Abstract
Auditory hair cell defect is a major cause of hearing impairment, often leading to spiral ganglia neuron (SGN) degeneration. The cell loss that follows is irreversible in mammals, because inner ear hair cells (HCs) have a limited capacity to regenerate. Here, we report that in the adult brain of both rodents and humans, the ependymal layer of the lateral ventricle contains cells with proliferative potential, which share morphological and functional characteristics with HCs. In addition, putative neural stem cells (NSCs) from the subventricular zone of the lateral ventricle can differentiate into functional SGNs. Also important, the NSCs can incorporate into the sensory epithelia, demonstrating their therapeutic potential. We assert that NSCs and edendymal cells can undergo an epigenetic functional switch to assume functional characteristics of HCs and SGNs. This study suggests that the functional plasticity of renewable cells and conditions that promote functional reprogramming can be used for cell therapy in the auditory setting.
Keywords: cochlea, ependymal cells, hearing restoration, neural stem cells, spiral ganglia neurons
In the mammalian auditory system, hair cells (HCs), the sensory receptor cell for sound and acceleration, are terminally differentiated cells. Degeneration of these cells, due to overstimulation, ototoxic drugs and aging, are the most common cause of hearing loss affecting approximately 10% of the worldwide population. Because HCs provide survival promoting stimuli (1) to spiral ganglia neurons (SGNs), a secondary effect of HC loss is the gradual degeneration and death of SGNs, leading to structural and electrical remodeling of the cochlear nucleus (CN). Recent reports have demonstrated that limited new HCs may be regenerated de novo (2) or via phenotypical transdifferentiation (3, 4) within the adult mammalian inner ear. Moreover, a small number of new SGNs can also be generated from the mature inner ear (5). However, the production of new HCs and SGNs is a rare event. Thus, considerable efforts have been made to identify a renewable cell source able to reconstruct damaged inner ears, with a special focus on various progenitor cells (2, 6–8), albeit limited success.
The embryonic germinal zone in the adult forebrain lateral ventricle (LV) region contains two morphologically distinct cell layers: The ependymal layer contains ciliated epithelial cells and the subventricular zone(SVZ), which is beneath the ependymal layer and hosts multipotential neural stem cells of active neurogenesis (9). A subpopulation of cells with astrocytic characteristics within the SVZ (10–13) has become the source of adult neural stem cells (NSCs) lining the LV, to produce both neurons and glia. Most intriguingly, there are phylogenetic lineage relationships between the adult forebrain germinal zone cells and the sensory and nonsensory epithelia of the inner ear. Both are derived from the neural ectodermal layer and share certain protein markers that are expressed within the organ of Corti and SGNs (14, 15). In addition, the cilia of forebrain ependymal cells are microtubular in structure and have an actin-filled process as in the HCs. Thus, we surmise that cells of the adult forebrain germinal zone might be potential candidate cells to be used autologously for the replacement of nonrenewable HCs and SGNs.
Ependymal cells adjacent to the spinal canal proliferate extensively upon spinal cord injuries (16, 17). Proliferation of adult brain LV ependymal cells (18) can also be detected after a stroke. Although previous studies failed to detect cell proliferation in these ependymal cells under physiological conditions (19), active proliferation of LV ependymal cells has been confirmed in several experiments in vitro (11, 20). In the present study, we present evidence that LV ependymal cells demonstrate proliferative capacity both in vitro and in vivo; most importantly, they have the potential to give rise to inner ear hair cell-like phenotypes. These cells share many morphological and functional characteristics with inner ear HCs, including; stereociliary and kinociliary bundles, expression of HC markers, selective uptake of FM1–43 dye, and are also able to establish functional synapses with primary SGNs. Moreover, the SGN-like neuronal progenies could be derived from SVZ NSCs residing underneath the ependymal layer. These neuronal progenies establish functional synapses with HCs and deafferentated SGNs. We propose that within the adult forebrain germinal zone, ependymal and subependymal cells can undergo an epigenetic functional switch that could potentially enable them to replace damaged HCs and SGNs in the auditory setting.
Results
Ependymal Layer of the LV Contains Cells That Display HC Characteristics and Proliferative Potential.
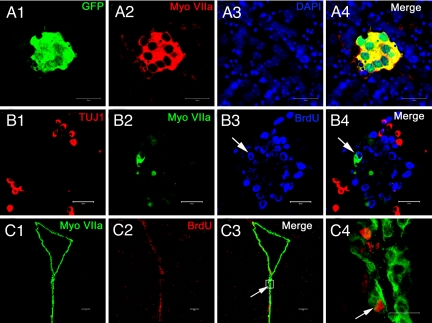
Myosin VIIA has been previously identified as a HC marker (21, 22) and is widely used in HC differentiation and regeneration studies (23). Unexpectedly, in in vitro cell culture characterization and expansion studies, neurospheres obtained from the LV of transgenic mice expressing the green fluorescent protein (GFP) under the control of MyoVIIA promoter (21), contained small GFP-positive colonies (Fig. 1A1). Expression of myosin VIIA in these colonies was confirmed with immunofluorescent staining (Fig. 1A2–4). To provide evidence that the ependymal cells may proliferate, we performed BrdU immunocytochemistry with these cultures. As shown in Fig. 1B1–4, some of the myosin VIIA-positive cells were also BrdU positive, indicating their in vitro proliferative capacity. However, they were distinct from the newly differentiated neurons derived from the same neurosphere, because those neurons expressed the neuronal marker, TuJ1. Next, to identify the cell type that expresses myosin VIIA and to determine whether they have proliferative potential in situ, we examined the lateral ventricular cells in BrdU-treated adult mice. Robust and specific staining of myosin VIIA was only observed in the polarized ependymal cells (Fig. S1), some of which were also BrdU-positive, demonstrating that the cells proliferate in vivo (Fig. 1C1–4).
