Abstract
Glucocorticoid (GC) is an adrenal steroid with diverse physiological effects. It undergoes a robust daily oscillation, which has been thought to be driven by the master circadian clock in the suprachiasmatic nucleus of the hypothalamus via the hypothalamus–pituitary–adrenal axis. However, we show that the adrenal gland has its own clock and that the peripheral clockwork is tightly linked to steroidogenesis by the steroidogenic acute regulatory protein. Examination of mice with adrenal-specific knockdown of the canonical clock protein BMAL1 reveals that the adrenal clock machinery is required for circadian GC production. Furthermore, behavioral rhythmicity is drastically affected in these animals, together with altered expression of Period1, but not Period2, in several peripheral organs. We conclude that the adrenal peripheral clock plays an essential role in harmonizing the mammalian circadian timing system by generating a robust circadian GC rhythm.
Keywords: adrenal gland, steroidogenic acute regulatory protein, BMAL1
Glucocorticoid [GC; corticosterone (CS) in rodents and cortisol in primates] is secreted by steroidogenic cells of the adrenal cortex and has widespread effects on the body. It modulates gluconeogenesis, lipid metabolism, cardiovascular tone, inflammation, and immune functions. Importantly, GC mediates behavioral adaptations to external cues, because its level responds sensitively to stress and to circadian rhythm (1, 2).
Most physiological and behavioral events in mammals are subject to well-controlled daily oscillations generated by an internal timekeeping system composed of clock genes with interacting positive and negative feedback loops. The hypothalamic suprachiasmatic nucleus (SCN) is believed to harbor a master clock that synchronizes and maintains the circadian rhythms of the periphery (3, 4). GC functions as a key humoral mediator transmitting resetting signals from the SCN to the peripheral clocks (4, 5).
GC exhibits a robust daily rhythm, and this oscillation has generally been attributed to the SCN modulating the hypothalamus–pituitary–adrenal (HPA) neuroendocrine axis (6). However, the daily oscillation of GC may not depend on the periodicity of its upstream hormonal regulators, corticotropin-releasing hormone and adrenocorticotropic hormone (ACTH), because GC rhythm was still present when ACTH was administered continuously to hypophysectomized rats (7). Thus, the source of the inherent circadian rhythm of GC synthesis and secretion from the adrenal gland is a long-standing issue.
One of the most plausible explanations for this complex aspect of the HPA axis is circadian modulation of the gland's responsiveness to its upstream regulators. In this context, it has been shown that the SCN can directly regulate GC secretion via the splanchnic nerve system (8, 9). Also, extensive analyses of the adrenal transcriptome have revealed the presence of the canonical molecular clock machinery in the adrenocortical cells, and suggested a gating mechanism for it (8, 10, 11). However, there is still conflicting evidence on the issue of the daily alterations in adrenal responsiveness and accompanying HPA axis reactivity. For instance, it has been argued that the diurnal changes in GC require splanchnic nerve integrity but are not mediated by differential responsiveness to ACTH (12). It has also been shown that the response to a mild stress failed to support increased HPA axis reactivity during the active period (13).
More importantly, previous findings do not fully account for the autonomous rhythmicity in steroid biosynthesis and secretion that was demonstrated in early studies with isolated adrenal glands (14–16). This strongly suggests that there remains an as-yet unresolved adrenal-intrinsic mechanism controlling the GC rhythm and involving ACTH-independent steroid production. Therefore, the present study was initiated to test the hypothesis that the adrenal peripheral clock plays a pivotal role in the oscillatory GC biosynthesis by an autonomous mechanism.
Results
Circadian Rhythms of CS Production and Adrenal Steroidogenic Acute Regulatory Protein (StAR) Expression.
We initially hypothesized that the daily rhythm in circulating CS was closely associated with its synthetic profile in vivo. To test this idea, we compared CS levels in the plasma and adrenal glands of male C57BL/6J mice entrained to a 24-h light–dark cycle (LD; 12:12). In accord with previous studies (11), plasma CS showed a robust daily variation, with a peak around the onset of night. This daily oscillation was closely paralleled by changes in adrenal CS content, suggesting that adrenal CS biosynthesis also exhibits a daily rhythm(Fig. 1A).
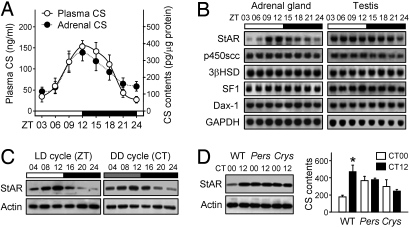
Fig. 1.
Circadian rhythms of CS levels and adrenal StAR expression. (A) Daily profiles of CS in plasma (n = 8–9 per point) and adrenal lysates (n = 8–10) were expressed as mean ± SE. CS content in adrenal lysates was normalized with protein content. ZT, Zeitgeber time. (B) The mRNA profiles for the indicated steroidogenesis-related genes in adrenal gland and testis obtained by Northern blotting. StAR indicates steroidogenic acute regulatory protein; P450scc, P450 side-chain cleavage enzyme; 3βHSD, 3β-hydroxysteroid dehydrogenase; SF-1, steroidogenic factor-1; Dax-1, dosage-sensitive sex reversal adrenal hypoplasia congenital critical region on the X chromosome-1; and GAPDH, glyceraldehyde-3-phosphate dehydrogenase as an internal control. (C) Adrenal StAR protein levels under LD and DD conditions measured by immunoblotting. CT, Circadian time. (D) StAR protein level (Left) and adrenal CS content (Right) were examined in wild-type (WT), Per1−/−Per2−/− (Pers) and Cry1−/−Cry2−/− (Crys) mice (n = 6 for WT and Pers mice, and n = 3 for Crys mice; *, P < 0.05 between CT00 and CT12).
