Abstract
Calcium oscillations suppress mitochondrial movements along the microtubules to support on-demand distribution of mitochondria. To activate this mechanism, Ca2+ targets a yet unidentified cytoplasmic factor that does not seem to be a microtubular motor or a kinase/phosphatase. Here, we have studied the dependence of mitochondrial dynamics on the Miro GTPases that reside in the mitochondria and contain two EF-hand Ca2+-binding domains, in H9c2 cells and primary neurons. At resting cytoplasmic [Ca2+] ([Ca2+]c), movements of the mitochondria were enhanced by Miro overexpression irrespective of the presence of the EF-hands. The Ca2+-induced arrest of mitochondrial motility was also promoted by Miro overexpression and was suppressed when either the Miro were depleted or their EF-hand was mutated. Miro also enhanced the fusion state of the mitochondria at resting [Ca2+]c but promoted mitochondrial fragmentation at high [Ca2+]c. These effects of Miro on mitochondrial morphology seem to involve Drp1 suppression and activation, respectively. In primary neurons, Miro also caused an increase in dendritic mitochondrial mass and enhanced mitochondrial calcium signaling. Thus, Miro proteins serve as a [Ca2+]c-sensitive switch and bifunctional regulator for both the motility and fusion-fission dynamics of the mitochondria.
Mitochondria are dynamically distributed in the cell to optimize the utilization of a limited amount of discrete organelles (1, 2). Cytoskeletal tracks and motor proteins have been identified for mitochondrial transport (3–6) but the signaling mechanisms that control motility and positioning remain to be solved. TNFα-and NGF receptor-activated pathways and plasma membrane phospholipids have been implicated in the control of mitochondrial movements (7–10). Recently, it has been shown in several cell types that physiological rises of [Ca2+]c arrest mitochondrial motility (11–14), effectively creating a homeostatic feedback circuit that positions these organelles near Ca2+ sources (12) enhancing Ca2+ buffering and ATP production where demand is high (15–18). Notably, in rat cortical neurons both the presence (11) and absence (19) of sensitivity to Ca2+ was described for mitochondrial motility. Although the calcium signal can stimulate the formation of multiple factors that affect mitochondrial motility [e.g., adenine nucleotides (13, 20)], Ca2+ by itself can control movement activity (12). Ca2+ does not seem to activate Ca2+/calmodulin-dependent kinases or the Ca2+-dependent protein phosphatase and does not seem to target directly the microtubular motors, dynein and kinesin to establish control over mitochondrial motility (12). Thus, we have proposed that a distinct Ca2+ sensor molecule is required to translate the Ca2+ signal for the microtubular motor proteins (12).
A subfamily of the Ras GTPases (Miro 1 and 2 proteins) is localized at the outer mitochondrial membrane (OMM) and has two potential Ca2+ binding domains, so called EF-hands (21). Both proteins consist of 618 amino acid residues and were found to be 60% identical (21). Miro is present in yeast (Gem1p) (22), Drosophila (dMiro) (23), and mammalian cells as well (21). Miro interacts with the kinesin-binding proteins, GRIF-1/Milton 2 and OIP106/Milton 1, suggesting that Miro forms a link between the mitochondria and the trafficking apparatus of the microtubules (24, 25). Both the GTPase domains and EF-hand motifs of Miro are exposed to the cytoplasm and are required for yeast Miro function in mitochondrial morphology (22). Here, we have tested the hypothesis that Miro serves as a Ca2+-sensitive regulator of mitochondrial motility and fusion-fission dynamics.
Results
Miro Proteins Support Mitochondrial Motility Along the Microtubules.
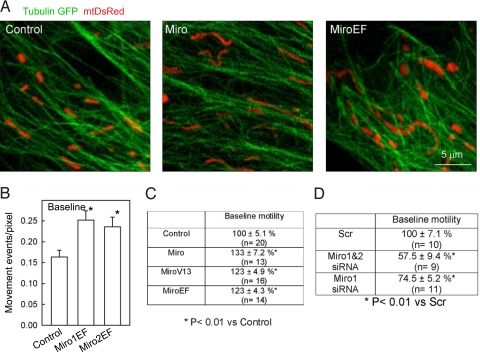
First we evaluated the effect of Miro1&2 (Miro) and Miro1&2-EF-hand mutants (24)(MiroEF) on basal mitochondrial movement in resting H9c2 cells. Cells were transiently transfected with Miro and MiroEF cDNAs and (i) the spatial relationship between mitochondria and microtubules as well as (ii) basal mitochondrial motility was measured (12). Mitochondria were aligned with and moved along the microtubules in mock-transfected cells as described before (12) and similar relation between mitochondria and microtubules was observed in Miro- and MiroEF-transfected cells (Fig. 1A). However, when [Ca2+]c was kept at resting level (<100 nM), cells overexpressing Miro or MiroEF or a constitutively active form of Miro1&2 (MiroV13) (24) displayed an increase in mitochondrial movements (Fig. 1 B and C). Furthermore, Miro-depleted cells (Miro1 or Miro1&2 siRNA) showed a decrease in mitochondrial motility (Fig. 1D). Because Miro 1 and 2 show structural homology including their GTPase and EF-hand domains (21), exerted similar effects on mitochondrial motility (Fig. 1B) and display similar mitochondrial distribution in H9c2 cells (Fig. S1), indicating that these proteins may substitute each other, in many subsequent experiments the expression of both Miro1 and 2 was altered. The overexpression and silencing of Miro1 and Miro2 was confirmed by both anti-Miro1 and anti-Miro2-specific antibodies in western blotting (Fig. S2) and in immunocytochemistry (data not shown). For the Myc tagged Miro constructs anti-Myc antibodies were also used (SI 1 and 2). These results indicate that the Miro proteins facilitate the mitochondrial movements along the microtubules in low [Ca2+]c environment and this effect does not require the EF-hand Ca2+-binding site.
