Abstract
Chk1 is widely known as a DNA damage checkpoint signaling protein. Unlike many other checkpoint proteins, Chk1 also plays an essential but poorly defined role in the proliferation of unperturbed cells. Activation of Chk1 after DNA damage is known to require the phosphorylation of several C-terminal residues, including the highly conserved S317 and S345 sites. To evaluate the respective roles of these individual sites and assess their contribution to the functions of Chk1, we used a gene targeting approach to introduce point mutations into the endogenous human CHK1 locus. We report that the essential and nonessential functions of Chk1 are regulated through distinct phosphorylation events and can be genetically uncoupled. The DNA damage response function of Chk1 was nonessential. Targeted mutation of S317 abrogated G2/M checkpoint activation, prevented subsequent phosphorylation of Chk1, impaired efficient progression of DNA replication forks, and increased fork stalling, but did not impact viability. Thus, the nonessential DNA damage response function of Chk1 could be unambiguously linked to its role in DNA replication control. In contrast, a CHK1 allele with mutated S345 did not support viability, indicating an essential role for this residue during the unperturbed cell cycle. A distinct, physiologic mode of S345 phosphorylation, initiated at the centrosome during unperturbed mitosis was independent of codon 317 status and mechanistically distinct from the ordered and sequential phosphorylation of serine residues on Chk1 induced by DNA damage. Our findings suggest an essential regulatory role for Chk1 phosphorylation during mitotic progression.
Keywords: ATR, centrosome, phosphorylation, mitosis, gene targeting
Chk1 is a critical transducer of signals that arise at exogenously induced DNA strand breaks, but is also required for normal cell growth (1). Chk1 appears to function during at least two phases of the unperturbed cell cycle: S phase and mitosis. During unperturbed S phase, Chk1 controls the progress of DNA replication. Reduced Chk1 activity results in increased replication-associated DNA strand breaks (2), impaired replication fork progression, and increased fork stalling (3, 4). It has been suggested that the role of Chk1 in DNA replication control may be critical for cell proliferation (2).
As a checkpoint protein, Chk1 is known to negatively regulate the G2/M transition (1, 5). Recent evidence suggests that, in addition to regulating S phase progression and mitotic entry, Chk1 is required for completion of unperturbed mitosis (6). Transient depletion of Chk1 by RNAi in synchronized cells results in a block at the subsequent metaphase (7). It would therefore appear likely that the role of Chk1 in promoting mitotic progression beyond metaphase is essential for cell division and continued proliferation. Whether the essential role of Chk1 is during S phase, mitosis, or both remains an open question (2, 7–9).
Although cells synchronized with nocodazole exhibit increased levels of Chk1 phosphoprotein (10, 11), a functional requirement for Chk1 phosphorylation during unperturbed mitosis has not been shown. Notably, the upstream Chk1 kinase ATR phosphorylates Chk1 on both S317 and S345 (12), and is similarly essential for cellular viability (13). Phosphorylation of Chk1 regulates its intracellular location (14–16). After DNA damage, Chk1 is phosphorylated upon multiple C-terminal residues, including S317 and S345 (1, 12), released from chromatin, and accumulates at the centrosome, where it is thought to prevent activation of CDK1 and entry into mitosis (17, 18). It is unknown if Chk1 is similarly regulated after DNA damage and during the unperturbed cell cycle.
We have taken a genetic approach to evaluate the individual roles of the S317 and S345 Chk1 phosphorylation sites in human cells. By targeting endogenous CHK1 alleles, we were able to functionally uncouple the essential and nonessential functions of Chk1 and distinguish a new mechanism for Chk1 activation during normal cell division, one that is qualitatively distinct from its regulation in response to DNA damage. Cumulatively, our findings support an essential role for Chk1 during mitotic progression.
Results
Targeting Chk1 Phosphorylation Sites in Human Cells.