To identify adrenal-specific factors responsible for oscillatory CS biosynthesis, we compared the mRNA expression profiles of a subset of major steroidogenic genes in the adrenal gland and testis. The latter was chosen because it is a steroidogenic organ, but its molecular clockwork is known to be less rhythmic (17). One of the gene transcripts examined was StAR; this was found to be expressed in a rhythmic fashion in the adrenal gland, but not in the testis (Fig. 1B). StAR acts as a rate-limiting step in steroidogenesis by transferring cholesterol from the outer mitochondrial membrane to the inner membrane, where the steroidogenic enzymatic reactions occur (18). Adrenal StAR protein levels also showed striking daily variations that were maintained even after 2 days of constant dark–dark (DD) conditions (Fig. 1C). The circadian rhythms of StAR expression and CS content of the adrenal gland were absent in arrhythmic Per1−/−Per2−/− (Pers) and Cry1−/−Cry2−/− (Crys) mutant mice (Fig. 1D). These findings indicate that the molecular clock is tightly linked to steroidogenesis in the adrenal gland and generates circadian rhythmicity of StAR gene expression and CS production.
Clock-Controlled StAR Expression Mediating Circadian Steroid Production.
The next set of experiments was aimed at confirming the crucial role of StAR as a molecular link between the adrenal peripheral clock and steroidogenesis. For this purpose we used an immortalized mouse adrenocortical steroidogenic cell line, Y1. As with fibroblasts (19), a 2-h serum shock was able to synchronize the molecular clock in the Y1 cells. The serum shock caused rhythmic steroid production and cyclic StAR mRNA accumulation with ≈24-h periodicity that persisted for up to 60 h. These features clearly depended on an intact cellular clock, because overexpression of CLOCKΔ19, a defective mutant of CLOCK (20), dampened the cyclic profiles of both steroid production and StAR mRNA expression (Fig. 2 A and B). Conversely, overexpression of CLOCK and BMAL1 increased both StAR protein level and steroid production, and this CLOCK:BMAL1-evoked steroid production was significantly attenuated by application of antisense oligodeoxynucleotides against StAR (Fig. 2C), confirming that StAR links the molecular clock with the steroidogenic pathway.
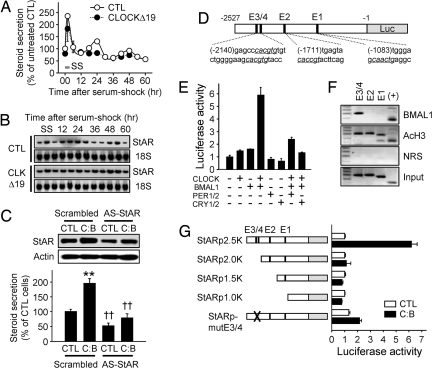
Fig. 2.
Transcriptional regulation of StAR expression by the cellular clock and the role of StAR in steroid production. (A) Adrenocortical Y1 cells were transfected with backbone plasmid (CTL) or the CMV promoter-driven CLOCKΔ19 plasmid (CLOCKΔ19). After serum shock (SS), hourly steroid secretion rates were measured and are expressed as percent (mean ± SE; n = 4–5). (B) With the same SS procedure, StAR mRNA levels were examined by Northern blotting. (C) Y1 cells were transfected with CTL or a 1:1 mixture of CLOCK and BMAL1-expressing constructs (C:B). Ten micrograms of scrambled (5′-GCTCTATGACTCCCAG-3′) or AS-StAR (5′-CATTTGGGTTCCACTC-3′) oligodeoxynucleotide (ODN) was included at the same time. Forty-eight hours after transfection, StAR protein levels (Upper) and steroid production (Lower) were measured (n = 4–5; **, P < 0.05 vs. CTL cells; ††, P < 0.05 vs. scrambled ODN-treated cells). (D) Schematic diagram of the mouse StAR promoter fused to the luciferase reporter (StARp2.5K). (E) Y1 cells were transfected with StARp2.5K in combination with various clock gene-expressing plasmids as indicated. Normalized luciferase activities are presented as mean ± SE of arbitrary unit (n = 6 for each group). (F) To reveal the binding of endogenous BMAL1 on the StAR promoter, chromatin immunoprecipitation assays were performed with anti-BMAL1 antibody, anti-acetylated histone H3 (AcH3) antibody, or preimmune normal rabbit serum (NRS). (+) indicates the functional E box-containing region on the D box-binding protein (DBP) promoter (48). (G) Effect of CLOCK:BMAL1 overexpression on luciferase activities driven by serially deleted or E box-mutated StAR promoters (n = 6–10 for each group).
These findings in turn raised the question of how the molecular clock governs StAR gene expression. Rhythmic transcription can be controlled through transcriptional enhancers, such as E boxes (binding sites for CLOCK:BMAL1 heterodimers; ref. 21). Analysis of the promoter region of the mouse StAR gene revealed 4 putative E boxes within the 2.5-kb region upstream of the transcription start site (Fig. 2D; denoted as E1–E4 from the proximal). To test whether StAR expression is directly regulated by the CLOCK:BMAL1 heterodimer, we measured luciferase activity driven by the 2.5-kb mouse StAR promoter in the presence of various sets of clock genes. Simultaneous expression of both CLOCK and BMAL1 increased StAR promoter activity up to 6-fold, and this induction was attenuated by coexpression of negative regulators, such as PERs or CRYs (Fig. 2E). Chromatin immunoprecipitation experiments revealed that endogenous BMAL1 in Y1 cells binds to E3 and/or E4 but not to E1 or E2 (Fig. 2F). In agreement with the binding experiments, elimination of E3 and E4 by truncation of the promoter region or by site-directed deletion of a core nucleotide (CACGTG to CACTG; ref. 22) abolished CLOCK:BMAL1-induced StAR promoter activity (Fig. 2G). Because mutation of either E3 or E4 singly lowered CLOCK:BMAL1-evoked StAR promoter activity somewhat, it is likely that the 2 E boxes function additively [supporting information (SI) Fig. S1]. Although the proximal region of the mammalian StAR promoter (up to −1.0 kb from the transcription start site), which contains binding motifs for SF-1, DAX-1, SP1, and CREB, is sufficient for tissue-specific and hormone-responsive StAR expression (23), our data suggest that the more distal region is required for appropriate temporal oscillation of StAR expression. It thus appears that StAR transcription is regulated by the CLOCK:BMAL1 heterodimer via the distal E box elements, and hence that StAR can be regarded as a clock-controlled gene in adrenocortical steroidogenic cells.