Fig. 1.
In vivo and in vitro proliferation of ependymal cells. (A1–4) Adult NSCs were isolated from the lateral wall of the LV (obtained from Myosin VIIA-GFP mice). Dissociated cells proliferated into neurospheres. Within the neurosphere a small cell colony was colabeled with GFP (A1) and myosin VIIA (A2), indicating the possible proliferation of myosin VIIA positive cells. Nuclei were labeled with DAPI (A3 and 4). (B1–4) After the neurospheres attached to the coverslips, some of the progenies expressed the early neuronal maker β tubulin III (TUJ1) (B1) and myosin VIIA (B2), respectively. Newly generated cells were labeled with BrdU. Arrows indicate an in vitro proliferated cell, which was simultaneously labeled with myosin VIIA and BrdU (B3 and 4). (C1–4) Cryosection of an adult brain taken from a BrdU treated mouse. The ependymal layer of the LV was clearly and specifically labeled with myosin VIIA (C1). Nuclei of proliferated cells were labeled with BrdU (C2). Arrows indicate an ependymal cell that was colabeled with myosin VIIA and BrdU. Higher magnification of the colabeled ependymal cell within the box area can be found in panel (C4), which indicates the possible in vivo proliferation. (Scale bars: A1–B4 and C1–3, 100 μm.)
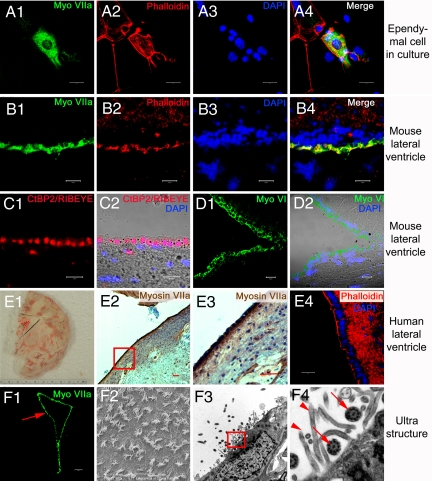
To test whether ependymal cells can assume the HC-structural phenotype, we performed immunostaining for myosin VIIA and phalloidin staining for F-actin. In culture, ependymal cells are columnar shape, remain myosin VIIA-positive, and extend appendages that are labeled with phalloidin, partially resembling HCs (Fig. 2A1–4). However, because the in vitro culture environment may be slightly different from in vivo conditions (24), we examined the expression of myosin VIIA and actin-based appendages in the ependymal cell layer of brain slices. Consistent with the in vitro scenario, the apical cellular layer of the LV was positively labeled with myosin VIIA and phalloidin (Fig. 2B1–4). To provide further evidence that these ependymal cells resemble inner ear HCs, we performed immunostaining with additional HC markers including ribeye, a HC synaptic protein (25), and myosin VI (22). As shown in Fig. 2 C1–D2, ribeye and myosin VI were also expressed by cells of ependymal layer (Fig. 2 C1–D2). Furthermore, the expression of myosin VIIA and clusters of actin-based appendages in the ependymal layer cell were not restricted to the nervous system of mice alone, but were found in humans as well, providing assurance that these findings transcend species-specific phenomena (Fig. 2E1–4). Moreover, we used scanning and transmission electron microscopy to examine the ultra structures at the apical aspects of ependymal cells. This analysis confirmed that the ependymal cell is lined with cillary appendages made of stereocilia and kinocilia, reminiscent of vestibular HCs in the inner ear (Fig. 2F1–4).
Fig. 2.
In vitro and in vivo structural profile of adult ependymal cells. (A1–4) Cultured adult ependymal cells remain myosin VIIA-positive (A1). Phalloidin-labeled actin-rich sterocilia-like appendages were found on the apical surface of the cells (A2). (B1–D2) Cryosection of the lateral wall of the LV of adult mice. Ependymal cells were clearly and specifically labeled with myosin VIIA (B1) and phalloidin (B2–4). Hair cell synaptic protein CtBP2/RIBEYE was observed in ependymal cells of the LV (C1 and 2). The ependymal cell layer of the LV also expressed myosin VI, an early HC marker (D1 and 2). Myosin VI-positive cells are shown in green (D1), whereas the nuclei-stain is in blue. The light microscope and merged image are represented in (D2). (E1–4) Myosin VIIA was expressed in adult human ependymal cells. Panel (E1) is a photomicrograph of a normal adult human brain sliced and frozen within 18 h of death. Postmortem, a wedge around the LV region was taken from the brain then fixed, sectioned and stained to provide panels (E2–4). The red dashed lines indicate the LV region (E1). Adult human ependymal cells also expressed myosin VIIA (E2). The boxed area of panel (E2) is enlarged in panel (E3) to demonstrate myosin VIIA expression. Phalloidin-labeled actin stereocilia-like appendages were found on the apical aspects of human ependymal cells (E4). Scanning (F2) and transmission electron microscopy (F3) of the lateral ventricle region (red arrow in F1) demonstrated that ependymal cells are also equipped with structural profiles of stereocilia and kinocilia, similar to HCs. The boxed area of panel (F3) was enlarged in panel (F4), showing stereociliary appendages (arrow heads) and the characteristic 9 + 2 microtuble structure of kinocilia (arrows). The nuclei were labeled with DAPI. (A3, A4, B3, B4, C2, D2, and E4). (Scale bars: A1–D2, E4, and F2, 20 μm; F1 and E2 and 3, 100 μm.)