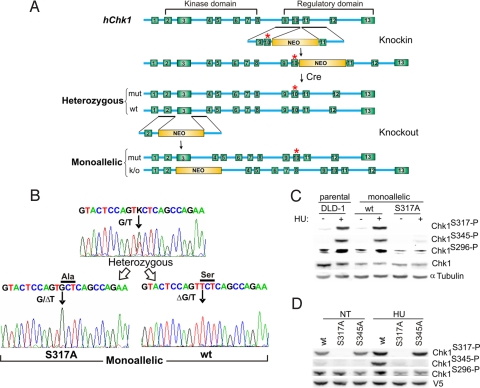
To assess the functional roles of the S317 and S345 Chk1 phosphorylation sites in human cells, we used a knockin/knockout approach to alter endogenous CHK1 alleles (Fig. 1A). This strategy was applied to the colorectal cancer cell line DLD-1, which has been extensively used for cell cycle analysis and has been shown to exhibit a high rate of recombinant adeno-associated virus (rAAV)-mediated gene targeting (19). DLD-1 cells are near diploid and harbor two wild-type CHK1 alleles. In the first gene-targeting step, S to A codon substitutions were introduced into endogenous CHK1 alleles by knockin vectors. Multiple heterozygous cell lines harboring single S317A or S345A alleles were obtained (supporting information (SI) Fig. S1). A second rAAV vector, designed to delete exon 3, which encodes the majority of the kinase domain, was then used to inactivate one CHK1 allele, resulting in monoallelic cell lines exclusively expressing either wild-type or mutated Chk1 (Fig. 1A). In cells harboring a single knockin S317A allele, the subsequent CHK1 knockout attempt resulted in the generation of four homologous recombinants, of which two clones exclusively expressed the S317A mutant (Fig. 1B). The successful derivation of both predicted cell types confirmed that the knockout construct integrated into both CHK1 alleles present in DLD-1 cells at a similar frequency.
Fig. 1.
S317-dependent sequential phosphorylation of Chk1 serine residues after HU treatment. (A) Generation of human cancer cells expressing a single CHK1 allele by homologous recombination via a knockin/knockout approach. rAAV targeting vectors harboring engineered S to A mutations in codon 317 (S317A) or codon 345 (S345A) were used to derive clones containing one targeted (*mutant) allele and one wild-type allele. Excision of the selectable marker by transient expression of the Cre recombinase resulted in expression of both mutant and wild-type alleles. Integration of a knockout vector directed against the kinase domain encoded by exon 3 resulted in monoallelic expression of Chk1. (B) Allelic Chk1 expression. Heterozygous cells expressing both wild-type and S317A alleles, and monoallelic cells were genotyped by RT-PCR and DNA sequencing. Cells exclusively expressing either G or T in position 1128 (NM_001274) were recovered. (C) Endogenous Chk1 phosphorylation in parental and monoallelic cell lines, as detected by immunoblot. Cells treated with 0.5 mM HU for 3 h were lysed and probed with phospho-Chk1-specific antibodies, or with an antibody against total Chk1 protein, as indicated. α-Tubulin was assessed as a loading control. (D) Phosphorylation of exogenous Chk1-V5 proteins in HEK293 cells. Cells treated with 0.5 mM HU for 3 h and untreated controls (NT) were lysed, and precipitated proteins were probed as in (C). Protein expression and recovery was evaluated by immunoblotting with an antibody directed against the V5 epitope.
DLD-Chk1S317A monoallelic cells were viable and showed no obvious growth defects. In contrast, attempts to knock out the remaining wild-type allele in S345A heterozygous cells were unsuccessful; no clones that exclusively expressed Chk1 S345A were recovered after multiple knockout experiments that generated a total of 15 homologous recombinants (P < 0.02). Rather, all clones inactivated the mutant S345A allele and expressed only the wild-type transcript. These findings suggest that the Chk1 S317A allele was able to support viability in the absence of wild-type Chk1, and that the S345A mutation may affect an essential function of Chk1. These findings are consistent with recent work by Niida et al. (10), who showed that the lethal phenotype of CHK1 knockout embryonic mouse cells could be rescued by exogenously expressed Chk1 mutated at S317A but not Chk1 mutated at S345A.
Loss of Signaling to S317 Impairs Chk1 Phosphorylation and Responses to DNA Damage.
Parental DLD-1 and monoallelic DLD-Chk1WT cells exhibited robust phosphorylation on S317, S345, and S296 after treatment with the DNA replication inhibitor hydroxyurea (HU) (Fig. 1C). DLD-Chk1S317A monoallelic cells were deficient in S317 phosphorylation, as expected, but also exhibited strikingly reduced HU-dependent phosphorylation on the flanking sites S296 and S345. We tested the phosphorylation status of S345A-Chk1 mutant by expressing epitope-tagged Chk1 proteins (Chk1-V5) in HEK293 cells. A low level of Chk1 S317 and S296 phosphorylation could be detected in wild-type Chk1-V5 in the absence of a stimulus; however, phosphorylation on S296, S317, and S345 all increased significantly after treatment with HU (Fig. 1D). The Chk1-V5 protein containing the S317A mutation exhibited markedly defective HU-dependent phosphorylation at the S296 and S345 sites, thus recapitulating the effects observed in DLD-Chk1S317A cells (Fig. 1C). Notably, levels of HU-dependent phosphorylation of S317 in the Chk1-V5 wild-type and S345A mutant proteins were similar (Fig. 1D). Therefore, though phosphorylation of S317 was required for phosphorylation of the flanking sites S296 and S345 in response to DNA replication inhibition, this relationship was not reciprocal. These findings suggest the phosphorylation of Chk1 after DNA damage is a cooperative process, with S317 phosphorylation required for subsequent phosphorylation of S345 and S296. Phosphorylation of all these residues may be required for Chk1-dependent DNA damage responses.