Adrenal Gland-Specific Disruption of BMAL1 Expression Dampens the Circadian CS Rhythm.
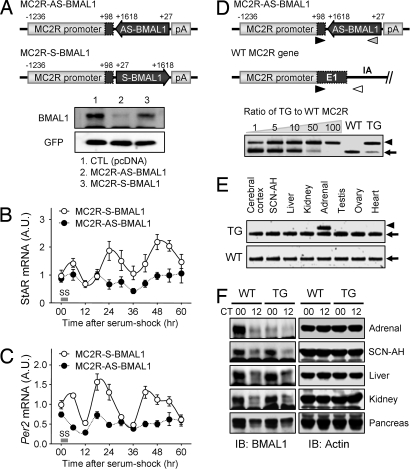
Next, we tested the role of the adrenal peripheral clock in producing the daily CS rhythm in vivo by ablating the molecular clockwork specifically in the adrenal CS-producing cells. We chose BMAL1 as a target for knockdown because our previous studies show that it plays the determining role in posttranslational regulation and function of the CLOCK:BMAL1 heterodimer (24, 25) and, more importantly, CLOCK has an overlapping role with NPAS2, another member of core clock components (26). We generated a transgenic (TG) mouse line in which a part of the BMAL1 coding region was expressed in an antisense orientation under tissue-specific control by using the 1.3-kb ACTH receptor (MC2R) promoter (MC2R-AS-BMAL1; see Figs. S2 and S3 for more information on this construct). Transfection of MC2R-AS-BMAL1 into Y1 cells completely blocked the serum shock-evoked oscillations of StAR and Per2 mRNA expression, whereas the control construct with the sense orientation (MC2R-S-BMAL1) did not (Fig. 3 A–C). The copy number of the MC2R-AS-BMAL1 transgene was estimated by competitive PCR genotyping and by constructing a standard curve from mixtures with different molar ratios of the purified PCR products of the transgene and endogenous MC2R gene. The deduced copy number in the heterozygous mutant mice was ≈60 (Fig. 3D and Fig. S4). In accordance with the adrenal gland-specific expression of the AS-BMAL1 transgene as revealed by competitive RT-PCR (Fig. 3E), immunoblot analyses showed that BMAL1 protein levels at CT00 and CT12 were dramatically reduced in an adrenal gland-specific fashion (Fig. 3F). The amount of adrenal BMAL1 protein expression in the TG mice at CT00 was comparable with that of their wild-type (WT) littermates at CT12, when BMAL1 levels were close to a minimum (27.8% ± 8.3% of WT at CT00 from 3 independent experiments). By contrast, BMAL1 protein expression in other tissues, including the SCN-anterior hypothalamus (SCN-AH), liver, kidney, and pancreas, was the same in the TG and WT mice (Fig. 3F). These results show that the MC2R promoter-driven AS-BMAL1 transgene knocks down endogenous BMAL1 protein in an adrenal gland-specific manner.
Fig. 3.
Generation of MC2R-AS-BMAL1 TG mice. (A) Schematic diagrams of the MC2R-AS-BMAL1 and MC2R-S-BMAL1 constructs (Upper). pA indicates the poly(A) signal from the bovine growth hormone gene. Effect of pMC2R-AS-BMAL1 or pMC2R-S-BMAL1 on endogenous BMAL1 protein levels in Y1 cells (Lower). Cotransfected GFP was used as an internal control (CTL). (B and C) Y1 cells were transfected with MC2R-S-BMAL1 or MC2R-AS-BMAL1. After serum shock, StAR (B) and Per2 (C) mRNA levels were examined. Data were normalized with TATA-binding protein (TBP) and are expressed as mean ± SE arbitrary units (A.U.), where the mean value of the MC2R-S-BMAL1 group at 0 h was set at 1 (n = 3). (D) Schematic diagrams of the MC2R-AS-BMAL1 transgene and the endogenous MC2R gene (Upper). Primer binding sites for genotyping are indicated by arrowheads. The copy number of MC2R-AS-BMAL1 TG was determined by competitive PCR genotyping (Lower). The arrowhead indicates PCR products derived from the MC2R-AS-BMAL1, and arrows indicate those from the endogenous MC2R gene. (E) Adrenal gland-specific mRNA expression of AS-BMAL1 TG as revealed by competitive RT-PCR. Arrowhead indicates PCR products derived from MC2R-AS-BMAL1, and arrows from endogenous Bmal1 mRNA. (F) Adrenal gland-specific knockdown of BMAL1 expression. BMAL1 protein levels at circadian time (CT)00 and CT12 were examined in the indicated tissues by immunoblotting.