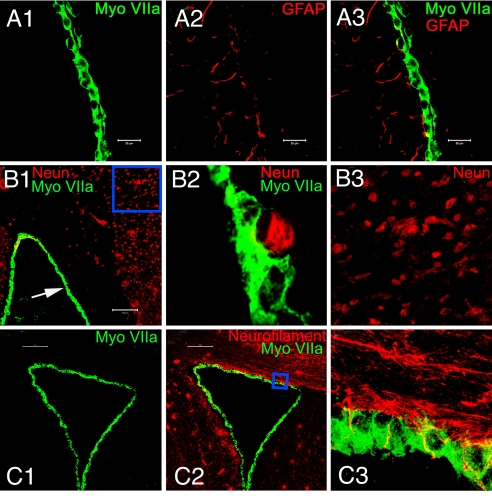
It is important to emphasize that the myosin VIIA-positive cells were only found in the ependymal layer of the LV. The cells are neither glial cells nor neurons. Instead, they are columnar, and take the shape of polarized epithelial cells (Fig. S1). These myosin VIIA-positive cells are distinct from glial cells and neurons, because they did not stain positively for the glial cell marker, glial fibrillary acidic protein (GFAP) (Fig. 3A1–3), and neuronal markers, such as NeuN (Fig. 3B1–3) or neurofilament (Fig. 3C1–3). Only a few end feet of glial cells could be seen extending to the ependymal cell layer, which surrounded the myosin VIIA labeled cell bodies, but did not penetrate into the cytoplasmic region of the cells. These results clearly indicate that these myosin VIIA-positive ependymal cells are neither neurons nor glial cells, but rather distinct epithelial cell-types.
Fig. 3.
Ependymal cells that were HC-marker positive, did not, in general, express glial cell and neuronal markers. Ependymal cells were labeled only with myosin VIIA (A1). GFAP demonstrated a distinct staining pattern in cells around ependymal cells (A2). Some GFAP positive cells extended to myosin VIIA positive ependymal cells (A3). Panel (B1) demonstrates that ependymal cells are not labeled with the mature neuronal marker, NeuN. Arrow indicates the nucleus of NeuN staining near an ependymal cell. The indicated area was enlarged in panel (B2), which shows that the NeuN labeling did not colocalize with ependymal cells. The rectangle area in panel (B1) was enlarged in panel (B3) to display the NeuN labeled neuronal cells. (C1–3) To verify that ependymal cells display the HC-phenotype and not the neuronal phenotype, an adult mouse brain section was double stained with myosin VIIA and another mature neuronal marker, neurofilament. Ependymal cells were specifically labeled with myosin VIIA (C1), but not neurofilament (C2). The panel shows neurofilament-positive nerve fibers in the LV. The rectangular area in panel (C2) was rescanned and enlarged in panel (C3), showing a projected image of serial scanned image frames. Panel (C3) not only demonstrates that ependymal cells do not express neuronal markers, it shows that nerve fibers make contacts with ependymal cells, in vivo. (Scale bars: A1–3, 20 μm; B1 and C1 and 2, 100 μm.)
Myosin VIIA-Positive Ependymal Cells Show Functional Characteristics of HCs and Can Incorporate into Cochlear Sensory Epithelia.
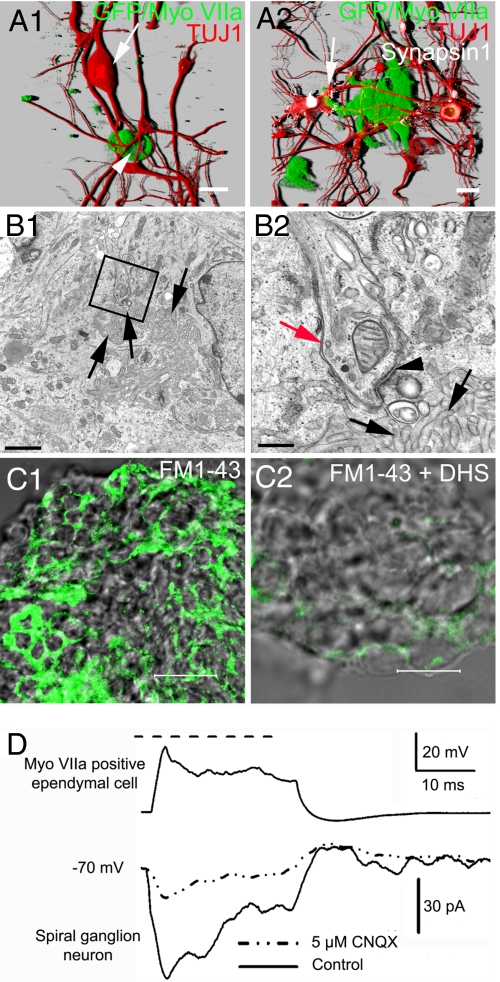
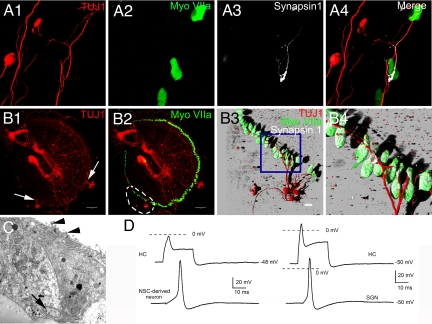
To further identify functional similarities between myosin VIIA-positive ependymal cells and inner ear HCs, we cocultured the ependymal cells from myosin VIIA-GFP transgenic mice with SGNs prepared from wild type mice. Ependymal cells established synapse-like contacts with SGNs (Fig. 4A1). Robust staining of synapsin 1 was observed at the sites of contact (Fig. 4A2; see enlarged image in Fig. S2). Transmission electron micrographs of serially sectioned cells illustrated characteristic synaptic structures such as; presynaptic vesicles, pre/postsynaptic membrane-associated density and synaptic thickening, and a specialized synaptic cleft (Fig. 4B1 and 2). Another similarity between ependymal cells and HCs is that ependymal cells express partially open large-conductance cation channels that are permeable to FM1–43 akin to mechano-sensitive channels in HCs. Ependymal cells show rapid (<60 s) FM1–43 uptake, which was inhibited by dihydrostreptomycin (26, 27) (Fig. 4C1 and 2). Finally, we recorded exemplary responses between myosin VIIA-positive ependymal cells (in 3 of 7 synapses) and SGNs (Fig. 4D). The fact that these synaptic responses were sensitive to a glutamate receptor blocker, CNQX (Fig. 4D), gives further credence to the tantalizing possibility that these in vitro results may mimic in vivo conditions (28).