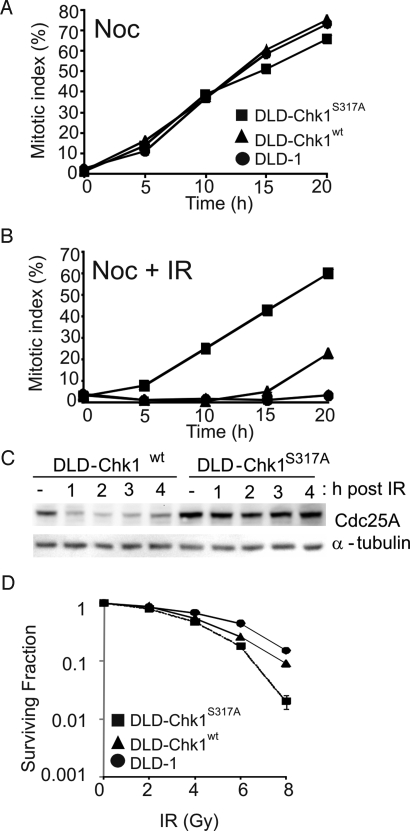
The generation of viable monoallelic cells expressing only the Chk1 S317A mutant allowed us to examine the functional consequences of S317 phosphorylation. Chk1 is known to be a critical component of G2/M arrest triggered by ionizing radiation (IR) (1, 5). DLD-Chk1S317A cells entered mitosis normally and were trapped effectively by nocodazole, a microtubule inhibitor (Fig. 2A). These cells exhibited near complete loss of the IR-dependent G2/M checkpoint compared with wild-type monoallelic and parental controls (Fig. 2B). Critical substrates of activated Chk1 are the Cdc25 phosphatases, which remove inhibitory phosphates from cyclin-dependent kinases and thereby facilitate cell cycle transitions (20). IR triggers rapid degradation of Cdc25A via activated Chk1 (21, 22). DLD-Chk1S317A cells were strikingly defective in Cdc25A degradation in response to IR (Fig. 2C). Viability after IR was modestly lower in DLD-Chk1S317A cells (Fig. 2D), to a similar extent to what has been previously observed in isogenic cells deficient in ATR (23).
Fig. 2.
S317 is required for the G2/M checkpoint in response to IR. (A and B) Activation of the G2/M checkpoint. Parental DLD-1 and Chk1 monoallelic cells DLD-Chk1WT and DLD-Chk1S317A were incubated in the presence of nocodazole (0.2 μg/ml) for the times indicated. Cells were untreated (A) or treated with 12 Gy IR (B). Cells were fixed and stained with Hoechst 33258. The mitotic index was determined by fluorescence microscopy. Approximately 200 cells were counted for each time point. (C) Degradation of Cdc25A. Following exposure to 12 Gy IR, monoallelic cells DLD-Chk1WT and DLD-Chk1S317A were lysed and probed with anti-Cdc25A antibody. α-Tubulin was assessed as a loading control. (D) Clonogenic survival following IR treatment. Cells were plated at low density and treated with the indicated doses of IR. Colony formation was assessed 14 d after treatment. Surviving fraction was determined after normalization to untreated controls. Each data point was derived from three independent plates.