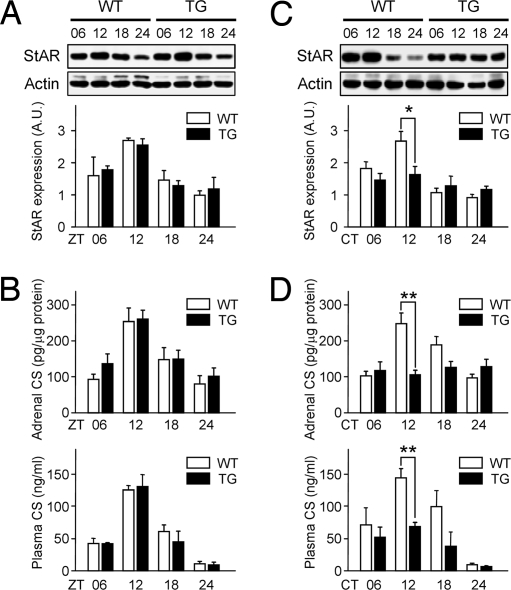
We used these TG mice to determine the effect of BMAL1 knockdown on the adrenal functions. In the presence of light cue, TG mice showed normal daily variations in adrenal StAR protein expression, CS contents, and plasma CS levels (Fig. 4 A and B). However, adrenal StAR and CS rhythms were completely abolished in the TG mice under DD conditions (Fig. 4 C and D Upper), indicating the exclusive role of the adrenal peripheral clock in these rhythms in vivo. The plasma CS oscillations also were strongly attenuated, although some daily variation was retained (Fig. 4D Lower). Despite impaired circadian periodicities, plasma CS levels and StAR induction evoked by a 30-min immobilization were not significantly influenced by the disruption of adrenal local clock (Fig. S5), suggesting that the physiological pathway mediating acute stress response is independent of the adrenal peripheral clock machinery. This is well in accordance with the previous reports showing that increase in circulating CS by stress was normal even in global BMAL1−/− mice (27, 28). Furthermore, TG mice also showed no apparent alterations in sympathoadrenal functions in terms of plasma norepinephrine and epinephrine levels, suggesting confined functioning of the transgene in ACTHR-positive adrenocortical cells (Table S1). Collectively, our findings indicate that an intact adrenocortical clock is required for the inherent circadian profiles of basal CS levels by evoking rhythmic steroidogenesis, and that StAR is an intrinsic peripheral regulator of the adrenal rhythm.
Fig. 4.
Dampened circadian CS rhythm in MC2R-AS-BMAL1 TG mice. WT and TG mice housed under 12 h of LD photoperiod (A and B) or DD conditions (C and D; 6–7 days after lights-off) were killed at ZT or CT 6, 12, 18, and 24 h. StAR protein levels in the adrenal glands were determined by immunoblotting (A and C; n = 4; *, P < 0.05 between WT and TG). (B and D) Adrenal CS contents (Upper) and plasma CS levels (Lower) in WT and TG mice were measured by RIA and are expressed as mean ± SE (n = 4–11 for DD and 4–6 for LD; **, P < 0.01 between WT and TG).
Role of the Adrenal Oscillator in Harmonizing the Circadian Timing System.
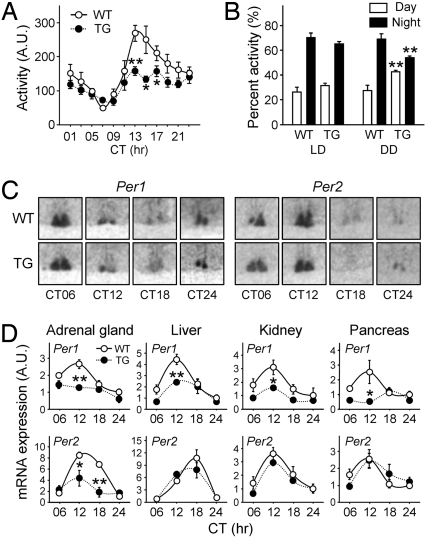
Recently, several genetic models in which molecular clocks were modulated in a cell type-specific manner have been developed to account for the tissue- or organ-specific regulation and function of clock genes (29–31). In this context, our adrenal-specific BMAL1 knockdown mice provide a useful system for testing the unique role of the adrenal peripheral clock in harmonizing and maintaining the circadian timing system, because GC has been proposed to be a Zeitgeber for peripheral oscillators (4). We therefore compared the home-cage activities, variations in body temperature, and cyclic expression of a subset of clock genes in the WT and TG mice. Circadian variations in locomotor activities under both LD and DD conditions were still observed in the BMAL1 knockdown mice (Fig. 5 A and B; P < 0.01 between day and night in all groups), with a free-running period similar to that of the WT under DD conditions (τ = 23.73 ± 0.07 for WT and 23.70 ± 0.05 for TG; P = 0.50). However, in the TG mice the amplitude of the behavioral rhythm was greatly attenuated, with weakened periodicity after 1 wk of constant darkness (Fig. 5A and Fig. S6). Because of a significant reduction in locomotor activity during active periods (subjective nighttime) but similar activity during rest periods (subjective daytime), the day/night differences in locomotor activity were significantly affected: the WT mice displayed 71.46% ± 4.32% of their activity during the active period, whereas this figure was reduced to 55.84% ± 1.29% for the TG mice after 1 wk of constant darkness (Fig. 5B). Interestingly, this reduction was not apparent in the presence of light cues. These results show that the amplitude of the behavioral rhythm under DD conditions was significantly influenced by ablation of the adrenal oscillator, along with the hypoactivity in their locomotor behaviors. In contrast to their behavioral rhythm, the circadian changes in body temperature of the TG mice were virtually the same as those of their WT littermates (Fig. S6), indicating that the adrenal peripheral clock is selective in its effects on circadian physiology.
Fig. 5.