Fig. 1.
Miro promotes mitochondrial movements along microtubules at basal [Ca2+]c in H9c2 cells. (A) Confocal images of TubulinGFP and mtDsRed taken in control (Left), Miro1&2 (Middle), and Miro1&2EF-expressing cells (Right). (B) Actual basal mitochondrial motility values (Left) in cells transfected with mitoYFP alone or mitoYFP+Miro1EF or mitoYFP+Miro2EF. *, P < 0.01. (C) Summarized data of baseline mitochondrial motility (Mito-motility) in cells transfected with mitoYFP (Control; n = 20 cells), mitoYFP+Miro (n = 13), mitoYFP+MiroV13 (n = 16), and mitoYFP+MiroEF (n = 14). Before imaging, cells were pretreated with thapsigargin (2 μM) in a Ca2+-free ECM for 7 min to eliminate both intracellular Ca2+ mobilization and Ca2+ entry and in turn to stabilize [Ca2+]c under the basal level (≈40 nM). Data are shown as % of control. (D) Summarized data of baseline Mito-motilities in MitoYFP expressing cells transfected with either scrambled control (Scr; n = 10), Miro1-siRNA (Miro1; n = 11), or Miro1&2-siRNA (Miro1&2; n = 9). The mitochondrial movements were quantitated in a CCD time-series recorded at the resting [Ca2+]c (≈40 nM). Data present % of Scr.
Miro Dependence of the [Ca2+]c-Induced Mitochondrial Motility Inhibition.
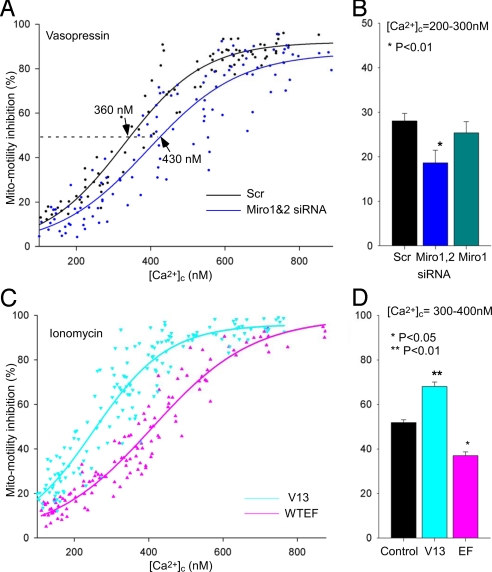
Because we have previously shown that an increase in [Ca2+]c leads to inhibition of basal mitochondrial motility (12), next we tested the effect of stimulation with vasopressin (VP), a Ca2+ mobilizing hormone on mitochondrial movements in control, Miro- and MiroEF-overexpressing cells. Strikingly, whereas in controls cells the VP (100 nM)-induced Ca2+ signal led to substantial inhibition of mitochondrial movements (by 68 ± 4%), in MiroEF expressing cells a significant part of this inhibition was lost (inhibition by 40 ± 4% and 51 ± 4% in Miro1EF and Miro2EF expressing cells, respectively, Fig. 2A). Miro1EF-and Miro2EF-expression did not affect significantly either the resting [Ca2+]c or the VP-induced [Ca2+]c spike, although it showed a tendency to suppress the latter one (P = 0.06 and 0.05, respectively, Fig. 2A). The reversal of the inhibition of mitochondrial movements was more pronounced at lower agonist concentrations (e.g., 0.25 nM VP evoked a [Ca2+]c rise but failed to inhibit mitochondrial movements in MiroEF expressing cells, Fig. 2B). Thus, next mitochondrial motility inhibition was plotted as a function of [Ca2+]c measured after the application of a range of VP doses. The extent of inhibition of mitochondrial movements showed a sigmoid function of [Ca2+]c reached during VP stimulation (Fig. 2C), and 50% inhibition was observed at 380 nM [Ca2+]c in control cells. MiroEF overexpression caused a right shift of Ca2+ sensitivity curve, indicating decreased sensitivity (Fig. 2C), whereas in Miro-V13 (Fig. 2C) and in Miro-overexpressing cells (data not shown) showed increased sensitivity. Indeed, in the 300–400 nM [Ca2+]c range, the decrease in mitochondrial motility was 45.6 ± 3.6% in the control, 70.0 ± 0.3% (P < 0.01) in MiroV13-expressing, 54.9 ± 3.1% (P = 0.05) in Miro-overexpressing and 34.5 ± 3.6% (P < 0.05) in MiroEF-expressing cells (n = 10–13) (Fig. 2D). Moreover, similarly to the expression of the MiroEF mutant, depletion of Miro by siRNA also attenuated the Ca2+-dependent mitochondrial motility inhibition in VP-stimulated cells (Fig. 3 A and B). The [Ca2+]c versus the motility inhibition relationship in the different Miro expression conditions indicated no change in cooperativity (Hill slopes were between 3.4–4.6). These data provide the first evidence that the Miro may be involved in the motility inhibition during the Ca2+ signal.
Fig. 2.