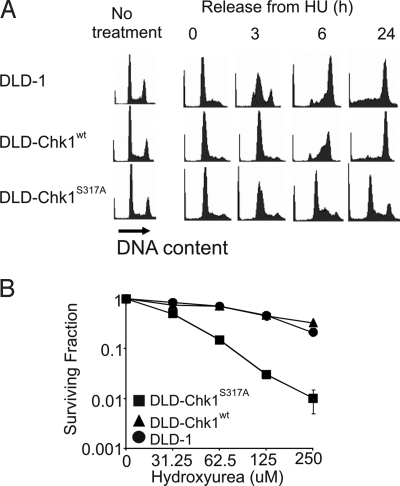
To examine the role of S317A phosphorylation in the overall progression of the cell cycle after DNA replication inhibition, we arrested cells in G1 and S phases by treatment with HU and then released them into fresh medium containing nocodazole. Irrespective of CHK1 genotype, all cells exhibited similar cell cycle profiles both before and immediately following HU treatment (Fig. 3A). The mitotic indices of all cells were less than 5% at 24 h in HU (data not shown). Following release from HU, cells harboring two copies of wild-type CHK1 (DLD-1) progressed rapidly through S phase and attained 4N DNA content, whereas cells harboring one active copy of CHK1 appeared to progress somewhat more slowly, as was most apparent at the 3 h time point (Fig. 3A). This effect is consistent with CHK1 haploinsufficiency, which has previously been observed in other Chk1-dependent phenotypes (24). Though all cells expressing wild-type Chk1 attained 4N DNA content by 24 h, DLD-Chk1S317A cells largely failed to progress through the cell cycle even at this late time point, and most cells in this population persistently exhibited a DNA content of 2N. DLD-Chk1S317A cells were similarly defective for recovery from a double block with thymidine and aphidicolin, suggesting progression through early S phase was impaired (data not shown). The S317A mutant protein was stable in response to HU treatment (Fig. S2), indicating that the impaired cell cycle progression in DLD-Chk1S317A cells was not due to abnormal protein turnover. This defective response to HU was mirrored by markedly decreased clonogenic survival of DLD-Chk1S317A cells over a range of HU doses (Fig. 3B).
Fig. 3.
Monoallelic cells expressing Chk1 S317A are defective for recovery from the DNA replication inhibitor HU. (A) Cell cycle distribution following HU treatment and release. Parental and monoallelic cells were treated with HU for 24 h, then washed and incubated in fresh medium containing nocodazole. DNA content was assessed by flow cytometry. (B) Clonogenic survival following HU treatment. Cells were treated with HU at the indicated concentrations for 48 h. Following treatment, washed cells were replated in drug-free medium. Colony formation was assessed 14 d following replating. Surviving fraction was determined after normalization to untreated controls. Each data point was derived from three independent plates.
S317 Is Required for DNA Replication Fork Progression and Stability.
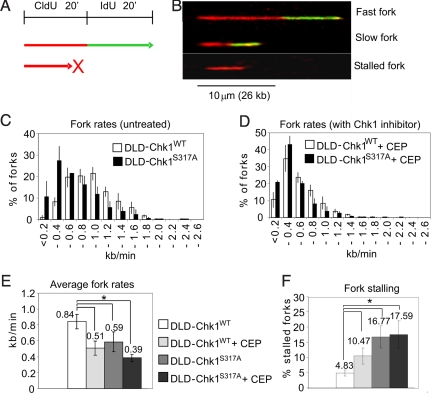
Previous studies have shown that Chk1 is required during normal S phase to control initiation of DNA replication (2) and replication fork progression (4). To examine whether the DNA damage response function of Chk1 is required during unperturbed DNA replication, we measured replication fork progression in our cell panel by DNA combing. Asynchronous DLD-Chk1S317A cells and DLD-Chk1WT controls were sequentially pulse labeled with CldU and IdU (Fig. 4A). The length of DNA replication tracts in combed DNA fibers was measured by immunofluorescence microscopy, allowing the assessment of fork speed and fork stalling (Fig. 4B). Compared with monoallelic controls, DLD-Chk1S317A cells exhibited slower replication fork progression (Fig. 4 C and E). The average fork rate of both cell lines was reduced to a similar extent by CEP-3891, a Chk1 inhibitor (Fig. 4 D and E), confirming that fork rate was highly dependent on Chk1 activity. DLD-Chk1S317A cells also exhibited a significantly increased proportion of stalled forks from 4.83% in DLD-Chk1wt to 16.77% in DLD-Chk1S317A (Fig. 4F). In contrast, origin firing density appeared to be similar in DLD-Chk1S317A and DLD-Chk1WT cells (Fig. S3). These findings suggest that phosphorylation at Chk1 S317 is required for efficient DNA replication fork progression. Though the defects observed in DLD-Chk1S317A cells are clearly not lethal during unperturbed cell growth, they likely contribute to the inability of these cells to restart the cell cycle after HU treatment. To further assess the functional significance of Chk1 phosphorylation during S-phase progression, we examined the role of ATR. Isogenic cells harboring biallelic hypomorphic mutations in ATR (23) exhibited an intermediate phenotype relative to DLD-Chk1WT and DLD-Chk1S317A cells (Fig. S4), including slow fork rates and increased fork stalling. These findings are commensurate with reduced levels of ATR protein and S317 phosphorylation in these cells (23) as well as defective recovery from HU treatment (25).
Fig. 4.