Behavioral rhythm and clock gene expression in MC2R-AS-BMAL1 TG mice. (A) Mean home-cage activities over the 7–14 days after the beginning of a DD cycle are presented as means ± SE, at 2-h intervals (n = 6 for each group; *, P < 0.05 and **, P < 0.01 between WT and TG). (B) Relative home-cage activities during rest (ZT or CT00–CT12) and activity periods (ZT or CT12–CT24) under LD or DD conditions. Activities under DD conditions were obtained during the second week of constant darkness. Data are presented as means ± SE % of total activity per day (n = 10 for each group; **, P < 0.01 vs. WT over the same period). (C and D) WT and TG mice housed under DD conditions for 6–7 days were killed at CT 6, 12, 18, and 24 h. (C) Per1 and Per2 expression in the SCN as revealed by in situ hybridization. (D) Per1 and Per2 mRNA expression profiles were obtained by real-time RT-PCR in the indicated organs. Data are normalized with TBP and expressed as means ± SE of A.U., where the mean WT value at CT24 is defined as 1 (n = 4–6, *, P < 0.05 and **, P < 0.01 vs. WT at the same time points).
Together with the behavioral anomalies, the expression profiles of the clock genes in several peripheral organs of the TG mice were affected. As shown in Fig. 5C, the Per1 and Per2 transcript levels in the SCN were apparently unaltered in the TG mice. This is consistent with the normal period lengths of locomotor activities and body temperature. However, the cyclic expression of both transcripts was significantly dampened in the adrenal gland of the mutant mice, along with the severely reduced BMAL1 protein levels, once again confirming that this knockdown is sufficient to functionally ablate the adrenal molecular clock (Fig. 5D). Interestingly, transcripts of Per1 but not Per2 were reduced, especially at the peak times, in a variety of peripheral organs, including liver, kidney, and pancreas (Fig. 5D), despite the normal BMAL1 expression in these organs. Taken together, these behavioral and molecular findings strongly suggest that the adrenal peripheral oscillator plays a crucial role in tuning the mammalian circadian rhythm by mediating and/or strengthening the time cues generated by the master clock.
Discussion
The present study has addressed the longstanding issue of the source of the inherent circadian rhythm in GC biosynthesis in, and secretion from, the adrenal gland. It demonstrates that the adrenal peripheral oscillator plays an essential role in producing an intrinsic circadian rhythm of GC by causing rhythmic steroid production.
In the adrenal gland, canonical clock genes are rhythmically expressed (8, 11). We showed above that StAR is a molecular link between the adrenal clock genes and circadian steroid biosynthesis in GC-producing cells. StAR expression evidently mediates molecular clock-evoked steroid production (Fig. 2), and its periodicity is maintained even in the absence of light cues, but it requires a functional oscillator (Figs. 1 and 4). We also established that StAR expression is controlled by the action of CLOCK:BMAL1 heterodimers on the upstream cis-elements of the murine promoter and, most importantly, that this regulation is required for a circadian steroidogenesis in the adrenal gland in vivo. Although recent studies on reproductive systems have suggested a possible link between StAR and the molecular clockwork (32, 33), we have provided compelling evidence, by adrenal-specific ablation of the molecular clock machinery, for circadian control of adrenal StAR expression and for its physiological relevance.
It should be noted that StAR is the only gene among the major steroid biosynthesis genes showing a robust adrenal-specific rhythmic expression (Fig. 1 B and C). Functions and expression profiles of StAR strongly suggest its pivotal role in the circadian steroid production. First, it is well established that StAR is rate-limiting for steroidogenesis, because modulating StAR gene expression alone causes substantial changes in the rate of steroid production (18, 34). Second, both StAR mRNA and protein levels increase in late daytime (Fig. 1 B and C), in contrast to other previously suggested steroidogenesis-related genes with cyclic expression, which reach their deduced peaks several hours after subjective lights-off time (10). This is in accord with the adrenal and plasma CS profiles, which peak just before the onset of subjective nighttime in rodents (35). Finally, our TG mice show that the circadian rhythm of StAR expression is evidently adrenal-autonomic in origin, although it can be also driven by a potent environmental cue, such as light–dark cycle.
Numerous studies on the daily GC rhythm have revealed quite complex features of the adrenal rhythm, primarily due to the relatively restricted roles of the upstream hormonal regulators of the HPA axis (7, 9, 35). Therefore, it has been proposed that the central clock influences the adrenal gland via SCN-derived neural and humoral inputs (8, 9, 36, 37). However, the SCN appears most important for the nadir of the CS rhythm. Although strong inhibition of basal CS release during the rest period by the SCN seems clear, requirement of an additional stimulatory/activating mechanism has been also implicated to fully account for the circadian peak of the CS rhythm (9, 35, 38). In this context, our findings suggest the presence of an adrenal-autonomous and steroidogenesis-dependent activating mechanism. As mentioned above, the cyclic accumulation of StAR mRNA and the accompanying steroid production are under the control of the adrenal peripheral clock.
However, we cannot exclude a central stimulatory mechanism, because an attenuated but not flattened circulating CS profile was still observed in the TG mice despite nearly flattened StAR and adrenal CS rhythms. Furthermore, both rhythmic StAR protein expression and subsequent CS production can be driven by light cue as well as the intrinsic oscillator. It should be noted that light could directly activate the adrenal gland in an SCN-dependent manner (8). Indeed, Ishida et al. (8) showed that photic signal could activate several signaling cascades in the adrenal gland, which can lead to enhanced CS production. Thus, it is plausible that adrenal steroidogenic machinery by itself can be continuously entrained or reset by regular LD cycles, regardless of the local clock. However, when such a resetting cue is weakened or disturbed, the adrenal oscillator appears to become dominant to drive rhythmic steroid production and subsequent circulating GC rhythm. Collectively, our findings indicate that rhythmic steroidogenesis by an intrinsic oscillator in the adrenal gland is required to generate an inherent circadian rhythm of GC, and that it may cooperate with systemic inputs to the gland that presumably originated from the central pacemaker by hormonal and neural mechanism.