Fig. 4.
Spiral ganglion neurons targeted myosin VIIA-positive ependymal cells to establish functional synaptic contacts. Adult SGNs and ependymal cells were cocultured to test whether they could recognize each other as the targets. To eliminate the possibility that the cocultured myosin VIIA-positive cells may originate from inner ear HCs, ependymal cells were collected from myosin VIIA-GFP transgenic mice and SGNs were collected from wild type mice (C57BL/6j), therefore, GFP staining in this figure indicates myosin VIIA positive ependymal cells. (A1) Shown is a 3-D reconstruction image of an adult SGN (arrow) projecting neurites to an ependymal cell (arrowhead). (A2) This panel demonstrates an enlarged nerve ending of an adult SGN connected to a cluster of ependymal cells. The accumulation of synapsin 1 at the enlarged nerve endings (arrow) of the adult SGN suggests that connections between SGNs and ependymal cells may form synapses. (B1 and 2) Ultra structure of synapses was found between cocultured SGNs and ependymal cells. The boxed area in panel (B1) demonstrates the synaptic ultra structure between a SGN and an ependymal cell, which is enlarged in panel (B2) to show detail. The black arrowhead indicates the postsynaptic thickens. The ependymal cell can be identified by its signature structure; the clustered cilia (black arrows). The red arrow indicates a nerve ending of the SGN. (C1) Merged transparent and fluorescence images of ependymal cells obtained after 60-sec exposure to FM1–43. Similar results were obtained by using shorter exposure time (<30 s; data not shown). (C2) FM1–43 loading of ependymal cells was inhibited after preexposure of the transduction channel blocker, dihydrostreptomycin (DHS) (240 sec; n = 6). (D) Dual recording from the connected myosin VIIA-positive ependymal cell (in current-clamp mode; 0.4 nA current injection) and SGN (in voltage-clamp at −70 mV holding potential). The synaptic current recorded in the SGN was sensitive to CNQX (dashed line), an AMPA receptor blocker. (Scale bars: A1 and 2, C1 and 2, 20 μm; B1, 2 μm; B2, 7 μm.)
Finally, to test whether ependymal cells can incorporate into cochlear sensory epithelia, we dissected from wild-type mouse (C57 BL/6), and the residual HCs were eliminated by using streptomycin treatment. As shown in Fig. S3 and Movie S1, ependymal cells incorporated well into the sensory epithelia, demonstrating their therapeutic potential.
NSCs from LV Differentiate into Functional Neurons with Defining Characteristics of SGNs.
Here, we tested whether NSCs from the SVZ, the very close neighbor of ependymal cell layer, can differentiate into neurons that share functional characteristics with SGNs. After in vitro differentiation, 55 ± 9% (mean ± SD, n = 9) of the NSCs isolated from the SVZ differentiated into neurons (Fig. S4). When cocultured with inner ear HCs, these neurons projected neurites to HCs and synapsin 1 accumulated at the nerve ending, suggesting the development of real synapses (Fig. 5A1–4). To further ascertain that NSC-derived neurons could establish synaptic contacts with HCs at the organ level, we first dissected the organ of Corti from the SGNs. The dissected organs of Corti were then incubated in β-bungarotoxin for 48 h to eliminate a substantial portion of the residual SGNs (29). Next, we carefully placed seeds of predifferentiated NSCs at the abneural aspects of the organ culture (Fig. 5B1–4). In accord with previous reports (29), β-bungarotoxin treatment eliminated most of the residual SGNs, as is reflected in minimal TUJ1 positive staining at the neural aspects of the organ of Corti (Fig. 5B1 and 2). After seven days in vitro, NSC-derived neurons extended neurites to innervate HCs (Fig. 5B2–4), fibers of NSC-derived neurons penetrated the organ of Corti making precise contact with HCs then stopping their growth and extension after reaching their targets. Also important, the branching pattern of neurites of NSC-derived neurons resembles a classic report by Retzius (30), whereby SGNs form multiple branches that undergo subsequent differential pruning. Hence, the in vitro innervation pattern of NSC-derived neurons on HCs resembles a microcosm of early development of cochlear ganglion neurons wherein neuronal fibers extend additional side branches, which are ultimately pruned in later neonatal stages (31). Synaptic connections between HCs and NSC-derived neurons were further verified by electron microscopic study. (Fig. 5C) Moreover, synaptic connections between HCs and NSC-derived neurons appeared functionally viable (Fig. 5D). Depolarization of HCs could elicit action potentials in neighboring NSC-derived neurons making synaptic contact. Similar responses were seen in adult SGNs (Fig. 5D), further establishing the authenticity and viability of these studies.