Monoallelic cells expressing Chk1 S317A exhibit slowed replication fork progression and increased replication fork stalling in unperturbed cells. (A) Schematic representation of double-labeling strategy. Nascent DNAs were sequentially labeled with CldU and IdU for 20 m each. (B) Labeled DNA fibers were measured via confocal microscopy (4) allowing the discrimination of fast forks (long labeled tracts), slow forks (short tracts), and stalled forks (red-only tracts). (C and D) Distribution of fork rates in monoallelic cells, DLD-Chk1WT and DLD-Chk1S317A. Cells were untreated (C) or treated with the Chk1 inhibitor CEP-3891 (D) before labeling. (E) Average fork rates in DLD-Chk1WT and DLD-Chk1S317A cells treated or not with CEP-3891. Values connected by lines are significantly different (Student's t test, P < 0.03). (F) Stalled forks as percentage of all CldU-labeled tracks. Values connected by lines are significantly different (P < 0.04). The means and standard deviations of three independent repeats are shown.
Chk1 S345 Is Phosphorylated during Unperturbed Mitosis.
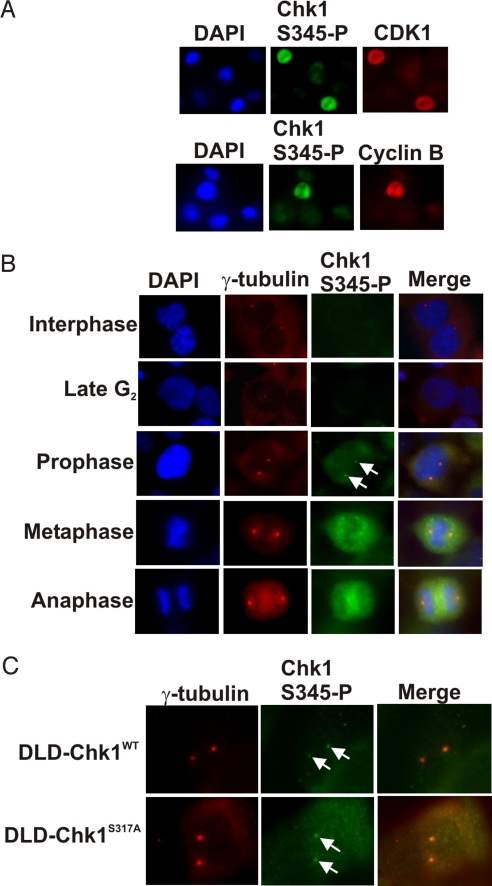
It is unclear whether phosphorylation of Chk1 is required during normal cell cycles. Our inability to derive cells expressing only the Chk1 S345A mutant suggested that phosphorylation of the highly conserved S345 is related to an essential function of Chk1. To identify clues as to the physiologic role of S345 phosphorylation, we assessed endogenous Chk1 S345 phosphoprotein (Chk1S345-P) during the unperturbed cell cycle by immunofluorescence. The abundance of Chk1S345-P was generally low (Fig. 5 A and B). A significantly greater intensity of staining was observed in cells that were in mitosis (Fig. 5A). Furthermore, foci of Chk1S345-P were observed during prophase (Fig. 5B). These foci corresponded to the positions of centrosomes, as assessed by costaining with an antibody directed against γ-tubulin. Centrosomal localization of Chk1S345-P appeared to be tightly restricted to prophase; such colocalization was not observed during G2, when centrosomes begin to separate before chromosome condensation, or during subsequent stages of mitosis (Fig. 5B). We reasoned that if phosphorylation of S345 is a critical step in an essential process, centrosomal localization of Chk1S345-P during prophase should be observable in DLD-Chk1S317A cells. Indeed, DLD-Chk1S317A cells exhibited centrosomal foci of Chk1S345-P during prophase (Fig. 5C). Therefore, in contrast to the phosphorylation of S345 that occurred following DNA damage or DNA replication inhibition, physiological phosphorylation of S345 at the centrosome was independent of codon 317 status.
Fig. 5.
Phosphorylation of Chk1 S345 during unperturbed mitosis. (A) Asynchronous H1299 lung carcinoma cells were stained with a DNA stain (DAPI) and antibodies specific for active CDK1, Cyclin B, and Chk1 phosphorylated on S345 (Chk1S345-P), as indicated. (B) H1299 cells were stained for Chk1S345-P and the centrosomal protein γ-tubulin. Shown are representative images from various phases of the cell cycle, as determined by nuclear morphology. Chk1S345-P foci are indicated (arrows). (C) Colocalizing foci of γ-tubulin and Chk1S345-P (arrows) are shown during prophase in monoallelic DLD-Chk1WT and DLD-Chk1S317A cells.
