split the IEIs into 2 groups (short and long) and estimate the probability that an IEI was short (see SI Methods). We then combined the spatial and temporal information to designate each IEI as either a WBI or an IBI by averaging the probability that the mouse remained at the feeder with the probability that the IEI was short (Fig. S6 and S7). To classify locomotion bouts, we developed a supervised learning algorithm that utilized our prior classification of inactive states and intake bouts (Fig. S8; see SI Methods). Our automated classification of ISs and bouts were in accord with manually-scored visual observation data (see SI Methods).
Comparison Clustering.
We tested the null hypothesis that a control and test group had the same pattern of AS onset times and durations by (i) combining data for all mouse days in the 2 groups, (ii) assigning each AS in the combined data to one of a number of clusters, and (iii) calculating a χ2 statistic for each cluster based on our null hypothesis that both groups contributed equal proportions of ASs to each cluster (Fig. S9; see SI Methods). The sum of the chi-squares over all clusters was used as the measure of overall difference between the two groups. The significance of any difference was determined by permuting the mice between the 2 groups and recalculating the sum of chi-squares. If a significant difference between groups was present, regions that contributed significantly (P < = 0.05) were found by obtaining a P value for each cluster adjusted for multiple comparisons, SR3 (55).
General Statistics.
Comparisons between groups were made for multiple variables using t tests or repeated measures (RM) ANOVA. For a given level of analysis, a Bonferroni correction for multiple comparisons was used. For instance, in comparing the daily amount of food, water, and movement a correction was made for 3 tests. All values reported are mean ± SE unless otherwise noted. Significance of time in all RM ANOVA, P < 0.001 unless otherwise noted.
Supplementary Material
Acknowledgments.
We thank Dr. Craig Van Dyke for helpful comments and support; Ross Henderson for helpful discussions and adapting data collection hardware and software for our purposes; Dr. Charles McCulloch for helpful discussions and statistical consultation; Lana Bogdanova for animal care; and Dr. Samuel Barondes, Dr. Louis Ptáček, Dr. Alan Basbaum, Dr. Jon Levine, Dr. Stephen Bonasera, Dr. Elaine Storm, Dr. Bruce Cree, and Scott Anderson for insightful feedback on the manuscript. This work was supported by the National Institutes of Health Grants MH71671 (to E.H.G.), NIA-AG026043 (to A.K.S.), MH61624 (to L.H.T.), MH1949 (to L.H.T.), MH077128 (to L.H.T.), and MH079145 (to L.H.T.); a National Alliance for Schizophrenia and Depression Artworks Young Investigator Award (to E.H.G.); a Howard Hughes Medical Institute Physician Postdoctoral Fellowship (to E.H.G.); the Stephen D. Bechtel, Jr. Foundation (A.K.S.), the University of California San Francisco Department of Neurology Alcohol and Addiction Research Program (to L.H.T.); the Bristol Myers Squibb Foundation Freedom to Discover Program (L.H.T.); the Sandler Foundation (L.H.T.); and the Sarah and William Hambrecht Foundation (L.H.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 20563.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809053106/DCSupplemental.
References
- 1.de Visser L, van den Bos R, Kuurman WW, Kas MJ, Spruijt BM. Novel approach to the behavioural characterization of inbred mice: Automated home cage observations. Genes Brain Behav. 2006;5:458–466. doi: 10.1111/j.1601-183X.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- 2.Tecott LH, Nestler EJ. Neurobehavioral assessment in the information age. Nat Neurosci. 2004;7:462–466. doi: 10.1038/nn1225. [DOI] [PubMed] [Google Scholar]
- 3.McFarland DJ. Decision making in animals. Nature. 1977;269:15–21. [Google Scholar]
- 4.Oatley K. Brain mechanisms and motivation. Nature. 1970;225:797–801. doi: 10.1038/225797a0. [DOI] [PubMed] [Google Scholar]
- 5.Prescott TJ, Redgrave P, Gurney K. Layered control architectures in robots and vertebrates. Adaptive Behav. 1999;7:99–127. [Google Scholar]
- 6.Casadesus G, Shukitt-Hale B, Joseph JA. Automated measurement of age-related changes in the locomotor response to environmental novelty and home-cage activity. Mechan Age Develop. 2001;122:1887–1897. doi: 10.1016/s0047-6374(01)00324-4. [DOI] [PubMed] [Google Scholar]
- 7.Gannon KS, Smith JC, Henderson R, Hendrick P. A system for studying the microstructure of ingestive behavior in mice. Physiol Behav. 1992;51:515–521. doi: 10.1016/0031-9384(92)90173-y. [DOI] [PubMed] [Google Scholar]