Because our TG mice show normal stress-induced secretion of both CS and catecholamines, this can be considered a valuable animal model for the hypocortisolism with attenuated basal GC rhythm and the unique role of the adrenal oscillator. Moreover, normal cyclicity of CS under LD conditions might minimize a potential developmental problem. In this regard, it is of interest that adrenal gland-specific ablation of the molecular clock could cause abnormalities in behavioral rhythm under DD cycles; the MC2R-AS-BMAL1 TG mice clearly displayed dampened amplitude of their behavioral rhythm in terms of day–night differences in home-cage activities. It is plausible that the dampened plasma CS rhythm was responsible for the changes, because it has been demonstrated that flattened GC profiles could attenuate circadian locomotor activities (39, 40). Because the body temperature is not significantly affected in our TG mice, the behavioral alterations are likely due to a selective effect of cyclic GC on brain functions dealing with the expression of periodic locomotor behaviors, such as coordinating motor functions or maintaining sleep–wake cycles (41).
In addition to altering behavioral rhythms, ablation of the adrenal clock also attenuated Per1 but not Per2 rhythmicity in several peripheral organs (Fig. 5D). In connection with such selectivity, it should be noted that the expression of clock genes can be differentially regulated by GC signaling; Per1 expression can be directly modulated via a distal GC-responsive promoter element (39, 42). Therefore, it is likely that the reduced circulating CS found in the TG mice is responsible for the alteration of cyclic accumulation of Per1 in peripheral organs as well as the dampened behavioral rhythm. By contrast, it was proposed recently that hepatic Per2 expression in vivo was profoundly influenced by a daily fluctuation in body temperature, rather than hepatic clock machinery (30). Therefore, the attenuated GC rhythm but normal cyclicities in body temperature of the TG mice suggest that the circadian expression of Per1 and Per2 may differentially respond to different systemic cues in cooperation with a local clock. The widespread influences of knockdown of adrenocortical BMAL1 expression indicate that the hierarchical organization of the mammalian circadian system needs to be reevaluated. The simplistic division into one central and several subsidiary peripheral oscillators is no longer tenable: our findings indicate that the adrenal clock is not simply one of the peripheral oscillators; rather, it plays a pivotal role in harmonizing the circadian rhythms of metabolism and behavior, and in this way may add flexibility to the dominant rhythm provided by the master clock in the SCN, as illustrated in Fig. S7.
One may also suppose that the adrenal clock by itself can produce the rhythmic physiological outputs from other tissues in a more direct fashion via GC signaling, and thus can contribute to generating and harmonizing the body's rhythms. This could depend on the diverse actions of GC on physiological processes (1, 2, 41), the clock-resetting activity of the hormone (5), and rapid regulatory inputs from the SCN to reset the phase of the gland (8). Moreover, there exist 2 types of GC receptors with distinctive affinities and capacities, and these are believed to be differentially activated at the circadian peak and nadir of circulating ligand levels (2). The observations that the expression of at least 10% of all genes is influenced by GC (2), and that the cyclicities of some hepatic genes can be affected by an intact adrenal gland and/or GC signaling (43, 44), strongly support this notion. Also, the circadian rhythm of certain discrete brain regions is dependent on GC signaling, implying that even higher brain functions can be directly influenced by the adrenal rhythm (40, 45). Recent studies indicate that subtle but sustained changes in GC levels, as well as drastic hypersecretion or hyposecretion, are potentially dangerous and may contribute to the onset of common metabolic disorders, such as insulin resistance, hypertension, obesity, and type 2 diabetes (1, 2). This emphasizes the potential harmful effects of dysregulation of the circadian GC rhythm due to genetic and/or environmental causes.
In conclusion, the present study demonstrates that the intrinsic adrenal peripheral clock can contribute to generating a robust circadian GC rhythm both in vitro and in vivo. The adrenal oscillator, in turn, plays a crucial role in maintaining the behavioral rhythm, as well as cyclic clock gene expression in other peripheral organs. Clock-controlled StAR gene expression in the adrenal gland may provide the molecular basis for this circadian steroid production, indicating that StAR links clock machinery and steroidogenesis (Fig. S7). This interlocking system is comparable to the relationship between cell cycle regulators and the molecular clockwork (46) and provides a valuable indication of how a subset of key cellular pathways can be linked to the circadian timing system at the molecular level.
Materials and Methods
Animal Care and Handling.
Male C57BL/6J mice at 9–12 weeks of age were kept in temperature-controlled (22 °C–23 °C) quarters under a 12-h light and 12-h dark (LD) photoperiod (lights on at 8:00 AM). For dark–dark (DD) conditions, mice were kept in constant darkness for the indicated duration from the lights-off time after entrainment for more than 10 days under LD conditions. All animal procedures were approved by the Institutional Animal Care and Use Committee of Seoul National University.
Generation of MC2R-AS-BMAL1 TG Mice.
The inserted fragment containing the MC2R promoter, AS-BMAL1, and the bovine growth hormone poly(A) signal was cut out by NdeI/NcoI digestion and purified by agarose gel electrophoresis. The TG mice were generated by microinjection of the purified DNA into the pronuclei of fertilized eggs of C57BL/6J mice.
Cell Culture and Transfection.
Materials for cell culture were obtained from Invitrogen. For serum shock, cells at 80–90% confluence were serum-starved for 24 h and incubated with 50% horse serum for 2 h, and then were returned to serum-free DMEM-F12K medium. The beginning of serum shock was defined as time 0.