Discussion
Chk1 is a critical mediator of the cell cycle. Insights into Chk1 regulation are therefore of biological importance and clinical relevance. Here, we genetically dissect the mechanism of activation of Chk1 in response to DNA damage and describe a novel mode of Chk1 regulation during unperturbed cell cycle.
We observed S317-dependent, ordered, and sequential phosphorylation events in response to DNA damage (Fig. 1 C and D). These events were required for normal IR-dependent G2/M checkpoint responses (Fig. 2) and recovery from HU-mediated DNA replication inhibition (Fig. 3). In addition, we observed slower replication fork progression and increased fork stalling during unperturbed S phase in DLD-Chk1S317A cells (Fig. 4). It has been shown previously that Chk1 is required to maintain normal rates of replication fork progression in the absence of DNA damage (4). Here we show that this function was regulated by S317-dependent phosphorylation and thus tightly linked to the ability of Chk1 to respond to DNA damage. This coupled relationship presumably reflects a role during S phase in which Chk1 responds to endogenous lesions or DNA tracts that are difficult to replicate. Our findings do not exclude the possibility that an S317-independent but essential role for Chk1 in DNA replication control may exist.
Our exhaustive attempts to create cell lines expressing only Chk1-S345A were unsuccessful despite clear verification that the same approach, applied in parallel, reliably yielded cells monoallelic for Chk-S317A. This finding strongly implicates S345 as a critical element of the essential function of Chk1. Our observation of S317-independent phosphorylation of S345 during mitosis, initiating at the centrosome, suggests a distinct role for Chk1 in promoting the progression of normal cell division. The centrosome is a primary site of mitotic regulation (26). During interphase a fraction of intracellular Chk1 protein is located at the centrosome where it is thought to prevent premature activation of Cyclin B/cyclin-dependent kinase 1 (Cdk1) (9). During unperturbed cell cycles, this inhibition is relieved at the onset of mitosis, during prophase, when Chk1 disassociates from the centrosome. Exogenous DNA damage causes an increase in centrosomal Chk1, which is thought to contribute to the G2/M checkpoint (9, 10, 18). In summary, though DNA damage-associated phosphorylation of Chk1 appears to promote its centrosomal localization (10, 15), our findings show that phosphorylation of Chk1 at S345 during unperturbed mitosis is coincident with the previously observed dissociation of Chk1 from the centrosome (9). Accordingly, we do not observe significant localization of pChk1S345 at the centrosome after prophase (Fig. 5B).
We propose that by two distinct mechanisms, distinguishable by phosphorylation site requirements, Chk1 can either impede mitotic entry or promote progression to metaphase. Notably, Cyclin B-Cdk1 activity increases on the centrosome during prophase (26), exactly the point in the cell cycle at which we observe Chk1 S345 phosphorylation (Fig. 5B), and others have observed its disassociation (9). In addition to the centrosome-associated Chk1 pool, a separate fraction of Chk1 in unperturbed cells is bound to chromatin (14). It has been shown that phosphorylation of multiple Chk1 sites causes dissociation of this chromatin-bound Chk1 pool (14). Our findings suggest, but do not prove, that a distinct mode of Chk1 phosphorylation may similarly be required for centrosome dissociation.
Following DNA damage and replication inhibition, Chk1 is phosphorylated by ATR and ATM (1, 12, 22, 27). Though it is possible that a distinct kinase is the activator of Chk1 during unperturbed mitosis, ATR, like Chk1, is essential for unperturbed growth and would therefore be the most likely candidate for the physiological Chk1 kinase. S345 is commonly used as a biomarker of Chk1 activation. We show that S345 phosphorylation is highly dependent on prior phosphorylation of S317 in response to DNA damage. It would appear likely that phosphorylation of S317 after DNA damage creates a conformational change that favors subsequent phosphorylation of neighboring sites and rapid Chk1 activation. Structural studies of Chk1 have thus far focused on the N-terminal kinase domain (28). Our study suggests that detailed analysis of the C terminus of human Chk1 and the consequences of individual phosphorylation events are likely to provide new insights into Chk1 regulation.
Chk1 has been a molecular target for the development of novel cancer therapeutics (29). The finding that the essential functions of Chk1 can be uncoupled from its DNA damage response functions has clear relevance to these ongoing efforts. All of the Chk1 inhibitors currently in clinical trials target sites directly involved in kinase function or ATP binding (29). Pharmacological uncoupling of the DNA damage response of Chk1 from its essential function would be a alternative strategy that might yield therapeutics with lower toxicity. Inhibition of phosphorylation of S317 but not S345 would inhibit the ability of Chk1 to respond to DNA damage from DNA-damaging drugs or radiation therapy, but preserve essential Chk1 activities. Loss of the S317 phosphorylation site enhanced the effects of HU on long-term survival (Fig. 3B) and may similarly potentiate the effects of commonly used therapies.