MiroEF suppresses and MiroV13 promotes the VP-induced Mito-motility inhibition. (A) Actual mitochondrial motility values (Left) and [Ca2+]c (Right) during stimulation with VP (100 nM) in cells transfected with mitoYFP alone or mitoYFP+Miro1EF or mitoYFP+Miro2EF. For VP the lowest motility and highest [Ca2+]c were calculated (n = 27–33). *, P < 0.01. For motility the baseline values were shown in Fig. 1B. (B) Simultaneous measurements of Mito-motility and [Ca2+]c in H9c2 cells expressing mitoYFP (Upper) and coexpressing mitoYFP with MiroEF (Lower). Left images show mitoYFP fluorescence (grayscale); Middle and Right images show the sites of mitochondrial movement calculated by subtraction of sequential images (ΔF: change in fluorescence between the two time points; red for positive changes, green for negative changes) before and after application of 0.25 nM VP in each condition. Graphs show both [Ca2+]c (Upper) and motility inhibition in cells expressing mitoYFP (Control; black) or coexpressing mitoYFP and MiroEF (red). (C) Dose-response relationships between Mito-motility and [Ca2+]c. Vector (black, n = 58), MiroEF (pink, n = 24) and MiroV13 (cyan, n = 27) expressing cells. Mito-motility decrease during VP stimulation was normalized to baseline motility in each cell (% inhibition) and is plotted against the corresponding [Ca2+]c elevations. The IC50 and Hillslope values were in each condition: Control; 380 ± 10 nM and −4.6, V13; 300 ± 20 nM and −4.4, EF; 450 ± 20 nM and −4.5. (D) Mito-motility inhibition at the range of [Ca2+]c = 300–400 nM were calculated in Control (n = 13), V13 (n = 10), and EF (n = 11).
Fig. 3.
Effect of Miro-silencing on the VP-induced and V13, EF overexpression on Ca2+-induced mitochondrial motility inhibition (A) Dose-response relationship between [Ca2+]c and motility inhibition in Miro1&2 siRNA (blue, n = 51) and scrambled control (Scr; black, n = 41) transfected cells. The IC50 and Hillslope values were 360 ± 10 nM and −4.2 in Scr and 430 ± 20 nM and −3.6 in Miro1&2 siRNA expressing cells. (B) Summarized data of mitochondrial motility inhibition in scrambled control (n = 17), Miro1siRNA (n = 7), and Miro1&2siRNA transfected cells (n = 19) in the range of [Ca2+]c = 200–300 nM. *, P < 0.01 vs. Scr. (C) Dose-response relationship between [Ca2+]c and motility in MiroEF (pink, n = 26) and MiroV13 (cyan, n = 31) expressing cells. For the quantitative estimation of [Ca2+]c-dependent mitochondrial motility inhibition, [Ca2+]c and motility were measured in cells that were incubated in a Ca2+-free buffer supplemented with EGTA (2 mM), thapsigargin (2 μM), an inhibitor of the sarco-endoplasmic reticulum Ca2+ pump, and ionomycin (10 μM) to ensure rapid equilibration of the cytosol with the extracellular [Ca2+], and then varying amounts of CaCl2 were added. (D) Summarized data of mitochondrial motility inhibitions in Control (n = 54), V13 (n = 36), and EF expressing cells (n = 29) at the range of [Ca2+]c = 300–400 nM.
To further clarify whether Miro played a role downstream to the [Ca2+]c elevation, [Ca2+]c and motility were measured in cells that were incubated in a Ca2+-free buffer supplemented with EGTA, thapsigargin, an inhibitor of the sarco-endoplasmic reticulum Ca2+ pump and ionomycin, a Ca2+ ionophore to ensure rapid equilibration of the cytosol with the extracellular [Ca2+], and then varying amounts of CaCl2 were added to set [Ca2+]c at different levels (Fig. 3 C and D). The data confirmed that the [Ca2+]c vs. motility relationships were left shifted in MiroV13-expressing, while showing a right shift in MiroEF-expressing cells (Fig. 3C). Again, in the 300–400 nM [Ca2+]c range, the decrease in mitochondrial motility was 56.7 ± 3.2% in the control, 72.2 ± 3.1% (P < 0.01) in MiroV13-expressing, and 42.8 ± 2.5% (P < 0.05) in MiroEF-expressing cells (n = 29–54). Importantly, using this experimental model we have also showed that both Miro1&2 N18 (a dominant negative lack of function GTPase mutant of Miro) and ΔTM (a mutant that lacks its transmembrane and mitochondrial targeting domain) attenuated [Ca2+]c-induced motility inhibition [the inhibition was 49.4 ± 2.7% in the control, whereas 33.9 ± 3.9% (P < 0.01) in N18-expressing and 33.0 ± 3.5% (P < 0.01) in ΔTM-expressing cells (n = 43–51cells)]. Thus, according to the results obtained in cells that were either stimulated with a Ca2+-mobilizing hormone (Figs. 2 and 3 A and B) or were directly perfused with Ca2+ (Fig. 3 C and D) we concluded that (i) the Miro proteins are important for Ca2+-induced movement inhibition of mitochondria and (ii) this effect requires both intact EF-hand and GTPase domains. Our results do not exclude the possibility that other (possibly lower affinity) Ca2+ sensors are involved in the process, because Miro knockdown caused only a shift in the Ca2+ dose-response with only a small suppression of the maximal motility inhibition. Alternatively, the residual Miro activity (e.g., the 25–30% remaining Miro expression in the silencing studies, or endogenous Miro in MiroEF expressing cells) was sufficient to mediate the motility inhibition when saturated by Ca2+.