Fig. 5.
NSC-derived neurons demonstrated defining characteristics of SGNs. (A1–4) Adult NSCs were cocultured with HCs. Nerve endings of a NSC-derived neuron in contact with a cocultured HC. The accumulation of synapsin 1 at the nerve ending suggests that the contact between NSC-derived neurons and HCs may develop into a real synapse. (B1–4) NSC-derived neurons also established synapse-like contacts with HCs at the organ level. The organ of Corti was collected from P3 mice and SGNs were removed. To eliminate the residual SGNs, the dissected organ of Corti was treated with β-bungarotoxin (0.5 μM) for 48 h then cocultured with NSCs. Neuronal cells were labeled with TUJ1 and HCs were labeled with myosin VIIA. As panel (B1) demonstrates, residual SGNs were selectively removed from the organ of Corti after pretreatment with β-bungarotoxin. Nerve fibers of NSC-derived neurons (arrows) penetrated the organ of Corti and established contacts with HCs (B2). The dashed line marks region in panel (B2) that was enlarged and reconstructed into a 3-D image in panel (B3); a cluster of NSC-derived neurons projected fibers into the organ of Corti and integrated with HCs. The accumulation of synapsin 1 indicates that the contacts between HCs and NSC-derived neurons may develop into synapses. The boxed area in panel (B3) was further enlarged in panel (B4) to show the synapse-like contacts. (C) Ultra structure of the nerve endings of a NSC derived-neuron that contacted cocultured HCs (arrowheads indicate the stereocilia). Synaptic vesicles were found within the nerve ending (arrow). (D, left panel) Simultaneous current-clamp recordings from a HC and a NSC-derived neuron in close contact. The HC was injected with 0.7-nA positive current. The NSC-derived neuron was injected with a sustained negative current to establish a membrane potential of −83 mV. Under these conditions, sufficient depolarization of the HC elicited action potentials in the NSC-derived neuron. (D, right panel) Similar results could be seen by a HC depolarization of 0.8 nA, leading to a corresponding action potential from a SGN. For SGNs in culture, action potentials could be generated at −50 mV resting potential. (Scale bars: A1–4, B3, C1–4, 20 μm; B1 and 2, 100 μm.)
NSCs Establish Functional Synapses with Target-Deprived SGNs.
We determined whether NSC-derived neurons could establish functional synaptic connections with adult SGNs. To accomplish this, first we cocultured NSCs with adult SGNs (Fig. S5). The SGNs and NSC derived neurons can be handily distinguished by their sizes; SGNs are approximately 4-fold larger than NSC-derived neurons (Fig. S5A). Dendro-dentritic synapses were formed. Additionally, NSC-derived neurons appeared to act as interneurons, linking the deafferentated SGNs (Fig. S6). The expression of synapsin 1 was invariably restricted to the axo-dendritic and axo-somatic contacts, suggesting that these may be genuine synapses (Fig. S5 B1–C3). To test whether the synapse-like connections between SGNs and NSC-derived neurons could occur at the organ level, we cocultured cochlear explants containing SGNs and NSC-derived neurons. As shown in Fig. S5D1–3, NSC-derived neurons extended their neurites through the cochlear explant to establish connections with SGNs. The corresponding expression of synapsin 1 at the site of contact between the two neuronal subtypes suggested the formation of synapses. An ultrastructural study showed characteristic membrane-associated density and synaptic thickening. Moreover, a sizable proportion (85 ± 6%; n = 3) of the synapses were recognizable by other features, such as the apposition of the pre- and postsynaptic membranes, the presynaptic vesicle clusters and the specialization of the synaptic cleft (Fig. S5E1–3). These analyses verified that the synapses formed between NSC-derived neurons and SGNs are equipped with the synaptic machinery to be functional.
From this baseline, we used electrophysiological analysis to establish the operational status of synapses identified in vitro and to determine whether their properties are consistent with a bona fide synapse, albeit in culture. Despite the prolonged culture conditions (7–9 days), NSC-derived neurons and adult SGNs were electrically healthy, with mean resting membrane potentials of (in mV) −49 ± 6 and −56 ± 5 (n = 17), respectively. However, injection of positive current (1–50 pA) did not suffice to elicit action potentials in NSC-derived neurons. Because cell culture conditions can greatly influence the functional expression of ionic channels, in particular the down regulation of inward rectifier K+ currents that clamp the resting membrane voltage toward the K+ equilibrium potential (approximately −80 mV) (32), we expected that the measured resting membrane potential may have inactivated inward Na+ and Ca2+ currents that are responsible for the depolarization phase of action potentials. Predictably, injection of negative current to release the inward currents from inactivation resulted in the generation of robust action potentials. As illustrated in Fig. S5F, during dual recordings from NSC-derived neurons, that projected axons unto adult SGNs, elicited action potentials resulted in excitatory postsynaptic inward currents in the SGN. Analysis of the delays in synaptic events (0.6 ± 0.3 ms; n = 9) suggested that the synaptic activity may be mediated by a classic fast neurotransmitter. In 17 of 26 dual recordings, SGNs served as the postsynaptic neurons, whereas in the remaining nine, they operated as presynaptic neurons (data not shown).