Materials and Methods
Gene Targeting.
Endogenous Chk1 alleles were altered in the colorectal cancer cell line DLD-1 by recombinant adeno-associated virus (rAAV)-based gene targeting (30). For the construction of targeting vector homology arms, genomic regions of 1–1.6 kb were amplified from genomic DNA, and site-directed mutagenesis was used to introduce point mutations within the codons for S317 or S345, thereby converting them into A codons (S317A and S345A, respectively). Homology arms were ligated to the SEPT selectable marker (30) to create the AAV shuttle constructs pAAV-Chk1S317A and pAAV-Chk1S345A. Virus production, infection, selection, the screening of homologous integrants, and Cre-mediated excision of the selectable marker was performed as described (19). A second round of gene targeting was performed with an rAAV-based knockout construct designed to disrupt exon 3, pAAV-ΔChk1. RNA was extracted from identified knockout clones (19) with the RNAeasy kit (Invitrogen), and cDNA amplification was performed with SuperScript II (Invitrogen).
Supporting Information.
Cell culture, transfection protocols, antibodies, immunoblotting, immunoflourescence, and DNA combing methods are described in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We gratefully acknowledge the expert assistance of the staff of the Kimmel Cancer Center Cell Imaging Core Facility. This work was supported by grants to F.B. from the National Institutes of Health (CA104253) and the Flight Attendant Medical Research Institute. D.W. is the recipient of a Ruth L. Kirchstein National Research Service Award (CA119724).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806917106/DCSupplemental.
References
- 1.Liu Q, et al. Chk1 is an essential kinase that is regulated by ATR and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14:1448–1459. [PMC free article] [PubMed] [Google Scholar]
- 2.Syljuasen RG, et al. Inhibition of human Chk1 causes increased initiation of DNA replication, phosphorylation of ATR targets, and DNA breakage. Mol Cell Biol. 2005;25:3553–3562. doi: 10.1128/MCB.25.9.3553-3562.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maya-Mendoza A, Petermann E, Gillespie DA, Caldecott KW, Jackson DA. Chk1 regulates the density of active replication origins during the vertebrate S phase. EMBO J. 2007;26:2719–2731. doi: 10.1038/sj.emboj.7601714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petermann E, et al. Chk1 requirement for high global rates of replication fork progression during normal vertebrate S phase. Mol Cell Biol. 2006;26:3319–3326. doi: 10.1128/MCB.26.8.3319-3326.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown EJ, Baltimore D. Essential and dispensable roles of ATR in cell cycle arrest and genome maintenance. Genes Dev. 2003;17:615–628. doi: 10.1101/gad.1067403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zachos G, Gillespie DA. Exercising restraints: Role of Chk1 in regulating the onset and progression of unperturbed mitosis in vertebrate cells. Cell Cycle. 2007;6:810–813. doi: 10.4161/cc.6.7.4048. [DOI] [PubMed] [Google Scholar]
- 7.Tang J, Erikson RL, Liu X. Checkpoint kinase 1 (Chk1) is required for mitotic progression through negative regulation of polo-like kinase 1 (Plk1) Proc Natl Acad Sci USA. 2006;103:11964–11969. doi: 10.1073/pnas.0604987103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naruyama H, et al. Essential role of Chk1 in S phase progression through regulation of RNR2 expression. Biochem Biophys Res Commun. 2008;374:79–83. doi: 10.1016/j.bbrc.2008.06.112. [DOI] [PubMed] [Google Scholar]
- 9.Kramer A, et al. Centrosome-associated Chk1 prevents premature activation of cyclin-B-Cdk1 kinase. Nat Cell Biol. 2004;6:884–891. doi: 10.1038/ncb1165. [DOI] [PubMed] [Google Scholar]