- 8.Chen SA, et al. Unlimited access to heroin self-administration: Independent motivational markers of opiate dependence. Neuropsychopharmacol. 2006;31:2692–2707. doi: 10.1038/sj.npp.1301008. [DOI] [PubMed] [Google Scholar]
- 9.Millecamps M, et al. Circadian pattern of spontaneous behavior in monarthritic rats: A novel global approach to evaluation of chronic pain and treatment effectiveness. Arthritis Rheumatism. 2005;52:3470–3478. doi: 10.1002/art.21403. [DOI] [PubMed] [Google Scholar]
- 10.Steele AD, Jackson WS, King OD, Lindquist S. The power of automated high-resolution behavior analysis revealed by its application to mouse models of Huntington's and prion diseases. Proc Natl Acad Sci USA. 2007;104:1983–1988. doi: 10.1073/pnas.0610779104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamborini P, Sigg H, Zbinden G. Quantitative analysis of rat activity in the home cage by infrared monitoring. Application to the acute toxicity testing of acetanilide and phenylmercuric acetate. Arch Toxicol. 1989;63:85–96. doi: 10.1007/BF00316429. [DOI] [PubMed] [Google Scholar]
- 12.Tang X, Sanford LD. Home cage activity and activity-based measures of anxiety in 129P3/J, 129X1/SvJ and C57BL/6J mice. Physiol Behav. 2005;84:105–115. doi: 10.1016/j.physbeh.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 13.Zorrilla EP, et al. Measuring meals: Structure of prandial food and water intake of rats. Am J Physiol. 2005;288:R1450–R1467. doi: 10.1152/ajpregu.00175.2004. [DOI] [PubMed] [Google Scholar]
- 14.Bonasera SJ, Schenk AK, Luxenberg EJ, Tecott LH. A novel method for automatic quantification of psychostimulant-evoked route-tracing stereotypy: Application to Mus musculus. Psychopharmacol. 2008;196:591–602. doi: 10.1007/s00213-007-0994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunner D, Nestler E, Leahy E. In need of high-throughput behavioral systems. Drug Discovery Today. 2002;7:S107–S112. doi: 10.1016/s1359-6446(02)02423-6. [DOI] [PubMed] [Google Scholar]
- 16.Donohue KD, Medonza DC, Crane ER, O'Hara BF. Assessment of a non-invasive high-throughput classifier for behaviours associated with sleep and wake in mice. Biomed Engineering Online. 2008;7:14. doi: 10.1186/1475-925X-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galsworthy MJ, et al. A comparison of wild-caught wood mice and bank voles in the Intellicage: Assessing exploration, daily activity patterns and place learning paradigms. Behav Brain Res. 2005;157:211–217. doi: 10.1016/j.bbr.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 18.Kas MJH, Malsen AD, Olivier B, Spruijt BM, van Ree JM. Differential genetic regulation of motor activity and anxiety-related behaviors in mice using an automated home cage task. Behav Neurosci. 2008;122:769–776. doi: 10.1037/0735-7044.122.4.769. [DOI] [PubMed] [Google Scholar]
- 19.Risbrough VB, et al. Differential contributions of dopamine D-1, D-2, and D-3 receptors to MDMA-induced effects on locomotor behavior patterns in mice. Neuropsychopharmacol. 2006;31:2349–2358. doi: 10.1038/sj.npp.1301161. [DOI] [PubMed] [Google Scholar]
- 20.Herbers JM. Time resources and laziness in animals. Oecologia. 1981;49:252–262. doi: 10.1007/BF00349198. [DOI] [PubMed] [Google Scholar]
- 21.Pyke GH. The economics of territory size and time budget in the Golden-Winged Sunbird. Am Nat. 1979;114:131–145. [Google Scholar]
- 22.Halle S, Stenseth NC, editors. Activity Patterns in Small Mammals: An Ecological Approach. Berlin: Springer; 2000. [Google Scholar]
- 23.Burt WH. Territoriality and home range concepts as applied to mammals. J Mamalogy. 1943;24:346–352. [Google Scholar]
- 24.Gray SJ, et al. Microhabitat and spatial dispersion of the grassland mouse (Mus spretus Lataste) J Zool. 1998;246:299–308. [Google Scholar]
- 25.Samuel MD, Pierce DJ, Garton EO. Identifying areas of concentrated use within the home range. J Animal Ecol. 1985;54:711–719. [Google Scholar]
- 26.Adams L, Davis SD. The internal anatomy of home range. J Mamal. 1967;48:529–536. [Google Scholar]
- 27.Lima SL, Dill LM. Behavioral decisions made under the risk of predation—A review and prospectus. Can J Zool. 1990;68:619–640. [Google Scholar]
- 28.Berdoy M. Defining bouts of behavior: A three-process model. Animal Behav. 1993;46:387–396. [Google Scholar]
- 29.Machlis L. Analysis of the temporal patterning of pecking in chicks. Behaviour. 1977;63:1–70. [Google Scholar]