Miro-Dependent Changes in Mitochondrial Morphology in H9c2 Cells.
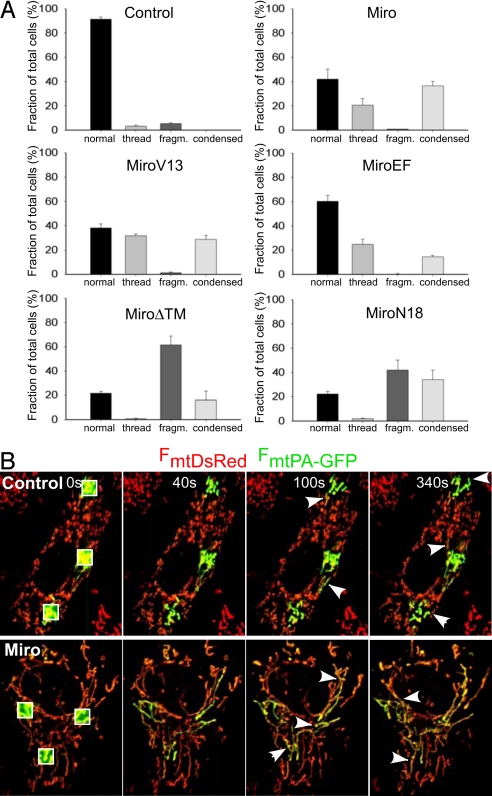
Previous studies have described Miro expression-dependent changes in mitochondrial morphology (22, 24, 25), which may be linked to the altered motility. In addition, the present results on the Ca2+ sensitivity of Miro activity necessitated further studies of the Miro dependence of mitochondrial morphology determined by the balance between fusion and fission. In H9c2 cells, Miro1-GFP colocalized with the mitochondria and induced mitochondrial thread formation and condensation (Fig. S1), as previously described in other cell types (24). Myc-tagged wild type Miro1&2, Miro1&2-V13, and Miro1&2EF also showed similar mitochondrial distribution (Fig. S1) and evoked mitochondrial thread formation and condensation (Fig. 4A). Notably, immunostaining did not show a difference in Miro overexpression levels between cells showing thread formation and condensation, and the ratio of the cells showing thread formation and condensation did not change from 24 h to 48 h overexpression (data not shown). On the other hand, dominant-negative Miro constructs (N18, ΔTM) and Miro knockdown (data not shown, n = 3) caused mitochondrial fragmentation and condensation (Fig. 4A). Silencing of Miro also evoked mitochondrial fragmentation (data not shown, n = 3). Thus, we confirmed that the connectivity state and thus the length of the mitochondria is directly proportional to the availability of Miro at resting [Ca2+]c, depending on its intact GTPase domain but not requiring the EF hands.
Fig. 4.
Mitochondrial morphology and fusion-fission activity in H9c2 cells overexpressing Miro constructs. (A) Mitochondrial patterns were classified (normal, thread, fragmented, and condensed) (24). H9c2 cells were transfected mitoYFP (Control), or cotransfected mitoYFP with Miro DNAs (WT; Miro1&2, V13; Miro1&2-V13, EF; Miro1&2-EF, ΔTM; Miro1&2-ΔTM, N18; Miro1&2-N18). The Myc-tagged Miro proteins were visualized by using a Myc-specific monoclonal antibody. For each experiment at least 300 cells were scored. (B) Visualization of the mitochondrial connectivity and fusion-fission activity in real time. Labeling of three subsets of mitochondria by photoactivation of PA-GFP in an H9c2 cell expressing mtPA-GFP, mtDsRed and Miro (lower row of images), MiroEF (data not shown) or pcDNA (Control, upper row of images). Irreversible photoactivation was achieved at 0 s as described in Materials and Methods. The rapid spreading (40-s images) of the mtPA-GFP fluorescence (green) indicates the matrix connectivity in the Miro and Control mitochondria. Images obtained at 100 s and 300 s illustrate several fusion events in Miro expressing cells and several fission events in the control cells.
Disruption of the dynein motor complex in HeLa cells has been shown to cause mitochondrial condensation around the nucleus and mitochondrial elongation that was attributed to Drp1 inhibition (26), prompting us to determine whether the Miro effects on mitochondrial structure were due to increased fusion or decreased fission activity. First, we showed that overexpression of a dominant negative form of Drp1 (Drp1K38A) prevented the mitochondrial fragmentation induced by Miro1&2-N18 or ΔTM in H9c2 cells (Fig. S3), demonstrating that an intact fission machinery is required for the effect. Then we directly assessed the fusion/fission activity in Miro and MiroEF overexpressing cells, by cotransfecting them with a matrix-targeted photoactivated GFP (mtPA-GFP) construct (Fig. 4B) that allowed quantification of the number and duration of fusion events in a subset of mitochondria following its photoactivation. Whereas in control cells PA-GFP fluorescence remained confined to the area of the photoactivation, it rapidly filled large complex networks in Miro- and MiroEF-overexpressing cells (Fig. 4B), confirming the increased continuity of the matrix space in the elongated mitochondria. However, the increased connectivity of mitochondria in those cells was not due to the increased number of fusion events, but rather to an increased duration of the transient fusion events [time elapsed between PA-GFP transfer and apparent separation of the organelles for control, Miro and MiroEF were: 49 ± 5, 75 ± 8, and 96 ± 13 s, respectively; n = 64 − 120]. Because the duration of fusion events is determined by the occurrence of subsequent fission, our results indicated a decrease in the incidence of mitochondrial fission. Thus, Miro appears to exert its effect on mitochondrial morphology at least partially by suppressing Drp1-mediated mitochondrial fission, although recruitment of fusion promoting cytoplasmic proteins may also be affected. Finally, our results also showed that long mitochondria show less movement activity than the short ones in H9c2 cells (X. Liu and G.H., unpublished work), indicating that the Miro effect on mitochondrial fusion-fission cannot account for the Miro effect on motility.