- 10.Niida H, Katsuno Y, Banerjee B, Hande MP, Nakanishi M. Specific role of Chk1 phosphorylations in cell survival and checkpoint activation. Mol Cell Biol. 2007;27:2572–2581. doi: 10.1128/MCB.01611-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiromizu T, et al. Regulation of mitotic function of Chk1 through phosphorylation at novel sites by cyclin-dependent kinase 1 (Cdk1) Genes Cells. 2006;11:477–485. doi: 10.1111/j.1365-2443.2006.00955.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhao H, Piwnica-Worms H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol Cell Biol. 2001;21:4129–4139. doi: 10.1128/MCB.21.13.4129-4139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown EJ, Baltimore D. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev. 2000;14:397–402. [PMC free article] [PubMed] [Google Scholar]
- 14.Smits VA, Reaper PM, Jackson SP. Rapid PIKK-dependent release of Chk1 from chromatin promotes the DNA-damage checkpoint response. Curr Biol. 2006;16:150–159. doi: 10.1016/j.cub.2005.11.066. [DOI] [PubMed] [Google Scholar]
- 15.Loffler H, et al. DNA damage-induced accumulation of centrosomal Chk1 contributes to its checkpoint function. Cell Cycle. 2007;6:2541–2548. doi: 10.4161/cc.6.20.4810. [DOI] [PubMed] [Google Scholar]
- 16.Puc J, et al. Lack of PTEN sequesters CHK1 and initiates genetic instability. Cancer Cell. 2005;7:193–204. doi: 10.1016/j.ccr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Smits VA. Spreading the signal: Dissociation of Chk1 from chromatin. Cell Cycle. 2006;5:1039–1043. doi: 10.4161/cc.5.10.2761. [DOI] [PubMed] [Google Scholar]
- 18.Loffler H, Lukas J, Bartek J, Kramer A. Structure meets function—centrosomes, genome maintenance and the DNA damage response. Exp Cell Res. 2006;312:2633–2640. doi: 10.1016/j.yexcr.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Rago C, Vogelstein B, Bunz F. Genetic knockouts and knockins in human somatic cells. Nat Protoc. 2007;2:2734–2746. doi: 10.1038/nprot.2007.408. [DOI] [PubMed] [Google Scholar]
- 20.Donzelli M, Draetta GF. Regulating mammalian checkpoints through Cdc25 inactivation. EMBO Rep. 2003;4:671–677. doi: 10.1038/sj.embor.embor887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mailand N, et al. Rapid destruction of human Cdc25A in response to DNA damage. Science. 2000;288:1425–1429. doi: 10.1126/science.288.5470.1425. [DOI] [PubMed] [Google Scholar]
- 22.Sorensen CS, et al. Chk1 regulates the S phase checkpoint by coupling the physiological turnover and ionizing radiation-induced accelerated proteolysis of Cdc25A. Cancer Cell. 2003;3:247–258. doi: 10.1016/s1535-6108(03)00048-5. [DOI] [PubMed] [Google Scholar]
- 23.Hurley PJ, Wilsker D, Bunz F. Human cancer cells require ATR for cell cycle progression following exposure to ionizing radiation. Oncogene. 2007;26:2535–2542. doi: 10.1038/sj.onc.1210049. [DOI] [PubMed] [Google Scholar]
- 24.Lam MH, Liu Q, Elledge SJ, Rosen JM. Chk1 is haploinsufficient for multiple functions critical to tumor suppression. Cancer Cell. 2004;6:45–59. doi: 10.1016/j.ccr.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 25.Wilsker D, Bunz F. Loss of ataxia telangiectasia mutated- and Rad3-related function potentiates the effects of chemotherapeutic drugs on cancer cell survival. Mol Cancer Ther. 2007;6:1406–1413. doi: 10.1158/1535-7163.MCT-06-0679. [DOI] [PubMed] [Google Scholar]
- 26.Jackman M, Lindon C, Nigg EA, Pines J. Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat Cell Biol. 2003;5:143–148. doi: 10.1038/ncb918. [DOI] [PubMed] [Google Scholar]
- 27.Gatei M, et al. Ataxia-telangiectasia-mutated (ATM) and NBS1-dependent phosphorylation of Chk1 on ser-317 in response to ionizing radiation. J Biol Chem. 2003;278:14806–14811. doi: 10.1074/jbc.M210862200. [DOI] [PubMed] [Google Scholar]
- 28.Chen P, et al. The 1.7 Å crystal structure of human cell cycle checkpoint kinase Chk1: Implications for Chk1 regulation. Cell. 2000;100:681–692. doi: 10.1016/s0092-8674(00)80704-7. [DOI] [PubMed] [Google Scholar]
- 29.Bucher N, Britten CD. G2 checkpoint abrogation and checkpoint kinase-1 targeting in the treatment of cancer. Br J Cancer. 2008;98:523–528. doi: 10.1038/sj.bjc.6604208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Topaloglu O, Hurley PJ, Yildirim O, Civin CI, Bunz F. Improved methods for the generation of human gene knockout and knockin cell lines. Nucleic Acids Res. 2005;33:e158. doi: 10.1093/nar/gni160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.