Discussion
This article reports on the first extensive analyses using structural, molecular, and functional criteria to demonstrate that adult brain germinal zone cells, derived from the same neuro-ectodermal layer as the otic vesicle epithelial cells, preserve the potential to undergo a functional switch to replace the nonrenewable inner ear sensory cells, that is, HCs and SGNs.
Previous reports have demonstrated that the regenerative potential of HCs after damage is largely restricted to self-repair of stereocilary bundles (33, 34). Regenerative proliferation in inner ear sensory epithelia has been reported, but is limited due to the paucity of putative new HCs production (35). Drosophila atonal homologs, essential genes for inner HC development (36), have been used to stimulate HCs production from supporting cells (3, 23) and to provide modest improvements in the hearing function of guinea pigs (3). Overexpression of Math1 in postnatal rat cochlear explant cultures induces the production of extra HCs (23). Hes1 can negatively regulate HC differentiation by antagonizing Math1 (37, 38). It has been suggested that the sensory epithelia of the inner ear may be the only conducive niche for HC differentiation (2); however, the mechanisms underlying HC differentiation are so far not fully understood. Cell replacement therapy is one potential way to repopulate damaged HCs. Pluripotent inner ear stem cells and transplanted exogenous progenitor cells have been verified as candidate cells (2, 6–8, 39), but the controlled differentiation of stem cells into functional HCs is essential for hearing restoration.
In the present study, we provide evidence suggesting that ependymal cells of the LV have proliferative potential and that these cells have essential characteristics that liken them to HCs. They are polarized with actin-based stereocilia and microtubule-based kinocilia, can be identified by well-characterized and commonly used HC markers (40) and express large-conductance FM1–43-permeable channels that are blocked by dihydrostreptomycin (26). Indeed, our initial assessment of the electrophysiology of ependymal cells suggests that they may be mechanically sensitive. However, future experiments that allow precise mechanical stimulation of short and less-defined polarized stereociliary appendages are required. Also notably important, cells of the ependymal layer have several of the defining electrophysiological characteristics of HCs: They are electrically active, send synaptic input to target-deprived SGNs and are capable of releasing glutamate in response to membrane depolarization. The identity of myosin VIIA-positive cells in the LV remains unclear, but they are not likely to be of neuronal or astrocytic origin, because they are essentially nonreactive to antibodies for neuronal and glial markers. Since the ependymal cells are sculpted with substantial components of HC phenotypes, we propose that ependymal cells may undergo a functional switch to serve H-C roles in the inner ear.
In inner ears, SGNs depend on neurotrophic factors released by HCs for survival (41). The ensuing degeneration of neurons after HC loss renders the need to replace or regenerate deafferented SGNs. In cases of primary SGN loss, auditory HCs remain intact. Repopulation of lost SGNs with NSC-derived neurons may provide improvement of hearing rehabilitation. Previous studies have attempted to replace lost SGNs by transplanting neurons from other ganglia or stem cells from exogenous sources, such as embryonic stem cells and neural stem cells (42, 43). None of these preliminary studies have demonstrated functional targeting to HCs. NSCs show extensive self-renewal capacity and differentiate spontaneously into neural cells. Transplantation studies have demonstrated the role of environmental factors in the fate decisions of adult NSCs. Adult NSCs differentiate into glia when transplanted into nonneurogenic regions (e.g., spinal cord); however, they adopt a neuronal fate when transplanted into neurogenic niches (44). The mature inner ear is not an enriched environment for neuronal differentiation (42) and the transplantation of predifferentiated NSCs is more likely to provide an effective functional replacement. Our experiments demonstrate that, given favorable conditions, some NSCs from the SVZ of the LV (45) can develop into neurons with essential features of SGNs; they are bipolar neurons that form synapses with HCs. More importantly, they respond to synaptic inputs from HC and fire action potentials. These findings not only demonstrate that adult-derived stem cells retain biochemical and functional potentials akin to embryonic stem cells (46), they also reveal the immensely unknown potentials of NSCs in the auditory setting. In addition to neurotrophic factors, synaptic activities likely activate multiple prosurvival signaling pathways that regulate SGNs survival and neurite growth (47). In this study, NSC-derived neurons form synapses with SGNs and are capable of generating electrical activities, which may provide prosurvival signals to promote SGN survival and neurite growth and targeting.
To repopulate lost HCs in the auditory setting, we assert that ependymal cells can be introduced into the damaged inner ear, where they may reprogram their functions to replace lost HCs. Cotransplantation with NSCs from the same brain germinal zone may further facilitate the reconstitution of sensorineural circuits to achieve hearing restoration. The functional plasticity of renewable cells revealed in this study may open a new therapeutic avenue for other neural degenerative diseases.
Methods
See SI Materials and Methods for additional information, including Movie S1.
The care and use of animals in this study was approved by the Ethical Committees at the University of California, Davis. C57BL/6j mice were purchased from Charles River Laboratories. Myosin VIIA-GFP mice were generated by using standard methods (21). Cells of the lateral ventricular layers were isolated and neurospheres were generated and cultured as previously described (42). Neuronal differentiation was enhanced with 2 μM retinoic acid. Target deprived adult SGNs (from C57BL/6j mice) were isolated as described in ref. 5 and cocultured with ependymal cells prepared from myosin VIIA-GFP mice. To coculture of NSC derived neurons and HCs, NSC-derived neurons were obtained from the lateral ventricle, and the organ of Corti was isolated from p3–5 mice. Residual SGNs were eliminated by treatment with β-bungarotoxin (Sigma, 0.5 μM) for 48 h.