Miro Modulates Mitochondrial Morphology in Resting Neurons.
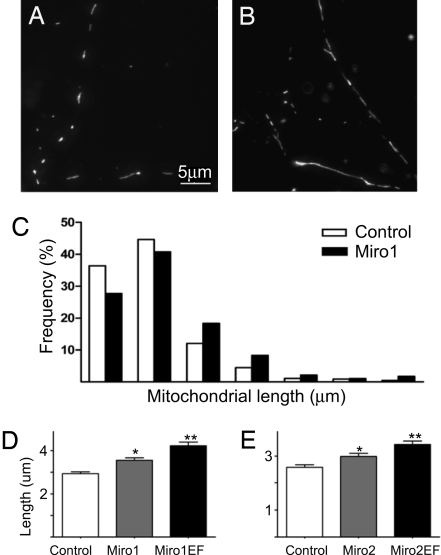
Our studies provided evidence that Miro proteins are implicated in increasing mitochondrial connectivity and in Ca2+-induced mitochondrial arrest in cultured cell lines, but these models did not allow us to study whether the protein and its EF-hand domain is also involved in distribution of mitochondria in distinct cellular domains. Thus, in further studies we used primary cortical neurons, where distinct signaling domains exist in the cell body, axon and dendrites and the mitochondrial distribution among these compartments is precisely controlled. First, primary cortical neurons were transfected with different Miro constructs together with mitochondrially targeted DsRed2 (mtDsRed) and GFP, and neuronal processes and cell bodies were analyzed for mitochondrial length and occupancy (Fig. 5 A and B). Mitochondria in neuronal processes were mostly tubular in shape and highly variable in size consistent with previous reports (11, 27). To analyze mitochondrial distribution in processes pyramidal neurons were chosen and their dendrite regions ≈10–100 μm away from the soma where mitochondria are mostly separated from each other. Compared with controls, Miro1-overexpressing neurons showed a right-shifted pattern in frequency distribution of mitochondrial length (Fig. 5C). Indeed, the mean length of mitochondria in both Miro1 and Miro2-overexpressing neurons was ≈20% higher than in the corresponding control groups. Importantly, Miro1EF and Miro2EF overexpression caused even larger increase in mitochondrial length in the processes (Fig. 5 D and E), indicating that dendritic Ca2+ levels under basal neuronal activity exert an inhibitory effect on Miro-dependent mitochondrial fusion. Moreover, the mitochondrial index [ratio of total mitochondrial length in the dendrite to dendritic length in a given dendritic segment (27)] was also significantly increased in Miro1 (2)-overexpressing group (Fig. S4 A and E), and showed a tendency to increase in MiroEF cells (Fig. S4 B and F). Because the number of mitochondria per 10 μm of dendrite was unchanged (data not shown), the increase in mitochondrial index reflected the presence of elongated mitochondria in the dendrites. The length of individual mitochondria in neuronal cell bodies could not be quantified because of the complexity of mitochondrial network in this region, but the total area occupied by mitochondria in the cell body was unchanged in Miro-overexpressing neurons (data not shown).
Fig. 5.
Mitochondrial distribution and length in Miro overexpressing neurons. (A–E) mtDsRed-labeled mitochondria (grayscale) in neuronal processes of control (A) and Miro1-over-expressing (B) neurons. Wide-field fluorescence micrograph shows the best focus slice of z-stack. Panel C shows the different frequency distribution of mitochondrial lengths in the Miro1 group, compared with the control. *, P < 0.001 vs. control, **, P < 0.001 vs. control and P < 0.05 vs. Miro1 (2) wild-type group (Dunn's multiple comparison test).
Repetitive Depolarization-Induced Changes in Mitochondrial Length Are Dependent on Miro's Ca2+-Binding Activity in Neurons.
Our findings indicated that Miro increases mitochondrial mass in the dendrites of resting neurons, which process is inhibited by the presence of EF-hand domains. To further evaluate the role of Ca2+ occupancy of Miro EF-hands in regulation of mitochondrial dynamics we used a model of repeated depolarizations, which is associated with transient [Ca2+]c increase, previously shown to evoke changes in neuronal plasticity (28) and mitochondrial redistribution (27). Mitochondria in neuronal processes were examined 1 h after four repetitive depolarizations with 90 mM KCl and compared with a parallel set of nonstimulated neurons. In control cultures no changes in mitochondrial morphology, index, or number were observed (Fig. S4A). In contrast, in Miro1-overexpressing neurons, mitochondria became shorter, dendritic mitochondrial index slightly decreased (Fig. S4 B and C) and the number of mitochondria was decreased (1.33 ± 0.36 mitochondria per 10 μm of dendrite in control, 1.26 ± 0.26 in Miro1-overexpressing versus 1.06 ± 0.29 in Miro1 after stimulations, P < 0.05, n ≥ 20), indicating mitochondrial redistribution after repetitive stimulation. A similar tendency was observed in the experiments with Miro1V13 (Fig. S4 C and D) and with Miro2 (Fig. S4 E and F). Most importantly, EF-hand mutations repressed these effects, and over-expression of Miro1EF and Miro2EF even caused a small but significant increase in mitochondrial length and number in the processes after prestimulation (Fig. S4). Thus, we concluded that overexpression of Miro, bearing intact Ca2+ binding EF-hand domains, mediates Ca2+-dependent fragmentation of dendritic mitochondria, which leads to their elimination or repositioning into the cell body. Abolition of Ca2+ binding by EF-hand mutations reversed the effect, probably by unmasking an opposing, independent Ca2+ mediated process. Importantly, endogenous Miro levels under physiological Ca2+ signaling events were not sufficient to support Ca2+-induced fragmentation and redistribution.