Analyses of Hormones, mRNA, and Protein Levels.
Measuring hormones, mRNA expression, and protein levels was carried out as described previously with modifications (24, 47). Detailed materials and methods are described in the SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Dr. D. R. Weaver (University of Massachusetts Medical School, Worcester, MA) and Dr. H. Okamura (Kobe University Graduate School of Medicine, Kobe, Japan) for kindly providing Per1−/−Per2−/− and Cry1−/−Cry2−/− mutant mice, respectively. This work was supported by grants from the Korea Ministry of Education, Science and Technology (MEST) through the Brain Research Center of the 21st Century Frontier Research Program. G. H. Son, S. Chung, H. K. Choe, H.-D. Kim and K. H. Lee were supported by Brain Korea 21 Research Fellowships from the Korea Ministry of Education, Science and Technology (MEST). G. H. Son was also supported by a Young Investigator's Award from the Korean Society for Endocrinology, and S. Chung by a Seoul Science Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806962106/DCSupplemental.
References
- 1.Wang M. The role of glucocorticoid action in the pathophysiology of the metabolic syndrome. Nutr Metab. 2005;2:3. doi: 10.1186/1743-7075-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buckingham JC. Glucocorticoids: Exemplars of multi-tasking. Br J Pharmacol. 2006;147:S258–S268. doi: 10.1038/sj.bjp.0706456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15:R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 4.Stratmann M, Schibler U. Properties, entrainment, and physiological functions of mammalian peripheral oscillators. J Biol Rhythms. 2006;21:494–506. doi: 10.1177/0748730406293889. [DOI] [PubMed] [Google Scholar]
- 5.Balsalobre A, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 6.Buijs RM, Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nat Rev Neurosci. 2001;2:521–526. doi: 10.1038/35081582. [DOI] [PubMed] [Google Scholar]
- 7.Meier AH. Daily variation in concentration of plasma corticosteroid in hypophysectomized rats. Endocrinology. 1976;98:1475–1479. doi: 10.1210/endo-98-6-1475. [DOI] [PubMed] [Google Scholar]
- 8.Ishida A, et al. Light activates the adrenal gland: Timing of gene expression and glucocorticoid release. Cell Metab. 2005;2:297–307. doi: 10.1016/j.cmet.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Ulrich-Lai YM, Arnhold MM, Engeland WC. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. Am J Physiol. 2006;290:R1128–R1135. doi: 10.1152/ajpregu.00042.2003. [DOI] [PubMed] [Google Scholar]
- 10.Oster H, Damerow S, Hut RA, Eichele G. Transcriptional profiling in the adrenal gland reveals circadian regulation of hormone biosynthesis genes and nucleosome assembly genes. J Biol Rhythms. 2006;21:350–361. doi: 10.1177/0748730406293053. [DOI] [PubMed] [Google Scholar]
- 11.Oster H, et al. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 2006;4:163–173. doi: 10.1016/j.cmet.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Dijkstra I, Binnekade R, Tilders FJ. Diurnal variation in resting levels of corticosterone is not mediated by variation in adrenal responsiveness to adrenocorticotropin but involves splanchnic nerve integrity. Endocrinology. 1996;137:540–547. doi: 10.1210/endo.137.2.8593800. [DOI] [PubMed] [Google Scholar]
- 13.Atkinson HC, Wood SA, Kershaw YM, Bate E, Lightman SL. Diurnal variation in the responsiveness of the hypothalamic-pituitary-adrenal axis of the male rat to noise stress. J Neuroendocrinol. 2006;18:526–533. doi: 10.1111/j.1365-2826.2006.01444.x. [DOI] [PubMed] [Google Scholar]
- 14.Andrews RV, Folk GE., Jr Circadian metabolic patterns in cultured hamster adrenal glands. Comp Biochem Physiol. 1964;11:393–409. doi: 10.1016/0010-406x(64)90006-4. [DOI] [PubMed] [Google Scholar]
- 15.Andrews RV. Phase response profile of hamster adrenal organ cultures treated with ACTH and exogenous steroid. Comp Biochem Physiol. 1980;67:275–277. [Google Scholar]
- 16.Lehoux JG, Lefebvre A. De novo synthesis of corticosteroids in hamster adrenal glands. J Steroid Biochem. 1980;12:479–485. doi: 10.1016/0022-4731(80)90310-6. [DOI] [PubMed] [Google Scholar]
- 17.Morse D, Cermakian N, Brancorsini S, Parvinen M, Sassone-Corsi P. No circadian rhythms in testis: Period1 expression is clock independent and developmentally regulated in the mouse. Mol Endocrinol. 2003;17:141–151. doi: 10.1210/me.2002-0184. [DOI] [PubMed] [Google Scholar]
- 18.Stocco DM. Steroidogenic acute regulatory protein. Vitam Horm. 1999;55:399–441. doi: 10.1016/s0083-6729(08)60940-1. [DOI] [PubMed] [Google Scholar]
- 19.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 20.Hong, et al. Inducible and reversible Clock gene expression in brain using the tTA system for the study of circadian behavior. PLoS Genet. 2007;3:e33. doi: 10.1371/journal.pgen.0030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gekakis N, et al. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 22.Jung H, et al. Involvement of CLOCK:BMAL1 heterodimer in serum-responsive mPer1 induction. NeuroReport. 2003;14:15–19. doi: 10.1097/00001756-200301200-00003. [DOI] [PubMed] [Google Scholar]
- 23.Stocco DM, Wang X, Jo Y, Manna PR. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: More complicated than we thought. Mol Endocrinol. 2005;19:2647–2659. doi: 10.1210/me.2004-0532. [DOI] [PubMed] [Google Scholar]