- 30.Morgan CA, Emmans GC, Tolkamp BJ, Kyriazakis I. Analysis of the feeding behavior of pigs using different models. Physiol Behav. 2000;68:395–403. doi: 10.1016/s0031-9384(99)00195-x. [DOI] [PubMed] [Google Scholar]
- 31.Tolkamp BJ, Kyriazakis II. To split behaviour into bouts, log-transform the intervals. Anim Behav. 1999;57:807–817. doi: 10.1006/anbe.1998.1022. [DOI] [PubMed] [Google Scholar]
- 32.Langton SD, Collett D, Sibly RM. Splitting behavior into bouts; A maximum likelihood approach. Behavior. 1995;132:781–799. [Google Scholar]
- 33.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 34.Wade JM, et al. Synergistic impairment of glucose homeostasis in ob/ob mice lacking functional serotonin 2C receptors. Endocrinology. 2008;149:955–961. doi: 10.1210/en.2007-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nonogaki K, Abdallah L, Goulding EH, Bonasera SJ, Tecott LH. Hyperactivity and reduced energy cost of physical activity in serotonin 5-HT2C receptor mutant mice. Diabetes. 2003;52:315–320. doi: 10.2337/diabetes.52.2.315. [DOI] [PubMed] [Google Scholar]
- 36.Nonogaki K, Strack AM, Dallman MF, Tecott LH. Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nat Med. 1998;4:1152–1156. doi: 10.1038/2647. [DOI] [PubMed] [Google Scholar]
- 37.Davison M. Interresponse times and the structure of choice. Behaval Processes. 2004;66:173–187. doi: 10.1016/j.beproc.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Levine JA. Nonexercise activity thermogenesis - liberating the life-force. J Intern Med. 2007;262:273–287. doi: 10.1111/j.1365-2796.2007.01842.x. [DOI] [PubMed] [Google Scholar]
- 39.de Vries MW, editor. The Experience of Psychopathology: Investigating Mental Disorders in Their Natural Settings. Cambridge, UK: Cambridge Univ Press; 1992. [Google Scholar]
- 40.Aschoff J. Circadian activity pattern with two peaks. Ecol. 1966;47:657–662. [Google Scholar]
- 41.Azen R, Budescu DV. The dominance analysis approach for comparing predictors in multiple regression. Psychol Methods. 2003;8:129–148. doi: 10.1037/1082-989x.8.2.129. [DOI] [PubMed] [Google Scholar]
- 42.Drai D, Golani I. SEE: A tool for the visualization and analysis of rodent exploratory behavior. Neurosci Biobehav R. 2001;25:409–426. doi: 10.1016/s0149-7634(01)00022-7. [DOI] [PubMed] [Google Scholar]
- 43.Kramer DL, McLaughlin RL. The behavioral ecology of intermittent locomotion. Am Zool. 2001;41:137–153. [Google Scholar]
- 44.Antin J, Gibbs J, Holt J, Young RC, Smith GP. Cholecystokinin elicits the complete behavioral sequence of satiety in rats. J Comp Physiol Psychol. 1975;89:784–790. doi: 10.1037/h0077040. [DOI] [PubMed] [Google Scholar]
- 45.Ishii Y, Blundell JE, Halford JC, Rodgers RJ. Effects of systematic variation in presatiation and fasting on the behavioural satiety sequence in male rats. Physiol Behav. 2003;79:227–238. doi: 10.1016/s0031-9384(03)00066-0. [DOI] [PubMed] [Google Scholar]
- 46.Collier G, Hirsch E, Hamlin PH. The ecological determinants of reinforcement in the rat. Physiol Behav. 1972;9:705–716. doi: 10.1016/0031-9384(72)90038-8. [DOI] [PubMed] [Google Scholar]
- 47.Johnson KG, Cabanac M. Homeostatic competition in rats fed at varying distances from a thermoneutral refuge. Physiol Behav. 1982;29:715–720. doi: 10.1016/0031-9384(82)90244-x. [DOI] [PubMed] [Google Scholar]
- 48.O'Reardon JP, et al. A randomized, placebo-controlled trial of sertraline in the treatment of night eating syndrome. Am J Psychiatry. 2006;163:893–898. doi: 10.1176/ajp.2006.163.5.893. [DOI] [PubMed] [Google Scholar]
- 49.Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez MC, Hidalgo CA, Barabasi AL. Understanding individual human mobility patterns. Nature. 2008;453:779–782. doi: 10.1038/nature06958. [DOI] [PubMed] [Google Scholar]
- 51.Haynes SN, Yoshioka DT. Clinical assessment applications of ambulatory biosensors. Psychol Assessment. 2007;19:44–57. doi: 10.1037/1040-3590.19.1.44. [DOI] [PubMed] [Google Scholar]
- 52.Hurling R, et al. Using internet and mobile phone technology to deliver an automated physical activity program: Randomized controlled trial. J Med Internet Res. 2007;9:25. doi: 10.2196/jmir.9.2.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laube P, Dennis T, Forer P, Walker M. Movement beyond the snapshot—Dynamic analysis of geospatial lifelines. Comput Environ Urban Syst. 2007;31:481–501. [Google Scholar]
- 54.Mayes E, Duncan P. Temporal patterns of feeding behavior in free-ranging horses. Behav. 1986;96:105–129. [Google Scholar]
- 55.Troendle JF. A stepwise resampling method of multiple hypothesis-testing. J Am Stat Assoc. 1995;90:370–378. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.