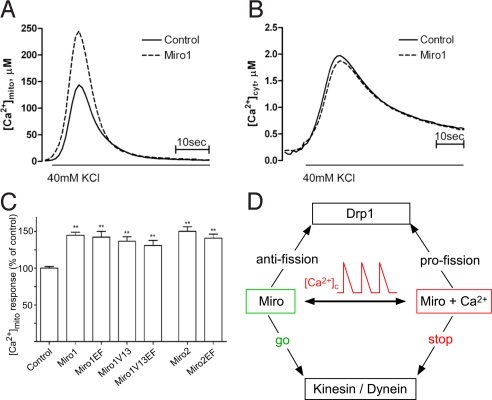
Effect of Miro Proteins on Mitochondrial Ca2+ Uptake in Neurons.
Finally, to test the consequences on physiological Ca2+ signals of Miro-dependent mitochondrial redistribution, evidence was sought whether Miro affects mitochondrial Ca2+ uptake. We cotransfected neurons with mitochondrially targeted aequorin (29) and Miro1, and induced plasma membrane depolarization by perfusing neurons with 40 mM KCl containing Krebs-Ringer buffer. The peak [Ca2+]m response was significantly increased in Miro1-overexpressing cells (143.6 ± 11.2 μM in control versus 245 ± 8.9 μM in Miro1-overexpressing cells, P < 0.001, Fig. 6A). The increasein mitochondrial Ca2+ uptake was not a consequence of changes in [Ca2+]c signaling because the peak of [Ca2+]c response was unchanged in Miro1 overexpressing neurons as measured in cytosolic aequorin expressing cells (Fig. 6B). Similar results were obtained by overexpressing either Miro2 or Miro1/V13 (Fig. 6C). When MiroEF was overexpressed in neuronal cultures, similarly to wild-type Miro overexpression, MiroEF increased [Ca2+]m upon plasma-membrane depolarization (Fig. 6C). Thus, Miro mediated redistribution and fusion of mitochondria increased their ability to accumulate Ca2+ independently of the Ca2+ binding activity of the protein.
Fig. 6.
Effect of Miro on [Ca2+]c and [Ca2+]m signaling in neurons. (A and B) Miro1 over-expression increases 40 mM KCl-induced [Ca2+]m uptake. Cortical cultures were cotransfected with aequorin targeted to mitochondria (A) or cytosol (B) and Miro1WT and Ca2+ uptake was measured luminometrically as described in Materials and Methods. The [Ca2+]c peak was 1.97 ± 0.16 μM in control versus 1.87 ± 0.08 μM in Miro1-overexpressing neurons (n = 8, P = 0.579). (C) Changes in mitochondrial Ca2+ uptake induced by overexpression of Miro1 and Miro2 mutants. Data presented as % of control, from 3–6 independent experiment (n ≥ 15), **, P < 0.001 vs. control group. (D) Scheme illustrating the bidirectional Ca2+-dependent control of mitochondrial motility and fusion-fission dynamics by Miro proteins.
Discussion
This work revealed that Miro proteins mediate multiple effects of [Ca2+]c signals on mitochondrial motility, fusion-fission dynamics and function (summarized in Fig. 6D). Miro, in resting nonpolarized cells, facilitates mitochondrial movements presumably by optimizing the anchorage of the kinesin/dynein motor complexes to the mitochondrial surface through GRIF-1/Milton 2 and OIP106/Milton 1 (24, 25). In neurons, this mechanism may be involved in directional transport of mitochondria into dendrites. However, exposure of the Miro's EF-hand to a [Ca2+]c rise relays a stop signal to the motors, and redirects dendritic transport of mitochondria in neurons. Importantly, this regulation takes place in the physiological range of global [Ca2+]c. Miro seems to be competent to confer the Ca2+ effect only if its GTPase domain is intact. Down-regulation of Miro resulted in a rightward shift in the Ca2+ dose-response for mitochondrial motility inhibition, whereas up-regulation exerted an opposite effect, indicating that a direct relationship exists between the availability of Miro and Ca2+ sensitivity. Thus, Miro is a Ca2+-sensing element of the molecular complex controlling mitochondrial motility. Miro may collaborate with another Ca2+ sensor that can mediate the high [Ca2+]c-induced maximal motility inhibition in Miro-depleted cells. Immunocytochemistry data showed fairly homogeneous distribution of Miro proteins in the mitochondria, indicating that Miro can mediate the arrest of the mitochondrial movements wherever [Ca2+]c rises. Because the presence of Miro is required for both mitochondrial motility at low [Ca2+]c and for mitochondrial arrest at sites of a [Ca2+]c elevation, it emerges as a key molecule for the homeostatic control of the mitochondrial distribution that allows strategic positioning of mitochondria where for example, ATP supply or Ca2+ buffering is needed. This represents a novel mechanism for remodeling of the Ca2+ signaling system (30).