Standard scanning and transmission electron microscopic methods were used to examine the ultrastructure of cells. In addition, immunohistochemical techniques were used to examine the expression of cell-specific proteins. Assay for mechanosensory transduction used in our previous report (27) was adopted for ependymal cell.
Patch-clamp recordings in the current and voltage clamp modes were carried by using appropriate recording solutions as we have described in ref. 48.
Supplementary Material
Acknowledgments.
Drs. N. Chiamvimonvat and Hong Qiu provided constructive comments on the manuscript. This work was supported by a grant from the NIDCD (National Institutes of Health) (to E.N.Y.), California Institute of Regenerative Medicine Grant S-CIRMTG1-GSTDW (to D.W.); and RS1-00453 to (E.N.Y.), and Judy and David Wachs Grant in Auditory Science from National Organization of Hearing Research. The frozen normal adult human brain slice was obtained from the Pritzker/Conte human brain bank held at University of California, Davis.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808044105/DCSupplemental.
References
- 1.Ernfors P, Duan ML, ElShamy WM, Canlon B. Protection of auditory neurons from aminoglycoside toxicity by neurotrophin-3. Nat Med. 1996;2:463–467. doi: 10.1038/nm0496-463. [DOI] [PubMed] [Google Scholar]
- 2.Li H, Roblin G, Liu H, Heller S. Generation of hair cells by stepwise differentiation of embryonic stem cells. Proc Natl Acad Sci USA. 2003;100:13495–13500. doi: 10.1073/pnas.2334503100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Izumikawa M, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- 4.Shou J, Zheng JL, Gao WQ. Robust generation of new hair cells in the mature mammalian inner ear by adenoviral expression of Hath1. Mol Cell Neurosci. 2003;23:169–179. doi: 10.1016/s1044-7431(03)00066-6. [DOI] [PubMed] [Google Scholar]
- 5.Wei D, Jin Z, Jarlebark L, Scarfone E, Ulfendahl M. Survival, synaptogenesis, and regeneration of adult mouse spiral ganglion neurons in vitro. Dev Neurobiol. 2007;67:108–122. doi: 10.1002/dneu.20336. [DOI] [PubMed] [Google Scholar]
- 6.Jeon SJ, Oshima K, Heller S, Edge AS. Bone marrow mesenchymal stem cells are progenitors in vitro for inner ear hair cells. Mol Cell Neurosci. 2007;34:59–68. doi: 10.1016/j.mcn.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakagawa T, Ito J. Cell therapy for inner ear diseases. Curr Pharm Des. 2005;11:1203–1207. doi: 10.2174/1381612053507530. [DOI] [PubMed] [Google Scholar]
- 8.Doyle KL, Kazda A, Hort Y, McKay SM, Oleskevich S. Differentiation of adult mouse olfactory precursor cells into hair cells in vitro. Stem Cells. 2007;25:621–627. doi: 10.1634/stemcells.2006-0390. [DOI] [PubMed] [Google Scholar]
- 9.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 10.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 11.Laywell ED, Rakic P, Kukekov VG, Holland EC, Steindler DA. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc Natl Acad Sci USA. 2000;97:13883–13888. doi: 10.1073/pnas.250471697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morshead CM, Garcia AD, Sofroniew MV, van Der Kooy D. The ablation of glial fibrillary acidic protein-positive cells from the adult central nervous system results in the loss of forebrain neural stem cells but not retinal stem cells. Eur J Neurosci. 2003;18:76–84. doi: 10.1046/j.1460-9568.2003.02727.x. [DOI] [PubMed] [Google Scholar]
- 13.Sanai N, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 14.Stankovic K, et al. Survival of adult spiral ganglion neurons requires erbB receptor signaling in the inner ear. J Neurosci. 2004;24:8651–8661. doi: 10.1523/JNEUROSCI.0733-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zecevic N. Specific characteristic of radial glia in the human fetal telencephalon. Glia. 2004;48:27–35. doi: 10.1002/glia.20044. [DOI] [PubMed] [Google Scholar]
- 16.Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- 17.Ke Y, Chi L, Xu R, Luo C, Gozal D, Liu R. Early response of endogenous adult neural progenitor cells to acute spinal cord injury in mice. Stem Cells. 2006;24:1011–1019. doi: 10.1634/stemcells.2005-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang RL, et al. Stroke induces ependymal cell transformation into radial glia in the subventricular zone of the adult rodent brain. J Cereb Blood Flow Metab. 2007;27:1201–1212. doi: 10.1038/sj.jcbfm.9600430. [DOI] [PubMed] [Google Scholar]
- 19.Spassky N, Merkle FT, Flames N, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiasson BJ, Tropepe V, Morshead CM, van der Kooy D. Adult mammalian forebrain ependymal and subependymal cells demonstrate proliferative potential, but only subependymal cells have neural stem cell characteristics. J Neurosci. 1999;19:4462–4471. doi: 10.1523/JNEUROSCI.19-11-04462.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boeda B, Weil D, Petit C. A specific promoter of the sensory cells of the inner ear defined by transgenesis. Hum Mol Genet. 2001;10:1581–1589. doi: 10.1093/hmg/10.15.1581. [DOI] [PubMed] [Google Scholar]