- 24.Kwon I, et al. BMAL1 shuttling controls transactivation and degradation of the CLOCK/BMAL1 heterodimer. Mol Cell Biol. 2006;26:7318–7330. doi: 10.1128/MCB.00337-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shim HS, et al. Rapid activation of CLOCK by Ca2+-dependent protein kinase C mediates resetting of the mammalian circadian clock. EMBO Rep. 2007;8:366–371. doi: 10.1038/sj.embor.7400920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeBruyne JP, Weaver DR, Reppert SM. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nat Neurosci. 2007;10:543–545. doi: 10.1038/nn1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudic RD, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curtis, et al. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci USA. 2007;104:3450–3455. doi: 10.1073/pnas.0611680104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDearmon EL, et al. Dissecting the functions of the mammalian clock protein BMAL1 by tissue-specific rescue in mice. Science. 2006;314:1304–1308. doi: 10.1126/science.1132430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kornmann B, Schaad O, Bujard H, Takahashi JS, Schibler U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol. 2007;5:e34. doi: 10.1371/journal.pbio.0050034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westgate EJ, et al. Genetic components of the circadian clock regulate thrombogenesis in vivo. Circulation. 2008;117:2087–2095. doi: 10.1161/CIRCULATIONAHA.107.739227. [DOI] [PubMed] [Google Scholar]
- 32.Nakao N, et al. Circadian clock gene regulation of steroidogenic acute regulatory protein gene expression in preovulatory ovarian follicles. Endocrinology. 2007;148:3031–3038. doi: 10.1210/en.2007-0044. [DOI] [PubMed] [Google Scholar]
- 33.Alvarez JD, et al. The circadian clock protein BMAL1 is necessary for fertility and proper testosterone production in mice. J Biol Rhythms. 2008;23:26–36. 15. doi: 10.1177/0748730407311254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR) J Biol Chem. 1994;269:28314–28322. [PubMed] [Google Scholar]
- 35.Kalsbeek A, van Heerikhuize JJ, Wortel J, Buijs RM. A diurnal rhythm of stimulatory input to the hypothalamo-pituitary-adrenal system as revealed by timed intrahypothalamic administration of the vasopressin V1 antagonist. J Neurosci. 1996;16:5555–5565. doi: 10.1523/JNEUROSCI.16-17-05555.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalsbeek A, van der Vliet J, Buijs RM. Decrease of endogenous vasopressin release necessary for expression of the circadian rise in plasma corticosterone: A reverse microdialysis study. J Neuroendocrinol. 1996;8:299–307. doi: 10.1046/j.1365-2826.1996.04597.x. [DOI] [PubMed] [Google Scholar]
- 37.Jasper MS, Engeland WC. Splanchnicotomy increases adrenal sensitivity to ACTH in nonstressed rats. Am J Physiol. 1997;273:E363–E368. doi: 10.1152/ajpendo.1997.273.2.E363. [DOI] [PubMed] [Google Scholar]
- 38.Buijs RM, Kalsbeek A, van der Woude TP, van Heerikhuize JJ, Shinn S. Suprachiasmatic nucleus lesion increases corticosterone secretion. Am J Physiol. 1993;264:R1186–R1192. doi: 10.1152/ajpregu.1993.264.6.R1186. [DOI] [PubMed] [Google Scholar]
- 39.Koyanagi S, et al. Chronic treatment with prednisolone represses the circadian oscillation of clock gene expression in mouse peripheral tissues. Mol Endocrinol. 2006;20:573–583. doi: 10.1210/me.2005-0165. [DOI] [PubMed] [Google Scholar]
- 40.Malek ZS, Sage D, Pévet P, Raison S. Daily rhythm of tryptophan hydroxylase-2 messenger ribonucleic acid within raphe neurons is induced by corticoid daily surge and modulated by enhanced locomotor activity. Endocrinology. 2007;148:5165–5172. doi: 10.1210/en.2007-0526. [DOI] [PubMed] [Google Scholar]
- 41.Chrousos GP, Kino T. Glucocorticoid action networks and complex psychiatric and/or somatic disorders. Stress. 2007;10:213–219. doi: 10.1080/10253890701292119. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto T, et al. Acute physical stress elevates mouse period1 mRNA expression in mouse peripheral tissues via a glucocorticoid-responsive element. J Biol Chem. 2005;280:42036–42043. doi: 10.1074/jbc.M509600200. [DOI] [PubMed] [Google Scholar]
- 43.Oishi K, et al. Genome-wide expression analysis reveals 100 adrenal gland-dependent circadian genes in the mouse liver. DNA Res. 2005;12:191–202. doi: 10.1093/dnares/dsi003. [DOI] [PubMed] [Google Scholar]
- 44.Reddy AB, et al. Glucocorticoid signaling synchronizes the liver circadian transcriptome. Hepatology. 2007;45:1478–1488. doi: 10.1002/hep.21571. [DOI] [PubMed] [Google Scholar]
- 45.Lamont EW, Robinson B, Stewart J, Amir S. The central and basolateral nuclei of the amygdala exhibit opposite diurnal rhythms of expression of the clock protein Period2. Proc Natl Acad Sci USA. 2005;102:4180–4184. doi: 10.1073/pnas.0500901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsuo T, et al. Control mechanism of the circadian clock for timing of cell division in vivo. Science. 2003;302:55–59. doi: 10.1126/science.1086271. [DOI] [PubMed] [Google Scholar]
- 47.Chung S, et al. Differential adaptive responses to chronic stress of maternally stressed male mice offspring. Endocrinology. 2005;146:3202–3210. doi: 10.1210/en.2004-1458. [DOI] [PubMed] [Google Scholar]
- 48.Ripperger JA, Schibler U. Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat Genet. 2006;38:369–374. doi: 10.1038/ng1738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.