At resting low [Ca2+]c levels, when the EF-hands are presumably not occupied by Ca2+ Miro also facilitates the formation of elongated mitochondria. Real time analysis of mitochondrial fusion/fission and the use of a dominant negative Drp1 construct provided evidence that (i) the Miro-mediated elongation of mitochondria involved suppression of Drp1-mediated fission and (ii) the shortening after Miro depletion is the result of Drp1-mediated fission, respectively. Notably, Miro-overexpressing cells lack mitochondrial necklaces that are present when a dominant negative Drp1 was expressed, suggesting that the Miro effect is not restricted to Drp1 inhibition. Collectively, the effects on mitochondrial motility and morphology show a unique capacity of Miro to maintain the mobility of the organelles while their size is increasing. However, extreme mitochondrial elongation may be accompanied by condensation because elongated organelles might present oversized cargo for the transport machinery (31). In contrast to resting conditions, repetitive or prolonged high [Ca2+]c signals trigger Miro-dependent shortening, mediated by the EF-hand domains, as shown in neurons expressing supraphysiological levels of the Miro proteins.
Finally, mitochondrial function can be modulated through the fusion state of the mitochondria and the activity of fusion-fission proteins (32, 33). Recently, docking of mitochondria by syntaphilin in the axons has been shown to affect local calcium signaling (34). The present study also uncovered that Miro supports distribution of mitochondria to the dendrites close to the Ca2+ entry sites and promotes the calcium signal propagation to the mitochondria. The latter effect does not seem to result from an increase in Ca2+ entry or mobilization but may be due to the mitochondrial distribution close to the Ca2+ source or to facilitation of the mitochondrial Ca2+ uniport. Increased Ca2+ transfer by the mitochondria provides a means to enhance the mitochondrial Ca2+ buffering and to stimulate the Ca2+-dependent reactions in mitochondrial energy production. Thus, Miro proteins both mediate the effects and regulate calcium signaling to coordinate the mitochondrial dynamics and contribution to cell function.
Materials and Methods
Detailed methods are described in SI Methods. Constructs of Miro and antibodies have been described previously (21, 24, 29). H9c2 cells were cultured and were cotransfected with Miro DNAs and mtYFP employing electroporation or with siRNAs with GeneSilencer (Genlantis). Primary cultures of cortical neurons were prepared from the cortices of neonatal Wistar rats, were grown in Neurobasal A medium, were transfected using Lipofectamine 2000TM with mtDsRed, GFP or aequorin and imaging and aequorin measurements were performed 6–10 days later (29). Simultaneous measurements of [Ca2+]c with mitochondrial motility in H9c2 cells were carried out by using a fluorescence or a confocal imaging setup as described before (12). For PA-GFP photoactivation three 25-μm2 areas were chosen per cell and illuminated with maximum power 442-nm excitation. PA-GFP fluorescence was then monitored using 488-nm excitation. The change in mitochondrial motility was evaluated as described previously (12). The length of processes and mitochondria in the neuronal cultures was measured manually with the aid of MetaMorph software.
Supplementary Material
Acknowledgments.
This work was supported by grants from the Estonian Science Foundation Grant 7175 and European Community Contract MTKD-CT-2004-517176 (to D.S.), Swedish Cancer Society and the Swedish Research Council (to P.A.), the Italian Association for Cancer Research, Telethon, local funds from the University of Ferrara, the Italian University Ministry, the EU (fondi strutturali Obiettivo 2), the PRRIITT program of the Emilia Romagna Region, and the Italian Space Agency and by National Institutes of Health Grants 1P01AG025532–01A1 (to R.R.) and DK51526 and GM59419 (to G.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808953105/DCSupplemental.
References
- 1.Rice SE, Gelfand VI. Paradigm lost: Milton connects kinesin heavy chain to miro on mitochondria. J Cell Biol. 2006;173:459–461. doi: 10.1083/jcb.200604071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reynolds IJ, Rintoul GL. Mitochondrial stop and go: Signals that regulate organelle movement. Sci STKE. 2004;2004:PE46. doi: 10.1126/stke.2512004pe46. [DOI] [PubMed] [Google Scholar]
- 3.Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vale RD. The molecular motor toolbox for intracellular transport. Cell. 2003;112:467–480. doi: 10.1016/s0092-8674(03)00111-9. [DOI] [PubMed] [Google Scholar]
- 5.Frederick RL, Shaw JM. Moving mitochondria: Establishing distribution of an essential organelle. Traffic. 2007;8:1668–1675. doi: 10.1111/j.1600-0854.2007.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anesti V, Scorrano L. The relationship between mitochondrial shape and function and the cytoskeleton. Biochim Biophys Acta. 2006;1757:692–699. doi: 10.1016/j.bbabio.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 7.De Vos K, et al. Tumor necrosis factor induces hyperphosphorylation of kinesin light chain and inhibits kinesin-mediated transport of mitochondria. J Cell Biol. 2000;149:1207–1214. doi: 10.1083/jcb.149.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chada SR, Hollenbeck PJ. Nerve growth factor signaling regulates motility and docking of axonal mitochondria. Curr Biol. 2004;14:1272–1276. doi: 10.1016/j.cub.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 9.De Vos KJ, Sable J, Miller KE, Sheetz MP. Expression of phosphatidylinositol (4,5) bisphosphate-specific pleckstrin homology domains alters direction but not the level of axonal transport of mitochondria. Mol Biol Cell. 2003;14:3636–3649. doi: 10.1091/mbc.E02-10-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minin AA, Kulik AV, Gyoeva FK, Li Y, Goshima G, Gelfand VI. Regulation of mitochondria distribution by RhoA and formins. J Cell Sci. 2006;119:650–670. doi: 10.1242/jcs.02762. [DOI] [PubMed] [Google Scholar]