- 22.Hasson T, et al. Unconventional myosins in inner-ear sensory epithelia. J Cell Biol. 1997;137:1287–1307. doi: 10.1083/jcb.137.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- 24.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 25.Knirsch M, et al. Persistence of Ca2+ channels in mature outer hair cells supports outer hair cell afferent signaling. J Neurosci. 2007;27:6442–6451. doi: 10.1523/JNEUROSCI.5364-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyers JR, et al. Lighting up the senses: FM1–43 loading of sensory cells through nonselective ion channels. J Neurosci. 2003;23:4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Si F, Brodie H, Gillespie PG, Vazquez AE, Yamoah EN. Developmental assembly of transduction apparatus in chick basilar papilla. J Neurosci. 2003;23:10815–10826. doi: 10.1523/JNEUROSCI.23-34-10815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glowatzki E, Fuchs PA. Transmitter release at the hair cell ribbon synapse. Nat Neurosci. 2002;5:147–154. doi: 10.1038/nn796. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Monedero R, Corrales CE, Cuajungco MP, Heller S, Edge AS. Reinnervation of hair cells by auditory neurons after selective removal of spiral ganglion neurons. J Neurobiol. 2006;66:319–331. doi: 10.1002/neu.20232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Retzius G. To the development of the cells of spiral ganglia neurons and for the ending way of the Gehornerven (dye) with the Saugethieren. Biol. Examine. 1893;4:52–57. [Google Scholar]
- 31.Huang LC, Thorne PR, Housley GD, Montgomery JM. Spatiotemporal definition of neurite outgrowth, refinement and retraction in the developing mouse cochlea. Development. 2007;134:2925–2933. doi: 10.1242/dev.001925. [DOI] [PubMed] [Google Scholar]
- 32.Nichols CG, Lopatin AN. Inward rectifier potassium channels. Annu Rev Physiol. 1997;59:171–191. doi: 10.1146/annurev.physiol.59.1.171. [DOI] [PubMed] [Google Scholar]
- 33.Zheng JL, Keller G, Gao WQ. Immunocytochemical and morphological evidence for intracellular self-repair as an important contributor to mammalian hair cell recovery. J Neurosci. 1999;19:2161–2170. doi: 10.1523/JNEUROSCI.19-06-02161.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gale JE, Meyers JR, Periasamy A, Corwin JT. Survival of bundleless hair cells and subsequent bundle replacement in the bullfrog's saccule. J Neurobiol. 2002;50:81–92. doi: 10.1002/neu.10002. [DOI] [PubMed] [Google Scholar]
- 35.Warchol ME, Lambert PR, Goldstein BJ, Forge A, Corwin JT. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science. 1993;259:1619–1622. doi: 10.1126/science.8456285. [DOI] [PubMed] [Google Scholar]
- 36.Bermingham NA, et al. Math1: An essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- 37.Lim DA, Tramontin AD, Trevejo JM, Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 38.Ishibashi M, Ang SL, Shiota K, Nakanishi S, Kageyama R, Guillemot F. Targeted disruption of mammalian hairy and Enhancer of split homolog-1 (HES-1) leads to up-regulation of neural helix-loop-helix factors, premature neurogenesis, and severe neural tube defects. Genes Dev. 1995;9:3136–3148. doi: 10.1101/gad.9.24.3136. [DOI] [PubMed] [Google Scholar]
- 39.Li H, Liu H, Heller S. Pluripotent stem cells from the adult mouse inner ear. Nat Med. 2003;9:1293–1299. doi: 10.1038/nm925. [DOI] [PubMed] [Google Scholar]
- 40.Hasson T, Heintzelman MB, Santos-Sacchi J, Corey DP, Mooseker MS. Expression in cochlea and retina of myosin VIIa, the gene product defective in Usher syndrome type 1B. Proc Natl Acad Sci USA. 1995;92:9815–9819. doi: 10.1073/pnas.92.21.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fritzsch B, Silos-Santiago I, Bianchi LM, Farinas I. Effects of neurotrophin and neurotrophin receptor disruption on the afferent inner ear innervation. Semin Cell Dev Biol. 1997;8:277–284. [PubMed] [Google Scholar]
- 42.Hu Z, et al. Survival and neural differentiation of adult neural stem cells transplanted into the mature inner ear. Exp Cell Res. 2005;302:40–47. doi: 10.1016/j.yexcr.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 43.Regala C, Duan M, Zou J, Salminen M, Olivius P. Xenografted fetal dorsal root ganglion, embryonic stem cell and adult neural stem cell survival following implantation into the adult vestibulocochlear nerve. Exp Neurol. 2005;193:326–333. doi: 10.1016/j.expneurol.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 44.Shihabuddin LS, Horner PJ, Ray J, Gage FH. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J Neurosci. 2000;20:8727–8735. doi: 10.1523/JNEUROSCI.20-23-08727.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alvarez-Buylla A, Lim DA. For the long run: Maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 46.Song HJ, Stevens CF, Gage FH. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat Neurosci. 2002;5:438–445. doi: 10.1038/nn844. [DOI] [PubMed] [Google Scholar]
- 47.Roehm PC, Hansen MR. Strategies to preserve or regenerate spiral ganglion neurons. Curr Opin Otolaryngol Head Neck Surg. 2005;13:294–300. doi: 10.1097/01.moo.0000180919.68812.b9. [DOI] [PubMed] [Google Scholar]
- 48.Levic S, Nie L, Tuteja D, Harvey M, Sokolowski BH, Yamoah EN. Development and regeneration of hair cells share common functional features. Proc Natl Acad Sci USA. 2007;104:19108–19113. doi: 10.1073/pnas.0705927104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.