- 11.Rintoul GL, Filiano AJ, Brocard JB, Kress GJ, Reynolds IJ. Glutamate decreases mitochondrial size and movement in primary forebrain neurons. J Neurosci. 2003;23:7881–7888. doi: 10.1523/JNEUROSCI.23-21-07881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yi M, Weaver D, Hajnoczky G. Control of mitochondrial motility and distribution by the calcium signal: A homeostatic circuit. J Cell Biol. 2004;167:661–672. doi: 10.1083/jcb.200406038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brough D, Schell MJ, Irvine RF. Agonist-induced regulation of mitochondrial and endoplasmic reticulum motility. Biochem J. 2005;392:291–297. doi: 10.1042/BJ20050738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quintana A, Schwarz EC, Schwindling C, Lipp P, Kaestner L, Hoth M. Sustained activity of CRAC channels requires translocation of mitochondria to the plasma membrane. J Biol Chem. 2006;281:40302–40309. doi: 10.1074/jbc.M607896200. [DOI] [PubMed] [Google Scholar]
- 15.Jouaville LS, Ichas F, Holmuhamedov EL, Camacho P, Lechleiter JD. Synchronization of calcium waves by mitochondrial substrates in Xenopus laevis oocytes. Nature. 1995;377:438–441. doi: 10.1038/377438a0. [DOI] [PubMed] [Google Scholar]
- 16.Hoth M, Button DC, Lewis RS. Mitochondrial control of calcium-channel gating: A mechanism for sustained signaling and transcriptional activation in T lymphocytes. Proc Natl Acad Sci USA. 2000;97:10607–10612. doi: 10.1073/pnas.180143997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park MK, Ashby MC, Erdemli G, Petersen OH, Tepikin AV. Perinuclear, perigranular and sub-plasmalemmal mitochondria have distinct functions in the regulation of cellular calcium transport. EMBO J. 2001;20:1863–1874. doi: 10.1093/emboj/20.8.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malli R, et al. Sustained Ca2+ transfer across mitochondria is Essential for mitochondrial Ca2+ buffering, sore-operated Ca2+ entry, and Ca2+ store refilling. J Biol Chem. 2003;278:44769–44779. doi: 10.1074/jbc.M302511200. [DOI] [PubMed] [Google Scholar]
- 19.Beltran-Parrazal L, Lopez-Valdez H, Brennan KC, Diaz-Munoz M, Vellis JD, Charles AC. Mitochondrial transport in processes of cortical neurons is independent of intracellular calcium. Am J Physiol Cell Physiol. 2006;291:C1193–1197. doi: 10.1152/ajpcell.00230.2006. [DOI] [PubMed] [Google Scholar]
- 20.Mironov SL. Spontaneous and evoked neuronal activities regulate movements of single neuronal mitochondria. Synapse. 2006;59:403–411. doi: 10.1002/syn.20256. [DOI] [PubMed] [Google Scholar]
- 21.Fransson A, Ruusala A, Aspenstrom P. Atypical Rho GTPases have roles in mitochondrial homeostasis and apoptosis. J Biol Chem. 2003;278:6495–6502. doi: 10.1074/jbc.M208609200. [DOI] [PubMed] [Google Scholar]
- 22.Frederick RL, McCaffery JM, Cunningham KW, Okamoto K, Shaw JM. Yeast Miro GTPase, Gem1p, regulates mitochondrial morphology via a novel pathway. J Cell Biol. 2004;167:87–98. doi: 10.1083/jcb.200405100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo X, et al. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005;47:379–393. doi: 10.1016/j.neuron.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 24.Fransson S, Ruusala A, Aspenstrom P. The atypical Rho GTPases Miro-1 and Miro-2 have essential roles in mitochondrial trafficking. Biochem Biophys Res Commun. 2006;344:500–510. doi: 10.1016/j.bbrc.2006.03.163. [DOI] [PubMed] [Google Scholar]
- 25.Glater EE, Megeath LJ, Stowers RS, Schwarz TL. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol. 2006;173:545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varadi A, Cirulli V, Rutter GA. Mitochondrial localization as a determinant of capacitative Ca2+ entry in HeLa cells. Cell Calcium. 2004;36:499–508. doi: 10.1016/j.ceca.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Wu GY, Deisseroth K, Tsien RW. Spaced stimuli stabilize MAPK pathway activation and its effects on dendritic morphology. Nat Neurosci. 2001;4:151–158. doi: 10.1038/83976. [DOI] [PubMed] [Google Scholar]
- 29.Chiesa A, et al. Recombinant aequorin and green fluorescent protein as valuable tools in the study of cell signalling. Biochem J. 2001;355:1–12. doi: 10.1042/0264-6021:3550001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 31.Campello S, Lacalle RA, Bettella M, Manes S, Scorrano L, Viola A. Orchestration of lymphocyte chemotaxis by mitochondrial dynamics. J Exp Med. 2006;203:2879–2886. doi: 10.1084/jem.20061877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Youle RJ, Karbowski M. Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol. 2005;6:657–663. doi: 10.1038/nrm1697. [DOI] [PubMed] [Google Scholar]
- 33.Szabadkai G, et al. Mitochondrial dynamics and Ca2+ signaling. Biochim Biophys Acta. 2006;1763:442–449. doi: 10.1016/j.bbamcr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Kang JS, et al. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell. 2008;132:137–148. doi: 10.1016/j.cell.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.